Pulsed Discharge Plasma in High-Pressure Environment for Water Pollutant Degradation and Nanoparticle Synthesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
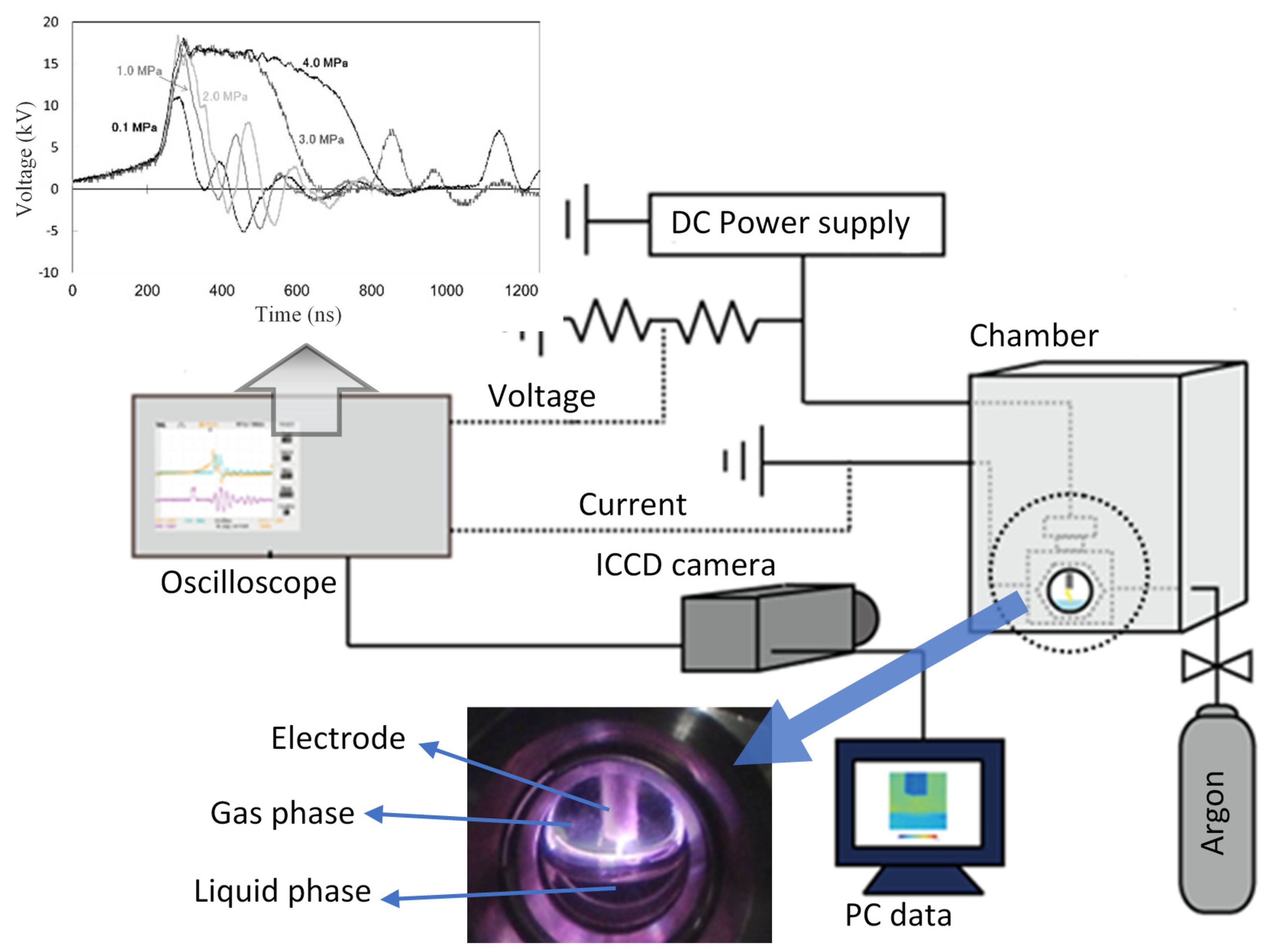
2.3. Experimental Procedure
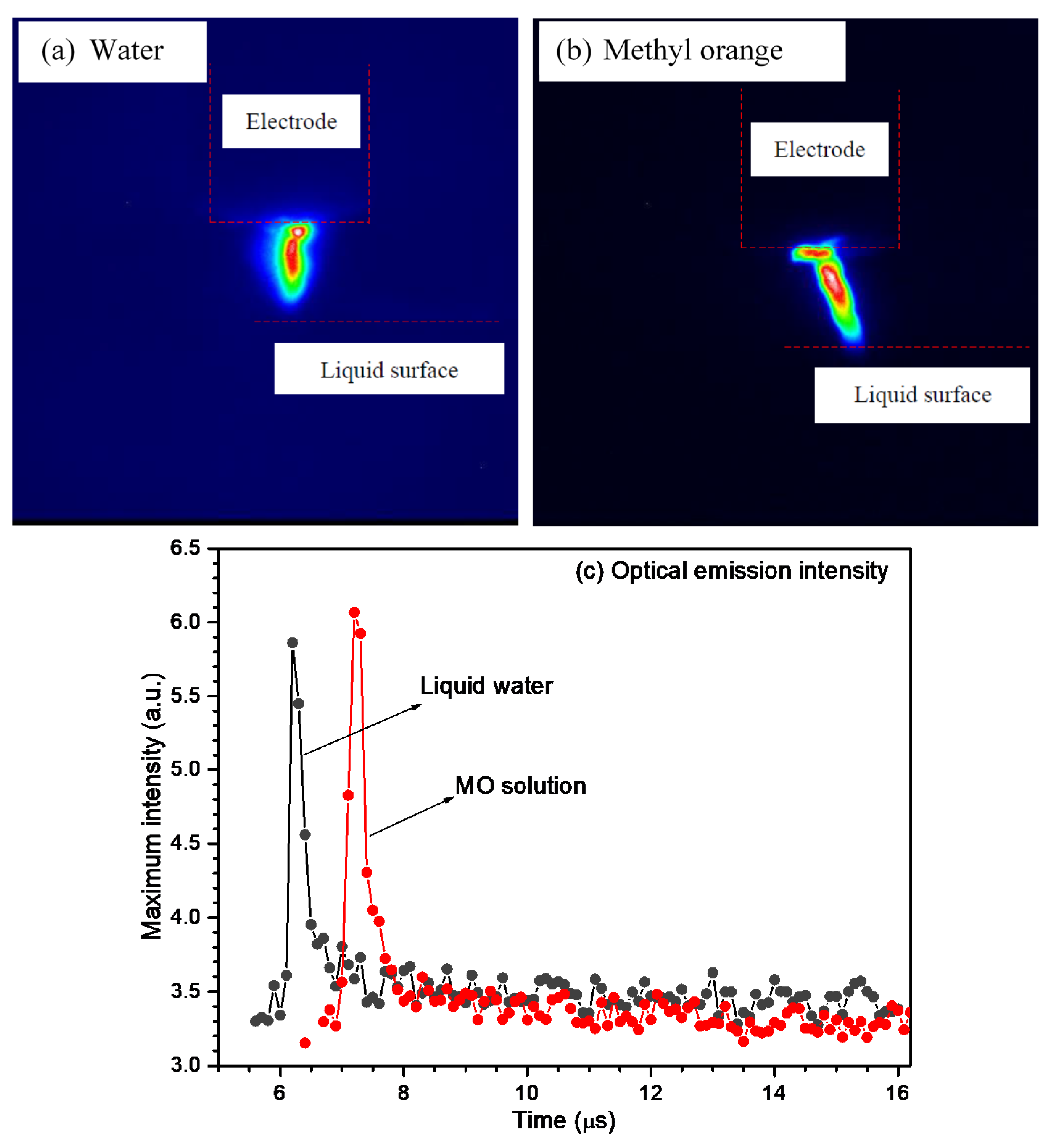
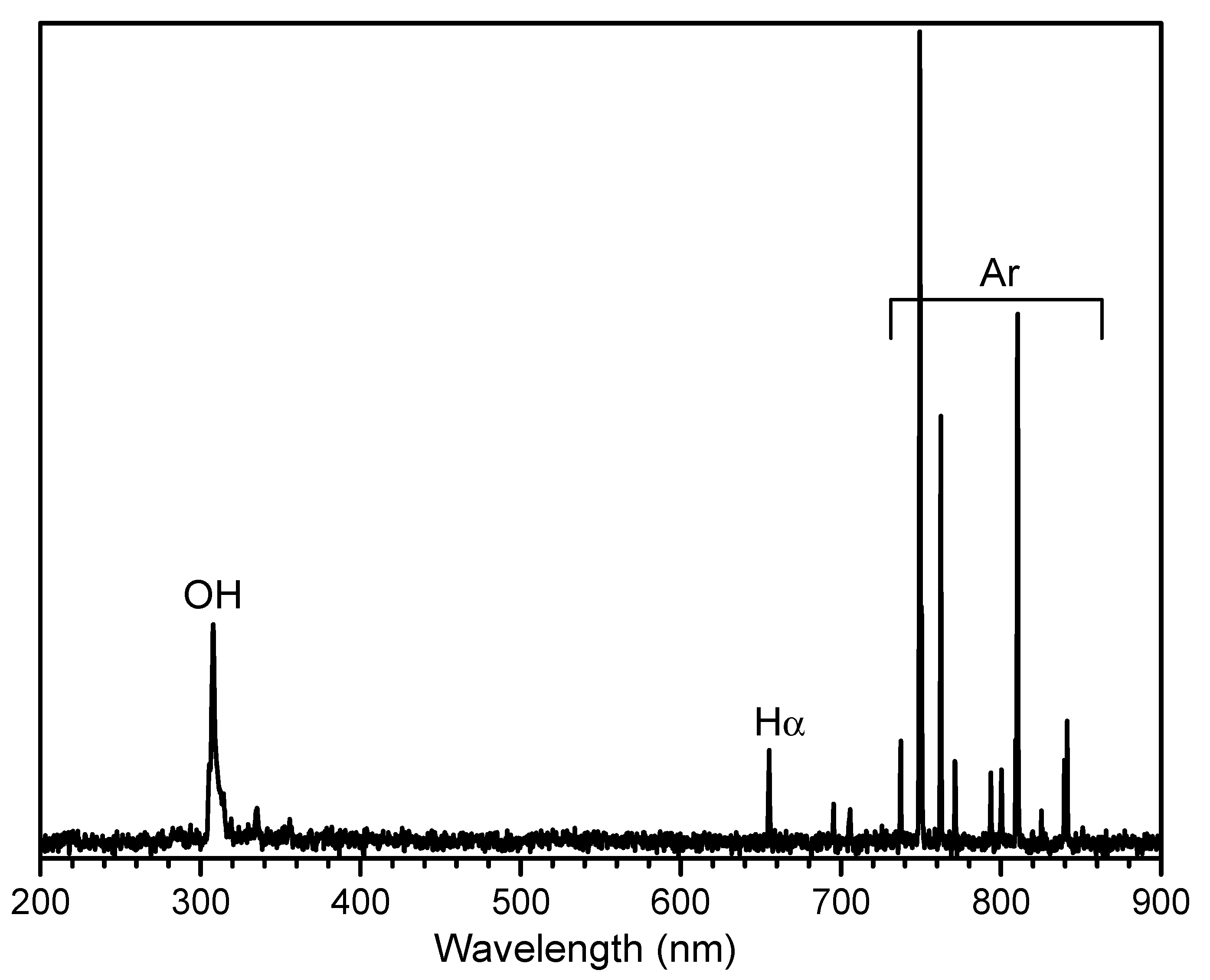
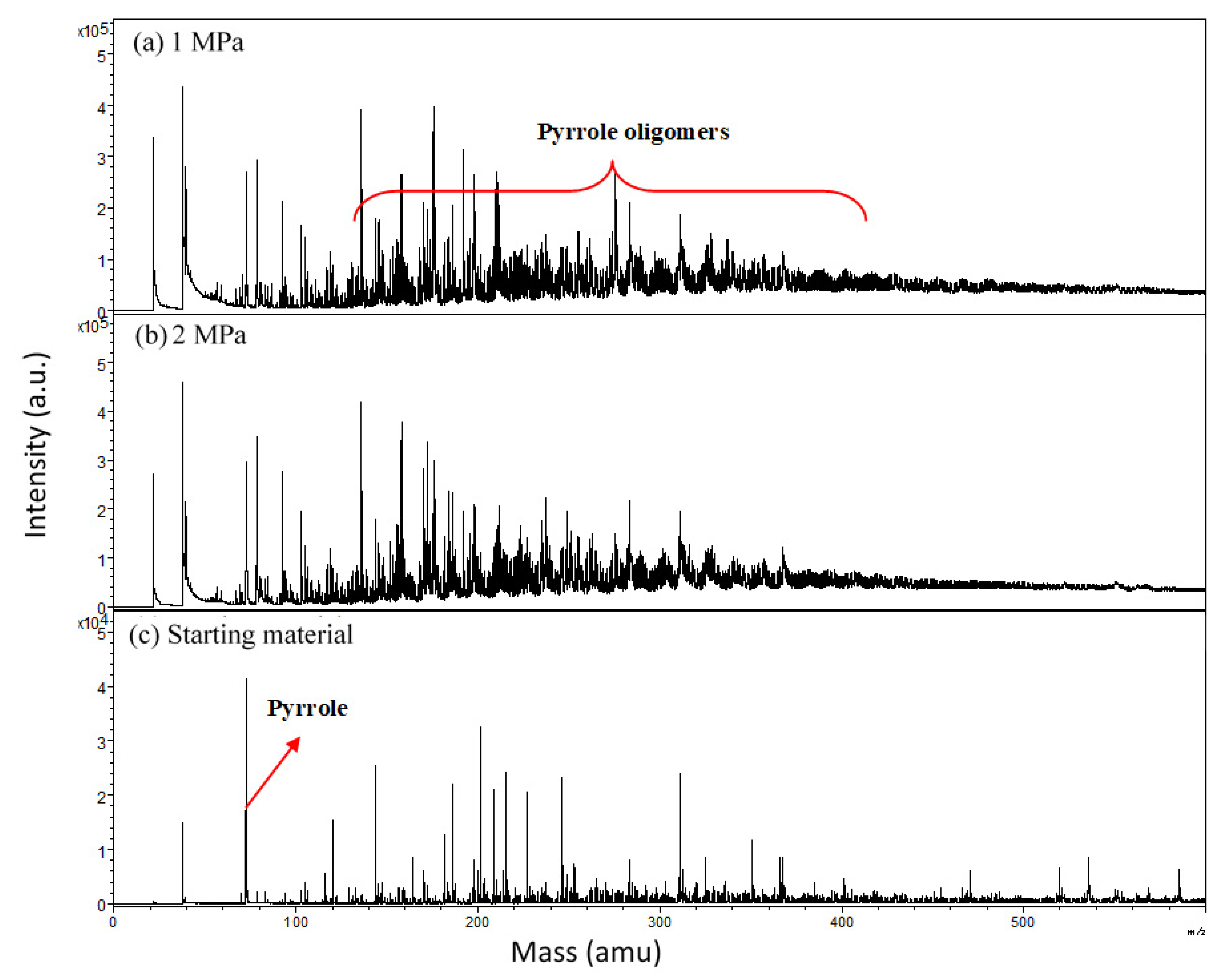
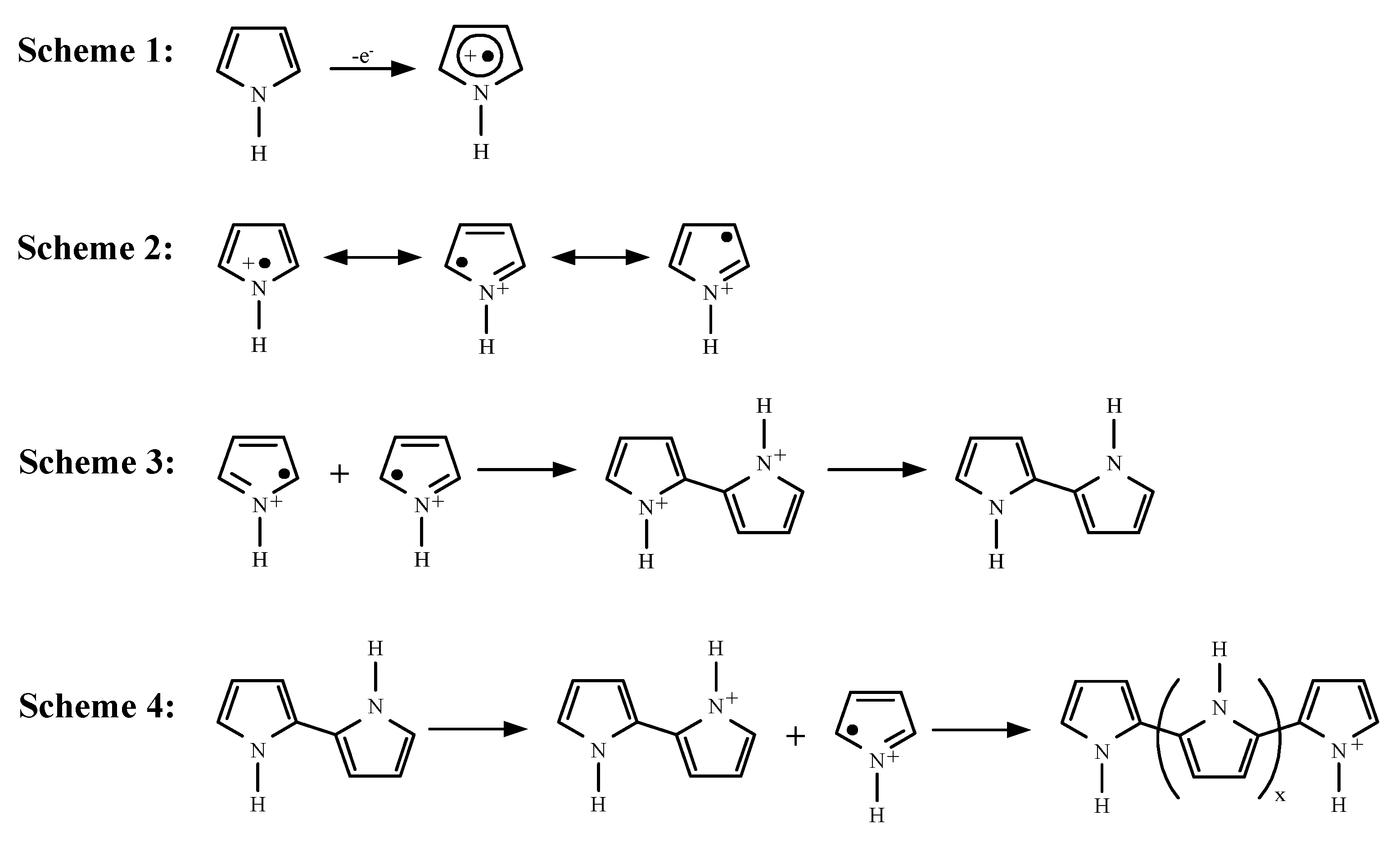
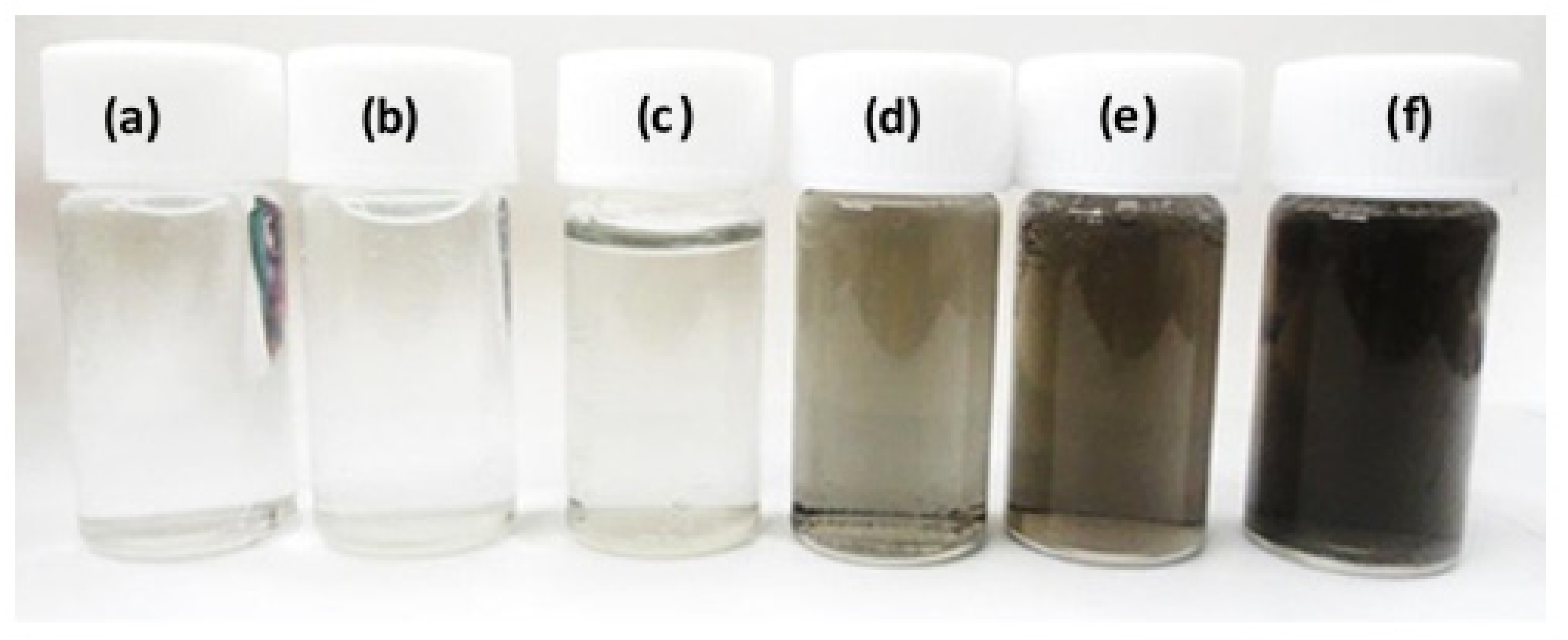
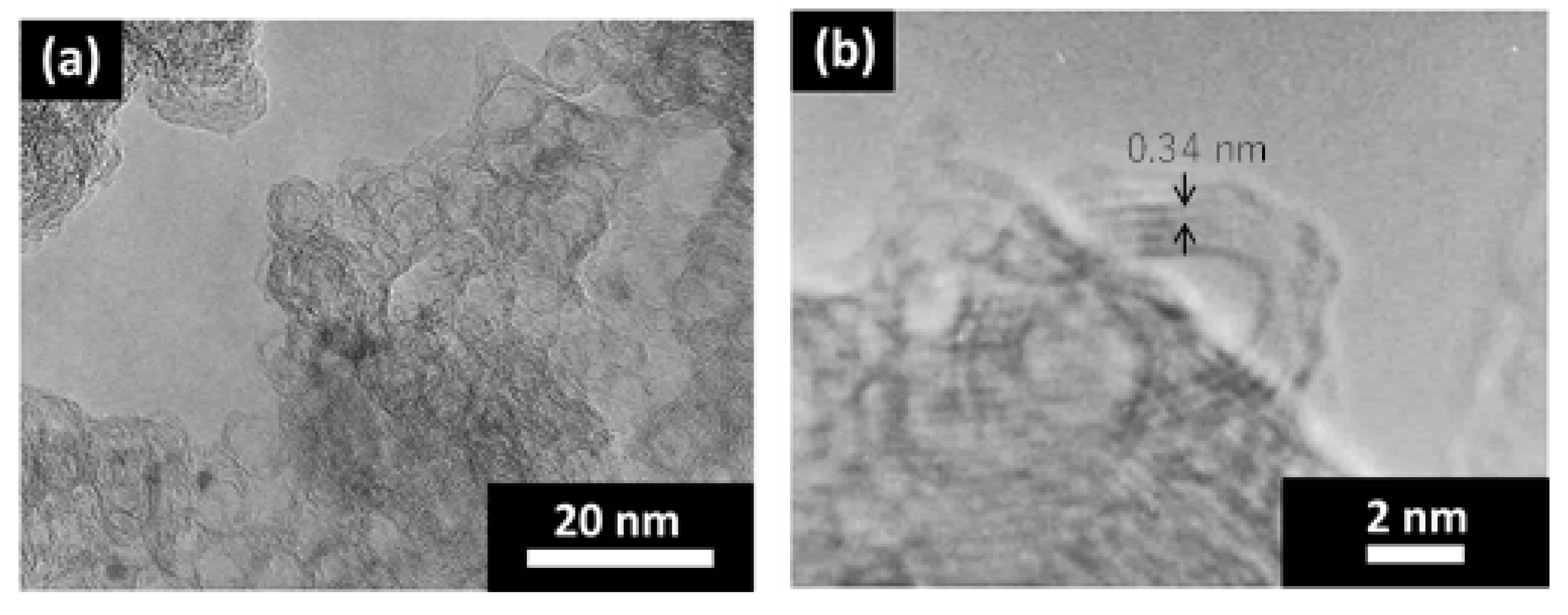
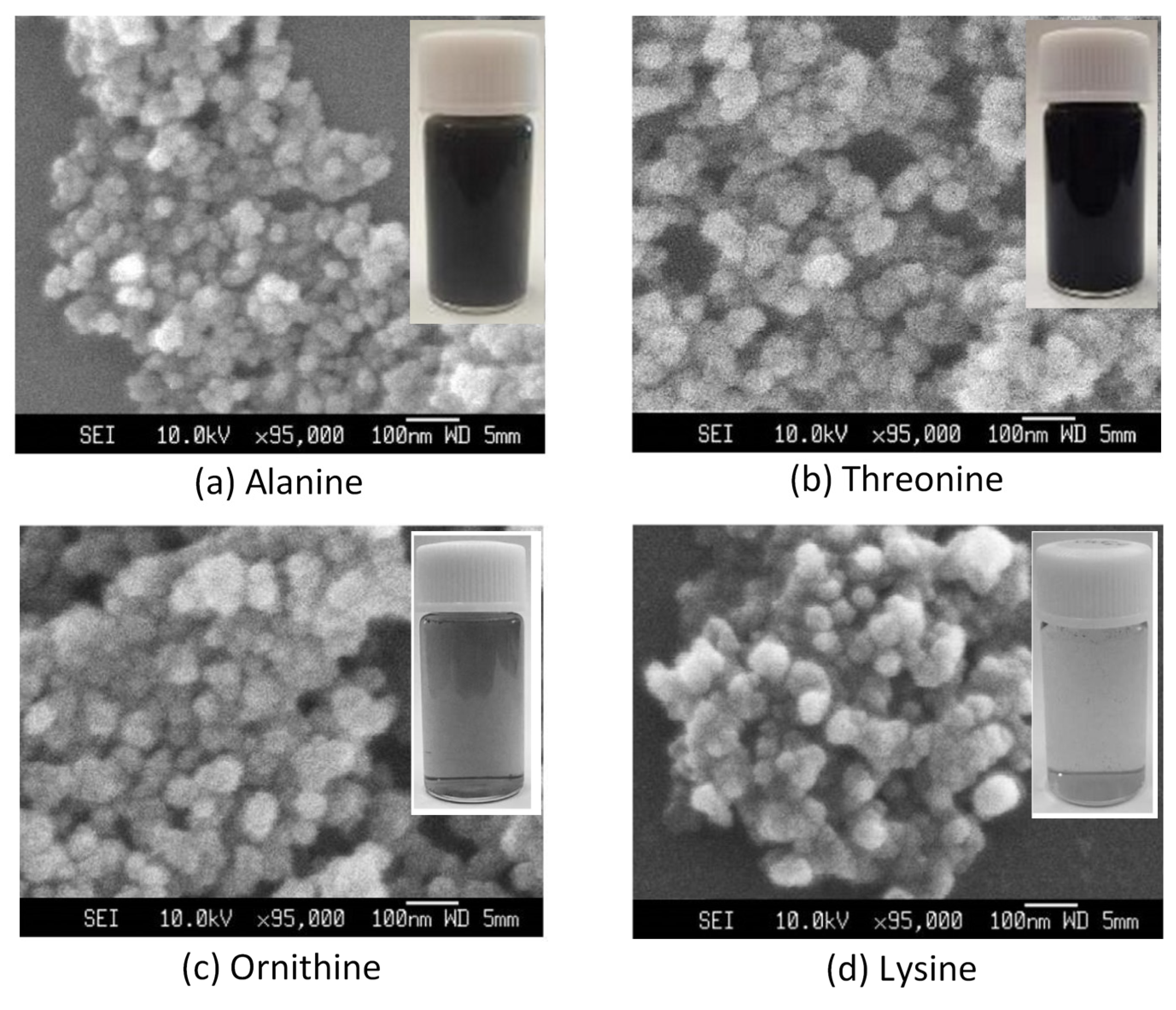
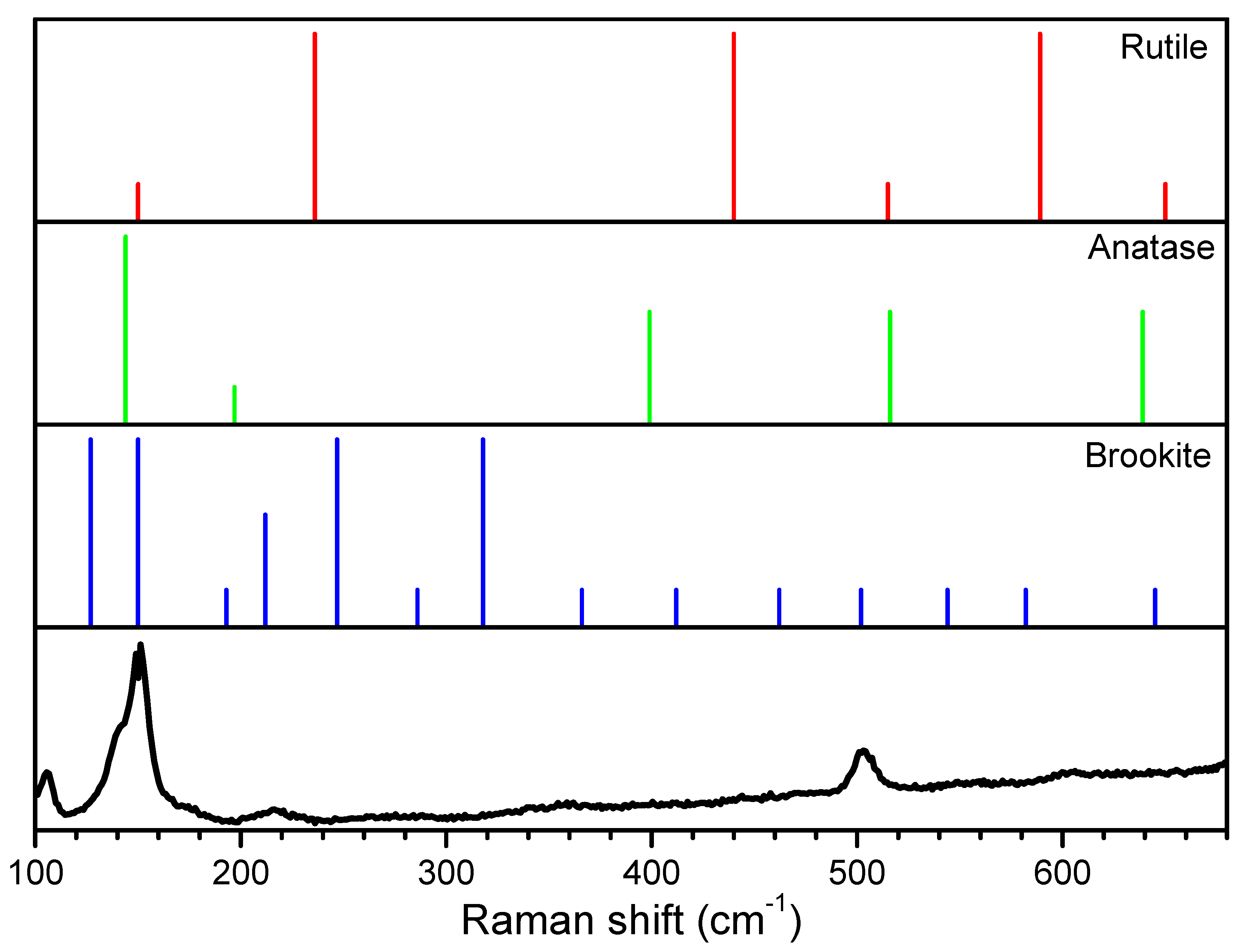
3. Results and Discussion
4. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Conrads, H.; Schmidt, M. Plasma generation and plasma sources. Plasma Sources Sci. Technol. 2000, 9, 441. [Google Scholar] [CrossRef]
- Lieberman, M.A.; Lichtenberg, A.J. Principles of Plasma Discharges and Materials Processing, 2nd ed.; John Wiley and Sons: Hoboken, NJ, USA, 2005; p. 7. [Google Scholar]
- Lu, X.; Reuter, S.; Laroussi, M.; Liu, D. Nonequilibrium atmospheric pressure plasma jets: Fundamentals, diagnostics, and medical applications; CRC Press: Boca Raton, FL, USA, 2019; pp. 9–26. [Google Scholar]
- Klimov, A.S.; Bakeev, I.Y.; Oks, E.M.; Tran, V.T.; Zenin, A.A. Plasma electron source for generating a ribbon beam in the forevacuum pressure range. Rev. Sci. Instrum. 2020, 91, 043505. [Google Scholar] [CrossRef]
- Zolotukhin, D.B.; Burdovitsin, V.A.; Oks, E.M. Plasma formation near the beam collector of a forevacuum−pressure plasma−cathode electron beam source. Phys. Plasma 2020, 27, 113509. [Google Scholar] [CrossRef]
- Locke, B.R.; Sato, M.; Sunka, P.; Hoffmann, M.R.; Chang, J.S. Electrohydraulic discharge and nonthermal plasma for water treatment. Ind. Eng. Chem. Res. 2006, 45, 882–905. [Google Scholar] [CrossRef]
- Bruggeman, P.; Leys, C. Non-thermal plasmas in and in contact with liquids. J. Phys. D: Appl. Phys. 2009, 42, 053001. [Google Scholar] [CrossRef]
- Parvulescu, V.I.; Magureanu, M.; Lukes, P. Plasma Chemistry and Catalysis in Gases and Liquids; John Wiley and Sons: Weinheim, Germany, 2012; pp. 185–188. [Google Scholar]
- Nishiyama, H.; Nagai, R.; Niinuma, K.; Takana, H. Characterization of DBD multiple bubble jets for methylene blue decolorization. JFST 2013, 8, 65–74. [Google Scholar] [CrossRef][Green Version]
- Guo, H.; Jiang, N.; Wang, H.; Lu, N.; Shang, K.; Li, J.; Wu, Y. Degradation of antibiotic chloramphenicol in water by pulsed discharge plasma combined with TiO2/WO3 composites: Mechanism and degradation pathway. J. Hazard. Mater. 2019, 371, 666–676. [Google Scholar] [CrossRef]
- Joshi, R.P.; Thagard, S.M. Streamer-Like Electrical Discharges in Water: Part II. Environmental Applications. Plasma Chem. Plasma Process 2013, 33, 17–49. [Google Scholar] [CrossRef]
- Fujita, H.; Kanazawa, S.; Ohtani, K.; Komiya, A.; Sato, T. Spatiotemporal analysis of propagation mechanism of positive primary streamer in water. J. Appl. Phys. 2013, 113, 113304. [Google Scholar] [CrossRef]
- Claverie, A.; Deroy, J.; Boustie, M.; Avrillaud, G.; Chuvatin, A.; Mazanchenko, E.; Demol, G.; Dramane, B. Experimental characterization of plasma formation and shockwave propagation induced by high power pulsed underwater electrical discharge. Rev. Sci. Instrum. 2014, 85, 063701. [Google Scholar] [CrossRef]
- Sun, B.; Xin, Y.; Zhu, X.; Gao, Z.; Yan, Z.; Ohshima, T. Effects of shock waves, ultraviolet light, and electric fields from pulsed discharges in water on inactivation of Escherichia coli. Bioelectrochemistry 2018, 120, 112–119. [Google Scholar] [CrossRef]
- Abramov, V.O.; Abramova, A.V.; Cravotto, G.; Nikonov, R.V.; Fedulov, I.S.; Ivanov, V.K. Flow−mode water treatment under simultaneous hydrodynamic cavitation and plasma. Ultrason. Sonochem. 2021, 70, 105323. [Google Scholar] [CrossRef]
- Fan, J.; Wu, H.; Liu, R.; Meng, L.; Sun, Y. Review on the treatment of organic wastewater by discharge plasma combined with oxidants and catalysts. Environ. Sci. Pollut. Res. 2020, 28, 2522–2548. [Google Scholar] [CrossRef]
- Akiyama, H.; Akiyama, M. Pulsed Discharge Plasmas in Contact with Water and their Applications. IEEJ Trans. Electr. Electron. Eng. 2021, 16, 6–14. [Google Scholar] [CrossRef]
