AI-Enabled Electrocardiogram Analysis for Disease Diagnosis
Abstract
:1. Introduction
1.1. Current Literature Survey
- Is ECG the main or only vital sign considered in the literature survey?
- Is artificial intelligence the focus technology for interpreting ECG?
- Were there significant challenges and future directions discussed?
1.2. Survey Goal
- What is the preprocessing required for ECG signal before using it with AI techniques?
- What are the ML/DL techniques applied in ECG-based heart diseases and other health issues?
- Are there any limitations and challenges in using ECG signals for ML/DL-based disease classification?
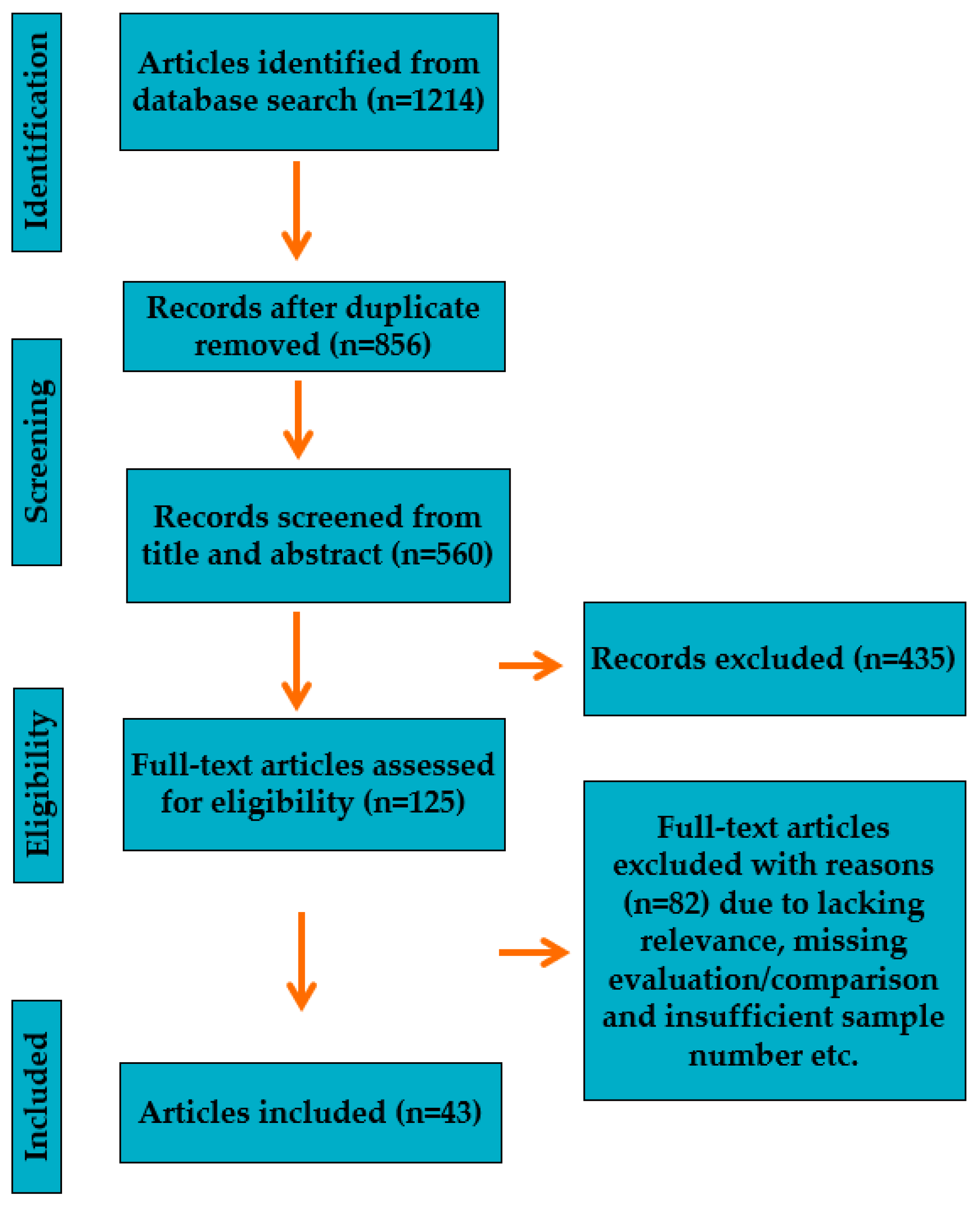
2. Framework for Reference Search and Selection Process
3. ECG Signal Analysis
- Time domain: The most significant is the time domain since the ECG presents the physical movement of the heart’s activities. However, the frequency and statistical domains provide helpful information that can remain unnoticed in time domain information. The time domain characteristics of the ECG waveform delineate its features based on time, which consist of amplitude, duration, P-wave duration, QRS duration, the QT interval, the RR interval, and the PR interval [52,53,54,55,56,57,58,59]. The height of the waveform, known as the amplitude, represents the strength of the electrical signal generated by the heart. Duration denotes the time the waveform takes to complete one cycle, calculated from the beginning of one waveform to the beginning of the next. P-wave duration measures the time taken for the first positive deflection of the waveform that shows atrial depolarization. QRS duration determines the time taken for the QRS complex, which displays ventricular depolarization. The QT interval represents the duration between the start of the QRS complex and the end of the T-wave, signifying the entire length of ventricular depolarization and repolarization. The RR interval indicates the duration between two consecutive R waves corresponding to the time between two heartbeats. Finally, the PR interval shows the time the electrical signal travels from the atria to the ventricles. These features provide crucial information about the heart’s electrical activity and help diagnose and monitor several cardiac conditions.
- Frequency domain: Frequency domain features of ECG refer to the characteristics of the ECG waveform in terms of frequency, obtained by converting the ECG signal from the time domain to the frequency domain using techniques like Fourier transform [59,60,61]. ECG’s most common frequency domain features are power spectral density (PSD), frequency bands, and heart rate variability (HRV). PSD measures the ECG signal’s power at different frequencies and provides information about the signal’s energy distribution over a broad range of frequencies. The frequency bands associated with ECG signals are shallow frequency (VLF), low frequency (LF), and High frequency (HF). VLF power is linked to long-term heart rate and blood pressure fluctuations. The autonomic nervous system’s sympathetic and parasympathetic branches control several physiological processes, including heart rate variability (HRV). Frequency domain methods, such as spectral analysis, which divides the HRV signal into several frequency bands, are frequently used to investigate HRV. In HRV analysis, the high-frequency (HF) band (usually 0.15–0.4 Hz) is linked to parasympathetic (vagal) activity, while the low-frequency (LF) band (generally 0.04–0.15 Hz) is thought to represent a combination of sympathetic and parasympathetic impulses. HRV measures the time intervals between successive heartbeat variations, helps to assess the risk of arrhythmia, and provides information about the autonomic nervous system’s function. By analyzing the ECG’s frequency domain features, medical professionals can gain insights into the heart’s functioning and the autonomic nervous system.
- Statistical domain: The statistical features of ECG are parameters that describe the distribution of the ECG signal in the time and frequency domains. These features include measures of central tendency, such as mean and median, measures of variability, such as variance and standard deviation, and measures of distribution, such as skewness and kurtosis. The mean of the ECG signal represents the average amplitude of the waveform over a specific time interval. At the same time, the variance measures the spread of the signal around the mean. High variability can indicate that the signal has many variabilities, while low variability indicates that the signal is relatively stable. Skewness measures the degree of asymmetry of the distribution, while kurtosis measures the degree of the peakedness of the distribution. Heart rate, also known as rate, is another statistical feature of ECG that describes the number of heartbeats per minute. This feature can assess the cardiovascular system’s overall health and detect rapid or slow heart rate abnormalities. These statistical features can be used to diagnose and monitor cardiovascular diseases by extracting them through mathematical techniques like signal processing and machine learning algorithms. They can also be used to develop automated ECG analysis tools for clinical applications.
- Filter-based: The filter-based method [62] evaluates each feature’s relevance independently of the machine learning algorithm by analyzing the correlation between each feature and the target variable. Filter-based methods include correlation-based feature selection (CFS), mutual information, and variance threshold.
- Wrapper-based methods assess the relevance of a subset of features by training and evaluating the machine learning algorithm on various feature subsets [62]. Although these methods are computationally expensive, they can yield better accuracy than filter-based methods. Recursive feature elimination (RFE) is a well-known wrapper-based method that recursively eliminates the least essential features until the desired number of features is selected.
- Embedded-based: Embedded-based methods are a combination of filter-based and wrapper-based methods. These methods perform feature selection during the machine learning algorithm’s training process [62]. Lasso (Least Absolute Shrinkage and Selection Operator) and Ridge regression are typical examples of embedded-based methods that use regularization techniques. Recently, there have been evolutionary algorithms that have been used for the selection of ECG features, some of the algorithms are genetic algorithm [63], particle swarm optimizer [64], firefly algorithm [64].
