The Promising Potential of Cold Atmospheric Plasma Therapies
Abstract
:1. Introduction
1.1. Establishment of the Subject Matter
1.2. Limitations and Current Problems in Plasma Medicine
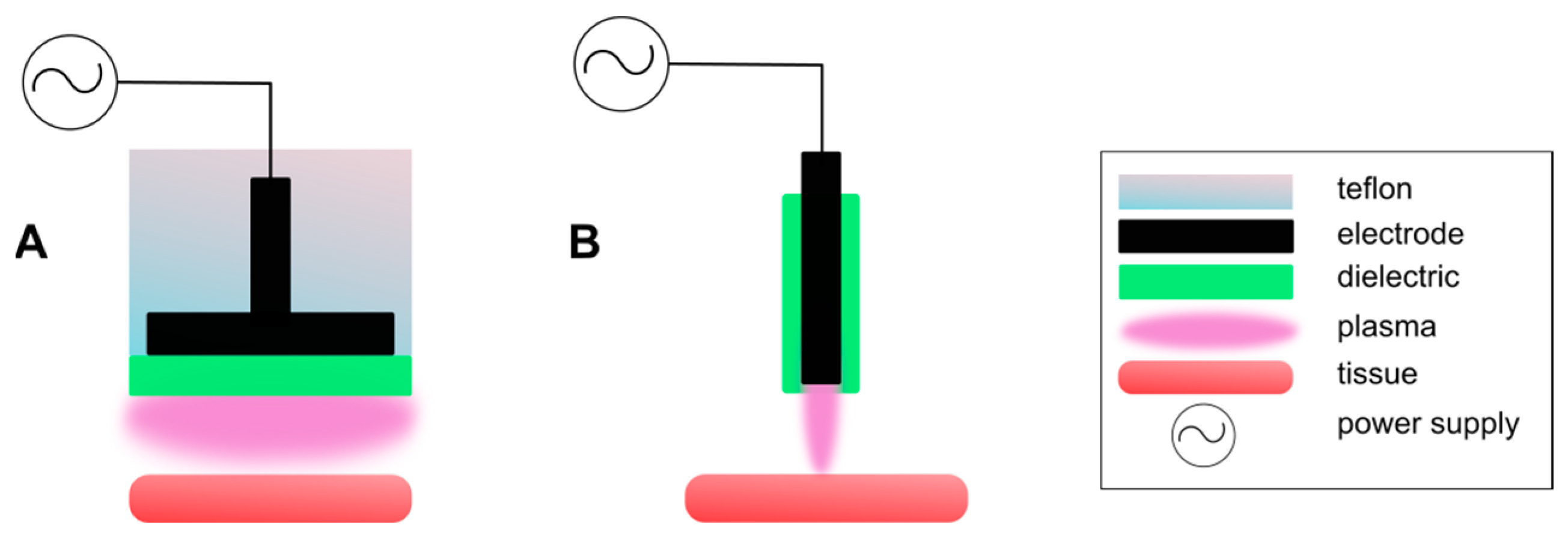
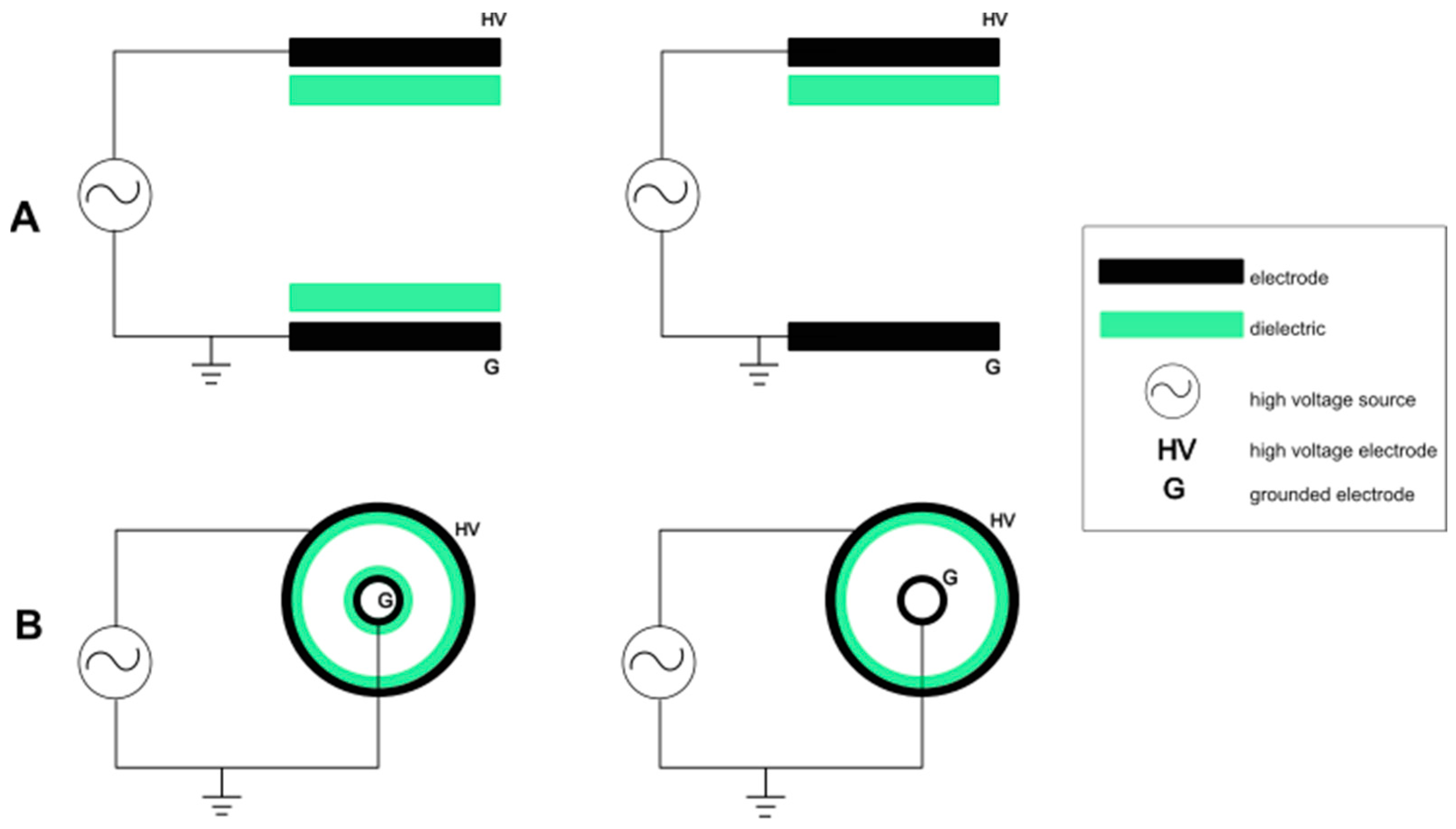
2. CAP Generation Techniques
2.1. Dielectric Barrier Discharge
2.2. Plasma Jet
3. Delivery Methods
- Long-lived reactive species;
- Short-lived reactive species;
- Physical considerations.