- Kosinskaya, I.V.; Polozova, L.P. A continuum source for the near vacuum region of the spectrum. J. Appl. Spectrosc. 1969, 11, 1151–1152. [Google Scholar] [CrossRef]
- Takamatsu, T.; Uehara, K.; Sasaki, Y.; Miyahara, H.; Matsumura, Y.; Iwasawa, A.; Ito, N.; Azuma, T.; Kohno, M.; Okino, A. Investigation of reactive species using various gas plasmas. RSC Adv. 2014, 4, 39901–39905. [Google Scholar] [CrossRef]
- Strutynski, C.; Teule-Gay, L.; Danto, S.; Cardinal, T. Optical Emission Detector Based on Plasma Discharge Generation at the Tip of a Multimaterial Fiber. Sensors 2020, 20, 2353. [Google Scholar] [CrossRef]
- Mohades, S.; Lietz, A.M.; Kushner, M.J. Generation of reactive species in water film dielectric barrier discharges sustained in argon, helium, air, oxygen and nitrogen. J. Phys. D Appl. Phys. 2020, 53, 435206. [Google Scholar] [CrossRef]
- Jaiswal, S.; Aguirre, E.M. Comparison of atmospheric pressure argon producing O (1S) and helium plasma jet on methylene blue degradation. AIP Adv. 2021, 11, 045311. [Google Scholar] [CrossRef]
- Park, G.Y.; Park, S.J.; Choi, M.Y.; Koo, I.G.; Byun, J.H.; Hong, J.W.; Sim, J.Y.; Collins, G.J.; Lee, J.K. Atmospheric−pressure plasma sources for biomedical applications. Plasma Sources Sci. Technol. 2012, 21, 043001. [Google Scholar] [CrossRef]
- Bruggeman, P.J.; Iza, F.; Brandenburg, R. Foundations of atmospheric pressure non−equilibrium plasmas. Plasma Sources Sci. Technol. 2017, 26, 123002. [Google Scholar] [CrossRef]
- Brany, D.; Dvorska, D.; Halasova, E.; Skovierova, H. Cold atmospheric plasma: A powerful tool for modern medicine. Int. J. Mol. Sci. 2020, 21, 2932. [Google Scholar] [CrossRef] [PubMed]
- Ussenov, Y.A.; Hansen, L.; Krueger, T.; Ramazanov, T.S.; Kersten, H. Particle formation during deposition of SiOx nanostructured thin films by atmospheric pressure plasma jet. Jpn. J. Appl. Phys. 2020, 59, SHHE06. [Google Scholar] [CrossRef]
- Banerjee, S.; Adhikari, E.; Sapkota, P.; Sebastian, A.; Ptasinska, S. Atmospheric Pressure Plasma Deposition of TiO2: A Review. Materials 2020, 13, 2931. [Google Scholar] [CrossRef] [PubMed]
- Youssef, N.A.; Shaban, S.A.; Ibrahim, F.A.; Mahmoud, A.S. Degradation of methyl orange using Fenton catalytic reaction. Egypt. J. Pet. 2016, 25, 317–321. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Vieira, C.L.; Wolfson, J.M.; Pingtian, G.; Huang, S. Review on the treatment of organic pollutants in water by ultrasonic technology. Ultrason. Sonochem. 2019, 55, 273–278. [Google Scholar] [CrossRef]
- Kaur, K.; Badru, R.; Singh, P.P.; Kaushal, S. Photodegradation of organic pollutants using heterojunctions: A review. J. Environ. Chem. Eng. 2020, 8, 103666. [Google Scholar]
- Onga, L.; Kornev, I.; Preis, S. Oxidation of reactive azo−dyes with pulsed corona discharge: Surface reaction enhancement. J. Electrostat. 2020, 103, 103420. [Google Scholar] [CrossRef]
- Barrera, H.; Cruz−Olivares, J.; Frontana−Uribe, B.A.; Gomez−Diaz, A.; Reyes−Romero, P.G.; Barrera−Diaz, C.E. Electro−Oxidation-Plasma Treatment for Azo Dye Carmoisine (Acid Red 14) in an Aqueous Solution. Materials 2020, 13, 1463. [Google Scholar] [CrossRef]
- Hafeez, A.; Shezad, N.; Javed, F.; Fazal, T.; ur Rehman, M.S.; Rehman, F. Synergetic Effect of Packed-bed Corona-DBD Plasma Micro-Reactor and Photocatalysis for Organic Pollutant Degradation. Sep. Pur. Technol. 2021, 269, 118728. [Google Scholar] [CrossRef]
- Saito, G.; Hosokai, S.; Tsubota, M.; Akiyama, T. Nickel nanoparticles formation from solution plasma using edge-shielded electrode. Plasma Chem. Plasma Process. 2011, 31, 719–728. [Google Scholar] [CrossRef][Green Version]
- Jiang, L.; Li, Q.; Zhu, D.; Attoui, M.; Deng, Z.; Tang, J.; Jiang, J. Comparison of nanoparticle generation by two plasma techniques: Dielectric barrier discharge and spark discharge. Aerosol Sci. Technol. 2017, 51, 206–213. [Google Scholar] [CrossRef][Green Version]
- Merciris, T.; Valensi, F.; Hamdan, A. Synthesis of nickel and cobalt oxide nanoparticles by pulsed underwater spark discharges. J. Appl. Phys. 2021, 129, 063303. [Google Scholar] [CrossRef]
- Watanabe, H.; Machmudah, S.; Kiyan, T.; Sasaki, M.; Akiyama, H.; Goto, M. Pyrrole conversion induced pulse discharge plasma over a water surface under high-pressure argon. Chem. Eng. Process. Process Intensif. 2012, 61, 51–57. [Google Scholar] [CrossRef]
