Spectroscopic Measurements of Dissolved O3, H2O2 and OH Radicals in Double Cylindrical Dielectric Barrier Discharge Technology: Treatment of Methylene Blue Dye Simulated Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Protocols
2.1.1. Dielectric Barrier Discharge Experimental Procedure
2.1.2. Quantification of Dissolved Ozone and Hydrogen Peroxide in DCDBD Reactor
- Solution 1: About 0.5 mL of phosphoric acid was mixed with 310 mg of indigo trisulfonate in a volumetric flask and filled up to 500 mL with Millipore water.
- Solution 2: Measured 14 g of sodium hydrogen phosphate was mixed with 17.5 g of H3PO4 in a volumetric flask and filled up with Millipore water.
2.1.3. Effect of pH on the Production of OH Radicals in the DBD Reactor
2.1.4. Effect of Scavengers and Terephthalic Acid Probe Concentration on the Quenching of OH Radicals in DBD Reactor
2.1.5. Dielectric Barrier Discharge Experimental Degradation Protocol of MB Simulated Wastewater
3. Results
3.1. Effect of pH on Dissolved Ozone Concentration in DCDBD Reactor
3.2. Effect of pH on Hydrogen Peroxide Production in DBD Reactor
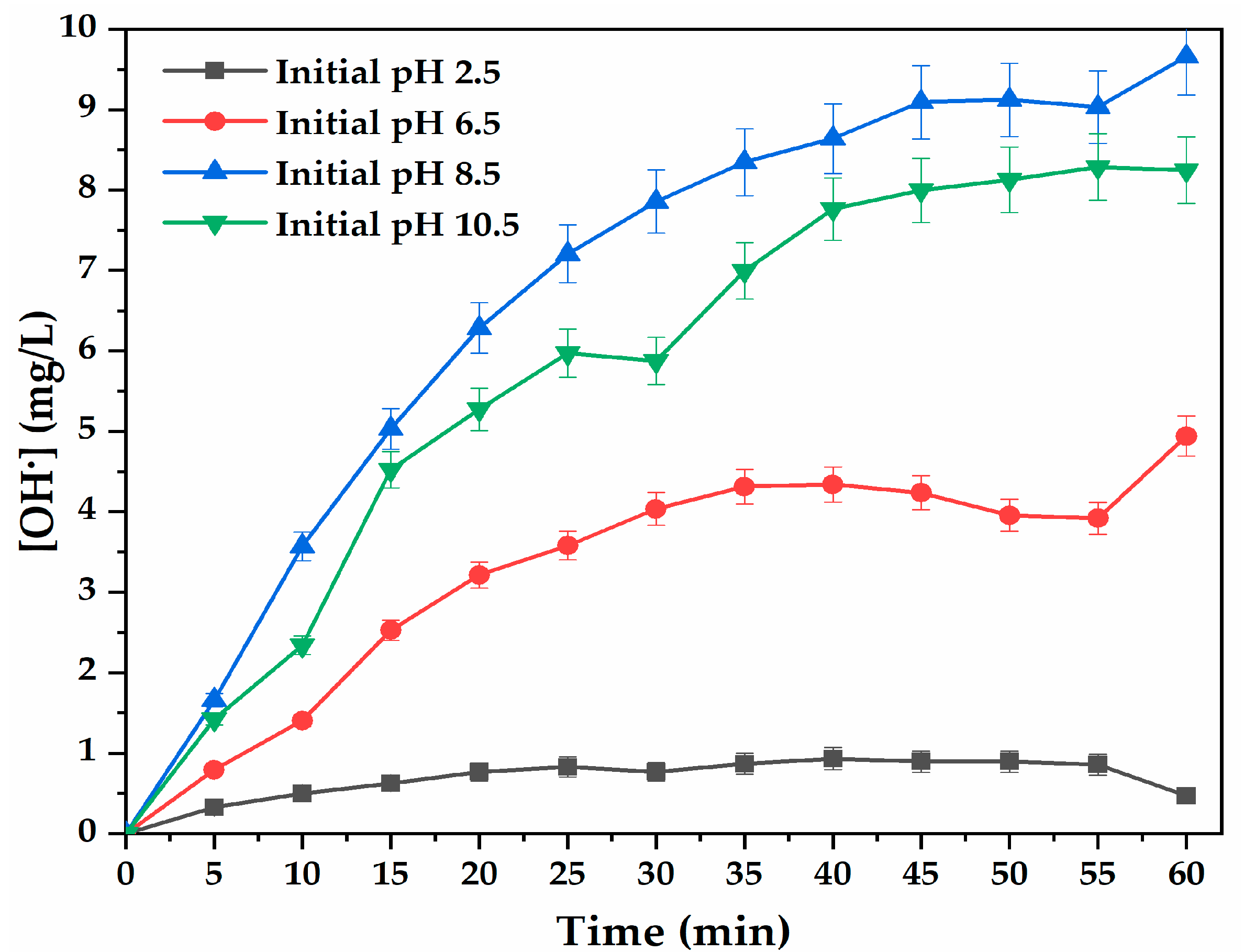
3.3. Effect of Solution pH on OH Production in the Absence of Buffer
3.4. Trends of Solution pH during Quantification of OH Radicals in the DBD Reactor
3.5. Effect of Initial Solution pH on OH Production in the Presence of Buffer
3.6. Effect of Sodium Carbonate on the Production of OH Radicals in DBD System
3.7. Effect of Sodium Chloride Scavenger on the Generation of OH Radicals
3.8. Effect of Chemical Probe Concentration on the Trapping of OH Radical
3.9. Decolouration of Simulated Methylene Blue by Optimized Double Cylindrical Dielectric Barrier Discharge: Effect of Working Parameters
3.9.1. Effect of Initial Concentration on Decolouration Rate of MB
3.9.2. Effect of pH on Decolouration of MB
3.9.3. Effect of Peak Voltage on Decolouration of MB
3.9.4. Ultraviolet-Visible Spectroscopy
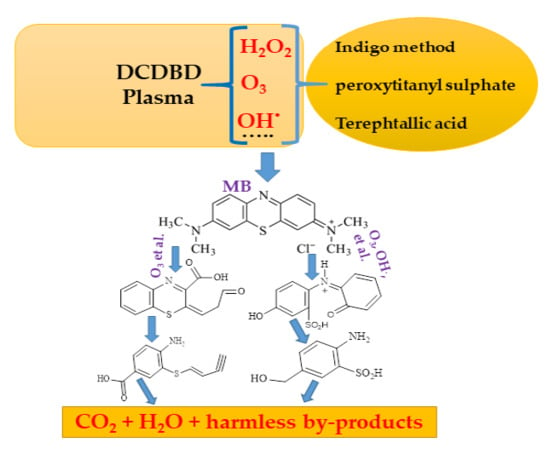
3.9.5. Liquid Chromatography/Mass Spectroscopy Analysis
4. Discussion
5. Conclusions
6. Novelty of the Study
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Weber, E.J.; Adams, R.L. Electrochemical monitoring of water remediation by metallic iron. Environ. Sci. Technol 1995, 29, 113. [Google Scholar]
- Mathur, N.; Bhatnagar, P. Mutagenicity assessment of textile dyes from Sanganer (Rajasthan). J.Environ. Biol. 2007, 28, 123–126. [Google Scholar] [PubMed]
- Wu, C.H.; Chern, J.M. Kinetics of photocatalytic decomposition of methylene blue. Ind. Eng. Chem. Res. 2006, 45, 6450–6457. [Google Scholar] [CrossRef]
- Rajeshwar, K.; Osugi, M.E.; Chanmanee, W.; Chenthamarakshan, C.R.; Zanoni, M.V.B.; Kajitvichyanukul, P.; Krishnan-Ayer, R. Heterogeneous photocatalytic treatment of organic dyes in air and aqueous media. J. Photochem. Photobiol. C Photochem. Rev. 2008, 9, 171–192. [Google Scholar] [CrossRef]
- Tan, B.H.; Teng, T.T.; Omar, A.K.M. Removal of dyes and industrial dye wastes by magnesium chloride. Water Res. 2000, 34, 597–601. [Google Scholar] [CrossRef]