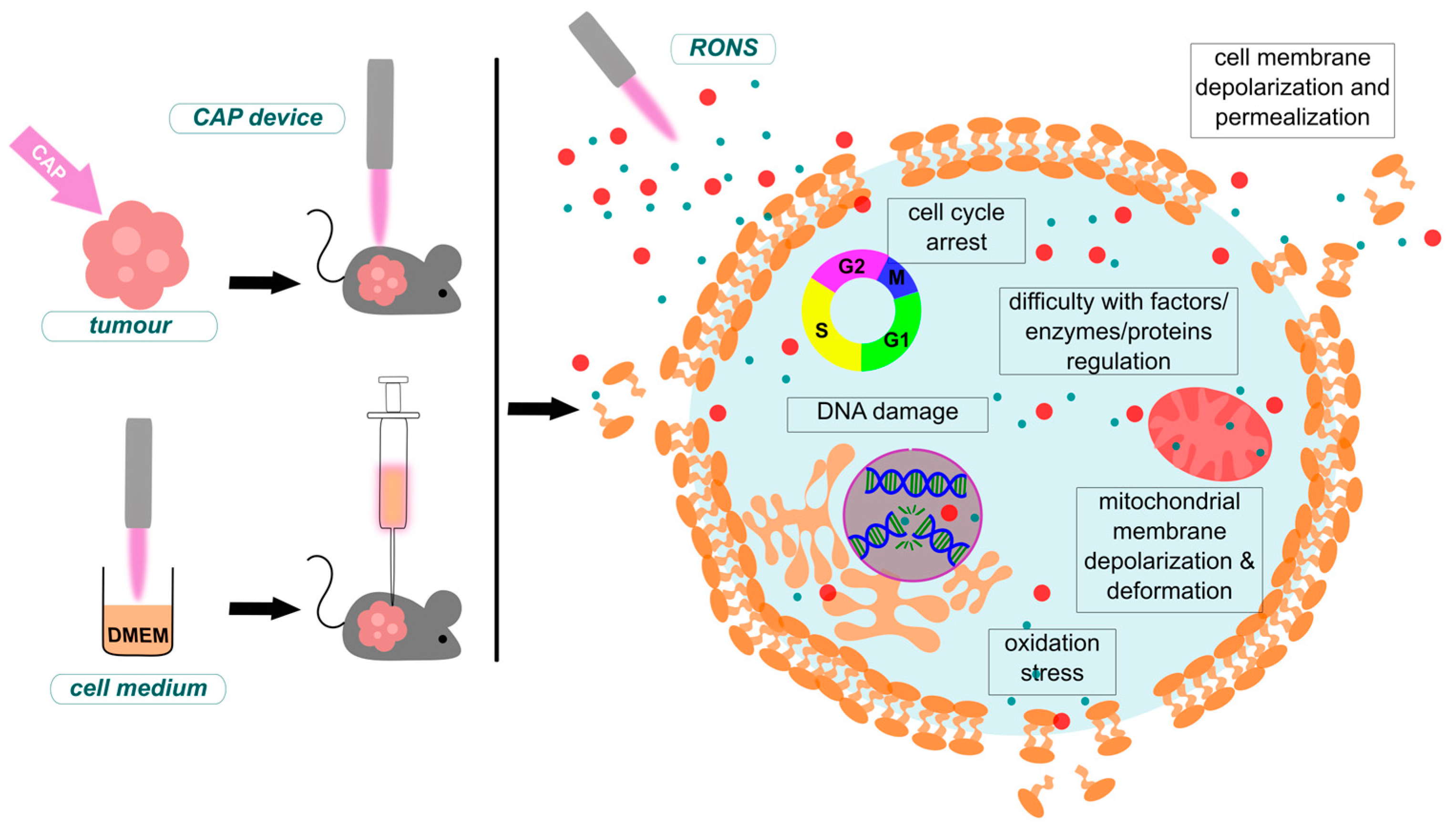
4. CAP in Medicine
- Cancer treatment—cold plasma has successfully demonstrated anti-cancer properties and is currently being explored as a potential therapy for certain types of cancer. It can induce apoptosis in cancer cells, slow down tumour growth or decrease its size, and sensitize cancer cells to other treatments like chemotherapy and radiation therapy [66].
- Immunology—CAP therapy has proved to modulate immune responses by influencing actions of innate and adaptive immune cells, potentially enhancing the body’s defence mechanisms against pathogens and tumour cells [2].
- Viral infections—cold plasma is being investigated for various applications in virology due to its ability to efficiently kill pathogens while sparing human cells. CAP-generated RONS have shown promise in the inactivation of viruses on surfaces and in the air, offering a novel approach to disinfection in healthcare and public spaces, as well as a modality for the treatment of infections [67].
- Neurology—CAP has shown potential in tumour treatment, including brain tumours like glioblastoma multiforme (GBM). Furthermore, it can serve as a beneficial treatment for neurodegenerative diseases like Alzheimer’s and Parkinson’s [68].
- Promoting wound healing—when applied to wounds, cold plasma works in two ways. First of all, it creates a cytotoxic environment for pathogens and even their spores by delivering reactive species like RNS and ROS. Secondarily it stimulates the process of wound regeneration by promoting tissue growth factors synthesis. It has successfully been used to treat chronic wounds, diabetic ulcers, and infected wounds, showing positive results in both accelerating healing and reducing infection rates [69,70].
- Stem cell—CAP displays the ability to influence the differentiation of stem cells and progenitor cells. Enhancing the growth rate while influencing cell differentiation processes is crucial for regenerative medicine applications [71].
- Dental applications—it is used for disinfection, treatment of gum disease (periodontitis), and promoting oral tissue regeneration. Plasma’s ability to kill bacteria results in the disinfection of dental implants and root canals. It also found an application in improving the bonding of dental materials to teeth [47].
4.1. Cancer Treatment
4.1.1. Mechanisms of CAP Anti-Cancer Effects and Selectivity
4.1.2. Differentiation of CAP-Induced Apoptosis and Autophagy Mechanisms
4.1.3. Selected CAP Anti-Cancer Studies
| Cancer Type | Cell Line | CAP Treatment Modality/ Device/Feed Gas | Main Observations |
|---|---|---|---|
| Lung | (SW900) [11] | Direct: * Plasma jet device [11] O2/N2 | High selectivity, death of 60–70% SW900 cancer cells [11]. Morphological changes at the cellular and subcellular levels, suppressing cancer cell growth [101]. |
| (A549) [117] | Indirect: Piezobrush PZ2 (Relyon Plasma GmbH, Regensburg, Germany) [117] Air | Disruption of the mitochondrial-nuclear network in cancer cells treated with PAM [117]. | |
| Colorectal | (HT29) [101] | Direct: Piezobrush PZ2 (Relyon Plasma GmbH, Regensburg, Germany) [101] Air | Morphological changes at the cellular and subcellular levels suppress cancer cell growth [101]. |
| Melanoma | (B16-F10) [115] | Direct: * Plasma jet device He Indirect: * Plasma jet device - | Viability reduced to 0% after 48 h of treatment [115]. Significant cell death and substantial reduction in tumour growth. |
| (DSMZ: ACC74) [118] | Indirect: miniFlatPlaSter (Terraplasma GmbH, Garching, Germany) [118] Air | CAP-treated solutions under acidic conditions caused protein nitration in cells [118]. | |
| Leukaemia | MOLM13 [110] | Direct: * Plasma jet device [110] He | Glutaminase activity of He plasma jet group was decreased [110]. |
| Breast | (MDA-MB-231) [119] | Indirect: * Plasma jet device [119] He Direct: kINPen IND plasma jet (Neoplas Tools GmbH, Greifswald, Germany) [120] Air | CAP-treated media displays anti-cancer capabilities [119]. Reduction in viability of cells and increase in apoptosis rate [120]. |
| (MCF-7) [119] | |||
| (HCC1806) [120] | Direct: kINPen IND plasma jet (Neoplas Tools GmbH, Greifswald, Germany) [120] Air | Reduction in viability of cells and increase in apoptosis rate [120]. | |
| Bladder | (SCaBer) [121] | Indirect: * Plasma jet device [121] Air | PAM, in a dose-dependent way, was considered to be an effective apoptotic agent lasting for several hours [121]. |
| (HT-1376) [122] | Direct: kINPen IND plasma jet (Neoplas Tools GmbH, Greifswald, Germany) [122] Air | Reduction in metabolic activity and protein content followed by a decrease in cell viability [122]. | |
| (TCCSUP) [122] | |||
| Cervical | (HeLa) [123] | Indirect: * Plasma jet device [123] - CAP-Jet (PlasmaMed Inc., New York, USA) [124] Ar | Inactivation of cancer cells [123]. Elevated ROS generation and induced substantial apoptosis in the cancer cells [124]. |
| (CaSki) [124] | Direct: CAP-Jet (PlasmaMed Inc., New York, USA) [124] Ar | Elevated ROS generation and induced substantial apoptosis in the cancer cells [124]. | |
| (HCT116) [124] | |||
| SIHA [97] | Indirect: * Portable plasma ‘corona pen’ [97] Air | Efficient apoptosis induction through the HOCl signalling pathway, finalized by lipid peroxidation [97]. | |
| Gastric | MKN-45 [97] | ||
| Sarcoma | SKN-MC [97] |
4.1.4. Comparison of CAP Anti-Cancer Therapy and Conventional Methods
4.2. CAP in Immunology
4.3. Viral Infections
4.4. Neurological Complications Therapy
4.5. Chronic and Acute Wound Treatment
4.6. Triggering Stem and Progenitor Cell Proliferation
| Treatment Target | CAP Device | Effect |
|---|---|---|
| Adipose-derived stromal cells [185] | * Self-made He/DBD device [186] | Halted cell growth and alteration in morphological characteristics occurred [186]. |
| Mouse neuroblastoma stem cells (N2a) [44] | * Air-CAP [44] | Increase in cell multiplication [44]. |
| Mouse neural stem cell (C17.2-NSC) [46] | * Self-made CAP jet device [46] | Increased cell multiplication and development [46]. |
| Osteoprogenitor cells (MC3T3-E1) [71,185] | * DBD NO-Plasma nozzle system [185] * Self-made He/plasma jet [71] | Dephosphorylation of FOXO1 transcription factor [185]. Stimulation of osteoblastic differentiation [71]. |
| Mesenchymal stem cells (MSCs) [187] | MicroPlaSter setup (Adtec Plasma Technology Co., Ltd., Hiroshima, Japan) [187] | Promoting cell growth through activation of genes responsible for proliferation expression [187]. |
4.7. Dental Medicine
5. CAP Devices for Medical Applications
6. Latest Trends
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
Abbreviations
| Ar | Argon |
| APPJ | Atmospheric Pressure Plasma Jet |
| ATM | Ataxia-telangiectasia mutated kinase |
| ATR | Ataxia telangiectasia and Rad3-related kinase |
| CAP | Cold Atmospheric Plasma |
| CNS | Central Nervous System |
| DA | Dopaminergic |
| DBD | Double Barrier Discharge |
| DC | Direct Current |
| DFE | Dielectric Free Electrode |
| ECM | Extracellular Matrix |
| EMT | Epithelial-to-Mesenchymal Transition |
| ER | Endoplasmatic Reticulum |
| FE-DBD | Floating Electrode Double Barrier Discharge |
| GBM | Glioblastoma Multiforme |
| GPF | Green Fluorescent Protein |
| H2O2 | Hydrogen Peroxide |
| HCC | Hepatocellular Cancer |
| He | Helium |
| HV | High Voltage |
| N2 | Nitrogen |
| NO | Nitric Oxide |
| NSC | Neural Stem Cells |
| NTP | Non-Thermal Plasma |
| O2 | Oxygen |
| OH | Hydroksyl Radical |
| RF | Radio Frequency |
| RNS | Reactive Nitrogen Species |
| RONS | Reactive Oxygen and Nitrogen Species |
| ROS | Reactive Oxygen Species |
| SE | Single Electrode |
| Te | Electron Temperature |
| Ti | Ion Temperature |
| UPR | Unfold Protein Response |
| UV | Ultraviolet |
| VEGF | Vascular Endothelial Growth Factor |
References
- Chen, Z.; Wirz, R.E. Cold Atmospheric Plasma (CAP) Technology and Applications; Morgan & Claypool Publishers: San Rafael, CA, USA, 2021; Volume 6. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, G.; Obenchain, R.; Zhang, R.; Bai, F.; Fang, T.; Wang, H.; Lu, Y.; Wirz, R.E.; Gu, Z. Cold atmospheric plasma delivery for biomedical applications. Mater. Today 2022, 54, 153–188. [Google Scholar] [CrossRef]
- Drake, G.W.F. Springer Series on Atomic, Optical, and Plasma Physics; Book series home; Springer: Berlin/Heidelberg, Germany; Available online: https://www.springer.com/series/411 (accessed on 19 December 2023).