- Hayashi, Y.; Machmudah, S.; Takada, N.; Kanda, H.; Sasaki, K.; Goto, M. Decomposition of methyl orange using pulsed discharge plasma at atmospheric pressure: Effect of different electrodes. Jpn. J. Appl. Phys. 2013, 53, 010212. [Google Scholar] [CrossRef]
- Hayashi, Y.; Machmudah, S.; Kanda, H.; Takada, N.; Sasaki, K.; Goto, M. Removal of water pollutants by pulsed discharge plasma and observation of its optical emission intensity at atmospheric pressure. Jpn. J. Appl. Phys. 2013, 52, 11NE02. [Google Scholar] [CrossRef]
- Hayashi, Y.; Takada, N.; Kanda, H.; Goto, M. One-step synthesis of water-dispersible carbon nanocapsules by pulsed arc discharge over aqueous solution under pressurized argon. Res. Chem. Intermed. 2017, 43, 4201–4211. [Google Scholar] [CrossRef]
- Hayashi, Y.; Takada, N.; Kanda, H.; Goto, M. Synthesis of hydrophilic carbon nanoparticles from amino acids by pulsed arc discharge over aqueous solution in argon under near-critical pressure. J. Supercrit. Fluids 2017, 120, 403–407. [Google Scholar] [CrossRef]
- Hayashi, Y.; Diono, W.; Takada, N.; Kanda, H.; Goto, M. Glycine Oligomerization by Pulsed Discharge Plasma over Aqueous Solution under Atmospheric Pressure. ChemEngineering 2018, 2, 17. [Google Scholar] [CrossRef]
- Kondo, H.; Yamada, M.; Takada, N.; Machmudah, S.; Kanda, H.; Goto, M. Dc−Plasma over Aqueous Solution for Synthesis of Titanium Dioxide Nanoparticles under Pressurized Argon. ACS Omega 2020, 5, 5443–5451. [Google Scholar]
- Joshi, R.P.; Thagard, S.M. Streamer-like electrical discharges in water: Part I. Fundamental mechanisms. Plasma Chem. Plasma Process. 2013, 33, 1–15. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, L.; Zhang, C.; Nie, Y.; Wu, Y.; Guan, Z. Spectroscopic investigation of the bipolar pulsed discharge in water-air mixture. IEEE Trans. Plasma Sci. 2006, 34, 1033–1037. [Google Scholar] [CrossRef]
- Friedl, R.; Fantz, U. Spectral intensity of the N2 emission in argon low-pressure arc discharges for lighting purposes. New J. Phys. 2012, 14, 043016. [Google Scholar] [CrossRef]
- Chou, S.L.; Lo, J.I.; Peng, Y.C.; Lu, H.C.; Cheng, B.M.; Ogilvie, J.F. Emission spectra of atomic and molecular nitrogen from photolysis of ammonia in solid neon. AIP Adv. 2019, 9, 055311. [Google Scholar] [CrossRef]
- Sarani, A.; Nikiforov, A.Y.; Leys, C. Atmospheric pressure plasma jet in Ar and Ar/H2O mixtures: Optical emission spectroscopy and temperature measurements. Phys. Plasma. 2010, 17, 063504. [Google Scholar] [CrossRef]
- Hsieh, K.C.; Wang, H.; Locke, B.R. Analysis of electrical discharge plasma in a gas−liquid flow reactor using optical emission spectroscopy and the formation of hydrogen peroxide. Plasma Process. Polym. 2016, 13, 908–917. [Google Scholar] [CrossRef]
- Mano, K.; Hayashi, Y.; Yamada, M.; Takahashi, S.; Takada, N.; Kanda, H.; Goto, M. Atmospheric-pressure pulsed discharge plasma in capillary slug flow system for dye decomposition. Chem. Eng. Process. Process Intensif. 2019, 135, 133–140. [Google Scholar]
- Horvatic, V.; Muller, S.; Veza, D.; Vadla, C.; Franzke, J. Atmospheric helium capillary dielectric barrier discharge for soft ionization: Broadening of spectral lines, gas temperature and electron number density. J. Anal. At. Spectrom. 2014, 29, 498–505. [Google Scholar] [CrossRef]
- Prasertsung, I.; Kaewcharoen, S.; Kunpinit, K.; Yaowarat, W.; Saito, N.; Phenrat, T. Enhanced degradation of methylene blue by a solution plasma process catalyzed by incidentally co-generated copper nanoparticles. Water Sci. Technol. 2019, 79, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Bozic, A.L.; Koprivanac, N.; Sunka, P.; Clupek, M.; Babicky, V. Organic synthetic dye degradation by modified pinhole discharge. Czech. J. Phys. 2004, 54, C958. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Quan, X. Decoloration of azo dye by a multi-needle-to-plate high-voltage pulsed corona discharge system in water. J. Electrostat. 2006, 64, 416–421. [Google Scholar] [CrossRef]
- Maehara, T.; Miyamoto, I.; Kurokawa, K.; Hashimoto, Y.; Iwamae, A.; Kuramoto, H.; Yamashita, H.; Mukasa, S.; Toyota, H.; Nomura, S.; et al. Degradation of methylene blue by RF plasma in water. Plasma Chem. Plasma Process. 2008, 28, 467–482. [Google Scholar] [CrossRef]