- Chu, W. Dye removal from textile dye wastewater using recycled alum sludge. Water Res. 2001, 35, 3147–3152. [Google Scholar] [CrossRef]
- Koyuncu, I. Reactive dye removal in dye/salt mixtures by nanofiltration membranes containing vinylsulphone dyes: Effects of feed concentration and cross flow velocity. Desalination 2002, 143, 243–253. [Google Scholar] [CrossRef]
- El-Daly, H.A.; Habib, A.F.M.; El-Din, M.A.B. Kinetics and mechanism of the oxidative color removal from Durazol Blue 8 G with hydrogen peroxide. Dye. Pigment. 2003, 57, 197–210. [Google Scholar] [CrossRef]
- Ge, J.; Qu, J. Ultrasonic irradiation enhanced degradation of azo dye on MnO2. Appl. Catal. B Environ. 2004, 87, 252. [Google Scholar] [CrossRef]
- Malik, P.K. Dye removal from wastewater using activated carbon developed from sawdust: Adsorption equilibrium and kinetics. J. Hazard. Mater. 2004, 113, 81–88. [Google Scholar] [CrossRef]
- Kurbus, T.; Slokar, Y.M.; Le Marechal, A.M. The study of the effects of the variables on H2O2/UVdecoloration of vinylsulphone dye: Part II. Dye. Pigment. 2002, 54, 67–78. [Google Scholar] [CrossRef]
- Mok, Y.S.; Jo, J.O.; Whitehead, J.C. Degradation of an azo dye Orange II using a gas phase dielectric barrier discharge reactor submerged in water. Chem. Eng. J. 2008, 142, 56–64. [Google Scholar] [CrossRef]
- Magureanu, M.; Piroi, D.; Mandache, N.B.; David, V.; Medvedovici, A.; Parvulescu, V.I. Degradation of pharmaceutical compound pentoxifylline in water by non-thermal plasma treatment. Water Res. 2010, 44, 3445–3453. [Google Scholar] [CrossRef] [PubMed]
- Rong, S.; Sun, Y.; Zhao, Z.; Wang, H. Dielectric barrier discharge induced degradation of diclofenac in aqueous solution. Water Sci. Technol. 2014, 69, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, Y.; Hu, J.; He, J.; Mei, S.; Xue, G.; Ognier, S. Removal of iopromide from an aqueous solution using dielectric barrier discharge. J. Chem. Technol. Biotechnol. 2013, 88, 468–473. [Google Scholar] [CrossRef]
- Mouele, E.S.M.; Tijani, J.O.; Fatoba, O.O.; Petrik, L.F. Degradation of organic pollutants and microorganisms from wastewater using different dielectric barrier discharge configurations—A critical review. Environ. Sci. Pollut. Res. 2015, 22, 18345–18362. [Google Scholar] [CrossRef]
- Mouele, E.S.M.; Fatoba, O.O.; Babajide, O.; Badmus, K.O.; Petrik, L.F. Review of the methods for determination of reactive oxygen species and suggestion for their application in advanced oxidation induced by dielectric barrier discharges. Environ. Sci. Pollut. Res. 2018, 25, 9265–9282. [Google Scholar] [CrossRef]
- Nehra, V.; Kumar, A.; Dwivedi, H.K. Atmospheric Non-Thermal Plasma Sources. Int. J. Eng. 2008, 2, 53–68. [Google Scholar]
- Calderon, P.B.; Roberfroid, M.B. Free Radicals and Oxidation Phenomena in Biological Systems; CRC Press: New York, NY, USA, 1995. [Google Scholar]
- Sawyer, D.T.; Valentine, J.S. How super is superoxide? Acc. Chem. Res. 1981, 14, 393–400. [Google Scholar] [CrossRef]