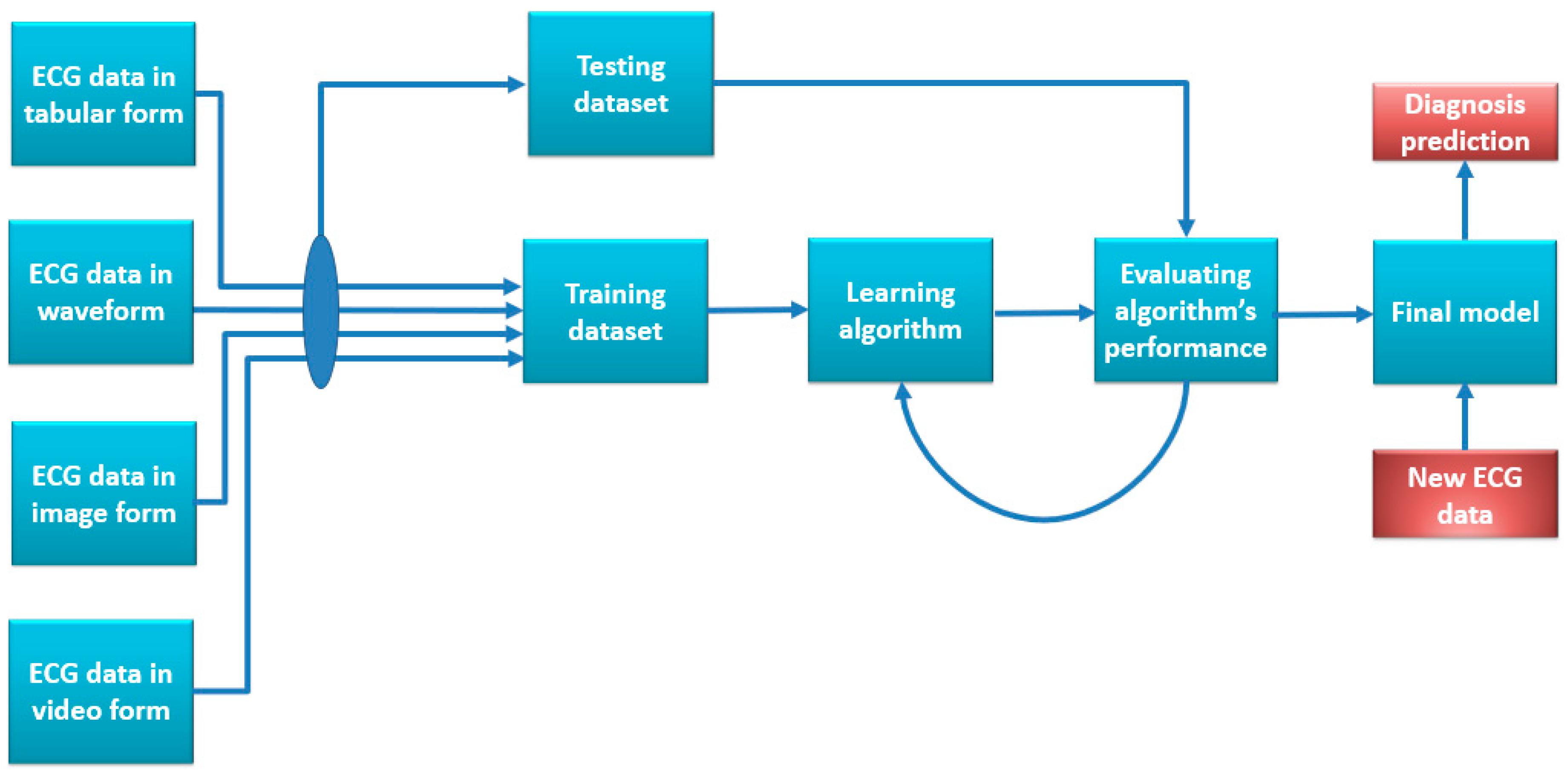
4. Use of ECG with AI for Clinical Analysis and Diagnosis
4.1. Use of ML with ECG for Heart Health Diagnosis
- Develop more robust machine learning algorithms: Often, the machine learning algorithms used today to diagnose ECGs are not resistant to signal noise and distortions. This is a significant drawback since real-world ECG recordings frequently contain noise and artifacts. Developing more robust machine learning algorithms that can better tolerate noise and artifacts should be the primary goal of future research.
- Use more extensive and diverse datasets: Most research on machine learning for ECG diagnosis has relied on relatively small datasets. The results’ generalizability is so constrained. Future research should use more extensive and varied datasets to increase the generalizability of the findings. A benchmark dataset should contain a sufficient number of features that are relevant to the class variable as well as a large number of samples from varied characteristics of patients.
- Integrate machine learning with other clinical data: Clinical data other than ECG signals can also be utilized to identify cardiac disorders. Information from patient medical records, lab results, and imaging investigations may also be utilized. Future studies should look at ways to better diagnose heart disease by combining machine learning with other clinical data.
- Develop mobile and wearable ECG devices: ECG equipment is becoming more wearable and portable. This enables the collection of ECG data outside of a hospital environment. Future studies should look at ways to enhance the early diagnosis of cardiac problems using mobile and wearable ECG sensors.
- Make machine learning algorithms more interpretable: Black boxes characterize many current machine learning techniques for ECG diagnosis. As a result, it is challenging to comprehend how the algorithms generate their predictions. Future studies should concentrate on creating interpretable machine learning algorithms so physicians can better comprehend the outcomes of the forecasts.
4.2. Use of DL with ECG for Heart Health Diagnosis
4.3. Use of ML/DL with ECG for Other Health Issues
5. Challenges and Future Recommendations
- External evaluation/standard system for evaluation: The first limitation of the current studies is an external evaluation or a standard system for evaluating the performance of the models produced by the researcher to create an acceptable benchmark. Also, when the disease differs between race and geographical area, the models do not have any answer or options for modification for those terms.
- Ability to be applied in clinical setups: Despite excellent experiment performance, none of the algorithms made it to a medical setup. The main reason behind this conclusion is that the use of ECG in most cases cannot be the only data source to achieve a conclusive diagnosis. Also, medical professionals are less likely to accept it for diagnosis unless the model’s performance is consistent over a different dataset with different characteristics and there is no regulatory problem.
- Limited resource in wearables for computation: The use of deep learning techniques in wearables, which use substantial neural networks, has another disadvantage. Significant computational resources are needed to handle and evaluate the data from these networks. This can be difficult for wearable technology or other edge computing devices since they do not have the capabilities to carry out such computationally demanding activities locally. In these circumstances, relying on cloud-based programs or distant servers to process data and make decisions can be necessary. This drawback emphasizes how crucial it is to consider the computing capabilities and resource needs when developing deep learning algorithms for ECG analysis in wearable technology or other situations with limited resources. Also, at the same time, using the cloud as an option to take care of the computation question comes to form the front of privacy and security. Sending personal information over the cloud makes it vulnerable to attacks or leaks. Therefore, the infrastructure needs to be there with proper security and privacy protocol before the data can be moved to another location for analysis or diagnosis.
- Imbalanced and limited labeled datasets: Imbalanced datasets are a big concern. Training deep learning algorithms requires significantly large amounts of data. However, not all cases or classes of diseases or health conditions have a large dataset with a balanced format. Therefore, the unavailability of a balanced dataset may provide accurate results, but that cannot be taken as a general rule and can result in overfitting or underfitting.
- Interpretability and transparency: In utilizing deep learning algorithms for ECG analysis to diagnose disorders, interpretability and transparency are significant problems. Convolutional neural networks (CNNs) are an example of a sophisticated deep learning model that is sometimes called a “black box” since it lacks explicit rules or explanations that humans can understand. It raises questions about the validity and dependability of the diagnostic judgments made by these models due to this lack of interpretability. Clinicians in conventional medicine rely on interpretability to comprehend the underlying causes of a diagnosis. They must be able to describe how specific characteristics or patterns in the ECG signal help to identify a particular condition. To track down and comprehend the precise elements or patterns that lead to a given diagnosis, deep learning models function by learning sophisticated representations from enormous volumes of data. Their lack of interpretability may hamper the acceptability and use of AI-based diagnostic tools in clinical settings.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shintomi, A.; Izumi, S.; Yoshimoto, M.; Kawaguchi, H. Effectiveness of the heartbeat interval error and compensation method on heart rate variability analysis. Healthc. Technol. Lett. 2022, 9, 9–15. [Google Scholar] [CrossRef]
- El-Baz, A.; Giridharan, G.A.; Shalaby, A.; Mahmoud, A.H.; Ghazal, M. Special Issue “Computer Aided Diagnosis Sensors”. Sensors 2022, 22, 8052. [Google Scholar] [CrossRef]
- Simegn, G.L.; Gebeyehu, W.B.; Degu, M.Z. Computer-Aided Decision Support System for Diagnosis of Heart Diseases. Res. Rep. Clin. Cardiol. 2022, 13, 39–54. [Google Scholar] [CrossRef]
- Marques, J.A.L.; Gois, F.N.B.; Madeiro, J.P.D.V.; Li, T.; Fong, S.J. Artificial neural network-based approaches for computer-aided disease diagnosis and treatment. In Cognitive and Soft Computing Techniques for the Analysis of Healthcare Data; Academic Press: Cambridge, MA, USA, 2022; pp. 79–99. [Google Scholar]
- He, Y.; Ren, K.; Shan, S. Design of Microcontroller-Based Heart Rate and Temperature Detection System. In Proceedings of the 2022 IEEE 5th International Conference on Information Systems and Computer Aided Education (ICISCAE), Dalian, China, 23–25 September 2022; pp. 22–25. [Google Scholar]
- Mamun, M.M.R.K.; Alouani, A. Arrhythmia Classification Using Hybrid Feature Selection Approach and Ensemble Learning Technique. In Proceedings of the 2021 IEEE Canadian Conference on Electrical and Computer Engineering (CCECE), Virtual, 12–17 September 2021; pp. 1–6. [Google Scholar]
- Mamun, M.M.R.K.; Alouani, A. Automatic Detection of Heart Diseases Using Biomedical Signals: A Literature Review of Current Status and Limitations. In Future of Information and Communication Conference; Springer: Cham, Switzerland, 2022; pp. 420–440. [Google Scholar]
- Rajpurkar, P.; Chen, E.; Banerjee, O.; Topol, E.J. AI in health and medicine. Nat. Med. 2022, 28, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Tadesse, G.A.; Ho, D.; Fei-Fei, L.; Zaharia, M.; Zhang, C.; Zou, J. Advances, challenges and opportunities in creating data for trustworthy AI. Nat. Mach. Intell. 2022, 4, 669–677. [Google Scholar] [CrossRef]
- Farina, E.; Nabhen, J.J.; Dacoregio, M.I.; Batalini, F.; Moraes, F.Y. An overview of artificial intelligence in oncology. Futur. Sci. OA 2022, 8, FSO787. [Google Scholar] [CrossRef]
- Benda, N.C.; Novak, L.L.; Reale, C.; Ancker, J.S. Trust in AI: Why we should be designing for APPROPRIATE reliance. J. Am. Med. Inform. Assoc. 2021, 29, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Busnatu, A.; Niculescu, A.; Bolocan, G.E.; Petrescu, D.N.; Păduraru, I.; Năstasă, M.; Lupușoru, M.; Geantă, O.; Andronic, A.M. Grumezescu Clinical Applications of Artificial Intelligence—An Updated Overview. J. Clin. Med. 2022, 11, 2265. [Google Scholar] [CrossRef] [PubMed]