- Mok, Y.S.; Jo, J.O.; Whitehead, J.C. Degradation of an azo dye Orange II using a gas phase dielectric barrier discharge reactor submerged in water. Chem. Eng. J. 2008, 142, 56–64. [Google Scholar] [CrossRef]
- Yano, T.; Shimomura, N.; Uchiyama, I.; Fukawa, F.; Teranishi, K.; Akiyama, H. Decolorization of indigo carmine solution using nanosecond pulsed power. IEEE Trans. Dielectr. Electr. Insul. 2009, 16, 1081–1087. [Google Scholar] [CrossRef]
- Stara, Z.; Krcma, F.; Nejezchleb, M.; Skalny, J.D. Organic dye decomposition by DC diaphragm discharge in water: Effect of solution properties on dye removal. Desalination 2009, 239, 283–294. [Google Scholar] [CrossRef]
- Li, J.; Wang, T.; Lu, N.; Zhang, D.; Wu, Y.; Wang, T.; Sato, M. Degradation of dyes by active species injected from a gas phase surface discharge. Plasma Sources Sci. Technol. 2011, 20, 034019. [Google Scholar] [CrossRef]
- Jiang, B.; Zheng, J.T.; Lu, X.; Liu, Q.; Wu, M.B.; Yan, Z.F.; Qiu, S.; Xue, Q.Z.; Wei, Z.X.; Xiao, H.J.; et al. Degradation of organic dye by pulsed discharge non−thermal plasma technology assisted with modified activated carbon fibers. Chem. Eng. J. 2013, 215, 969–978. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, L.; Shang, H.; Li, Q.; Zhou, W. Treatment of azo dye wastewater by the self−flocculating marine bacterium Aliiglaciecola lipolytica. Environ. Technol. Innov. 2020, 19, 100810. [Google Scholar] [CrossRef]
- Nawaz, N.; Ali, S.; Shabir, G.; Rizwan, M.; Shakoor, M.B.; Shahid, M.J.; Afzal, M.; Arslan, M.; Hashem, A.; Allah, E.F.A.; et al. Bacterial augmented floating treatment wetlands for efficient treatment of synthetic textile dye wastewater. Sustainability 2020, 12, 3731. [Google Scholar] [CrossRef]
- Cui, M.H.; Gao, L.; Lee, H.S.; Wang, A.J. Mixed dye wastewater treatment in a bioelectrochemical system-centered process. Bioresour. Technol. 2020, 297, 122420. [Google Scholar] [CrossRef]
- Ravadelli, M.; da Costa, R.E.; Lobo−Recio, M.A.; Akaboci, T.R.V.; Bassin, J.P.; Lapolli, F.R.; Belli, T.J. Anoxic/oxic membrane bioreactor assisted by electrocoagulation for the treatment of azo-dye containing wastewater. J. Environ. Chem. Eng. 2021, 9, 105286. [Google Scholar] [CrossRef]
- Gasparik, R.; Yamabe, C.; Ihara, S.; Satoh, S. Comparison of Copper and Stainless Steel Used for Low Voltage Electrode in Wire−to−Plane Electrode Configuration for NOx Treatment. Jpn. J. Appl. Phys. 1998, 37, 5786. [Google Scholar] [CrossRef]
- Morvova, M. Dc corona discharges in CO2-air and CO-air mixtures for various electrode materials. J. Phys. D Appl. Phys. 1998, 31, 1865. [Google Scholar] [CrossRef]
- Kornev, R.A.; Vorotyntsev, V.M.; Petukhov, A.N.; Razov, E.N.; Mochalov, L.A.; Trubyanov, M.M.; Vorotyntsev, A.V. Catalytic effects of electrode material on the silicon tetrachloride hydrogenation in RF-arc-discharge. RSC Adv. 2016, 6, 99816–99824. [Google Scholar] [CrossRef]
- Zhu, S.; Zhou, A.; Yu, F.; Dai, B.; Ma, C. Enhanced CO2 decomposition via metallic foamed electrode packed in self-cooling DBD plasma device. Plasma Sci. Technol. 2019, 21, 085504. [Google Scholar] [CrossRef]
- Wu, P.; Li, X.; Ullah, N.; Li, Z. Synergistic effect of catalyst and plasma on CO2 decomposition in a dielectric barrier discharge plasma reactor. Mol. Catal. 2021, 499, 111304. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Ramsden, C.A.; Joule, J.A.; Zhdankin, V.V. Handbook of Heterocyclic Chemistry, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2010; p. 420. [Google Scholar]
- Unaleroglu, C.; Temelli, B.; Tasgin, D.I. Access to pyrrole−based heterocyclic compounds via addition of pyrrole to C = C and C = N bonds. Pure Appl. Chem. 2014, 86, 925–932. [Google Scholar] [CrossRef]
- Sanchez, N.M.; de Klerk, A. Autoxidation of aromatics. Appl. Petrochem. Res. 2018, 8, 55–78. [Google Scholar] [CrossRef]
- Wetzel, S.J.; Guttman, C.M.; Girard, J.E. The influence of matrix and laser energy on the molecular mass distribution of synthetic polymers obtained by MALDI−TOF−MS. Int. J. Mass Spectrom. 2004, 238, 215–225. [Google Scholar] [CrossRef]
- Hosseini, S.; Martinez−Chapa, S.O. Fundamentals of MALDI−TOF−MS Analysis: Applications in Bio−Diagnosis, Tissue Engineering and Drug Delivery; Springer Nature: Singapore, 2016; pp. 1–32. [Google Scholar]