- Sies, H.; Menck, C.F.M. Singlet oxygen induced DNA damage. Mutat. Res. 1992, 275, 367–375. [Google Scholar] [CrossRef]
- Rehman, A.U.; Cser, K.; Sass, L.; Vass, I. Characterization of singlet oxygen production and its involvement in photodamage of Photosystem II in the cyanobacterium Synechocystis PCC 6803 by histidine-mediated chemical trapping. Biochim. Biophys. Acta—Bioenerg 2013, 1827, 689–698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madhu, G.M.; Lourdu, M.A.; Raj, A.; Vasantha, K.; Pai, K. Titamium oxide (TiO2 ) assisted photocatalytic degradation of methylene blue. J. Environ. Biol. 2009, 30, 259–264. [Google Scholar] [PubMed]
- Joshi, K.M.; Shrivastava, V.S. Removal of methylene blue dye aqueous solution using photocatalysis. Int. J. Nano Dimens. 2011, 2, 241–252. [Google Scholar]
- Lee, H.; Park, S.H.; Kim, B.H.; Kim, S.J.; Kim, S.C.; Seo, S.G.; Jung, S.C. Contribution of Dissolved Oxygen to Methylene Blue Decomposition by Hybrid Advanced Oxidation Processes System. Int. J. Photoenergy 2012, 2012, 6. [Google Scholar] [CrossRef]
- Hameed, B.H.; Din, A.M.; Ahmad, A.L. Adsorption of methylene blue onto bamboo-based activated Carbon: Kinetics and equilibrium studies. J. Hazard. Mater. 2007, 141, 819–825. [Google Scholar] [CrossRef]
- Huang, Z.Y.; Chen, F.L.; Wang, H. Analysis of the degradation mechanism of methylene blue by atmospheric pressure dielectric barrier discharge plasma. Chem. Eng. J. 2010, 162, 250–256. [Google Scholar] [CrossRef]
- Magureanu, M.; Bradu, C.; Piroi, D.; Mandache, N.B.; Parvulescu, V. Pulsed corona discharge for degradation of methylene Blue in Water. Plasma Chem. Plasma Process. 2013, 33, 51–64. [Google Scholar] [CrossRef]
- Reddy, P.M.K.; Raju, B.R.; Karuppiah, J.; Reddy, E.L.; Subrahmanyam, C. Degradation and mineralization of methylene blue by dielectric barrier discharge non-thermal plasma reactor. Chem. Eng. J. 2013, 217, 41–47. [Google Scholar] [CrossRef]
- Tang, S.; Yuan, D.; Rao, Y.; Li, M.; Shi, G.; Gu, J.; Zhang, T. Percarbonate promoted antibiotic decomposition in dielectric barrier discharge plasma. J. Hazard. Mater. 2019, 366, 669–676. [Google Scholar] [CrossRef]
- Atkinson, R. Kinetics and mechanisms of the gas-phase reactions of the hydroxyl radical with organic compounds under atmospheric conditions. Chem. Rev. 1985, 85, 69–201. [Google Scholar] [CrossRef]
- Haugland, R.P. Handbook of Fluorescence Probes, 6th ed.; Molecular Probes: Eugene, OR, USA, 1996. [Google Scholar]
- Glaze, W.H. Reaction products of ozone: A review. Environ. Health Perspect. 1986, 69, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Gurol, M.D.; Vatistas, R. Oxidation of phenolic compounds by ozone and ozone and u.v. radiation: A comparative study. Water Res. 1987, 21, 895–900. [Google Scholar] [CrossRef]
- Ullmann Encyclopedia of Industrial Chemistry, 5th ed.; Ozone; Verlag Chemie: Einheim, Germany, 1991; Volume A18, pp. 349–357. ISBN 3-527-20118-1.