- Laroussi, M.; Lu, X.; Keidar, M. Perspective: The Physics, Diagnostics, and Applications of Atmospheric Pressure Low Temperature Plasma Sources Used in Plasma Medicine. J. Appl. Phys. 2017, 122, 10201. [Google Scholar] [CrossRef]
- Hamouda, S.A.; Alshawish, N.K.; Abdalla, Y.K.; Ibrahim, M.K. Ultraviolet Radiation: Health Risks and Benefits. Saudi J. Eng. Technol. 2022, 7, 533–541. [Google Scholar] [CrossRef]
- Brandenburg, R. Dielectric barrier discharges: Progress on plasma sources and on the understanding of regimes and single filaments. Plasma Sources Sci. Technol. 2017, 26, 053001. [Google Scholar] [CrossRef]
- Miao, Y.; Yokochi, A.; Jovanovic, G.; Zhang, S.; von Jouanne, A. Application-oriented non-thermal plasma in chemical reaction engineering: A review. Green Energy Resour. 2023, 1, 100004. [Google Scholar] [CrossRef]
- Tornin, J.; Labay, C.; Tampieri, F.; Ginebra, M.P.; Canal, C. Evaluation of the effects of cold atmospheric plasma and plasma-treated liquids in cancer cell cultures. Nat. Protoc. 2021, 16, 2826–2850. [Google Scholar] [CrossRef] [PubMed]
- Miebach, L.; Freund, E.; Clemen, R.; Weltmann, K.D.; Metelmann, H.R.; von Woedtke, T.; Gerling, T.; Wende, K.; Bekeschus, S. Conductivity augments ROS and RNS delivery and tumor toxicity of an argon plasma jet. Free Radic. Biol. Med. 2022, 180, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Xu, X.; Zhang, S.; Cai, D.; Dai, X. Cold atmospheric plasma conveys selectivity on triple negative breast cancer cells both in vitro and in vivo. Free Radic. Biol. Med. 2018, 124, 205–213. [Google Scholar] [CrossRef]
- Keidar, M. Plasma for cancer treatment. Plasma Sources Sci. Technol. 2015, 24, 033001. [Google Scholar] [CrossRef]
- Yan, D.; Sherman, J.H.; Keidar, M. Cold atmospheric plasma, a novel promising anti-cancer treatment modality. Oncotarget 2017, 8, 15977–15995. [Google Scholar] [CrossRef]
- Metelmann, H.R.; Seebauer, C.; Miller, V.; Fridman, A.; Bauer, G.; Graves, D.B.; Pouvesle, J.M.; Rutkowski, R.; Schuster, M.; Bekeschus, S.; et al. Clinical experience with cold plasma in the treatment of locally advanced head and neck cancer. Clin. Plasma Med. 2018, 9, 6–13. [Google Scholar] [CrossRef]
- Leduc, M.; Guay, D.; Leask, R.L.; Coulombe, S. Cell permeabilization using a non-thermal plasma. New J. Phys. 2009, 11, 115021. [Google Scholar] [CrossRef]
- Keidar, M.; Beilis, I.I. Plasma Engineering; Elsevier: Amsterdam, The Netherlands, 2018; p. 567. [Google Scholar]
- Matsusaka, S. Control of particle charge by atmospheric pressure plasma jet (APPJ): A review. Adv. Powder Technol. 2019, 30, 2851–2858. [Google Scholar] [CrossRef]
- Lu, X.; Naidis, G.V.; Laroussi, M.; Reuter, S.; Graves, D.B.; Ostrikov, K. Reactive species in non-equilibrium atmospheric-pressure plasmas: Generation, transport, and biological effects. Phys. Rep. 2016, 630, 1–84. [Google Scholar] [CrossRef]
- Antimicrobial and Anticancer Efficacy of Atmospheric Pressure Cold Plasma Technology. Available online: https://www.researchgate.net/publication/358780755_Antimicrobial_and_Anticancer_Efficacy_of_Atmospheric_Pressure_Cold_Plasma_Technology (accessed on 19 December 2023).