- Chidambaram, S. IoT-based ECG monitoring system for smart healthcare data applications. In Recent Advancement of IoT Devices in Pollution Control and Health Applications; Woodhead Publishing: Sawston, UK, 2023; pp. 109–125. [Google Scholar]
- Devi, D.H.; Duraisamy, K.; Armghan, A.; Alsharari, M.; Aliqab, K.; Sorathiya, V.; Das, S.; Rashid, N. 5G Technology in Healthcare and Wearable Devices: A Review. Sensors 2023, 23, 2519. [Google Scholar] [CrossRef]
- Hughes, A.; Shandhi, M.H.; Master, H.; Dunn, J.; Brittain, E. Wearable Devices in Cardiovascular Medicine. Circ. Res. 2023, 132, 652–670. [Google Scholar] [CrossRef]
- Gawali, D.H.; Wadhai, V.M.; Patil, M.; Chanchlani, A.S. A Wearable ECG Sensor for Intelligent Cardiovascular Health Informatics. In Designing Intelligent Healthcare Systems, Products, and Services Using Disruptive Technologies and Health Informatics; CRC Press: Boca Raton, FL, USA, 2022; pp. 109–129. [Google Scholar]
- Gomes, N.; Pato, M.; Lourenço, A.R.; Datia, N. A Survey on Wearable Sensors for Mental Health Monitoring. Sensors 2023, 23, 1330. [Google Scholar] [CrossRef] [PubMed]
- Shumba, A.; Montanaro, T.; Sergi, I.; Fachechi, L.; De Vittorio, M.; Patrono, L. Leveraging IoT-Aware Technologies and AI Techniques for Real-Time Critical Healthcare Applications. Sensors 2022, 22, 7675. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.-W.; Lee, W.; Kim, Y.-J. A Real-Time Wearable Physiological Monitoring System for Home-Based Healthcare Applications. Sensors 2021, 22, 104. [Google Scholar] [CrossRef]
- Ouda, H.; Badr, A.; Rashwan, A.; Hassanein, H.S.; Elgazzar, K. Optimizing Real-Time ECG Data Transmission in Constrained Environments. In Proceedings of the ICC 2022—IEEE International Conference on Communications, Seoul, Republic of Korea, 16–20 May 2022; pp. 2114–2119. [Google Scholar]
- Mincholé, A.; Camps, J.; Lyon, A.; Rodríguez, B. Machine learning in the electrocardiogram. J. Electrocardiol. 2019, 57, S61–S64. [Google Scholar] [CrossRef] [PubMed]
- Wasimuddin, M.; Elleithy, K.; Abuzneid, A.-S.; Faezipour, M.; Abuzaghleh, O. Stages-Based ECG Signal Analysis From Traditional Signal Processing to Machine Learning Approaches: A Survey. IEEE Access 2020, 8, 177782–177803. [Google Scholar] [CrossRef]
- Hong, S.; Zhou, Y.; Shang, J.; Xiao, C.; Sun, J. Opportunities and challenges of deep learning methods for electrocardiogram data: A systematic review. Comput. Biol. Med. 2020, 122, 103801. [Google Scholar] [CrossRef]
- Somani, S.; Russak, A.J.; Richter, F.; Zhao, S.; Vaid, A.; Chaudhry, F.; De Freitas, J.K.; Naik, N.; Miotto, R.; Nadkarni, G.N.; et al. Deep learning and the electrocardiogram: Review of the current state-of-the-art. Europace 2021, 23, 1179–1191. [Google Scholar] [CrossRef]
- Saini, S.K.; Gupta, R. Artificial intelligence methods for analysis of electrocardiogram signals for cardiac abnormalities: State-of-the-art and future challenges. Artif. Intell. Rev. 2021, 55, 1519–1565. [Google Scholar] [CrossRef]
- Siontis, K.C.; Noseworthy, P.A.; Attia, Z.I.; Friedman, P.A. Artificial intelligence-enhanced electrocardiography in cardiovascular disease management. Nat. Rev. Cardiol. 2021, 18, 465–478. [Google Scholar] [CrossRef]
- Liu, X.; Wang, H.; Li, Z.; Qin, L. Deep learning in ECG diagnosis: A review. Knowl.-Based Syst. 2021, 227, 107187. [Google Scholar] [CrossRef]
- Chung, C.T.; Lee, S.; King, E.; Liu, T.; Armoundas, A.A.; Bazoukis, G.; Tse, G. Clinical significance, challenges and limitations in using artificial intelligence for electrocardiography-based diagnosis. Int. J. Arrhythmia 2022, 23, 24. [Google Scholar] [CrossRef] [PubMed]
- Petmezas, G.; Stefanopoulos, L.; Kilintzis, V.; Tzavelis, A.; Rogers, J.A.; Katsaggelos, A.K.; Maglaveras, N. State-of-the-Art Deep Learning Methods on Electrocardiogram Data: Systematic Review. JMIR Med. Inform. 2022, 10, e38454. [Google Scholar] [CrossRef] [PubMed]
- Rjoob, K.; Bond, R.; Finlay, D.; McGilligan, V.; Leslie, S.J.; Rababah, A.; Iftikhar, A.; Guldenring, D.; Knoery, C.; McShane, A. Machine learning and the electrocardiogram over two decades: Time series and meta-analysis of the algorithms, evaluation metrics and applications. Artif. Intell. Med. 2022, 132, 102381. [Google Scholar] [CrossRef]
- Ayano, Y.M.; Schwenker, F.; Dufera, B.D.; Debelee, T.G. Interpretable Machine Learning Techniques in ECG-Based Heart Disease Classification: A Systematic Review. Diagnostics 2022, 13, 111. [Google Scholar] [CrossRef] [PubMed]
- Merdjanovska, E.; Rashkovska, A. Comprehensive survey of computational ECG analysis: Databases, methods and applications. Expert Syst. Appl. 2022, 203, 117206. [Google Scholar] [CrossRef]
- Denysyuk, H.V.; Pinto, R.J.; Silva, P.M.; Duarte, R.P.; Marinho, F.A.; Pimenta, L.; Gouveia, A.J.; Gonçalves, N.J.; Coelho, P.J.; Zdravevski, E.; et al. Algorithms for automated diagnosis of cardiovascular diseases based on ECG data: A comprehensive systematic review. Heliyon 2023, 9, e13601. [Google Scholar] [CrossRef]
- Pradhan, B.K.; Neelappu, B.C.; Sivaraman, J.; Kim, D.; Pal, K. A Review on the Applications of Time-Frequency Methods in ECG Analysis. J. Healthc. Eng. 2023, 2023, 3145483. [Google Scholar] [CrossRef]
- Marouf, M.; Saranovac, L.; Vukomanovic, G. Algorithm for EMG noise level approximation in ECG signals. Biomed. Signal Process. Control 2017, 34, 158–165. [Google Scholar] [CrossRef]
- Christov, I.I.; Daskalov, I.K. Filtering of electromyogram artifacts from the electrocardiogram. Med. Eng. Phys. 1999, 21, 731–736. [Google Scholar] [CrossRef]
- Joy, J.; Manimegalai, P. Wavelet based EMG artifact removal from ECG signal. J. Eng. Comp. Appl. Sci. 2013, 2, 55–58. [Google Scholar]
- Belgurzi, A.N.S.; Elshafiey, I. A Power Line Interference Canceler using Wavelet Transform and Adaptive Filter for ECG Signal. In Proceedings of the 2017 International Conference on Computer and Applications (ICCA), Doha, United Arab Emirates, 6–7 September 2017; pp. 206–210. [Google Scholar]
- Dobrev, D.; Neycheva, T.; Krasteva, V.; Iliev, I. High-Q comb FIR filter for mains interference elimination. Annu. J. Electron. 2010, 4, 126–129. [Google Scholar]
- Kocoń, S.; Piskorowski, J. Digital finite impulse response notch filter with non-zero initial conditions, based on an infinite impulse response prototype filter. Metrol. Meas. Syst. 2012, 19, 767–776. [Google Scholar] [CrossRef]
- Rieta, J.J.; Zarzoso, V.; Millet-Roig, J.; Garcia-Civera, R.; Ruiz-Granell, R. Atrial activity extraction based on blind source separation as an alternative to QRST cancellation for atrial fibrillation analysis. In Computers in Cardiology 2000. Vol.27 (Cat. 00CH37163); IEEE: Piscataway, NJ, USA, 2020. [Google Scholar] [CrossRef]
- Warmerdam, G.J.J.; Vullings, R.; Schmitt, L.; Van Laar, J.O.E.H.; Bergmans, J.W.M. A Fixed-Lag Kalman Smoother to Filter Power Line Interference in Electrocardiogram Recordings. IEEE Trans. Biomed. Eng. 2016, 64, 1852–1861. [Google Scholar] [CrossRef]
- Marques, J.A.L.; Cortez, P.C.; Madeiro, J.P.D.V.; Fong, S.J.; Schlindwein, F.S.; De Albuquerque, V.H.C. Automatic Cardiotocography Diagnostic System Based on Hilbert Transform and Adaptive Threshold Technique. IEEE Access 2019, 7, 73085–73094. [Google Scholar] [CrossRef]
- Hamilton, P. A comparison of adaptive and nonadaptive filters for reduction of power line interference in the ECG. IEEE Trans. Biomed. Eng. 1996, 43, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Taralunga, D.D.; Gussi, I.; Strungaru, R. Fetal ECG enhancement: Adaptive power line interference cancellation based on Hilbert Huang Transform. Biomed. Signal Process. Control 2015, 19, 77–84. [Google Scholar] [CrossRef]
- Levkov, C.; Mihov, G.; Ivanov, R.; Daskalov, I.; Christov, I.; Dotsinsky, I. Removal of power-line interference from the ECG: A review of the subtraction procedure. Biomed. Eng. Online 2005, 4, 50. [Google Scholar] [CrossRef]
- Rieta, J.; Castells, F.; Sanchez, C.; Zarzoso, V.; Millet, J. Atrial Activity Extraction for Atrial Fibrillation Analysis Using Blind Source Separation. IEEE Trans. Biomed. Eng. 2004, 51, 1176–1186. [Google Scholar] [CrossRef]
- von Borries, R.; Pierluissi, J.; Nazeran, H. Wavelet Transform-Based ECG Baseline Drift Removal for Body Surface Potential Mapping. In Proceedings of the 2005 IEEE Engineering in Medicine and Biology 27th Annual Conference, Shanghai, China, 31 August–3 September 2005; pp. 3891–3894. [Google Scholar]
- Sayadi, O.; Shamsollahi, M.B. Multiadaptive Bionic Wavelet Transform: Application to ECG Denoising and Baseline Wandering Reduction. EURASIP J. Adv. Signal Process. 2007, 2007, 041274. [Google Scholar] [CrossRef]
- Tinati, B.M.A. ECG baseline wander elimination using wavelet packets. World Acad. Sci. Eng. Technol. 2005, 3, 14–16. [Google Scholar]