- Kirk, D.F.; Othmer, R.E. Encyclopedia of Chemical Technology, 4th ed.; Ozone; Wiley-Interscience: London, UK, 1996; pp. 953–994. ISBN 0-471-52686-X. [Google Scholar]
- Tarr, M. Chemical Degradation Methods for Wastes and Pollutants; Marcel Dekker Inc.: New York, NY, USA, 2003. [Google Scholar]
- Gupta, S.B. Investigation of a Physical Disinfection Process Based on Pulsed Underwater Corona Discharges. Ph.D. Thesis, Forschungszentrum Karlshrue, Karlshrue, Germany, 2007. ISSN 0947-8620 urn: nbn: de:0005-073362. [Google Scholar]
- Tichonovas, M.; Krugly, E.; Racys, V.; Hippler, R.; Kauneliene, V.; Stasiulaitiene, I.; Martuzevicius, D. Degradation of various textile dyes as wastewater pollutants under dielectric barrier discharge plasma treatment. Chem. Eng. J. 2013, 229, 9–19. [Google Scholar] [CrossRef]
- Marotta, E.; Schiorlin, M.; Ren, X.; Rea, M.; Paradisi, C. Advanced oxidation process for degradation of aqueous phenol in a dielectric barrier discharge reactor. Plasma Process. Polym. 2011, 8, 867–875. [Google Scholar] [CrossRef]
- Selma, M. Chemical Processes in Aqueous Phase Pulsed Electrical Discharges: Fundamental Mechanisms and Applications to Organic Compound Degradation. Ph.D. Thesis, Florida State University Libraries, Electronic Theses, Treatises and Dissertations, the Graduate School, Florida State University, Tallahassee, FL, USA, 2007. [Google Scholar]
- Kanazawa, S.; Kawano, H.; Watanabe, S.; Furuki, T.; Akamine, S.; Ichiki, R.; Ohkubo, T.; Kocik, M.; Mizeraczyk, J. Observation of OH radicals produced by pulsed discharges on the surface of a liquid. Plasma Sources Sci. Technol. 2011, 20, 034010. [Google Scholar] [CrossRef]
- Badmus, K.O.; Tijani, J.O.; Eze, C.P.; Fatoba, O.O.; Petrik, L.F. Quantification of Radicals Generated in a Sonicator. Analytical and Bioanalytical Chemistry Research. 2016, 3, 139–147. [Google Scholar]
- Sahni, M.; Locke, B.R. Quantification of hydroxyl radicals produced in aqueous phase pulsed electrical discharge reactors. Ind. Eng. Chem. Res. 2006, 45, 5819–5825. [Google Scholar] [CrossRef]
- Lopez, J.L. Dielectric Barrier Discharge, Ozone Generation, and their Applications. In Complex Plasmas Summer Institute; Saint Peter’s College: Jersey City, NJ, USA, 2008. [Google Scholar]
- Magureanu, M.; Dobrin, D.; Mandache, N.B.; Bradu, C.; Medvedovici, A.; Parvulescu, V.I. The mechanism of plasma destruction of enalapril and related metabolites in water. Plasma Process. Polym. 2013, 2013, 459 –468. [Google Scholar] [CrossRef]
- Massima, M.E.S. Water Treatment using Electrohydraulic Discharge System. MSc Thesis, University of the Western Cape, Western Cape, South Africa, 2014. [Google Scholar]
- Burlica, R.; Kirkpatrick, M.J.; Finney, W.C.; Clark, R.J.; Locke, B.R. Organic dye removal from aqueous solution by glidarc discharges. J. Electrostat. 2004, 62, 309–321. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, M.; Hao, X.; Lei, L. Degradation mechanisms of 4-chlorophenol in a novel gas–liquid hybrid discharge reactor by pulsed high voltage system with oxygen or nitrogen bubbling. Chemosphere 2007, 67, 702–711. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, H.; Zhang, Y. Decolorisation and mineralisation of CI Reactive Black 8 by the Fenton and ultrasound/ Fenton methods. Color Technol. 2007, 123, 101. [Google Scholar] [CrossRef]
- Dojčinović, B.P.; Roglić, G.M.; Obradović, B.M.; Kuraica, M.M.; Kostić, M.M.; Nešić, J.; Manojlović, D.D. Decolorization of reactive textile dyes using water falling film dielectric barrier discharge. J. Hazard. Mater. 2011, 192, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Tijani, J.O.; Mouele, M.E.S.; Tottito, T.C.; Fatoba, O.O.; Petrik, L.F. Degradation of 2-Nitrophenol by Dielectric Barrier Discharge System: The Influence of Carbon Doped TiO2 Photocatalyst Supported on Stainless Steel Mesh. Plasma Chem. Plasma Process. 2017, 37, 1343–1373. [Google Scholar] [CrossRef]
- Kochany, J.; Lipczynska-Kochany, E. Application of the EPR spin-trapping technique for the investigation of the reactions of carbonate, bicarbonate, and phosphate anions with hydroxyl radicals generated by the photolysis of H2O2. Chemosphere 1992, 25, 1769–1782. [Google Scholar] [CrossRef]
- Beltrán, F.J.; Aguinaco, A.; García-Araya, J.F.; Oropesa, A. Ozone and photocatalytic processes to remove the antibiotic sulfamethoxazole from water. Water Res. 2008, 42, 3799–3808. [Google Scholar] [CrossRef]