- Utsumi, F.; Kajiyama, H.; Nakamura, K.; Tanaka, H.; Mizuno, M.; Ishikawa, K.; Kondo, H.; Kano, H.; Hori, M.; Kikkawa, F. Effect of indirect nonequilibrium atmospheric pressure plasma on anti-proliferative activity against chronic chemo-resistant ovarian cancer cells in vitro and in vivo. PLoS ONE 2013, 8, e81576. [Google Scholar] [CrossRef] [PubMed]
- Boeckmann, L.; Berner, J.; Kordt, M.; Lenz, E.; Schäfer, M.; Semmler, M.-L.; Frey, A.; Sagwal, S.K.; Rebl, H.; Miebach, L.; et al. Synergistic effect of cold gas plasma and experimental drug exposure exhibits skin cancer toxicity in vitro and in vivo. J. Adv. Res. 2023, 57, 181–196. [Google Scholar] [CrossRef] [PubMed]
- Rafiei, A.; Sohbatzadeh, F.; Hadavi, S.; Bekeschus, S.; Alimohammadi, M.; Valadan, R. Inhibition of murine melanoma tumor growth in vitro and in vivo using an argon-based plasma jet. Clin. Plasma Med. 2020, 19–20, 100102. [Google Scholar] [CrossRef]
- Chatraie, M.; Torkaman, G.; Khani, M.; Salehi, H.; Shokri, B. In vivo study of non-invasive effects of non-thermal plasma in pressure ulcer treatment. Sci. Rep. 2018, 8, 5621. [Google Scholar] [CrossRef] [PubMed]
- Mirpour, S.; Piroozmand, S.; Soleimani, N.; Jalali Faharani, N.; Ghomi, H.; Fotovat Eskandari, H.; Sharifi, A.M.; Mirpour, S.; Eftekhari, M.; Nikkhah, M. Utilizing the micron sized non-thermal atmospheric pressure plasma inside the animal body for the tumor treatment application. Sci. Rep. 2016, 6, 29048. [Google Scholar] [CrossRef]
- Bekeschus, S.; Liebelt, G.; Menz, J.; Berner, J.; Sagwal, S.K.; Wende, K.; Weltmann, K.D.; Boeckmann, L.; von Woedtke, T.; Metelmann, H.R.; et al. Tumor cell metabolism correlates with resistance to gas plasma treatment: The evaluation of three dogmas. Free Radic. Biol. Med. 2021, 167, 12–28. [Google Scholar] [CrossRef]
- Semmler, M.L.; Bekeschus, S.; Schäfer, M.; Bernhardt, T.; Fischer, T.; Witzke, K.; Seebauer, C.; Rebl, H.; Grambow, E.; Vollmar, B.; et al. Molecular Mechanisms of the Efficacy of Cold Atmospheric Pressure Plasma (CAP) in Cancer Treatment. Cancers 2020, 12, 269. [Google Scholar] [CrossRef] [PubMed]
- Keidar, M.; Yan, D.; Beilis, I.I.; Trink, B.; Sherman, J.H. Plasmas for Treating Cancer: Opportunities for Adaptive and Self-Adaptive Approaches. Trends Biotechnol. 2018, 36, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Fridman, G.; Friedman, G.; Gutsol, A.; Shekhter, A.B.; Vasilets, V.N.; Fridman, A. Applied Plasma Medicine. Plasma Process. Polym. 2008, 5, 503–533. [Google Scholar] [CrossRef]
- Belkessa, N.; Bouzaza, A.; Assadi, A.A. Understanding of the synergy effect of DBD plasma discharge combined to photocatalysis in the case of Ethylbenzene removal: Interaction between plasma reactive species and catalyst. J. Environ. Chem. Eng. 2023, 11, 110640. [Google Scholar] [CrossRef]
- Royintarat, T.; Seesuriyachan, P.; Boonyawan, D.; Choi, E.H.; Wattanutchariya, W. Mechanism and optimization of non-thermal plasma-activated water for bacterial inactivation by underwater plasma jet and delivery of reactive species underwater by cylindrical DBD plasma. Curr. Appl. Phys. 2019, 19, 1006–1014. [Google Scholar] [CrossRef]
- Andhare, K.; Livingstone, D.; Arumugam, R.; Manivasakan, S.; Subramanian, B.; Subramanian, Y.; Pasupathy, A.; Devendran, R. Effect of non-thermal oxygen-DBD plasma treatment on reducing the phthalate leach and in improving the mechanical strength properties of polymethyl methacrylate denture base and denture liner. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Tripathy, S.; Srivastav, P.P. Effect of dielectric barrier discharge (DBD) cold plasma-activated water pre-treatment on the drying properties, kinetic parameters, and physicochemical and functional properties of Centella asiatica leaves. Chemosphere 2023, 332, 38901. [Google Scholar] [CrossRef] [PubMed]
- Maybin, J.A.; Thompson, T.P.; Flynn, P.B.; Skvortsov, T.; Hickok, N.J.; Freeman, T.A.; Gilmore, B.F. Cold atmospheric pressure plasma-antibiotic synergy in Pseudomonas aeruginosa biofilms is mediated via oxidative stress response. Biofilm 2023, 5, 100122. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Song, R.; Wang, Y.; Zhong, R.; Zhang, Y.; Zhou, J.; Wang, T.; Jia, H.; Zhu, L. Inhibited conjugative transfer of antibiotic resistance genes in antibiotic resistant bacteria by surface plasma. Water Res. 2021, 204, 117630. [Google Scholar] [CrossRef]
- Li, H.; Song, R.; Wang, Y.; Zhong, R.; Zhang, Y.; Zhou, J.; Wang, T.; Zhu, L. Simultaneous removal of antibiotic-resistant bacteria and its resistance genes in water by plasma oxidation: Highlights the effects of inorganic ions. Sep. Purif. Technol. 2021, 278, 119672. [Google Scholar] [CrossRef]
- Pina-Perez, M.C.; Martinet, D.; Palacios-Gorba, C.; Ellert, C.; Beyrer, M. Low-energy short-term cold atmospheric plasma: Controlling the inactivation efficacy of bacterial spores in powders. Food Res. Int. 2020, 130, 108921. [Google Scholar] [CrossRef] [PubMed]
- Bauer, G. Targeting Protective Catalase of Tumor Cells with Cold Atmospheric Plasma- Activated Medium (PAM). Anticancer. Agents Med. Chem. 2017, 18, 784–804. [Google Scholar] [CrossRef] [PubMed]
- Min, T.; Xie, X.; Ren, K.; Sun, T.; Wang, H.; Dang, C.; Zhang, H. Therapeutic Effects of Cold Atmospheric Plasma on Solid Tumor. Front. Med. 2022, 9, 884887. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.P.; Khumsupan, D.; Chou, Y.J.; Hsieh, K.C.; Hsu, H.Y.; Ting, Y.; Cheng, K.C. Applications of atmospheric cold plasma in agricultural, medical, and bioprocessing industries. Appl. Microbiol. Biotechnol. 2022, 106, 7737. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, T.; Carvajal Berrio, D.A.; Daum, R.; Reisenauer, C.; Weltmann, K.D.; Wallwiener, D.; Brucker, S.Y.; Schenke-Layland, K.; Brauchle, E.M.; Weiss, M. Molecular Effects and Tissue Penetration Depth of Physical Plasma in Human Mucosa Analyzed by Contact- and Marker-Independent Raman Microspectroscopy. ACS Appl. Mater. Interfaces 2019, 11, 42885–42895. [Google Scholar] [CrossRef] [PubMed]
- Dagogo-Jack, I.; Shaw, A.T. Tumour heterogeneity and resistance to cancer therapies. Nat. Rev. Clin. Oncol. 2017, 15, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Braný, D.; Dvorská, D.; Halašová, E.; Škovierová, H. Cold Atmospheric Plasma: A Powerful Tool for Modern Medicine. Int. J. Mol. Sci. 2020, 21, 2932. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, M.; Adhikari, B.; Adhikari, A.; Yan, D.; Soni, V.; Sherman, J.H.; Keidar, M. Combination drug delivery using cold atmospheric plasma technology. Nanocarriers Deliv. Comb. Drugs 2021, 11, 393–423. [Google Scholar] [CrossRef]
- Gangemi, S.; Petrarca, C.; Tonacci, A.; Di Gioacchino, M.; Musolino, C.; Allegra, A. Cold Atmospheric Plasma Targeting Hematological Malignancies: Potentials and Problems of Clinical Translation. Antioxidants 2022, 11, 1592. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.Y.; Hong, Y.J.; Lim, J.; Choi, J.S.; Choi, E.H.; Kang, S.; Rhim, H. Cold atmospheric plasma (CAP), a novel physicochemical source, induces neural differentiation through cross-talk between the specific RONS cascade and Trk/Ras/ERK signaling pathway. Biomaterials 2018, 156, 258–273. [Google Scholar] [CrossRef]