- Mamun, M.M.R.K.; Alouani, A.T. Cuffless Blood Pressure Measurement Using Linear and Nonlinear Optimized Feature Selection. Diagnostics 2022, 12, 408. [Google Scholar] [CrossRef]
- Petrenas, A.; Marozas, V.; Sornmo, L.; Lukosevicius, A. An Echo State Neural Network for QRST Cancellation During Atrial Fibrillation. IEEE Trans. Biomed. Eng. 2012, 59, 2950–2957. [Google Scholar] [CrossRef]
- Guzik, P.; Piskorski, J.; Krauze, T.; Wykretowicz, A.; Wysocki, H. Heart rate asymmetry by Poincaré plots of RR intervals. Biomed. Eng. 2006, 51, 272–275. [Google Scholar] [CrossRef]
- Yakut, Ö.; Bolat, E.D. An improved QRS complex detection method having low computational load. Biomed. Signal Process. Control 2018, 42, 230–241. [Google Scholar] [CrossRef]
- Park, J.; Lee, S.; Jeon, M. Atrial fibrillation detection by heart rate variability in Poincare plot. Biomed. Eng. Online 2009, 8, 38. [Google Scholar] [CrossRef]
- Lin, C.-C.; Chang, H.-Y.; Huang, Y.-H.; Yeh, C.-Y. A Novel Wavelet-Based Algorithm for Detection of QRS Complex. Appl. Sci. 2019, 9, 2142. [Google Scholar] [CrossRef]
- Lee, J.S.; Lee, S.J.; Choi, M.; Seo, M.; Kim, S.W. QRS detection method based on fully convolutional networks for capacitive electrocardiogram. Expert Syst. Appl. 2019, 134, 66–78. [Google Scholar] [CrossRef]
- Pan, J.; Tompkins, W.J. A real-time QRS detection algorithm. IEEE Trans. Biomed. Eng. 1985, BME-32, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Hussein, A.F.; Kumar, N.A.; Burbano-Fernandez, M.; Ramirez-Gonzalez, G.; Abdulhay, E.; De Albuquerque, V.H.C. An Automated Remote Cloud-Based Heart Rate Variability Monitoring System. IEEE Access 2018, 6, 77055–77064. [Google Scholar] [CrossRef]
- Marinho, L.B.; Nascimento, N.d.M.; Souza, J.W.M.; Gurgel, M.V.; Filho, P.P.R.; de Albuquerque, V.H.C. A novel electrocardiogram feature extraction approach for cardiac arrhythmia classification. Futur. Gener. Comput. Syst. 2019, 97, 564–577. [Google Scholar] [CrossRef]
- Nascimento, N.M.M.; Marinho, L.B.; Peixoto, S.A.; Madeiro, J.P.D.V.; de Albuquerque, V.H.C.; Filho, P.P.R. Heart Arrhythmia Classification Based on Statistical Moments and Structural Co-occurrence. Circuits Syst. Signal Process. 2019, 39, 631–650. [Google Scholar] [CrossRef]
- Mahajan, R.; Kamaleswaran, R.; Howe, J.A.; Akbilgic, O. Cardiac Rhythm Classification from a Short Single Lead ECG Recording via Random Forest. In Proceedings of the 2017 Computing in Cardiology (CinC), Rennes, France, 24–27 September 2017; pp. 1–4. [Google Scholar] [CrossRef]
- Kora, P. ECG based Myocardial Infarction detection using Hybrid Firefly Algorithm. Comput. Methods Programs Biomed. 2017, 152, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Goharrizi, M.A.B.; Teimourpour, A.; Falah, M.; Hushmandi, K.; Isfeedvajani, M.S. Multi-lead ECG heartbeat classification of heart disease based on HOG local feature descriptor. Comput. Methods Programs Biomed. Update 2023, 3, 100093. [Google Scholar]
- Nandanwar, J.; Singh, J.; Patidar, S. ECG Signals- Early detection of Arrhythmia using Machine Learning approaches. In Proceedings of the 2023 13th International Conference on Cloud Computing, Data Science & Engineering (Confluence), Noida, India, 19–20 January 2023; pp. 32–38. [Google Scholar]
- Dhanamjayulu, C.; Suraj, G.V.; Nikhil, M.; Kaluri, R.; Koppu, S. A Machine Learning Algorithm-Based IoT-Based Message Alert System for Predicting Coronary Heart Disease. In International Conference on Advancements in Smart Computing and Information Security; Springer: Cham, Switzerland, 2022; pp. 362–376. [Google Scholar]
- Ahamad, G.N.; Shafiullah; Fatima, H.; Imdadullah; Zakariya, S.M.; Abbas, M.; Alqahtani, M.S.; Usman, M. Influence of Optimal Hyperparameters on the Performance of Machine Learning Algorithms for Predicting Heart Disease. Processes 2023, 11, 734. [Google Scholar] [CrossRef]
- Geweid, G.G.; Chen, J.D. Automatic classification of atrial fibrillation from short single-lead ECG recordings using a Hybrid Approach of Dual Support Vector Machine. Expert Syst. Appl. 2022, 198, 116848. [Google Scholar] [CrossRef]
- Mazidi, M.H.; Eshghi, M.; Raoufy, M.R. Premature Ventricular Contraction (PVC) Detection System Based on Tunable Q-Factor Wavelet Transform. J. Biomed. Phys. Eng. 2022, 12, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Fu, G.; Struppa, D.; Abudayyeh, I.; Contractor, T.; Anderson, K.; Chu, H.; Rakovski, C. A High Precision Machine Learning-Enabled System for Predicting Idiopathic Ventricular Arrhythmia Origins. Front. Cardiovasc. Med. 2022, 9, 809027. [Google Scholar] [CrossRef]
- He, K.; Liang, W.; Liu, S.; Bian, L.; Xu, Y.; Luo, C.; Li, Y.; Yue, H.; Yang, C.; Wu, Z. Long-term single-lead electrocardiogram monitoring to detect new-onset postoperative atrial fibrillation in patients after cardiac surgery. Front. Cardiovasc. Med. 2022, 9, 1001883. [Google Scholar] [CrossRef]
- Naz, M.; Shah, J.H.; Khan, M.A.; Sharif, M.; Raza, M.; Damaševičius, R. From ECG signals to images: A transformation based approach for deep learning. PeerJ Comput. Sci. 2021, 7, e386. [Google Scholar] [CrossRef]
- Grogan, M.; Lopez-Jimenez, F.; Cohen-Shelly, M.; Dispenzieri, A.; Attia, Z.I.; Ezzedine, O.F.A.; Lin, G.; Kapa, S.; Borgeson, D.D.; Friedman, P.A.; et al. Artificial Intelligence–Enhanced Electrocardiogram for the Early Detection of Cardiac Amyloidosis. Mayo Clin. Proc. 2021, 96, 2768–2778. [Google Scholar] [CrossRef]
- Wang, X.; Li, R.; Wang, S.; Shen, S.; Zhang, W.; Zhou, B.; Wang, Z. Automatic diagnosis of ECG disease based on intelligent simulation modeling. Biomed. Signal Process. Control 2021, 67, 102528. [Google Scholar] [CrossRef]
- Houssein, E.H.; Ibrahim, I.E.; Neggaz, N.; Hassaballah, M.; Wazery, Y.M. An efficient ECG arrhythmia classification method based on Manta ray foraging optimization. Expert Syst. Appl. 2021, 181, 115131. [Google Scholar] [CrossRef]
- Chumrit, N.; Weangwan, C.; Aunsri, N. ECG-based Arrhythmia Detection using Average Energy and Zero-crossing Features with Support Vector Machine. In Proceedings of the 2020 5th International Conference on Information Technology (InCIT), Chonburi, Thailand, 21–22 October 2020; pp. 282–287. [Google Scholar]
- Mazaheri, V.; Khodadadi, H. Heart arrhythmia diagnosis based on the combination of morphological, frequency and nonlinear features of ECG signals and metaheuristic feature selection algorithm. Expert Syst. Appl. 2020, 161, 113697. [Google Scholar] [CrossRef]
- Subramanian, K.; Prakash, N.K. Machine Learning based Cardiac Arrhythmia detection from ECG signal. In Proceedings of the 2020 Third International Conference on Smart Systems and Inventive Technology (ICSSIT), Tirunelveli, India, 20–22 August 2020; pp. 1137–1141. [Google Scholar]
- Wang, Z.; Qian, L.; Han, C.; Shi, L. Application of multi-feature fusion and random forests to the automated detection of myocardial infarction. Cogn. Syst. Res. 2019, 59, 15–26. [Google Scholar] [CrossRef]
- Yang, W.; Si, Y.; Wang, D.; Zhang, G.; Liu, X.; Li, L. Automated intra-patient and inter-patient coronary artery disease and congestive heart failure detection using EFAP-Net. Knowl.-Based Syst. 2020, 201, 106083. [Google Scholar] [CrossRef]
- Bashar, S.K.; Ding, E.; Albuquerque, D.; Winter, M.; Binici, S.; Walkey, A.J.; McManus, D.D.; Chon, K.H. Atrial Fibrillation Detection in ICU Patients: A Pilot Study on MIMIC III Data. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 298–301. [Google Scholar]
- Kong, D.; Zhu, J.; Wu, S.; Duan, C.; Lu, L.; Chen, D. A novel IRBF-RVM model for diagnosis of atrial fibrillation. Comput. Methods Programs Biomed. 2019, 177, 183–192. [Google Scholar] [CrossRef]
- Mahmood, I.S.; Abdelrahman, I.A.M. A Comparison between Different Classifiers for Diagnoses of Atrial Fibrillation. In Proceedings of the 2019 International Conference on Computer, Control, Electrical, and Electronics Engineering (ICCCEEE), Khartoum, Sudan, 21–23 September 2019; pp. 1–6. [Google Scholar]
- Hannun, A.Y.; Rajpurkar, P.; Haghpanahi, M.; Tison, G.H.; Bourn, C.; Turakhia, M.P.; Ng, A.Y. Cardiologist-level arrhythmia detection and classification in ambulatory electrocardiograms using a deep neural network. Nat. Med. 2019, 25, 65–69. [Google Scholar] [CrossRef]
- Attia, Z.I.; Kapa, S.; Lopez-Jimenez, F.; McKie, P.M.; Ladewig, D.J.; Satam, G.; Pellikka, P.A.; Enriquez-Sarano, M.; Noseworthy, P.A.; Munger, T.M.; et al. Screening for cardiac contractile dysfunction using an artificial intelligence–enabled electrocardiogram. Nat. Med. 2019, 25, 70–74. [Google Scholar] [CrossRef]
- Lokhande, P.P.; Chinnaiah, K. Cardiac Disease Detection Using IoT-Enabled ECG Sensors and Deep Learning Approach. In International Conference on Advanced Communication and Intelligent Systems; Springer: Cham, Switzerland, 2022; pp. 195–204. [Google Scholar]