- Joshi, A.A.; Locke, B.R.; Arce, P.; Finney, W.C. Formation of hydroxyl radicals, hydrogen peroxide and aqueous electrons by pulsed streamer corona discharge in aqueous solution. J. Hazard. Mater. 1995, 41, 3–30. [Google Scholar] [CrossRef]
- Wang, J.; Qian, Q.; Chen, Q.; Liu, X.; Luo, Y.; Xue, H.; Li, Z. Significant role of carbonate radicals in tetracycline hydrochloride degradation based on solar light-driven TiO2-seashell composites: Removal and transformation pathways. Chin. J. Catal. 2020, 41, 1511–1521. [Google Scholar] [CrossRef]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical Review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (⋅OH/⋅O−in Aqueous Solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef] [Green Version]
- Wahyudiono; Mano, K.; Hayashi, Y.; Yamada, M.; Takahashi, S.; Takada, N.; Kanda, H.; Goto, M. Atmospheric-pressure pulsed discharge plasma in capillary slug flow system for dye decomposition. Chem. Eng. Process.-Process Intensif. 2019, 135, 133–140. [Google Scholar] [CrossRef]
- Şahin, S.; Demir, S.; Güҫer, C. Simultaneous UV-vis spectrophotometric determination of disperse dyes in textile wastewater by partial least squares and principal component regression. Dye. Pigm. 2007, 73, 368. [Google Scholar] [CrossRef]
- Abdullah, M.; Low, G.K.C.; Matthews, R.W. Effects of common inorganic anions on rates of photocatalytic oxidation of organic carbon over illuminated titanium dioxide. J. Phys. Chem. 1990, 94, 6820–6825. [Google Scholar] [CrossRef]
- Staehelin, J.; Hoigné, J. Reaktionsmechanismus und Kinetik des Ozonzerfalls in Wasser in Gegenwart organischer Stoffe. Vom Wasser 1983, 61, 337–348. [Google Scholar]
- Hoigne, J. The Chemistry of Ozone in Water. In Process Technologies for Water Treatment; Stucki, S., Ed.; Plenum Publishing Corporation: New York, NY, USA, 1988. [Google Scholar]
- Tschirch, J.; Dillert, R.; Bahnemann, D.; Proft, B.; Biedermann, A.; Goer, B. Photodegradation of methylene blue in water, a standard method to determine the activity of photocatalytic coatings? Res. Chem. Intermed. 2008, 34, 381–392. [Google Scholar] [CrossRef]
- Liebert, M.A.; Lee, B.; Liaw, W.; Lou, J. Photocatalytic Decolorization of Méthylène Blue in Aqueous TiO2 Suspension. Environ. Eng. Sci. 1999, 16, 165–175. [Google Scholar]
- Qifu, L.; Guohua, N.; Yiman, J.; Zhaojun, W.; Song, J.; Kefu, L. Special type of plasma dielectric barrier discharge reactor for direct ozonization of water and degradation of organic pollution Degradation of Alizarin Red by Hybrid Gas-Liquid Dielectric Barrier Discharge Treatment of Wastewater with High Conductivity by Pulsed Discharge Plasma. J. Phys. D Appl. Phys. 2008, 41, 85207. [Google Scholar]
- Xie, Y.; Chen, F.; He, J.; Zhao, J.; Wang, H. Photoassisted degradation of dyes in the presence of Fe3+ and H 2O2 under visible irradiation. J. Photochem. Photobiol. A Chem. 2000, 136, 235–240. [Google Scholar] [CrossRef]
- Neppolian, B.; Choi, H.C.; Sakthivel, S.; Arabindoo, B.; Murugesan, V. Solar light induced and TiO2 assisted degradation of textile dye reactive blue 4. Chemosphere 2002, 46, 1173–1181. [Google Scholar] [CrossRef]
- Haarstrick, E.H.A.; Kut, O.M. TiO2-Assisted Degradation of Environmentally Relevant Organic Compounds in Wastewater Using a Novel Fluidized Bed Photoreactor. Environ. Sci. Technol. 1996, 30, 817–824. [Google Scholar] [CrossRef]
- Lv, K.; Yu, J.; Deng, K.; Li, X.; Li, M. Effect of phase structures on the formation rate of hydroxyl radicals on the surface of TiO2. J. Phys. Chem. Solids 2010, 71, 519–522. [Google Scholar] [CrossRef]
- Hirakawa, T.; Yawata, K.; Nosaka, Y. Photocatalytic reactivity for O2{radical dot}-and OH{radical dot} radical formation in anatase and rutile TiO2 suspension as the effect of H2O2 addition. Appl. Catal. A Gen. 2007, 18, 3247–3254. [Google Scholar] [CrossRef]
- Liu, J.; Lagger, G.; Tacchini, P.; Girault, H.H. Generation of OH radicals at palladium oxide nanoparticle modified electrodes, and scavenging by fluorescent probes and antioxidants. J. Electroanal. Chem. 2008, 619, 131–136. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Jiang, X.; Hou, X. Dielectric barrier discharge molecular emission spectrometer as gas chromatographic detector for amines. Microchem. J. 2015, 119, 108–113. [Google Scholar] [CrossRef]
- Nakabayashi, Y.; Nosaka, Y. The pH dependence of OH radical formation in photo-electrochemical water oxidation with rutile TiO2 single crystals. Phys. Chem. Chem. Phys. 2015, 17, 30570–30576. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, H.; Akamine, S.; Ichiki, R.; Kanazawa, S. Comparison of OH Radical Concentration Generated by Underwater Discharge Using Two Methods. Int. J. Plasma Environ. Sci. Technol. 2016, 10, 24–28. [Google Scholar]