- Tan, F.; Wang, Y.; Zhang, S.; Shui, R.; Chen, J. Plasma Dermatology: Skin Therapy Using Cold Atmospheric Plasma. Front. Oncol. 2022, 12, 918484. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Zhao, S.; Yan, X. Nerve Stem Cell Differentiation by a One-step Cold Atmospheric Plasma Treatment In Vitro. J. Vis. Exp. 2019, 2019, 58663. [Google Scholar] [CrossRef] [PubMed]
- Lata, S.; Chakravorty, S.; Mitra, T.; Pradhan, P.K.; Mohanty, S.; Patel, P.; Jha, E.; Panda, P.K.; Verma, S.K.; Suar, M. Aurora Borealis in dentistry: The applications of cold plasma in biomedicine. Mater. Today Bio 2021, 13, 100200. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, C.; Berganza, C.; Zhang, J. Cold Atmospheric Plasma: Methods of production and application in dentistry and oncology. Med. Gas Res. 2013, 3, 21. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, B.; Bezbaruah, R.; Rynjah, D.; Newar, A.; Sengupta, S.; Pegu, P.; Dey, N.; Bora, S.; Barman, D. Cold Atmospheric Plasma: A Noteworthy Approach in Medical Science. Sci. Pharm. 2023, 2, 46–76. [Google Scholar] [CrossRef]
- Laroussi, M. A Brief Note on the History of the Dielectric Barrier Discharge and Its Application for Biological Decontamination. IEEE Trans. Radiat. Plasma Med. Sci. 2022, 6, 121–125. [Google Scholar] [CrossRef]
- Rueda, V.; Diez, R.; Bente, N.; Piquet, H. Combined Image Processing and Equivalent Circuit Approach for the Diagnostic of Atmospheric Pressure DBD. Appl. Sci. 2022, 12, 8009. [Google Scholar] [CrossRef]
- Elaissi, S.; Charrada, K. Simulation of Cold Atmospheric Plasma Generated by Floating-Electrode Dielectric Barrier Pulsed Discharge Used for the Cancer Cell Necrosis. Coatings 2021, 11, 1405. [Google Scholar] [CrossRef]
- Laroussi, M.; Stapelmann, K.; Longo, S.; Ranieri, P. The Resistive Barrier Discharge: A Brief Review of the Device and Its Biomedical Applications. Plasma 2021, 4, 75–80. [Google Scholar] [CrossRef]
- do Nascimento, F.; Gerling, T.; Kostov, K.G. On the gas heating effect of helium atmospheric pressure plasma jet. Phys. Scr. 2023, 98, 055013. [Google Scholar] [CrossRef]
- Armand, A.; Khani, M.; Asnaashari, M.; AliAhmadi, A.; Shokri, B. Comparison study of root canal disinfection by cold plasma jet and photodynamic therapy. Photodiagnosis Photodyn. Ther. 2019, 26, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Sremački, I.; Bruno, G.; Jablonowski, H.; Leys, C.; Nikiforov, A.; Wende, K. Influence of aerosol injection on the liquid chemistry induced by an RF argon plasma jet. Plasma Sources Sci. Technol. 2021, 30, 095018. [Google Scholar] [CrossRef]
- Lu, X.P.; Laroussi, M. Dynamics of an atmospheric pressure plasma plume generated by submicrosecond voltage pulses. J. Appl. Phys. 2006, 100, 063302. [Google Scholar] [CrossRef]
- Mashayekh, S.; Rajaee, H.; Akhlaghi, M.; Shokri, B.; Hassan, Z.M. Atmospheric-pressure plasma jet characterization and applications on melanoma cancer treatment (B/16-F10). Phys. Plasmas 2015, 22, 093508. [Google Scholar] [CrossRef]
- Miebach, L.; Mohamed, H.; Wende, K.; Miller, V.; Bekeschus, S. Pancreatic Cancer Cells Undergo Immunogenic Cell Death upon Exposure to Gas Plasma-Oxidized Ringers Lactate. Cancers 2023, 15, 319. [Google Scholar] [CrossRef] [PubMed]
- Mateu-Sanz, M.; Tornín, J.; Brulin, B.; Khlyustova, A.; Ginebra, M.P.; Layrolle, P.; Canal, C. Cold Plasma-Treated Ringer’s Saline: A Weapon to Target Osteosarcoma. Cancers 2020, 12, 227. [Google Scholar] [CrossRef] [PubMed]
- Labay, C.; Roldán, M.; Tampieri, F.; Stancampiano, A.; Bocanegra, P.E.; Ginebra, M.P.; Canal, C. Enhanced Generation of Reactive Species by Cold Plasma in Gelatin Solutions for Selective Cancer Cell Death. ACS Appl. Mater. Interfaces 2020, 12, 47256–47269. [Google Scholar] [CrossRef] [PubMed]
- Labay, C.; Hamouda, I.; Tampieri, F.; Ginebra, M.P.; Canal, C. Production of reactive species in alginate hydrogels for cold atmospheric plasma-based therapies. Sci. Rep. 2019, 9, 16160. [Google Scholar] [CrossRef]
- Graves, D.B. The emerging role of reactive oxygen and nitrogen species in redox biology and some implications for plasma applications to medicine and biology. J. Phys. D Appl. Phys. 2012, 45, 263001. [Google Scholar] [CrossRef]
- Laroussi, M. Cold Plasma in Medicine and Healthcare: The New Frontier in Low Temperature Plasma Applications. Front. Phys. 2020, 8, 528206. [Google Scholar] [CrossRef]
- Kong, M.G.; Kroesen, G.; Morfill, G.; Nosenko, T.; Shimizu, T.; Van Dijk, J.; Zimmermann, J.L. Plasma medicine: An introductory review. New J. Phys. 2009, 11, 115012. [Google Scholar] [CrossRef]
- Dai, X.; Shen, L.; Zhang, J. Cold atmospheric plasma: Redox homeostasis to treat cancers? Trends Biotechnol. 2023, 41, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Munoz, R. Infectious diseases: Overview, challenges, and perspectives. Smart Nanomater. Combat Spread Viral Infect. Adv. Strateg. Prev. Viral Infect. 2023, 1, 1–21. [Google Scholar] [CrossRef]
- Conway, G.E.; He, Z.; Hutanu, A.L.; Cribaro, G.P.; Manaloto, E.; Casey, A.; Traynor, D.; Milosavljevic, V.; Howe, O.; Barcia, C.; et al. Cold Atmospheric Plasma induces accumulation of lysosomes and caspase-independent cell death in U373MG glioblastoma multiforme cells. Sci. Rep. 2019, 9, 12891. [Google Scholar] [CrossRef] [PubMed]
- Babaeva, N.Y.; Kushner, M.J. Reactive fluxes delivered by dielectric barrier discharge filaments to slightly wounded skin. J. Phys. D Appl. Phys. 2012, 46, 025401. [Google Scholar] [CrossRef]
- Boeckmann, L.; Schäfer, M.; Bernhardt, T.; Semmler, M.L.; Jung, O.; Ojak, G.; Fischer, T.; Peters, K.; Nebe, B.; Müller-Hilke, B.; et al. Cold Atmospheric Pressure Plasma in Wound Healing and Cancer Treatment. Appl. Sci. 2020, 10, 6898. [Google Scholar] [CrossRef]
- Tominami, K.; Kanetaka, H.; Sasaki, S.; Mokudai, T.; Kaneko, T.; Niwano, Y. Cold atmospheric plasma enhances osteoblast differentiation. PLoS ONE 2017, 12, e0180507. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Zhao, S.; Mao, X.; Lu, X.; He, G.; Yang, G.; Chen, M.; Ishaq, M.; Ostrikov, K. Selective neuronal differentiation of neural stem cells induced by nanosecond microplasma agitation. Stem Cell Res. 2014, 12, 387–399. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Gong, F.; Jin, T.; Liu, Q.; Fang, H.; Chen, Y.; Wang, G.; Chu, P.K.; Wu, Z.; Ostrikov, K. Dose-Dependent Effects in Plasma Oncotherapy: Critical In Vivo Immune Responses Missed by In Vitro Studies. Biomolecules 2023, 13, 707. [Google Scholar] [CrossRef]
- Shojaei, E.; Zare, S.; Shirkavand, A.; Eslami, E.; Fathollah, S.; Mansouri, P. Biophysical evaluation of treating adipose tissue-derived stem cells using non-thermal atmospheric pressure plasma. Sci. Rep. 2022, 12, 11127. [Google Scholar] [CrossRef]
- Park, J.; Lee, H.; Lee, H.J.; Kim, G.C.; Kim, D.Y.; Han, S.; Song, K. Non-Thermal Atmospheric Pressure Plasma Efficiently Promotes the Proliferation of Adipose Tissue-Derived Stem Cells by Activating NO-Response Pathways. Sci. Rep. 2016, 6, 39298. [Google Scholar] [CrossRef]
- Griseti, E.; Kolosnjaj-Tabi, J.; Gibot, L.; Fourquaux, I.; Rols, M.P.; Yousfi, M.; Merbahi, N.; Golzio, M. Pulsed Electric Field Treatment Enhances the Cytotoxicity of Plasma-Activated Liquids in a Three-Dimensional Human Colorectal Cancer Cell Model. Sci. Rep. 2019, 9, 7583. [Google Scholar] [CrossRef]
- Azzariti, A.; Iacobazzi, R.M.; Di Fonte, R.; Porcelli, L.; Gristina, R.; Favia, P.; Fracassi, F.; Trizio, I.; Silvestris, N.; Guida, G.; et al. Plasma-activated medium triggers cell death and the presentation of immune activating danger signals in melanoma and pancreatic cancer cells. Sci. Rep. 2019, 9, 4099. [Google Scholar] [CrossRef]