- Yoo, H.J.; Kim, D.H.; Shin, D.J.; Oh, Y.S.; Lee, S.J.; Lee, J.Y.; Choi, Y.J.; Lee, S.H.; Lee, K.S.; Kim, Y.S.; et al. Recent developments in pre−treatment and analytical techniques for synthetic polymers by MALDI−TOF mass spectrometry. Anal. Methods 2020, 12, 5767–5800. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Goto, M. Recovery of phenolic compounds through the decomposition of lignin in near and supercritical water. Chem. Eng. Process: Process Intensif. 2008, 47, 1609–1619. [Google Scholar]
- Sasaki, M.; Goto, M. Thermal decomposition of guaiacol in sub- and supercritical water and its kinetic analysis. J. Mater. Cycles Waste Manage 2011, 13, 68–79. [Google Scholar]
- Ding, W.; Zhou, J.; Zeng, Y.; Wang, Y.N.; Shi, B. Preparation of oxidized sodium alginate with different molecular weights and its application for crosslinking collagen fiber. Carbohydr. Polym. 2017, 157, 1650–1656. [Google Scholar] [CrossRef] [PubMed]
- Changsuwan, P.; Paksung, N.; Inoue, S.; Inoue, T.; Kawai, Y.; Noguchi, T.; Tanigawa, H.; Matsumura, Y. Conversion of guaiacol in supercritical water gasification: Detailed effect of feedstock concentration. J. Supercrit. Fluids 2018, 142, 32–37. [Google Scholar] [CrossRef]
- Ziero, H.D.D.; Buller, L.S.; Mudhoo, A.; Ampese, L.C.; Mussatto, S.I.; Carneiro, T.F. An overview of subcritical and supercritical water treatment of different biomasses for protein and amino acids production and recovery. J. Environ. Chem. Eng. 2020, 8, 104406. [Google Scholar] [CrossRef]
- Genies, E.M.; Bidan, G.; Diaz, A.F. Spectro electrochemical study of polypyrrole films. J. Electroanal. Chem. Interfacial Electrochem. 1983, 149, 101–113. [Google Scholar] [CrossRef]
- Balint, R.; Cassidy, N.J.; Cartmell, S.H. Conductive polymers: Towards a smart biomaterial for tissue engineering. Acta Biomater. 2014, 10, 2341–2353. [Google Scholar] [CrossRef]
- Pawar, P.M.; Ronge, B.P.; Balasubramaniam, R.; Seshabhattar, S. Techno-Societal 2016: Proceedings of the International Conference on Advanced Technologies for Societal Applications; Springer Nature: Cham, Switzerland, 2018; p. 662. [Google Scholar]
- Stejskal, J.; Trchova, M. Surfactants and amino acids in the control of nanotubular morphology of polypyrrole and their effect on the conductivity. Colloid Polym. Sci. 2020, 298, 319–325. [Google Scholar] [CrossRef]
- Diaz, A.F.; Crowley, J.; Bargon, J.; Gardini, G.P.; Torrance, J.B. Electrooxidation of aromatic oligomers and conducting polymers. J. Electroanal. Chem. Interfacial Electrochem. 1981, 121, 355–361. [Google Scholar] [CrossRef]
- Wang, S.; Xu, D.; Guo, Y.; Tang, X.; Wang, Y.; Zhang, J.; Ma, H.; Qian, L.; Li, Y. Supercritical Water Processing Technologies for Environment, Energy and Nanomaterial Applications; Springer Nature: Singapore, 2019; p. 47. [Google Scholar]
- Jiang, Z.; Li, Y.; Wang, S.; Cui, C.; Yang, C.; Li, J. Review on Mechanisms and Kinetics for Supercritical Water Oxidation Processes. Appl. Sci. 2020, 10, 4937. [Google Scholar] [CrossRef]
- Ishigami, M.; Cumings, J.; Zettl, A.; Chen, S. A simple method for the continuous production of carbon nanotubes. Chem. Phys. Lett. 2000, 319, 457–459. [Google Scholar] [CrossRef]
- Sano, N.; Wang, H.; Alexandrou, I.; Chhowalla, M.; Teo, K.B.K.; Amaratunga, G.A.J.; Iimura, K. Properties of carbon onions produced by an arc discharge in water. J. Appl. Phys. 2002, 92, 2783–2788. [Google Scholar] [CrossRef]
- Suehiro, J.; Imasaka, K.; Ohshiro, Y.; Zhou, G.; Hara, M.; Sano, N. Production of carbon nanoparticles using pulsed arc discharge triggered by dielectric breakdown in water. Jpn. J. Appl. Phys. 2003, 42, L1483. [Google Scholar] [CrossRef]
- Wang, S.D.; Chang, M.H.; Cheng, J.J.; Chang, H.K.; Ming-Der Lan, K. Unusual morphologies of carbon nanoparticles obtained by arc discharge in deionized water. Carbon 2005, 43, 1322–1325. [Google Scholar] [CrossRef]
- Okada, T.; Kaneko, T.; Hatakeyama, R. Conversion of toluene into carbon nanotubes using arc discharge plasmas in solution. Thin Solid Films 2007, 515, 4262–4265. [Google Scholar] [CrossRef]