- Wang, Z.; Stavrakis, S.; Yao, B. Hierarchical deep learning with Generative Adversarial Network for automatic cardiac diagnosis from ECG signals. Comput. Biol. Med. 2023, 155, 106641. [Google Scholar] [CrossRef]
- Akbilgic, O.; Karabayir, I.; Butler, L.; Güntürkün, F.; Chinthala, L.; Jefferies, J.L.; Baykaner, T.; Herrington, D.M.; Soliman, E.Z.; Davis, R. A real world evidence for the performance of an ecg-ai based heart failure risk predictor. J. Am. Coll. Cardiol. 1985, 81 (Suppl. S8), 727. [Google Scholar] [CrossRef]
- Munawar, S.; Angappan, G.; Konda, S. Arrhythmia Classification Based on Bi-Directional Long Short-Term Memory and Multi-Task Group Method. Int. J. E-Collab. 2023, 19, 1–18. [Google Scholar] [CrossRef]
- Li, Y.; Luo, J.-H.; Dai, Q.-Y.; Eshraghian, J.K.; Ling, B.W.-K.; Zheng, C.-Y.; Wang, X.-L. A deep learning approach to cardiovascular disease classification using empirical mode decomposition for ECG feature extraction. Biomed. Signal Process. Control 2023, 79, 104188. [Google Scholar] [CrossRef]
- Bhaskarpandit, S.; Gade, A.; Dash, S.; Dash, D.K.; Tripathy, R.K.; Pachori, R.B. Detection of Myocardial Infarction From 12-Lead ECG Trace Images Using Eigendomain Deep Representation Learning. IEEE Trans. Instrum. Meas. 2023, 72, 1–12. [Google Scholar] [CrossRef]
- Mahajan, R.; Pundir, P.; Gambhir, A.; Adumala, S. ECG-based deep learning framework to identify ventricular arrhythmias in patients monitored with mct. J. Am. Coll. Cardiol. 2023, 81, 2176. [Google Scholar] [CrossRef]
- Leema, A.; Nagaraj, J. A deep learning framework for automatic cardiovascular classification from Electrocardiogram Images. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Wang, Z.; Jin, B.; Li, S.; Zhang, F.; Zhang, W. ECG-grained Cardiac Monitoring Using UWB Signals. Proc. Acm Interact. Mob. Wearable Ubiquitous Technol. 2022, 6, 1–25. [Google Scholar] [CrossRef]
- Ismail, A.R.; Jovanovic, S.; Ramzan, N.; Rabah, H. ECG Classification Using an Optimal Temporal Convolutional Network for Remote Health Monitoring. Sensors 2023, 23, 1697. [Google Scholar] [CrossRef] [PubMed]
- Maurya, J.P.; Manoria, M.; Joshi, S. Cardiac Arrhythmia Classification Using Cascaded Deep Learning Approach (LSTM & RNN). In Proceedings of the International Conference on Machine Learning, Image Processing, Network Security and Data Sciences, Virtual Event, 19–20 January 2023; pp. 3–13. [Google Scholar]
- Monaci, S.; Qian, S.; Gillette, K.; Puyol-Antón, E.; Mukherjee, R.; Elliott, M.K.; Whitaker, J.; Rajani, R.; O’neill, M.; Rinaldi, C.A.; et al. Non-invasive localization of post-infarct ventricular tachycardia exit sites to guide ablation planning: A computational deep learning platform utilizing the 12-lead electrocardiogram and intracardiac electrograms from implanted devices. Europace 2022, 25, 469–477. [Google Scholar] [CrossRef]
- Allam, J.P.; Samantray, S.; Ari, S. Patient-specific ECG beat classification using EMD and deep learning-based technique. In Advanced Methods in Biomedical Signal Processing and Analysis; Academic Press: Cambridge, MA, USA, 2023; pp. 87–108. [Google Scholar]
- Alsheikhy, A.; Said, Y.F.; Shawly, T.; Lahza, H. A Model to Predict Heartbeat Rate Using Deep Learning Algorithms. Healthcare 2023, 11, 330. [Google Scholar] [CrossRef]
- Zhao, X.; Huang, G.; Wu, L.; Wang, M.; He, X.; Wang, J.-R.; Zhou, B.; Liu, Y.; Lin, Y.; Liu, D.; et al. Deep learning assessment of left ventricular hypertrophy based on electrocardiogram. Front. Cardiovasc. Med. 2022, 9, 952089. [Google Scholar] [CrossRef]
- Liu, P.; Sun, X.; Han, Y.; He, Z.; Zhang, W.; Wu, C. Arrhythmia classification of LSTM autoencoder based on time series anomaly detection. Biomed. Signal Process. Control 2021, 71, 103228. [Google Scholar] [CrossRef]
- Radhakrishnan, T.; Karhade, J.; Ghosh, S.; Muduli, P.; Tripathy, R.; Acharya, U.R. AFCNNet: Automated detection of AF using chirplet transform and deep convolutional bidirectional long short term memory network with ECG signals. Comput. Biol. Med. 2021, 137, 104783. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Automated detection of premature ventricular contraction based on the improved gated recurrent unit network. Comput. Methods Programs Biomed. 2021, 208, 106284. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Paul, A.; Agarwal, A.; Jindal, S.K. Real Time Arrhythmia Detecting Wearable using a Novel Deep Learning Model. In Proceedings of the 2020 International Conference on Interdisciplinary Cyber Physical Systems (ICPS), Chennai, India, 28–29 December 2020; pp. 14–19. [Google Scholar]
- Rajpurkar, P.; Hannun, A.Y.; Haghpanahi, M.; Bourn, C.; Ng, A.Y. Cardiologist-level arrhythmia detection with convolutional neural networks. arXiv 2017, arXiv:1707.01836. [Google Scholar]
- Zhai, X.; Tin, C. Automated ECG Classification Using Dual Heartbeat Coupling Based on Convolutional Neural Network. IEEE Access 2018, 6, 27465–27472. [Google Scholar] [CrossRef]
- Huang, J.; Chen, B.; Yao, B.; He, W. ECG Arrhythmia Classification Using STFT-Based Spectrogram and Convolutional Neural Network. IEEE Access 2019, 7, 92871–92880. [Google Scholar] [CrossRef]
- Liu, W.; Huang, Q.; Chang, S.; Wang, H.; He, J. Multiple-feature-branch convolutional neural network for myocardial infarction diagnosis using electrocardiogram. Biomed. Signal Process. Control 2018, 45, 22–32. [Google Scholar] [CrossRef]
- Niu, J.; Tang, Y.; Sun, Z.; Zhang, W. Inter-Patient ECG Classification With Symbolic Representations and Multi-Perspective Convolutional Neural Networks. IEEE J. Biomed. Health Inform. 2019, 24, 1321–1332. [Google Scholar] [CrossRef]
- Acharya, U.R.; Fujita, H.; Oh, S.L.; Raghavendra, U.; Tan, J.H.; Adam, M.; Gertych, A.; Hagiwara, Y. Automated identification of shockable and non-shockable life-threatening ventricular arrhythmias using convolutional neural network. Futur. Gener. Comput. Syst. 2018, 79, 952–959. [Google Scholar] [CrossRef]
- Tan, J.H.; Hagiwara, Y.; Pang, W.; Lim, I.; Oh, S.L.; Adam, M.; Tan, R.S.; Chen, M.; Acharya, U.R. Application of stacked convolutional and long short-term memory network for accurate identification of CAD ECG signals. Comput. Biol. Med. 2018, 94, 19–26. [Google Scholar] [CrossRef]
- Yao, Q.; Wang, R.; Fan, X.; Liu, J.; Li, Y. Multi-class Arrhythmia detection from 12-lead varied-length ECG using Attention-based Time-Incremental Convolutional Neural Network. Inf. Fusion 2019, 53, 174–182. [Google Scholar] [CrossRef]
- Lih, O.S.; Jahmunah, V.; San, T.R.; Ciaccio, E.J.; Yamakawa, T.; Tanabe, M.; Kobayashi, M.; Faust, O.; Acharya, U.R. Comprehensive electrocardiographic diagnosis based on deep learning. Artif. Intell. Med. 2020, 103, 101789. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Sim, G.; Matuszewski, B. Inter-patient ECG classification with convolutional and recurrent neural networks. Biocybern. Biomed. Eng. 2019, 39, 868–879. [Google Scholar] [CrossRef]
- Andersen, R.S.; Peimankar, A.; Puthusserypady, S. A deep learning approach for real-time detection of atrial fibrillation. Expert Syst. Appl. 2018, 115, 465–473. [Google Scholar] [CrossRef]
- Liu, H.; Cui, S.; Zhao, X.; Cong, F. Detection of obstructive sleep apnea from single-channel ECG signals using a CNN-transformer architecture. Biomed. Signal Process. Control 2023, 82, 104581. [Google Scholar] [CrossRef]
- Srivastava, G.; Chauhan, A.; Kargeti, N.; Pradhan, N.; Dhaka, V.S. ApneaNet: A hybrid 1DCNN-LSTM architecture for detection of Obstructive Sleep Apnea using digitized ECG signals. Biomed. Signal Process. Control 2023, 84, 104754. [Google Scholar] [CrossRef]
- Tyagi, P.K.; Agrawal, D. Automatic detection of sleep apnea from single-lead ECG signal using enhanced-deep belief network model. Biomed. Signal Process. Control 2023, 80, 104401. [Google Scholar] [CrossRef]
- Yue, H.; Li, P.; Li, Y.; Lin, Y.; Huang, B.; Sun, L.; Ma, W.; Fan, X.; Wen, W.; Lei, W. Validity study of a multi-scaled fusion network using single-lead electrocardiogram signals for obstructive sleep apnea diagnosis. J. Clin. Sleep Med. 2023, 19, 1017–1025. [Google Scholar] [CrossRef]
- Jothi, E.S.J.; Anitha, J.; Priyadharshini, J.; Hemanth, D.J. Deep Learning Based Obstructive Sleep Apnea Detection for e-health Applications. In International Conference on Electronic Governance with Emerging Technologies; Springer: Cham, Switzerland, 2022; pp. 1–11. [Google Scholar]
- Rathore, K.S.; Sricharan, V.; Preejith, S.; Sivaprakasam, M. MRNet—A Deep Learning Based Multitasking Model for Respiration Rate Estimation in Practical Settings. In Proceedings of the 2022 IEEE 10th International Conference on Serious Games and Applications for Health(SeGAH), Sydney, Australia, 10–12 August 2022; pp. 1–6. [Google Scholar]