- Bianco, A.; Passananti, M.; Perroux, H.; Voyard, G.; Mouchel-Vallon, C.; Chaumerliac, N.; Mailhot, G.; Deguillaume, L.; Brigante, M. A better understanding of hydroxyl radical photochemical sources in cloud waters collected at the puy de Dôme station—Experimental versus modelled formation rates. Atmos. Chem. Phys. 2015, 15, 9191–9202. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, D.H.; Kuang, X.M.; Scott, J.A.; Rocha, G.O.; Paulson, S.E. Terephthalate Probe for Hydroxyl Radicals: Yield of 2-Hydroxyterephthalic Acid and Transition Metal Interference. Anal. Lett. 2018, 51, 2488–2497. [Google Scholar] [CrossRef]
- Lallement, A.; Vinatier, V.; Brigante, M.; Deguillaume, L.; Delort, A.M.; Mailhot, G. First evaluation of the effect of microorganisms on steady state hydroxyl radical concentrations in atmospheric waters. Chemosphere 2018, 212, 715–722. [Google Scholar] [CrossRef]
- Judée, F.; Simon, S.; Bailly, C.; Dufour, T. Plasma-activation of tap water Using DBD for agronomy applications: Identification and quantification of long lifetime chemical species and production/consumption mechanisms. Water Res. 2018, 133, 47–59. [Google Scholar] [CrossRef] [Green Version]
- Lide, D.R. Handbook of Chemistry and Physics, 79th ed.; Chemical Rubber Co.: Cleveland, OH, USA, ISBN Solubility Sel. Gases Water; 1999; Volume 8, pp. 8–86. [Google Scholar]
- Hoeben, W. Pulsed Corona-Iduced Degradation of Organic Materials in Water; Technische Universiteit Eindhoven: Eindhoven, The Netherlands, 2000. [Google Scholar]
- Jiang, B.; Zheng, J.; Qiu, S.; Wu, M.; Zhang, Q.; Yan, Z.; Xue, Q. Review on electrical discharge plasma technology for wastewater remediation. Chem. Eng. J. 2014, 236, 348–368. [Google Scholar] [CrossRef]
- Munter, R. Advanced oxidation processes –current status and prospects. Proc. Est. Acad. Sci. Chem. 2001, 50, 59–80. [Google Scholar]
- Jo, J.-O.; Mok, Y.S. In-situ production of ozone and ultraviolet light using a barrier discharge reactor for wastewater treatment. J. Zhejiang Univ. Sci. A 2009, 10, 1359–1366. [Google Scholar] [CrossRef]
- Inanloo, K.; Naddafi, K.; Mesdaghinia, A.; Nasseri, S.; Nodehi, R.N.; Rahimi, A. Optimization Of Operational Parameters for Decolorization And Degradation Of C. I. Reactive Blue 29 By Ozone. J. Environ. Health Sci. Eng. 2011, 8, 227–234. [Google Scholar]
- Reddy, P.M.K.; Subrahmanyam, C. Green approach for wastewater treatment-degradation and mineralization of aqueous organic pollutants by discharge plasma. Ind. Eng. Chem. Res. 2012, 51, 11097–11103. [Google Scholar] [CrossRef]
- Sugiarto, A.T.; Ito, S.; Ohshima, T.; Sato, M.; Skalny, J.D. Oxidative decoloration of dyes by pulsed discharge plasma in water. J. Electrostat. 2003, 58, 135–145. [Google Scholar] [CrossRef]
- Ince, N.H.; Tezcanli, G. Treatability of textile dye-bath effluents by advanced oxidation: Preparation for reuse. Water Sci. Technol. 1999, 40, 183. [Google Scholar] [CrossRef]
- Thagard, S.M.; Takashima, K.; Mizuno, A. Chemistry of the positive and negative electrical discharges formed in liquid water and above a gas-liquid surface. Plasma Chem. Plasma Process. 2009, 29, 455. [Google Scholar] [CrossRef]
- Lu, F.K.; Emanuel, G.; Panicker, P.K.; Satyanand, U.S.; Emanuel, G.; Svihel, B.T. Development of Corona Discharge Apparatus for Supersonic Flow Millimeter-scaled boats and flotillas View project Blast wave attenuation View project Development of Corona Discharge Apparatus for Supersonic Flow. AIAA Pap. 2003, 6925, 2003. [Google Scholar]
- Wang, C.; Yao, J. Decolorization of methylene blue with TiO2 sol via UV irradiation photocatalytic degradation. Int. J. Photoenergy 2010, 2010, 643182. [Google Scholar]
- Akishev, Y.; Trushkin, N.; Grushin, M.; Petryakov, A.; Karal’nik, V.; Kobzev, E.; Kholodenko, V.; Chugunov, V.; Kireev, G.; Rakitsky, Y.; et al. Inactivation of Microorganisms in Model Biofilms by an Atmospheric Pressure Pulsed Non-thermal Plasma. In Plasma for Bio-Decontamination, Medicine and Food Security; Machala, Z., Hensel, K., Akishev, Y., Eds.; NATO Science for Peace and Security Series A: Chemistry and Biology; Springer: Dordrecht, The Netherlands, 2012; ISBN 978-94-007-2851-6. [Google Scholar] [CrossRef]
- Magureanu, M.; Mandache, N.B.; Parvulescu, V.I. Degradation of organic dyes in water by electrical discharges. Plasma Chem. Plasma Process. 2007, 27, 589–598. [Google Scholar] [CrossRef]