- Scalese, S.; Scuderi, V.; Bagiante, S.; Simone, F.; Russo, P.; D’Urso, L.; Compagnini, G.; Privitera, V. Controlled synthesis of carbon nanotubes and linear C chains by arc discharge in liquid nitrogen. J. Appl. Phys. 2010, 107, 014304. [Google Scholar] [CrossRef]
- Kang, J.; Li, O.L.; Saito, N. Synthesis of structure−controlled carbon nano spheres by solution plasma process. Carbon 2013, 60, 292–298. [Google Scholar] [CrossRef]
- Zhong, R.; Hong, R. Continuous preparation and formation mechanism of few-layer graphene by gliding arc plasma. Chem. Eng. J. 2020, 387, 124102. [Google Scholar] [CrossRef]
- Puliyalil, H.; Jurkovic, D.L.; Dasireddy, V.D.; Likozar, B. A review of plasma-assisted catalytic conversion of gaseous carbon dioxide and methane into value-added platform chemicals and fuels. RSC Adv. 2018, 8, 27481–27508. [Google Scholar] [CrossRef]
- Vukusic, T.; Shi, M.; Herceg, Z.; Rogers, S.; Estifaee, P.; Thagard, S.M. Liquid-phase electrical discharge plasmas with a silver electrode for inactivation of a pure culture of Escherichia coli in water. Innov. Food Sci. Emerg. Technol. 2016, 38, 407–413. [Google Scholar] [CrossRef]
- Anikeev, V.; Fan, M. Supercritical Fluid Technology for Energy and Environmental Applications; Elsevier: Amsterdam, The Netherlands, 2013; p. 243. [Google Scholar]
- Wilhelm, E.; Battino, R.; Wilcock, R.J. Low-pressure solubility of gases in liquid water. Chem. Rev. 1977, 77, 219–262. [Google Scholar] [CrossRef]
- Kennan, R.P.; Pollack, G.L. Pressure dependence of the solubility of nitrogen, argon, krypton, and xenon in water. J. Chem. Phys. 1990, 93, 2724–2735. [Google Scholar] [CrossRef]
- Erofeev, M.V.; Kostyrya, I.D.; Tarasenko, V.F. Pulsed discharge in nitrogen and argon under an elevated pressure in a nonuniform electric field. Tech. Phys. 2007, 52, 1291. [Google Scholar] [CrossRef]
- De Gelder, J.; De Gussem, K.; Vandenabeele, P.; Moens, L. Reference database of Raman spectra of biological molecules. J. Raman Spectrosc. 2007, 38, 1133. [Google Scholar] [CrossRef]
- Kumar, C.S. Raman Spectroscopy for Nanomaterials Characterization; Springer Science and Business Media: Berlin/Heidelberg, Germany, 2012; p. 147. [Google Scholar]
- Thomas, S.; Thomas, R.; Zachariah, A.K.; Kumar, R. Spectroscopic Methods for Nanomaterials Characterization; Elsevier: Amsterdam, The Netherlands, 2017; Volume 2, pp. 95–127. [Google Scholar]
- Elashmawi, I.S.; Gaabour, L.H. Raman, morphology and electrical behavior of nanocomposites based on PEO/PVDF with multi−walled carbon nanotubes. Results Phys. 2015, 5, 105–110. [Google Scholar] [CrossRef]
- Rodriguez, L.A.A.; Pianassola, M.; Travessa, D.N. Production of TiO2 Coated Multiwall Carbon Nanotubes by the Sol-Gel Technique. Mater. Res. 2017, 20, 96–103. [Google Scholar] [CrossRef]
- Frost, R.L. An infrared and Raman spectroscopic study of the uranyl micas. Spectrochim. Acta Part A 2004, 60, 1469–1480. [Google Scholar] [CrossRef]
- Makarem, M.; Lee, C.M.; Kafle, K.; Huang, S.; Chae, I.; Yang, H.; Kubicki, J.D.; Kim, S.H. Probing cellulose structures with vibrational spectroscopy. Cellulose 2019, 26, 35–79. [Google Scholar] [CrossRef]
- Hadjiivanov, K.I.; Panayotov, D.A.; Mihaylov, M.Y.; Ivanova, E.Z.; Chakarova, K.K.; Andonova, S.M.; Drenchev, N.L. Power of Infrared and Raman Spectroscopies to Characterize Metal-Organic Frameworks and Investigate Their Interaction with Guest Molecules. Chem. Rev. 2021, 121, 1286–1424. [Google Scholar] [CrossRef]
- Panickar, R.; Sobhan, C.B.; Chakravorti, S. Chemical vapor deposition synthesis of carbon spheres: Effects of temperature and hydrogen. Vacuum 2020, 172, 109108. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Q.; Xu, K.; Kaneko, T.; Hatakeyama, R. Synthesis of graphene nanosheets from petroleum asphalt by pulsed arc discharge in water. Chem. Eng. J. 2013, 215, 45–49. [Google Scholar] [CrossRef]
- Kharissova, O.V.; Kharisov, B.I. Variations of interlayer spacing in carbon nanotubes. RSC Adv. 2014, 4, 30807–30815. [Google Scholar] [CrossRef]
- Bodnar, W.; Schiorlin, M.; Frank, A.; Schulz, T.; Wohrl, N.; Miron, C.; Scheu, C.; Kolb, J.F.; Kruth, A. Synthesis of grapheme-related carbon nanoparticles from a liquid isopropanol precursor by a one-step atmospheric plasma process. Appl. Surf. Sci. 2020, 514, 145926. [Google Scholar] [CrossRef]