- Kumar, A.K.; Ritam, M.; Han, L.; Guo, S.; Chandra, R. Deep learning for predicting respiratory rate from biosignals. Comput. Biol. Med. 2022, 144, 105338. [Google Scholar] [CrossRef]
- Liu, J.; Xu, H.; Wang, J.; Peng, X.; He, C. Non-invasive diagnosis of fetal arrhythmia based on multi-domain feature and hierarchical extreme learning machine. Biomed. Signal Process. Control 2023, 79, 104191. [Google Scholar] [CrossRef]
- Al-Saadany, D.; Attallah, O.; Elzaafarany, K.; Nasser, A.A.A. A Machine Learning Framework for Fetal Arrhythmia Detection via Single ECG Electrode. In International Conference on Computational Science; Springer: Cham, Switzerland, 2022; pp. 546–553. [Google Scholar]
- Baghel, N.; Burget, R.; Dutta, M.K. 1D-FHRNet: Automatic Diagnosis of Fetal Acidosis from Fetal Heart Rate Signals. Biomed. Signal Process. Control 2021, 71, 102794. [Google Scholar] [CrossRef]
- Nakatani, S.; Yamamoto, K.; Ohtsuki, T. Fetal Arrhythmia Detection based on Deep Learning using Fetal ECG Signals. In Proceedings of the GLOBECOM 2022—2022 IEEE Global Communications Conference, Rio de Janeiro, Brazil, 4–8 December 2022; pp. 2266–2271. [Google Scholar]
- Long, W.; Wang, X. BPNet: A multi-modal fusion neural network for blood pressure estimation using ECG and PPG. Biomed. Signal Process. Control 2023, 86, 105287. [Google Scholar] [CrossRef]
- Mamun, M.M.R.K.; Alouani, A. Using Photoplethysmography & ECG Towards a Non-Invasive Cuff less Blood Pressure Measurement Technique. In Proceedings of the 2019 IEEE Canadian Conference of Electrical and Computer Engineering (CCECE), Edmonton, AB, Canada, 5–8 May 2019; pp. 1–4. [Google Scholar]
- Bose S, S.N. Dual-Stage Learning Approach towards Continuous Cuffless Blood Pressure Monitoring; Innovations in Information and Communication Technology Series; IJAICT India Publications: Waterloo, ON, Canada, 2020; pp. 369–372. [Google Scholar] [CrossRef]
- Mamun, M.M.R.K.; Sherif, A. Advancement in the Cuffless and Noninvasive Measurement of Blood Pressure: A Review of the Literature and Open Challenges. Bioengineering 2022, 10, 27. [Google Scholar] [CrossRef]
- Mamun, M.M.R.K. Cuff-less blood pressure measurement based on hybrid feature selection algorithm and multi-penalty regularized regression technique. Biomed. Phys. Eng. Express 2021, 7, 065030. [Google Scholar] [CrossRef]
- Attia, Z.I.; Harmon, D.M.; Behr, E.R.; Friedman, P.A. Application of artificial intelligence to the electrocardiogram. Eur. Heart J. 2021, 42, 4717–4730. [Google Scholar] [CrossRef]
- Weil, E.L.; Noseworthy, P.A.; Lopez, C.L.; Rabinstein, A.A.; Friedman, P.A.; Attia, Z.I.; Yao, X.; Siontis, K.C.; Kremers, W.K.; Christopoulos, G.; et al. Artificial Intelligence–Enabled Electrocardiogram for Atrial Fibrillation Identifies Cognitive Decline Risk and Cerebral Infarcts. Mayo Clin. Proc. 2022, 97, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Park, S.; Kwon, S.-H.; Cho, K.-H.; Lee, H. AI-Based Stroke Disease Prediction System Using ECG and PPG Bio-Signals. IEEE Access 2022, 10, 43623–43638. [Google Scholar] [CrossRef]
- Chau, T.; Lin, C.-S.; Shang, H.-S.; Fang, W.-H.; Lee, D.-J.; Lee, C.-C.; Tsai, S.-H.; Wang, C.-H.; Lin, S.-H. Point-of-care artificial intelligence-enabled ECG for dyskalemia: A retrospective cohort analysis for accuracy and outcome prediction. NPJ Digit. Med. 2022, 5, 8. [Google Scholar] [CrossRef]
- Dash, D.P.; Kolekar, M.H.; Chakraborty, C.; Khosravi, M.R. Review of Machine and Deep Learning Techniques in Epileptic Seizure Detection using Physiological Signals and Sentiment Analysis. ACM Trans. Asian Low-Resour. Lang. Inf. Process. 2022. [Google Scholar] [CrossRef]
- Chen, S.; Zheng, R.; Wang, T.; Jiang, T.; Gao, F.; Wang, D.; Cao, J. Deterministic Learning-Based WEST Syndrome Analysis and Seizure Detection on ECG. IEEE Trans. Circuits Syst. II Express Briefs 2022, 69, 4603–4607. [Google Scholar] [CrossRef]
- McInnis, R.P.; Ayub, M.A.; Jing, J.; Halford, J.J.; Mateen, F.J.; Westover, M.B. Epilepsy diagnosis using a clinical decision tool and artificially intelligent electroencephalography. Epilepsy Behav. 2023, 141, 109135. [Google Scholar] [CrossRef] [PubMed]

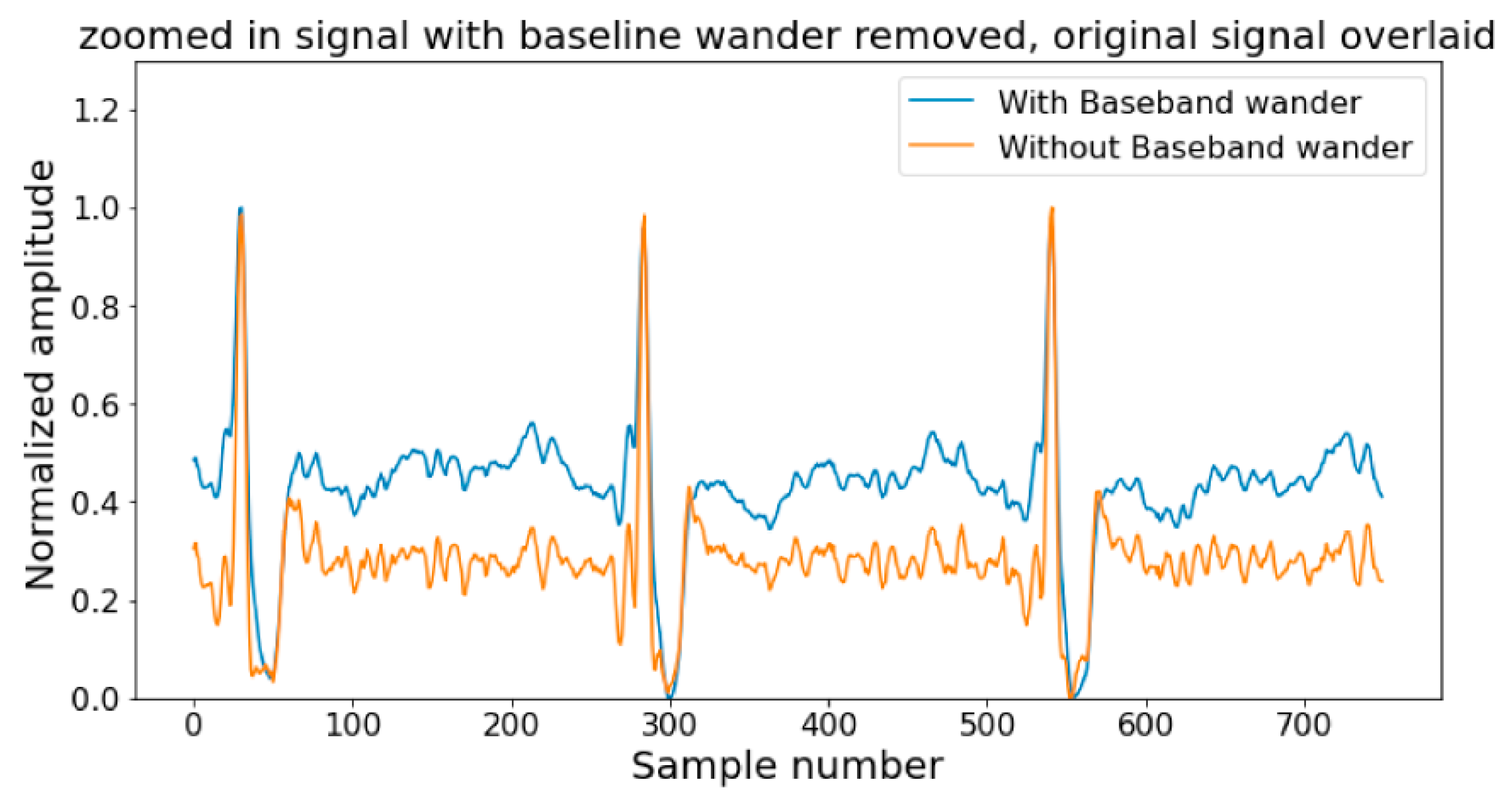

| Reference | Year | Aspects (First: ECG Is the Primary Signal, Second: AI Is the Focus Technology; Third: Sufficient Discussion on Challenges and Future Directions | Summary | Limitations |
|---|---|---|---|---|
| [21] | 2019 | 1 and 2 | Focus:
| Lacks:
|
| [22] | 2020 | 1 and 3 | Focus:
| Lacks:
|
| [23] | 2020 | 1, partial 2 and 3 | Focus:
| Lacks:
|
| [24] | 2020 | 1 and partial 2 | Focus:
| Lacks:
|
| [25] | 2021 | 1, partial 2 and patial 3 | Focus:
| Lacks:
|
| [26] | 2021 | 1, 2 and 3 | Focus:
| Lacks:
|
| [27] | 2021 | 1, partial 2 and 3 | Focus:
| Lacks:
|
| [28] | 2022 | 1, partial 2 and 3 | Focus:
| Lacks:
|
| [29] | 2022 | 1, partial 2 and 3 | Focus:
| Lacks:
|
| [30] | 2022 | 1 and 2 | Focus:
| Lacks:
|
| [31] | 2022 | 1, partial 2 and 3 | Focus:
| Lacks:
|
| [32] | 2022 | 1, partial 2 and partial 3 | Focus:
| Lacks:
|
| [33] | 2023 | 1 and 2 | Focus:
| Lacks:
|
| [34] | 2023 | 1, partial 2 and 3 | Focus:
| Lacks:
|
| Ref. | Year | Heart Disease | Algorithm | Summary | Limitations |
|---|---|---|---|---|---|
| [65] | 2023 | Bundle branch block, cardiomyopathy, Dysrhythmia, and myocardial infarction | Support vector machine | Assessing HOG-SVM for precise 15-lead ECG heartbeat disease classification. | Requires a large dataset of ECG signals to train the machine learning models. It is not always accurate, especially for rare or complex arrhythmias. |
| [66] | 2023 | Arrhythmia | Random forest classification, support vector machines | The study highlights the importance of ML in precisely detecting aberrant heartbeats. 98.26% and 100% accuracy are reached across a variety of datasets. | The study used a small dataset of ECG signals, which may not represent the wider population. The study did not consider the impact of noise and artifacts on the ECG signals. |
| [67] | 2023 | Cardiovascular infirmity | Decision tree, Naïve Bayes, KNN, SVM | With an F-measure of 0.95 and an accuracy rate of 95.5%, ML predicts cardiac disease. In comparison to current models, the integration of characteristics and ensembles enhances the prediction of coronary disease. | Small data: 300 patients A few features: Age, gender, blood pressure. |
| [68] | 2023 | General heart condition | Logistic regression, KNN, SVM, DT, RF, GB | Grid search CV innovation: improved accuracy. The results of the experiments found the most influential factors for predicting heart disease. | Small dataset: The study used a small dataset of 296 patients. Not generalizable: The results may not be generalizable to other datasets or populations. |
| [69] | 2022 | AF | Support vector machine | Atrial fibrillation was detected utilizing a hybrid technique with a Dual Support Vector Machine as the primary strategy. | Noisy data: The study did not consider the impact of noise and artifacts on the ECG signals. Single lead: The study only used a single lead ECG recording, which may not be enough to classify atrial fibrillation accurately. |
| [70] | 2022 | Premature ventricular contraction | Support vector machine, K nearest neighbor | 22 ECG recordings were provided via the MIT/BIH arrhythmia database. Q-factor wavelet generated sub-bands while reducing noise. ANOVA for feature extraction and a variety of ML methods for evaluation. | Noisy data: The study did not consider the impact of noise and artifacts on the ECG signals. Single algorithm: The study only evaluated the performance of a single algorithm, KNN. |
| [71] | 2022 | Arrhythmia | Gradient boosting | ECGs from 18,612 CA success patients were examined. Four classification schemes are created and compared by ML models. IVA origin prediction has a high 98.24% accuracy, outperforming previous studies and human experts. | Single type: The study only considered a single type of idiopathic ventricular arrhythmia. Real-world: The study was not conducted in a real-world clinical setting. |
| [72] | 2022 | AF | Support vector machine | P-wave characteristics that indicate postoperative atrial fibrillation are the focus of this study. | Single-lead ECG: The study used a single-lead ECG, which may not be as sensitive as a 12-lead ECG. Retrospective data: The data were collected retrospectively, which may introduce bias. |