- Magureanu, M.; Piroi, D.; Mandache, N.B.; Parvulescu, V. Decomposition of methylene blue in water using a dielectric barrier discharge: Optimization of the operating parameters. J. Appl. Phys. 2008, 104, 103306. [Google Scholar] [CrossRef]
- Wang, J.; Sun, Y.; Jiang, H.; Feng, J. Removal of caffeine from water by combining dielectric barrier discharge (DBD) plasma with goethite. J. Saudi Chem. Soc. 2017, 21, 545–557. [Google Scholar] [CrossRef] [Green Version]
- Bruno, G.; Heusler, T.; Lackmann, J.W.; von Woedtke, T.; Weltmann, K.D.; Wende, K. Cold physical plasma-induced oxidation of cysteine yields reactive sulfur species (RSS). Clin. Plasma Med. 2019, 14, 100083. [Google Scholar] [CrossRef]
- Patange, A.; Boehm, D.; Giltrap, M.; Lu, P.; Cullen, P.J.; Bourke, P. Assessment of the disinfection capacity and eco-toxicological impact of atmospheric cold plasma for treatment of food industry effluents. Sci. Total Environ. 2018, 631, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zhang, H.; Xu, Z.; Zhang, Z.; Cheng, C.; Ni, G.; Lan, Y.; Meng, Y.; Xia, W.; Chu, P.K. Preferential production of reactive species and bactericidal efficacy of gas-liquid plasma discharge. Chem. Eng. J. 2019, 362, 402–412. [Google Scholar] [CrossRef]
- Wang, B.; Dong, B.; Xu, M.; Chi, C.; Wang, C. Degradation of methylene blue using double-chamber dielectric barrier discharge reactor under different carrier gases. Chem. Eng. Sci. 2017, 168, 90–100. [Google Scholar] [CrossRef]
- Nguyen, D.B.; Trinh, Q.H.; Hossain, M.M.; Lee, W.G.; Mok, Y.S. Enhancement of plasma-assisted catalytic CO2 reforming of CH4 to syngas by avoiding outside air discharges from ground electrode. Int. J. Hydrog. Energy 2019. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, Q.; Ozkan, A.; Chattopadhyay, B.; Wallaert, G.; Baert, K.; Terryn, H.; Delplancke-Ogletree, M.P.; Geerts, Y.; Reniers, F. Atmospheric pressure dielectric barrier discharge synthesis of morphology-controllable TiO2 films with enhanced photocatalytic activity. Thin Solid Film. 2018, 664, 90–99. [Google Scholar] [CrossRef]
- Ramli, N.A.H.; Zaaba, S.K.; Mustaffa, M.T.; Zakaria, A.; Shahriman, A.B. Review on the development of plasma discharge in liquid solution. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2017; Volume 1824. [Google Scholar]
- Permata, Y.E.; Cahyani, R.A.; Karamah, E.F.; Bismo, S. Characteristic of phenol and 2,4-dichlorophenol synthetic wastewater degradation in a DBD (dielectric barrier discharge) reactor. J. Phys. Conf. Ser. 2019, 1349, 012073. [Google Scholar] [CrossRef]
- Suzuki, K.; Endo, T.; Fukushima, T.; Sato, A.; Suzuki, T.; Nakayama, T.; Suematsu, H.; Niihara, K. Controlling Oxygen Content by Varying Oxygen Partial Pressure in Chromium Oxynitride Thin Films Prepared by Pulsed Laser Deposition. Mater. Trans. 2013, 54, 1140–1144. [Google Scholar] [CrossRef] [Green Version]
- Fadda, A.; Barberis, A.; Sanna, D. Influence of pH, buffers and role of quinolinic acid, a novel iron chelating agent, in the determination of hydroxyl radical scavenging activity of plant extracts by Electron Paramagnetic Resonance (EPR). Food Chem. 2018, 240, 174–182. [Google Scholar] [CrossRef]
- Brandt, S.; Schütz, A.; Klute, F.D.; Kratzer, J.; Franzke, J. Dielectric barrier discharges applied for optical spectrometry. Spectrochim. Acta-Part B At. Spectrosc. 2016, 123, 6–32. [Google Scholar] [CrossRef]
- Brandt, S.; Klute, F.D.; Schütz, A.; Franzke, J. Dielectric barrier discharges applied for soft ionization and their mechanism. Anal. Chim. Acta 2017, 951, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Bianco, A.; Brigante, M.; Mailhot, G. UVA-UVB activation of hydrogen peroxide and persulfate for advanced oxidation processes: Efficiency, mechanism and effect of various water constituents. J. Hazard. Mater. 2018, 347, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Merouani, S.; Hamdaoui, O.; Saoudi, F.; Chiha, M.; Pétrier, C. Influence of bicarbonate and carbonate ions on sonochemical degradation of Rhodamine B in aqueous phase. J. Hazard. Mater. 2010, 175, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Pieczyńska, A.; Ossowski, T.; Bogdanowicz, R.; Siedlecka, E. Electrochemical degradation of textile dyes in a flow reactor: Effect of operating conditions and dyes chemical structure. Int. J. Environ. Sci. Technol. 2019, 16, 929–942. [Google Scholar] [CrossRef]