- Phan, P.Q.; Naraprawatphong, R.; Pornaroontham, P.; Park, J.; Chokradjaroen, C.; Saito, N. N-Doped few-layer graphene encapsulated Pt-based bimetallic nanoparticles via solution plasma as an efficient oxygen catalyst for the oxygen reduction reaction. Mater. Adv. 2021, 2, 322–335. [Google Scholar] [CrossRef]
- Chandra, V.; Park, J.; Chun, Y.; Lee, J.W.; Hwang, I.C.; Kim, K.S. Water-dispersible magnetite-reduced graphene oxide composites for arsenic removal. ACS Nano 2010, 4, 3979–3986. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Lu, Y.; Wang, Y.; Zhang, L.; Wang, W. Preparation and characterization of dopamine-decorated hydrophilic carbon black. Appl. Surf. Sci. 2012, 258, 5387–5393. [Google Scholar] [CrossRef]
- Sakudo, A.; Chou, H.; Ikuta, K.; Nagatsu, M. Integration of antibody by surface functionalization of graphite-encapsulated magnetic beads using ammonia gas plasma technology for capturing influenza A virus. Bioorg. Med. Chem. 2015, 25, 1876–1879. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Wei, J.; Pan, J.; Huang, P.; Guo, S.; Zhang, J.; Zhang, X.; Feng, B. The up-converted photoluminescence and cell imaging of water-soluble carbondots. Chem. Phys. Lett. 2015, 638, 196–200. [Google Scholar] [CrossRef]
- Fujii, N.; Takata, T.; Fujii, N.; Aki, K.; Sakaue, H. D−Amino acids in protein: The mirror of life as a molecular index of aging. Biochim. Biophys. Acta Proteins Proteom. 2018, 1866, 840–847. [Google Scholar] [CrossRef]
- Hemmler, D.; Roullier-Gall, C.; Marshall, J.W.; Rychlik, M.; Taylor, A.J.; Schmitt-Kopplin, P. Insights into the chemistry of non−enzymatic browning reactions in different ribose-amino acid model systems. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Goz, V.G.; Nagy, A.; Farkas, V.; Keszei, E.; Perczel, A. Unwanted hydrolysis or α/β-peptide bond formation: How long should the rate−limiting coupling step take? RSC Adv. 2019, 9, 30720–30728. [Google Scholar]
- Kumar, L.R.; Thimmalapura, V.; Panduranga, V.; Mahesh, M.; Ramana, P.V.; Sureshbabu, V.V. Copper catalyzed aryl amidation between Nα-Fmoc-protected amino-acid azides and aryl boronic acids. Synth. Commun. 2020, 50, 506–515. [Google Scholar] [CrossRef]
- Machmudah, S.; Sato, T.; Sasaki, M.; Goto, M. Silver nanoparticles generated by pulsed laser ablation in supercritical CO2 medium. High Press. Res. 2012, 32, 60–66. [Google Scholar] [CrossRef]
- Machmudah, S.; Takada, N.; Kanda, H.; Sasaki, K.; Goto, M. Fabrication of gold and silver nanoparticles with pulsed laser ablation under pressurized CO2. Adv. Nat. Sci. Nanosci. Nanotechnol. 2013, 4, 045011. [Google Scholar] [CrossRef]
- Tran, Q.H.; Nguyen, V.Q.; Le, A.T. Silver nanoparticles: Synthesis, properties, toxicology, applications and perspectives. Adv. Nat. Sci. Nanosci. Nanotechnol. 2013, 4, 033001. [Google Scholar] [CrossRef]
- Duhan, J.S.; Kumar, R.; Kumar, N.; Kaur, P.; Nehra, K.; Duhan, S. Nanotechnology: The new perspective in precision agriculture. Biotechnol. Rep. 2017, 15, 11–23. [Google Scholar] [CrossRef]
- Yamada, M.; Takahashi, S.; Takada, N.; Kanda, H.; Goto, M. Synthesis of Silver Nanoparticles by Atmospheric-Pressure Pulsed Discharge Plasma in Slug Flow System. Jpn. J. Appl. Phys. 2019, 58, 016001. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, H.M.N. Chemical, physical, and biological coordination: An interplay between materials and enzymes as potential platforms for immobilization. Coord. Chem. Rev. 2019, 388, 1–23. [Google Scholar] [CrossRef]
- Kolikov, V.A.; Kurochkin, V.E.; Panina, L.K.; Rutberg, A.F.; Rutberg, F.G.; Snetov, V.N.; Stogov, A.Y. Prolonged microbial resistance of water treated by a pulsed electrical discharge. Tech. Phys. 2007, 52, 263–270. [Google Scholar] [CrossRef]
- Saito, G.; Akiyama, T. Nanomaterial synthesis using plasma generation in liquid. J. Nanomater. 2015, 2015, 21. [Google Scholar] [CrossRef]
- Kim, K.S.; Kim, T.H. Nanofabrication by thermal plasma jets: From nanoparticles to low−dimensional nanomaterials. J. Appl. Phys. 2019, 125, 070901. [Google Scholar] [CrossRef]
- Arsov, L.D.; Kormann, C.; Plieth, W. Electrochemical synthesis and in situ Raman spectroscopy of thin films of titanium dioxide. J. Raman Spectrosc. 1991, 22, 573–575. [Google Scholar] [CrossRef]