| [73] | 2021 | Ventricular arrhythmia | Support vector machine | The suggested deep learning approach for diagnosing VA makes use of Cubic SVM. 97.6% accuracy is achieved on the MIT-BIH dataset. | Requires ECG signal preprocessing: The proposed method requires ECG signal preprocessing, which can be time-consuming and error-prone. Not generalizable to all arrhythmias: The method was only evaluated on a small number of arrhythmias, so it is unclear how generalizable it is to other arrhythmias. |
| [74] | 2021 | Amyloid builds up in organs | KNN | This research aims to create an AI-powered tool to recognize cardiac amyloidosis by analyzing a conventional 12-lead electrocardiogram. | Unclear generalizability: How the results would generalize to other populations or settings is unclear. Need for validation: The model needs to be validated in a more extensive and more diverse population before it can be used in clinical practice. |
| [75] | 2021 | CVD | ML | Simulation modeling is used in the work to diagnose ECG disorders automatically. Includes waveform localization, denoising, and ML-based disease identification and feature extraction. | Noisy data: The study did not consider the impact of noise and artifacts on the ECG signals. Single model: The study only used a single machine learning model, which may not be generalizable to other datasets or conditions. |
| [76] | 2021 | Arrhythmia | Support vector machine | ECG descriptors of several types (LBP, wavelet, HOS, morphological) were extracted. MRFO-SVM hybrid for feature selection and classification (MRFO + SVM). Performance enhanced using MIT-BIH Arrhythmia database training. | Limited features: The study only considered a limited number of features, such as the QRS complex and the ST segment. Not interpretable: The model is not interpretable, making it difficult to understand why it makes its predictions. |
| [77] | 2020 | Arrhythmia | Support vector machine | A method for arrhythmia identification using ECG readings is introduced. Uses average energy, zero-crossing values, feature extraction, and SVM classification during preprocessing. Evaluated using the QT, normal sinus rhythm, and MIT-BIH arrhythmia databases. | Limited features: The study only considered two features, average energy and zero-crossing, which may not be sufficient to detect all types of arrhythmias accurately. Noisy data: The study did not consider the impact of noise and artifacts on the ECG recordings, which could affect the accuracy of the detection algorithm. |
| [78] | 2020 | Arrhythmia | KNN | The study developed a computer-aided diagnostic system that automatically classifies and diagnoses seven types of cardiac arrhythmias by analyzing ECG signals. | Limited features: The study only considered a limited number of features, such as heart rate and rhythm. Noisy data: The study did not consider the impact of noise and artifacts on the ECG signals. |
| [79] | 2020 | Arrhythmia | Support vector machine | ECG data are used in the study to assess heart abnormalities that cause arrhythmias. After filtering and segmenting, the R-R interval, BPM, P wave, and QRS peak are extracted. SVM classifier validates the technique’s efficacy with 91% accuracy, improved precision, recall, and F1 score. | Noisy data: The study did not consider the impact of noise and artifacts on the ECG signals. Single classifier: The study only used a single machine learning classifier, which may not be the best approach for all arrhythmias. |
| [80] | 2020 | Myocardial Infarction | RF, SVM, KNN, and NN | The study proposes a new ECG-based MI detection method. Features that were extracted using PCA, statistics, entropy, and data augmentation. In terms of accuracy, sensitivity, specificity, and F1, random forests perform better than SVM, BPNN, and KNN. | Single lead: The study only used a single lead of the ECG signal, which may not be sufficient to detect myocardial infarction accurately. Noisy data: The study did not consider the impact of noise and artifacts on the ECG signals. |
| [81] | 2020 | CVD | Support vector machine | ECG fragment alignment and PCA classification method are introduced. ECG waveform analysis using the MIMIC III dataset is used for AF diagnosis in ICU patients. | Small dataset: The study used a small dataset of 200 patients, which may not represent the wider population. Limited features: The study only considered a limited number of features, such as ECG signals and medical records. |
| [82] | 2019 | AF. | Support vector machine, KNN. | The research described a technique for identifying AF in ICU patients by analyzing the ECG waveform data from the MIMIC III dataset. | The study only evaluated the algorithm’s performance on a single type of AF, so its performance may not be as good for other types of AF. The study did not consider the patient medical history or other clinical factors, which could also affect the diagnosis of AF. |
| [83] | 2019 | AF. | IRBF-RVM, SVM, KNN, Naïve Bayes, AdaBoost, RF | Research suggests using a 12-lead ECG to diagnose AF using the IRBF-RVM model. As diagnostic characteristics, RR intervals are employed. AF classification of 98.16% on ECG Channel II. | Not generalizable: The study was conducted on a single dataset and may not be generalizable to other populations. Sensitive to noise: The model is sensitive to noise in the ECG signals. |
| [84] | 2019 | AF | RF, KNN, SVM | Through comparison research, the study seeks to recommend the best AF classifier for coronary heartbeat detection. | Lack of diversity: The dataset was not diverse, consisting of only young, healthy patients. Noisy data: The ECG signals in the dataset were noisy, which could have affected the accuracy of the classifiers. |
| Reference | Year | Heart Disease | Deep Learning Algorithm | Summary |
|---|---|---|---|---|
| [87] | 2023 | General heart disease | CNN | The suggested system uses the ANOVA-F test as a feature selection technique to organize data efficiently. Using a Convolutional Neural Network Model, it classifies patient data, determines the severity of cardiac illnesses, and swiftly notifies clinicians. Accurate input data from ECG sensors and IoT devices are used. |
| [88] | 2023 | Arrhythmia | Generative Adversarial Network (GAN) | A hierarchical deep learning framework called MadeGAN is presented in this research for the interpretation of ECG signals. MadeGAN combines a Memory-Augmented Deep AutoEncoder with GAN to identify multi-class arrhythmias and anomaly detection. Experimental findings utilizing the MIT-BIH arrhythmia database show that the suggested model performs better than current approaches. |
| [89] | 2023 | Heart failure | ECG-AI model based on deep learning | The effectiveness of ECG-AI in predicting heart failure has been verified using real-world data; ECG-AI could potentially play a crucial role in accurately identifying patients. |
| [90] | 2023 | Arrhythmia | Bi-directional LSTM, CNN, intra LSTM | This study suggests the MTGBi-LSTM approach for categorizing arrhythmias to solve the overfitting issue in CNN. It uses multitask learning and global/intra-LSTM techniques to improve feature selection and avoid local optimum. The MTGBi-LSTM model outperforms the current AFibNet, achieving a 96.48% accuracy and a 97.73% sensitivity on the MIT-BIH dataset. |
| [91] | 2023 | Left/right BBB | CNN | This work emphasizes the usage of a feature picture. It provides a global feature extraction approach for categorizing multi-periodic heartbeats. Empirical Mode Decomposition (EMD) creates the feature picture, successfully depicting the "heartbeat condition." This feature picture is then used by a two-dimensional Convolutional Neural Network (CNN) to classify the patient’s heartbeat status accurately. The efficiency of the suggested technique is demonstrated by the experimental findings, which also indicate how well it can identify and analyze heartbeats. |
| [92] | 2023 | Myocardial infarction | EfficientNetV2B2-based transfer learning model | A new method for automatic detection of myocardial infarction (MI) using 12-lead ECG trace images is introduced in the article through an edge domain-based deep representation learning (DRL) approach. |
| [93] | 2023 | Ventricular arrhythmia | CNN | This study trained a 5-layer 1D convolutional neural network (CNN) using a selected dataset of 46,595 ECG recordings. The model outperformed comparable models in the literature when tested using cross-validation, held-out data, and an external dataset. Using brief, single-lead ECG data, the computationally effective CNN correctly diagnosed ventricular arrhythmia (VA), demonstrating the possibility for accelerated and enhanced patient treatment using MCT monitor data. |
| [94] | 2023 | Myocardial Infraction | DenseNet | Image augmentation techniques are applied to detect Normal, Abnormal Heartbeat and Myocardial infarction (MI) to balance the ECG image dataset classes. |