- Lei, Y.; Lu, J.; Zhu, M.; Xie, J.; Peng, S.; Zhu, C. Radical chemistry of diethyl phthalate oxidation via UV/peroxymonosulfate process: Roles of primary and secondary radicals. Chem. Eng. J. 2020, 379, 122339. [Google Scholar] [CrossRef]
- Damodar, R.A.; You, S.J.; Chou, H.H. Study the self cleaning, antibacterial and photocatalytic properties of TiO2 entrapped PVDF membranes. J. Hazard. Mater. 2009, 172, 1321–1328. [Google Scholar] [CrossRef]
- Zhang, R.; Liao, H.; Yang, J.; Fan, X.; Yang, B. A molecular beam mass spectrometric investigation of plasma assisted oxidation and pyrolysis of methane. Proc. Combust. Inst. 2019, 37, 5577–5586. [Google Scholar] [CrossRef]
- Turhan, Z.T.K.; Durukan, I.; Ozturkcan, S.A. Decolorization of textile basic dye in aqueous solution by ozone. Dye. Pigment. 2012, 92, 897–901. [Google Scholar] [CrossRef]
- Aziz, K.H.H.; Mahyar, A.; Miessner, H.; Mueller, S.; Kalass, D.; Moeller, D.; Khorshid, I.; Rashid, M.A.M. Application of a planar falling film reactor for decomposition and mineralization of methylene blue in the aqueous media via ozonation, Fenton, photocatalysis and non-thermal plasma: A comparative study. Process Saf. Environ. Prot. 2018, 113, 319–329. [Google Scholar] [CrossRef]
- Benzaouak, A.; Ellouzi, I.; Ouanji, F.; Touach, N.; Kacimi, M.; Ziyad, M.; El Mahi, M.; Lotfi, E.M. Photocatalytic degradation of Methylene Blue (MB) dye in aqueous solution by ferroelectric Li1-xTa1-xWxO3 materials. Colloids Surf. A Phys. Eng. Asp. 2018, 553, 586–592. [Google Scholar] [CrossRef]
- Son, G.; Kim, D.H.; Lee, J.S.; Kim, H.I.; Lee, C.; Kim, S.R.; Lee, H. Synchronized methylene blue removal using Fenton-like reaction induced by phosphorous oxoanion and submerged plasma irradiation process. J. Environ. Manag. 2018, 206, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Kim, D.W.; Park, D.W. Silver nanoparticle-loaded filter paper: Innovative assembly method by nonthermal plasma and facile application for the reduction of methylene blue. Surf. Coat. Technol. 2019, 366, 7–14. [Google Scholar] [CrossRef]
- Iervolino, G.; Vaiano, V.; Palma, V. Enhanced removal of water pollutants by dielectric barrier discharge non-thermal plasma reactor. Sep. Purif. Technol. 2019, 215, 155–162. [Google Scholar] [CrossRef]











| Compound | Molecular Structure | Chemical Structure | Retention Time (min) | M/Z |
|---|---|---|---|---|
| 7-isopropyl-N,N-dimethyl-5l4-phenothiazin-3-amine (I) | C17H20N2S |  | 4.138 | 284.4 |
| (E)-2-(3-oxopropylidene)-2H-benzo[b][1,4]thiazine-3-carboxylic acid (II) | C12H9NO3S |  | 1.922 | 247.3 |
| (Z)-6-hydroxy-2-(3-oxoprop-1-en-1-yl)-3-sulfinoquinolin-1-ium (III) | C12H10NO4S+ |  | 5.406 | 264.3 |
| (E)-4-amino-3-(but-1-en-3-yn-1-ylthio)benzoic acid (IV) | C11H9NO2S |  | 5.414 | 219.3 |
| 2-amino-5-(hydroxymethyl)benzenesulfinic acid (V) | C7H9NO3S |  | 5.790 | 187.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Massima Mouele, E.S.; Tijani, J.O.; Masikini, M.; Fatoba, O.O.; Eze, C.P.; Onwordi, C.T.; Zar Myint, M.T.; Kyaw, H.H.; Al-Sabahi, J.; Al-Abri, M.; et al. Spectroscopic Measurements of Dissolved O3, H2O2 and OH Radicals in Double Cylindrical Dielectric Barrier Discharge Technology: Treatment of Methylene Blue Dye Simulated Wastewater. Plasma 2020, 3, 59-91. https://doi.org/10.3390/plasma3020007
Massima Mouele ES, Tijani JO, Masikini M, Fatoba OO, Eze CP, Onwordi CT, Zar Myint MT, Kyaw HH, Al-Sabahi J, Al-Abri M, et al. Spectroscopic Measurements of Dissolved O3, H2O2 and OH Radicals in Double Cylindrical Dielectric Barrier Discharge Technology: Treatment of Methylene Blue Dye Simulated Wastewater. Plasma. 2020; 3(2):59-91. https://doi.org/10.3390/plasma3020007
Chicago/Turabian StyleMassima Mouele, Emile Salomon, Jimoh. O. Tijani, Milua Masikini, Ojo. O. Fatoba, Chuks P. Eze, Chionydua T. Onwordi, Myo Tay Zar Myint, Htet Htet Kyaw, Jamal Al-Sabahi, Mohammed Al-Abri, and et al. 2020. "Spectroscopic Measurements of Dissolved O3, H2O2 and OH Radicals in Double Cylindrical Dielectric Barrier Discharge Technology: Treatment of Methylene Blue Dye Simulated Wastewater" Plasma 3, no. 2: 59-91. https://doi.org/10.3390/plasma3020007
APA StyleMassima Mouele, E. S., Tijani, J. O., Masikini, M., Fatoba, O. O., Eze, C. P., Onwordi, C. T., Zar Myint, M. T., Kyaw, H. H., Al-Sabahi, J., Al-Abri, M., Dobretsov, S., Laatikainen, K., & Petrik, L. F. (2020). Spectroscopic Measurements of Dissolved O3, H2O2 and OH Radicals in Double Cylindrical Dielectric Barrier Discharge Technology: Treatment of Methylene Blue Dye Simulated Wastewater. Plasma, 3(2), 59-91. https://doi.org/10.3390/plasma3020007