- Uchida, G.; Mino, Y.; Suzuki, T.; Ikeda, J.-i.; Suzuki, T.; Takenaka, K.; Setsuhara, Y. Decomposition and oxidation of methionine and tryptophan following irradiation with a nonequilibrium plasma jet and applications for killing cancer cells. Sci. Rep. 2019, 9, 6625. [Google Scholar] [CrossRef]
- Freund, E.; Liedtke, K.R.; van der Linde, J.; Metelmann, H.R.; Heidecke, C.D.; Partecke, L.I.; Bekeschus, S. Physical plasma-treated saline promotes an immunogenic phenotype in CT26 colon cancer cells in vitro and in vivo. Sci. Rep. 2019, 9, 634. [Google Scholar] [CrossRef]
- Yan, D.; Xiao, H.; Zhu, W.; Nourmohammadi, N.; Zhang, L.G.; Bian, K.; Keidar, M. The role of aquaporins in the anti-glioblastoma capacity of the cold plasma-stimulated medium. J. Phys. D Appl. Phys. 2017, 50, 055401. [Google Scholar] [CrossRef]
- Agre, P.; King, L.S.; Yasui, M.; Guggino, W.B.; Ottersen, O.P.; Fujiyoshi, Y.; Engel, A.; Nielsen, S. Aquaporin water channels--from atomic structure to clinical medicine. J. Physiol. 2002, 542, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Beitz, E. Aquaporins with selectivity for unconventional permeants. Cell. Mol. Life Sci. 2007, 64, 2413–2421. [Google Scholar] [CrossRef] [PubMed]
- Almasalmeh, A.; Krenc, D.; Wu, B.; Beitz, E. Structural determinants of the hydrogen peroxide permeability of aquaporins. FEBS J. 2014, 281, 647–656. [Google Scholar] [CrossRef]
- Yusupov, M.; Lackmann, J.W.; Razzokov, J.; Kumar, S.; Stapelmann, K.; Bogaerts, A. Impact of plasma oxidation on structural features of human epidermal growth factor. Plasma Process. Polym. 2018, 15, 1800022. [Google Scholar] [CrossRef]
- Busco, G.; Robert, E.; Chettouh-Hammas, N.; Pouvesle, J.M.; Grillon, C. The emerging potential of cold atmospheric plasma in skin biology. Free Radic. Biol. Med. 2020, 161, 290–304. [Google Scholar] [CrossRef] [PubMed]
- De Meyer, F.; Smit, B. Effect of cholesterol on the structure of a phospholipid bilayer. Proc. Natl. Acad. Sci. USA 2009, 106, 3654–3658. [Google Scholar] [CrossRef]
- Van Der Paal, J.; Neyts, E.C.; Verlackt, C.C.W.; Bogaerts, A. Effect of lipid peroxidation on membrane permeability of cancer and normal cells subjected to oxidative stress. Chem. Sci. 2016, 7, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Glab, J.A.; Doerflinger, M.; Nedeva, C.; Jose, I.; Mbogo, G.W.; Paton, J.C.; Paton, A.W.; Kueh, A.J.; Herold, M.J.; Huang, D.C.S.; et al. DR5 and caspase-8 are dispensable in ER stress-induced apoptosis. Cell Death Differ. 2017, 24, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Lemmer, I.L.; Willemsen, N.; Hilal, N.; Bartelt, A. A guide to understanding endoplasmic reticulum stress in metabolic disorders. Mol. Metab. 2021, 47, 101169. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, M.; Li, Z.; Yue, J.; Xu, M.; Zhang, Y.; Yung, K.K.L.; Li, R. Fine particulate matter induces mitochondrial dysfunction and oxidative stress in human SH-SY5Y cells. Chemosphere 2019, 218, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, H.; Wang, H.G. CHOP is involved in endoplasmic reticulum stress-induced apoptosis by enhancing DR5 expression in human carcinoma cells. J. Biol. Chem. 2004, 279, 45495–45502. [Google Scholar] [CrossRef] [PubMed]
- Vítor, A.C.; Huertas, P.; Legube, G.; de Almeida, S.F. Studying DNA Double-Strand Break Repair: An Ever-Growing Toolbox. Front. Mol. Biosci. 2020, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Maréchal, A.; Zou, L. DNA damage sensing by the ATM and ATR kinases. Cold Spring Harb. Perspect. Biol. 2013, 5, a012716. [Google Scholar] [CrossRef]
- Bekeschus, S.; Schütz, C.S.; Nießner, F.; Wende, K.; Weltmann, K.D.; Gelbrich, N.; Von Woedtke, T.; Schmidt, A.; Stope, M.B. Elevated H2AX Phosphorylation Observed with kINPen Plasma Treatment Is Not Caused by ROS-Mediated DNA Damage but Is the Consequence of Apoptosis. Oxid. Med. Cell. Longev. 2019, 2019, 8535163. [Google Scholar] [CrossRef]
- de Carvalho, S.S.; Rodovalho, C.M.; Gaviraghi, A.; Mota, M.B.S.; Jablonka, W.; Rocha-Santos, C.; Nunes, R.D.; da Encarnação Sá-Guimarães, T.d.E.; Oliveira, D.S.; Melo, A.C.A.; et al. Aedes aegypti post-emergence transcriptome: Unveiling the molecular basis for the hematophagic and gonotrophic capacitation. PLoS Negl. Trop. Dis. 2021, 15, e0008915. [Google Scholar] [CrossRef] [PubMed]
- Apoptosis. Available online: https://www.genome.gov/genetics-glossary/apoptosis (accessed on 19 December 2023).
- Bauer, G.; Sersenová, D.; Graves, D.B.; Machala, Z. Cold Atmospheric Plasma and Plasma-Activated Medium Trigger RONS-Based Tumor Cell Apoptosis. Sci. Rep. 2019, 9, 14210. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M.; Gümbel, D.; Hanschmann, E.M.; Mandelkow, R.; Gelbrich, N.; Zimmermann, U.; Walther, R.; Ekkernkamp, A.; Sckell, A.; Kramer, A.; et al. Cold Atmospheric Plasma Treatment Induces Anti-Proliferative Effects in Prostate Cancer Cells by Redox and Apoptotic Signaling Pathways. PLoS ONE 2015, 10, e0130350. [Google Scholar] [CrossRef] [PubMed]
- Jawaid, P.; Rehman, M.U.; Zhao, Q.L.; Takeda, K.; Ishikawa, K.; Hori, M.; Shimizu, T.; Kondo, T. Helium-based cold atmospheric plasma-induced reactive oxygen species-mediated apoptotic pathway attenuated by platinum nanoparticles. J. Cell. Mol. Med. 2016, 20, 1737–1748. [Google Scholar] [CrossRef] [PubMed]
- Turrini, E.; Laurita, R.; Stancampiano, A.; Catanzaro, E.; Calcabrini, C.; Maffei, F.; Gherardi, M.; Colombo, V.; Fimognari, C. Cold Atmospheric Plasma Induces Apoptosis and Oxidative Stress Pathway Regulation in T-Lymphoblastoid Leukemia Cells. Oxid. Med. Cell. Longev. 2017, 2017, 4271065. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Mang, X.; Li, X.; Cai, Z.; Tan, F. Cold atmospheric plasma induces apoptosis in human colon and lung cancer cells through modulating mitochondrial pathway. Front. Cell Dev. Biol. 2022, 10, 915785. [Google Scholar] [CrossRef] [PubMed]
- Jalili, A.; Irani, S.; Mirfakhraie, R. Combination of cold atmospheric plasma and iron nanoparticles in breast cancer: Gene expression and apoptosis study. Onco. Targets. Ther. 2016, 9, 5911–5917. [Google Scholar] [CrossRef]
- Hua, D.; Cai, D.; Ning, M.; Yu, L.; Zhang, Z.; Han, P.; Dai, X. Cold atmospheric plasma selectively induces G0/G1 cell cycle arrest and apoptosis in AR-independent prostate cancer cells. J. Cancer 2021, 12, 5977–5986. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, M.; Adhikari, B.; Ghimire, B.; Baboota, S.; Choi, E.H. Cold Atmospheric Plasma and Silymarin Nanoemulsion Activate Autophagy in Human Melanoma Cells. Int. J. Mol. Sci. 2020, 21, 1939. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, J.; Cai, L.; Dai, X. Cold Atmospheric Plasma Conveys Selectivity Against Hepatocellular Carcinoma Cells via Triggering EGFR(Tyr1068)-Mediated Autophagy. Front. Oncol. 2022, 12, 895106. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, J.; Gao, L.; Ma, Y.; Xu, G.; Li, X.; Hao, Y.; Shi, X.; Zhang, G.J. Helium low temperature plasma induced HepG2 cells autophagy through ROS-mediated PI3K/AKT/mTOR/P70s6k signaling pathway. AIP Adv. 2019, 9, 1076059. [Google Scholar] [CrossRef]