| [95] | 2023 | Arrhythmia | Conditional generative adversarial network | An accurate ECG is produced using the contactless cardiac monitoring device, RF-ECG, employing ultra-wideband signals. In addition to mapping mechanical-to-electrical cardiac activity, complementary restoration techniques restore pulse signal fluctuation. Results from experiments support its usefulness in identifying heart problems. |
| [96] | 2023 | Abnormal heart rate type | Temporal convolutional network | The paper proposes a novel ML architecture using temporal convolution networks (TCN) for classifying five heart diseases based on ECG signals. |
| [97] | 2023 | Arrhythmia | LSTM and RNN | An LSTM and RNN cascaded model for categorizing ECG signal abnormalities is presented in this study. The suggested model yields a 93.46% sensitivity, an 84.36% specificity, and an 89.9% accuracy. The comparison findings show that LSTM with RNN successfully analyzes ECG signals and is ideal for real-time produced data sequences like ECG. |
| [98] | 2023 | Arrhythmia | MLP | The DL algorithms for locating the earliest systolic activation sites were trained using a library of simulated paced beats and post-infarct VTs. The platform successfully localized VTs from implanted device intracardiac EGMs (mean LE = 13.10 2.36 mm) and surface ECGs (mean LE = 9.61 2.61 mm). Clinical VT localization was in line with the targets for ablation. |
| [99] | 2023 | Abnormal beat classification | DNN | This work suggests a personalized deep learning method for identifying ECG beats. Preprocessing employs EMD to break down beats into intrinsic mode functions (IMFs) and segment the signal based on R-peaks. A deep learning-based bespoke model is used to recognize beats utilizing certain IMFs. The method accurately detects ECG beats by removing important data using convolutional layers with various steps. |
| [100] | 2023 | Abnormal heart rate type | AlexNet, CNN, LSTMs, and ResNet50V2 | The article introduces a practical and reliable approach for determining the average heart rate by analyzing the reflected light on the skin. |
| [101] | 2022 | LVH | LSTM and CNN | Using a 12-lead ECG, a CNN-LSTM DL model was created to identify left ventricular hypertrophy (LVH). One thousand eight hundred sixty-three patients’ echocardiogram and ECG data were examined, and the model was developed and evaluated using various patient subgroups according to gender and relative wall thickness. |
| [102] | 2022 | Arrhythmia | CNN, LSTM, autoencoder | Utilizing a network layer design based on LSTM, the team optimized an autoencoder structure that increases the precision of arrhythmia classification when used alongside their ECG preprocessing process. |
| [103] | 2021 | AF | CNN and LSTM | Using ECG signals and deep learning-based techniques in the time-frequency domain to identify AF and differentiate between terminating and non-terminating AF episodes. |
| [104] | 2021 | Premature ventricular contraction | G.R.U., CNN | In order to enhance the recognition of PVC signals, the paper presents a modified gated recurrent unit model that integrates a scale parameter into the current bidirectional GRU model. |
| [105] | 2021 | Arrhythmia | LSTM and CNN | The article aimed to develop a wearable application that uses signal preprocessing and a deep learning model comprising 1-D CNN and LSTM. |
| Ref. | Year | Health Problem | Algorithm | Vital Parameter | Summary |
|---|---|---|---|---|---|
| [117] | 2023 | Obstructive sleep apnea (OSA) | CNN-transformer | Single channel ECG | From single-channel ECG data, OSA was identified using a CNN-Transformer architecture. The model consists of two components: a Transformer for global context modeling and a CNN for feature learning. The approach obtained an 88.2% accuracy and a 0.95 AUC for segment classification, while for recording classification, it reached a 100% accuracy and a 4.33 MAE. |
| [118] | 2023 | Obstructive sleep apnea | 1D CNN-LSTM | ECG signal | The initial model, which used a modified Alexnet architecture with a long short-term memory layer added, had an accuracy rate of 90.87%. The accuracy of the second model, ApneaNet, was 90.13% while using fewer processing resources. Both models showed promising results and the real-time ability to identify sleep apnea. |
| [119] | 2023 | Obstructive sleep apnea | Deep Belief Networks (DBN) | ECG signal and ECG-derived respiration | The technique used two types of Deep Belief Network (DBN) structures with Restricted Boltzmann Machines (RBM). The suggested method outperformed previous SLA detection techniques, achieving an accuracy of 89.11% for per-segment detection and 97.17% for per-recording detection. |
| [120] | 2023 | Obstructive sleep apnea | Multi-scaled fusion network | Single channel ECG | On an FAH-ECG dataset, a squeeze-and-excitation and multiscale fusion network (SE-MSCNN) algorithm demonstrated strong agreement with manual scoring and attained an accuracy of 86.6%. The SE-MSCNN may make creating wearable technology for effective obstructive sleep apnea screening easier. |
| [121] | 2023 | Obstructive sleep apnea | CNN and LSTM | ECG and PPG | 200 ECG and PPG signals were recorded for the investigation using publicly accessible apnea databases. The DC-LSTM network attained a 98.2% accuracy rate, a 97.4% sensitivity rate, a 97.5% specificity rate, and a 0.92 Kappa coefficient. These outcomes are equivalent to those of fully automated algorithms, making it appropriate for OSA e-health monitoring carried out at home. |
| [122] | 2022 | Respiration rate | Deep learning | ECG, PPG, and accelerometer | The study proposes a new multitasking deep learning network that simultaneously forecasts respiratory rate and waveform, which ultimately helps reduce error rates across different activities. |
| [123] | 2022 | Respiration rate | CNN-LSTM | ECG, PPG, and EMG | The models were assessed using two window sizes—32 s and 64 s—based on mean absolute error (MAE). The 64 s timeframe produced more accurate forecasts, according to the results. Bi-LSTM with Bahdanau attention outperformed the other models in terms of how well it handled the bio-signals, especially when it came to sEMG-based data (MAE of 0.51 0.03) and PPG/ECG-based data (MAE of 0.24 0.03). |
| [124] | 2023 | Fetal arrhythmia | Hierarchical extreme learning machine | Fetal ECG | This study introduces a multi-domain feature extraction approach to predict fetal arrhythmia accurately. The technique entails utilizing neighborhood component analysis (NCA) to screen sensitive data and a stacked ELM-SAE network for high-level fusion features. |
| [125] | 2022 | Fetal arrhythmia | Machine learning algorithm | Abdomen ECG | The framework uses approaches for noise reduction and pulls out important characteristics from various domains. The boosted decision tree classifier, one of four machine learning classifiers, achieves the maximum accuracy of 93.12%. |
| [126] | 2022 | Fetal heart rate | 1D-CNN | Fetal ECG | Fetal acidosis is correctly identified from FHR signals by a diagnostic method that uses a 1D-CNN model. With signal preprocessing, accuracy rises to 99.09% on a large dataset. Real-time use is made possible by integration with inexpensive electrical components. |
| [127] | 2022 | Fetal arrhythmia | Deep learning | Fetal ECG | A deep learning model divides segmented signals into standard and arrhythmic categories. The approach provides improved accuracy compared to traditional methods by concentrating on labeled segments, which lessens the influence of heartbeat detection findings. |
| [128] | 2023 | Blood pressure | Box–Cox transformation | ECG and PPG | The paper introduces the Box–Cox transformation technique to rectify label distribution and an end-to-end model called “BPNet” with a cross-modal fusion module capable of accurately capturing the relationship between multi-level ECG and PPG features. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan Mamun, M.M.R.; Elfouly, T. AI-Enabled Electrocardiogram Analysis for Disease Diagnosis. Appl. Syst. Innov. 2023, 6, 95. https://doi.org/10.3390/asi6050095
Khan Mamun MMR, Elfouly T. AI-Enabled Electrocardiogram Analysis for Disease Diagnosis. Applied System Innovation. 2023; 6(5):95. https://doi.org/10.3390/asi6050095
Chicago/Turabian StyleKhan Mamun, Mohammad Mahbubur Rahman, and Tarek Elfouly. 2023. "AI-Enabled Electrocardiogram Analysis for Disease Diagnosis" Applied System Innovation 6, no. 5: 95. https://doi.org/10.3390/asi6050095
APA StyleKhan Mamun, M. M. R., & Elfouly, T. (2023). AI-Enabled Electrocardiogram Analysis for Disease Diagnosis. Applied System Innovation, 6(5), 95. https://doi.org/10.3390/asi6050095