- Tian, F.; Zhang, Y.; Zhang, J.; Pan, C. Raman spectroscopy: A new approach to measure the percentage of anatase TiO2 exposed (001) facets. J. Phys. Chem. C 2012, 116, 7515–7519. [Google Scholar] [CrossRef]
- Anitha, V.C.; Banerjee, A.N.; Joo, S.W. Recent developments in TiO2 as n-and p-type transparent semiconductors: Synthesis, modification, properties, and energy-related applications. J. Mater. Sci. 2015, 50, 7495–7536. [Google Scholar] [CrossRef]
- Monai, M.; Montini, T.; Fornasiero, P. Brookite: Nothing new under the sun? Catalysts 2017, 7, 304. [Google Scholar] [CrossRef]
- Srivatsa, K.M.K.; Bera, M.; Basu, A. Pure brookite titania crystals with large surface area deposited by plasma enhanced chemical vapour deposition technique. Thin Solid Films 2008, 516, 7443–7446. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Huang, X.; Li, Q.; Li, G. New insights into fluorinated TiO2 (brookite, anatase and rutile) nanoparticles as efficient photocatalytic redox catalysts. RSC Adv. 2015, 5, 34302–34313. [Google Scholar] [CrossRef]
- Alamelu, K.; Raja, V.; Shiamala, L.; Ali, B.J. Biphasic TiO2 nanoparticles decorated graphene nanosheets for visible light driven photocatalytic degradation of organic dyes. Appl. Surf. Sci. 2018, 430, 145–154. [Google Scholar] [CrossRef]
- Ge, M.; Cao, C.; Huang, J.; Li, S.; Chen, Z.; Zhang, K.Q.; Al-Deyab, S.S.; Lai, Y. A review of one-dimensional TiO2 nanostructured materials for environmental and energy applications. J. Mater. Chem. A 2016, 4, 6772–6801. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, H.; Andino, J.M.; Li, Y. Photocatalytic CO2 reduction with H2O on TiO2 nanocrystals: Comparison of anatase, rutile, and brookite polymorphs and exploration of surface chemistry. ACS Catal. 2012, 2, 1817–1828. [Google Scholar] [CrossRef]
- Zhou, P.; Xie, Y.; Liu, L.; Song, J.; Chen, T.; Ling, Y. Bicrystalline TiO2 heterojunction for enhanced organic photodegradation: Engineering and exploring surface chemistry. RSC Adv. 2017, 7, 16484–16493. [Google Scholar] [CrossRef]
- Di Paola, A.; Bellardita, M.; Palmisano, L. Brookite, the least known TiO2 photocatalyst. Catalysts 2013, 3, 36–73. [Google Scholar] [CrossRef]
- Jiang, B.; Zheng, J.; Qiu, S.; Wu, M.; Zhang, Q.; Yan, Z.; Xue, Q. Review on electrical discharge plasma technology for wastewater remediation. Chem. Eng. J. 2014, 236, 348. [Google Scholar] [CrossRef]
- Pfeiffer, T.V.; Feng, J.; Schmidt-Ott, A. New developments in spark production of nanoparticles. Adv. Powder Technol. 2014, 25, 56. [Google Scholar] [CrossRef]
- Kohut, A.; Villy, L.P.; Ajtai, T.; Geretovszky, Z.; Galbacs, G. The effect of circuit resistance on the particle output of a spark discharge nanoparticle generator. J. Aerosol Sci. 2018, 118, 59. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, X.; Qin, J.; Shen, X.; Yu, R.; Ma, M.; Liu, R. Porous carbon-doped TiO2 on TiC nanostructures for enhanced photocatalytic hydrogen production under visible light. J. Catal. 2017, 347, 36–44. [Google Scholar] [CrossRef]
- Haghighi, N.R.; Poursalehi, R. Effect of C/H and C/O ratios on the arc discharge synthesis of titanium carbide nano-particles in organic liquids. Appl. Nanosci. 2019, 9, 411–421. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diono, W.; Machmudah, S.; Kanda, H.; Zhao, Y.; Goto, M. Pulsed Discharge Plasma in High-Pressure Environment for Water Pollutant Degradation and Nanoparticle Synthesis. Plasma 2021, 4, 309-331. https://doi.org/10.3390/plasma4020021
Diono W, Machmudah S, Kanda H, Zhao Y, Goto M. Pulsed Discharge Plasma in High-Pressure Environment for Water Pollutant Degradation and Nanoparticle Synthesis. Plasma. 2021; 4(2):309-331. https://doi.org/10.3390/plasma4020021
Chicago/Turabian StyleDiono, Wahyu, Siti Machmudah, Hideki Kanda, Yaping Zhao, and Motonobu Goto. 2021. "Pulsed Discharge Plasma in High-Pressure Environment for Water Pollutant Degradation and Nanoparticle Synthesis" Plasma 4, no. 2: 309-331. https://doi.org/10.3390/plasma4020021
APA StyleDiono, W., Machmudah, S., Kanda, H., Zhao, Y., & Goto, M. (2021). Pulsed Discharge Plasma in High-Pressure Environment for Water Pollutant Degradation and Nanoparticle Synthesis. Plasma, 4(2), 309-331. https://doi.org/10.3390/plasma4020021