- Alimohammadi, M.; Golpur, M.; Sohbatzadeh, F.; Hadavi, S.; Bekeschus, S.; Niaki, H.A.; Valadan, R.; Rafiei, A. Cold Atmospheric Plasma Is a Potent Tool to Improve Chemotherapy in Melanoma In Vitro and In Vivo. Biomolecules 2020, 10, 1011. [Google Scholar] [CrossRef] [PubMed]
- Attri, P.; Kaushik, N.K.; Kaushik, N.; Hammerschmid, D.; Privat-Maldonado, A.; De Backer, J.; Shiratani, M.; Choi, E.H.; Bogaerts, A. Plasma treatment causes structural modifications in lysozyme, and increases cytotoxicity towards cancer cells. Int. J. Biol. Macromol. 2021, 182, 1724–1736. [Google Scholar] [CrossRef]
- Cha, J.-Y.; Lee, H.-J. Targeting Lipid Metabolic Reprogramming as Anticancer Therapeutics. J. Cancer Prev. 2016, 21, 209–215. [Google Scholar] [CrossRef]
- Xu, D.; Ning, N.; Xu, Y.; Xia, W.; Liu, D.; Chen, H.; Kong, M.G. Effect of He Plasma Jet Versus Surface Plasma on the Metabolites of Acute Myeloid Leukemia Cells. Front. Oncol. 2021, 11, 552480. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Song, X.; Yang, F.; Wang, J.; Sun, M.; Liu, G.; Ahmad, N.; Zhou, Y.; Zhang, Y.; Shi, G.; et al. Combined effects of vitamin C and cold atmospheric plasma-conditioned media against glioblastoma via hydrogen peroxide. Free Radic. Biol. Med. 2023, 194, 1–11. [Google Scholar] [CrossRef]
- Van Loenhout, J.; Freire Boullosa, L.; Quatannens, D.; De Waele, J.; Merlin, C.; Lambrechts, H.; Lau, H.W.; Hermans, C.; Lin, A.; Lardon, F.; et al. Auranofin and Cold Atmospheric Plasma Synergize to Trigger Distinct Cell Death Mechanisms and Immunogenic Responses in Glioblastoma. Cells 2021, 10, 2936. [Google Scholar] [CrossRef]
- Xiong, Z. Cold Atmospheric Plasmas: A Novel and Promising Way to Treat Neurological Diseases. Trends Biotechnol. 2018, 36, 582–583. [Google Scholar] [CrossRef]
- Bauer, G.; Sersenová, D.; Graves, D.B.; Machala, Z. Dynamics of Singlet Oxygen-Triggered, RONS-Based Apoptosis Induction after Treatment of Tumor Cells with Cold Atmospheric Plasma or Plasma-Activated Medium. Sci. Rep. 2019, 9, 13931. [Google Scholar] [CrossRef]
- Saadati, F.; Mahdikia, H.; Abbaszadeh, H.A.; Abdollahifar, M.A.; Khoramgah, M.S.; Shokri, B. Comparison of Direct and Indirect cold atmospheric-pressure plasma methods in the B16F10 melanoma cancer cells treatment. Sci. Rep. 2018, 8, 7689. [Google Scholar] [CrossRef]
- Lee, H.J.; Shon, C.H.; Kim, Y.S.; Kim, S.; Kim, G.C.; Kong, M.G. Degradation of adhesion molecules of G361 melanoma cells by a non-thermal atmospheric pressure microplasma. New J. Phys. 2009, 11, 115026. [Google Scholar] [CrossRef]
- Adachi, T.; Tanaka, H.; Nonomura, S.; Hara, H.; Kondo, S.I.; Hori, M. Plasma-activated medium induces A549 cell injury via a spiral apoptotic cascade involving the mitochondrial-nuclear network. Free Radic. Biol. Med. 2015, 79, 28–44. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.; Gebhardt, L.; Arndt, S.; Karrer, S.; Zimmermann, J.L.; Fischer, M.J.M.; Bosserhoff, A.K. Cold atmospheric plasma causes a calcium influx in melanoma cells triggering CAP-induced senescence. Sci. Rep. 2018, 8, 10048. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Talbot, A.; Nourmohammadi, N.; Cheng, X.; Canady, J.; Sherman, J.; Keidar, M. Principles of using Cold Atmospheric Plasma Stimulated Media for Cancer Treatment. Sci. Rep. 2015, 5, 18339. [Google Scholar] [CrossRef] [PubMed]
- Almeida-Ferreira, C.; Silva-Teixeira, R.; Gonçalves, A.C.; Marto, C.M.; Sarmento-Ribeiro, A.B.; Caramelo, F.; Botelho, M.F.; Laranjo, M. Cold Atmospheric Plasma Apoptotic and Oxidative Effects on MCF7 and HCC1806 Human Breast Cancer Cells. Int. J. Mol. Sci. 2022, 23, 1698. [Google Scholar] [CrossRef] [PubMed]
- Mohades, S.; Laroussi, M.; Sears, J.; Barekzi, N.; Razavi, H. Evaluation of the effects of a plasma activated medium on cancer cells. Phys. Plasmas 2015, 22, 122001. [Google Scholar] [CrossRef]
- Tavares-Da-silva, E.; Pereira, E.; Pires, A.S.; Neves, A.R.; Braz-Guilherme, C.; Marques, I.A.; Abrantes, A.M.; Gonçalves, A.C.; Caramelo, F.; Silva-Teixeira, R.; et al. Cold Atmospheric Plasma, a Novel Approach against Bladder Cancer, with Higher Sensitivity for the High-Grade Cell Line. Biology 2021, 10, 41. [Google Scholar] [CrossRef]
- Sato, T.; Yokoyama, M.; Johkura, K. A key inactivation factor of HeLa cell viability by a plasma flow. J. Phys. D Appl. Phys. 2011, 44, 372001. [Google Scholar] [CrossRef]
- Ha, J.H.; Kim, Y.J. Photodynamic and Cold Atmospheric Plasma Combination Therapy Using Polymeric Nanoparticles for the Synergistic Treatment of Cervical Cancer. Int. J. Mol. Sci. 2021, 22, 1172. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Bhardwaj, A.; Gupta, S. Cancer treatment therapies: Traditional to modern approaches to combat cancers. Mol. Biol. Rep. 2023, 50, 9663–9676. [Google Scholar] [CrossRef]
- Yeung, K.T.; Yang, J. Epithelial-mesenchymal transition in tumor metastasis. Mol. Oncol. 2017, 11, 28–39. [Google Scholar] [CrossRef]
- Karimian, A.; Ahmadi, Y.; Yousefi, B. Multiple functions of p21 in cell cycle, apoptosis and transcriptional regulation after DNA damage. DNA Repair 2016, 42, 63–71. [Google Scholar] [CrossRef]
- Naser, R.; Dilabazian, H.; Bahr, H.; Barakat, A.; El-Sibai, M. A guide through conventional and modern cancer treatment modalities: A specific focus on glioblastoma cancer therapy (Review). Oncol. Rep. 2022, 48, 190. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti, I.D.L.; Soares, J.C.S. Conventional Cancer Treatment. Adv. Cancer Treat. 2021, 29–56. [Google Scholar] [CrossRef] [PubMed]
- Debela, D.T.; Muzazu, S.G.Y.; Heraro, K.D.; Ndalama, M.T.; Mesele, B.W.; Haile, D.C.; Kitui, S.K.; Manyazewal, T. New approaches and procedures for cancer treatment: Current perspectives. SAGE Open Med. 2021, 9, 20503121211034366. [Google Scholar] [CrossRef] [PubMed]
- Sreedevi, P.R.; Suresh, K.; Yugeswaran, S. Cold atmospheric plasma-induced oxidative stress and ensuing immunological response—A Neo-Vista in immunotherapy. Free Radic. Res. 2022, 56, 498–510. [Google Scholar] [CrossRef]
- Bekeschus, S.; Clemen, R.; Metelmann, H.R. Potentiating anti-tumor immunity with physical plasma. Clin. Plasma Med. 2018, 12, 17–22. [Google Scholar] [CrossRef]
- Bekeschus, S.; Seebauer, C.; Wende, K.; Schmidt, A. Physical plasma and leukocytes—Immune or reactive? Biol. Chem. 2018, 400, 63–75. [Google Scholar] [CrossRef]
- Bekeschus, S.; Scherwietes, L.; Freund, E.; Liedtke, K.R.; Hackbarth, C.; von Woedtke, T.; Partecke, L.I. Plasma-treated medium tunes the inflammatory profile in murine bone marrow-derived macrophages. Clin. Plasma Med. 2018, 11, 1–9. [Google Scholar] [CrossRef]
- Freund, E.; Moritz, J.; Stope, M.; Seebauer, C.; Schmidt, A.; Bekeschus, S. Plasma-Derived Reactive Species Shape a Differentiation Profile in Human Monocytes. Appl. Sci. 2019, 9, 2530. [Google Scholar] [CrossRef]
- Liedtke, K.R.; Bekeschus, S.; Kaeding, A.; Hackbarth, C.; Kuehn, J.P.; Heidecke, C.D.; Von Bernstorff, W.; Von Woedtke, T.; Partecke, L.I. Non-thermal plasma-treated solution demonstrates antitumor activity against pancreatic cancer cells in vitro and in vivo. Sci. Rep. 2017, 7, 8319. [Google Scholar] [CrossRef]
- Shaul, M.E.; Fridlender, Z.G. Tumour-associated neutrophils in patients with cancer. Nat. Rev. Clin. Oncol. 2019, 16, 601–620. [Google Scholar] [CrossRef]
- Liedtke, K.R.; Freund, E.; Hackbarth, C.; Heidecke, C.D.; Partecke, L.I.; Bekeschus, S. A myeloid and lymphoid infiltrate in murine pancreatic tumors exposed to plasma-treated medium. Clin. Plasma Med. 2018, 11, 10–17. [Google Scholar] [CrossRef]
- Bonaventura, P.; Shekarian, T.; Alcazer, V.; Valladeau-Guilemond, J.; Valsesia-Wittmann, S.; Amigorena, S.; Caux, C.; Depil, S. Cold tumors: A therapeutic challenge for immunotherapy. Front. Immunol. 2019, 10, 440886. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, J.; Chen, Y.; Kang, Y.; Liao, Z.; He, Y.; Zhang, C. Novel Immunotherapies for Osteosarcoma. Front. Oncol. 2022, 12, 830546. [Google Scholar] [CrossRef]
- Lin, A.; Gorbanev, Y.; De Backer, J.; Van Loenhout, J.; Van Boxem, W.; Lemière, F.; Cos, P.; Dewilde, S.; Smits, E.; Bogaerts, A. Non-Thermal Plasma as a Unique Delivery System of Short-Lived Reactive Oxygen and Nitrogen Species for Immunogenic Cell Death in Melanoma Cells. Adv. Sci. 2019, 6, 1802062. [Google Scholar] [CrossRef] [PubMed]
- Van Loenhout, J.; Flieswasser, T.; Boullosa, L.F.; De Waele, J.; Van Audenaerde, J.; Marcq, E.; Jacobs, J.; Lin, A.; Lion, E.; Dewitte, H.; et al. Cold Atmospheric Plasma-Treated PBS Eliminates Immunosuppressive Pancreatic Stellate Cells and Induces Immunogenic Cell Death of Pancreatic Cancer Cells. Cancers 2019, 11, 1597. [Google Scholar] [CrossRef]
- Guo, L.; Xu, R.; Gou, L.; Liu, Z.; Zhao, Y.; Liu, D.; Zhang, L.; Chen, H.; Kong, M.G. Mechanism of virus inactivation by cold atmospheric-pressure plasma and plasmaactivated water. Appl. Environ. Microbiol. 2018, 84, 726–744. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liang, Y.; Wei, K.; Li, W.; Yao, M.; Zhang, J.; Grinshpun, S.A. MS2 virus inactivation by atmospheric-pressure cold plasma using different gas carriers and power levels. Appl. Environ. Microbiol. 2015, 81, 996–1002. [Google Scholar] [CrossRef]
- Aboubakr, H.A.; Gangal, U.; Youssef, M.M.; Goyal, S.M.; Bruggeman, P.J. Inactivation of virus in solution by cold atmospheric pressure plasma: Identification of chemical inactivation pathways. J. Phys. D Appl. Phys. 2016, 49, 204001. [Google Scholar] [CrossRef]
- Zimmermann, J.L.; Dumler, K.; Shimizu, T.; Morfill, G.E.; Wolf, A.; Boxhammer, V.; Schlegel, J.; Gansbacher, B.; Anton, M. Effects of cold atmospheric plasmas on adenoviruses in solution. J. Phys. D Appl. Phys. 2011, 44, 505201. [Google Scholar] [CrossRef]
- Jin, T.; Xu, Y.; Dai, C.; Zhou, X.; Xu, Q.; Wu, Z. Cold atmospheric plasma: A non-negligible strategy for viral RNA inactivation to prevent SARS-CoV-2 environmental transmission. AIP Adv. 2021, 11, 85019. [Google Scholar] [CrossRef]
- Cortázar, O.D.; Megía-Macías, A.; Moreno, S.; Brun, A.; Gómez-Casado, E. Vulnerability of SARS-CoV-2 and PR8 H1N1 virus to cold atmospheric plasma activated media. Sci. Rep. 2022, 12, 263. [Google Scholar] [CrossRef]
- Chen, Z.; Garcia, G.; Arumugaswami, V.; Wirz, R.E. Cold atmospheric plasma for SARS-CoV-2 inactivation. Phys. Fluids 2020, 32, 111702. [Google Scholar] [CrossRef]
- Volotskova, O.; Dubrovsky, L.; Keidar, M.; Bukrinsky, M. Cold Atmospheric Plasma Inhibits HIV-1 Replication in Macrophages by Targeting Both the Virus and the Cells. PLoS ONE 2016, 11, e0165322. [Google Scholar] [CrossRef] [PubMed]
- Goldman, S.A. Stem and Progenitor Cell-Based Therapy of the Central Nervous System: Hopes, Hype, and Wishful Thinking. Cell Stem Cell 2016, 18, 174–188. [Google Scholar] [CrossRef]
- Weltmann, K.D.; Von Woedtke, T. Plasma medicine—Current state of research and medical application. Plasma Phys. Control. Fusion 2016, 59, 014031. [Google Scholar] [CrossRef]
- Nam, M.K.; Kim, G.Y.; Yun, S.E.; Jang, J.Y.; Kim, Y.H.; Choi, E.H.; Kang, S.; Rhim, H. Harmless effects of argon plasma on caudal fin regeneration and embryogenesis of zebrafish: Novel biological approaches for safe medical applications of bioplasma. Exp. Mol. Med. 2017, 49. [Google Scholar] [CrossRef]
- Tan, F.; Rui, X.; Xiang, X.; Yu, Z.; Al-Rubeai, M. Multimodal treatment combining cold atmospheric plasma and acidic fibroblast growth factor for multi-tissue regeneration. FASEB J. 2021, 35, e21442. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Han, R.; Li, Y.; Lu, C.; Chen, X.; Xiong, Z.; Mao, X. Investigation of the mechanism of enhanced and directed differentiation of neural stem cells by an atmospheric plasma jet: A gene-level study. J. Appl. Phys. 2019, 125, 163301. [Google Scholar] [CrossRef]
- Tian, M.; Qi, M.; Liu, Z.; Xu, D.; Chen, H.; Kong, M.G. Cold Atmospheric Plasma Elicits Neuroprotection Against Glutamate Excitotoxicity by Activating Cellular Antioxidant Defense. Plasma Chem. Plasma Process. 2021, 41, 945–954. [Google Scholar] [CrossRef]
- Yan, X.; Yang, B.; Ouyang, J.; Zhang, C.; Lai, Y.; Shi, Z.; Han, R.; Zhang, W.; Yuan, F.; Ostrikov, K. Mechanisms of atmospheric pressure plasma protection of neuronal cells under simulated ischemic stroke conditions. AIP Adv. 2022, 12, 025114. [Google Scholar] [CrossRef]
- Razzokov, J.; Yusupov, M.; Bogaerts, A. Oxidation destabilizes toxic amyloid beta peptide aggregation. Sci. Rep. 2019, 9, 5476. [Google Scholar] [CrossRef] [PubMed]
- Sardella, E.; Mola, M.G.; Gristina, R.; Piccione, M.; Veronico, V.; De Bellis, M.; Cibelli, A.; Buttiglione, M.; Armenise, V.; Favia, P.; et al. A Synergistic Effect of Reactive Oxygen and Reactive Nitrogen Species in Plasma Activated Liquid Media Triggers Astrocyte Wound Healing. Int. J. Mol. Sci. 2020, 21, 3343. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.T.; Jang, Y.S.; Kim, U.K.; Kim, H.J.; Ryu, M.H.; Kim, G.C.; Hwang, D.S. Non-thermal plasma application enhances the recovery of transected sciatic nerves in rats. Exp. Biol. Med. 2021, 246, 1287–1296. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Cai, J.; Xu, G.; Ren, H.; Chen, S.; Chang, Z.; Liu, J.; Huang, C.; Zhang, G.; Wu, X. Effect of Cold Plasma on Cell Viability and Collagen Synthesis in Cultured Murine Fibroblasts. Plasma Sci. Technol. 2016, 18, 353–359. [Google Scholar] [CrossRef]
- Badr, G.; El-Hossary, F.M.; Lasheen, F.E.d.M.; Negm, N.Z.; Khalaf, M.; Salah, M.; Sayed, L.H.; Abdel-Maksoud, M.A.; Elminshawy, A. Cold atmospheric plasma induces the curing mechanism of diabetic wounds by regulating the oxidative stress mediators iNOS and NO, the pyroptotic mediators NLRP-3, Caspase-1 and IL-1β and the angiogenesis mediators VEGF and Ang-1. Biomed. Pharmacother. 2023, 169, 115934. [Google Scholar] [CrossRef] [PubMed]
- Fathollah, S.; Mirpour, S.; Mansouri, P.; Dehpour, A.R.; Ghoranneviss, M.; Rahimi, N.; Safaie Naraghi, Z.; Chalangari, R.; Chalangari, K.M. Investigation on the effects of the atmospheric pressure plasma on wound healing in diabetic rats. Sci. Rep. 2016, 6, 19144. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Liu, Y.; Li, J.; Zhang, N.; Zhou, M.; Li, Y.; Zhao, G.; Wang, N.; Wang, A.; Wang, Y.; et al. A novel atmospheric-pressure air plasma jet for wound healing. Int. Wound J. 2022, 19, 538–552. [Google Scholar] [CrossRef]
- Nasruddin; Nakajima, Y.; Mukai, K.; Rahayu, H.S.E.; Nur, M.; Ishijima, T.; Enomoto, H.; Uesugi, Y.; Sugama, J.; Nakatani, T. Cold plasma on full-thickness cutaneous wound accelerates healing through promoting inflammation, re-epithelialization and wound contraction. Clin. Plasma Med. 2014, 2, 28–35. [Google Scholar] [CrossRef]
- Kusakci-Seker, B.; Demirayak-Akdemir, M. The effect of non-thermal atmospheric pressure plasma application on wound healing after gingivectomy. Int. Wound J. 2020, 17, 1376–1383. [Google Scholar] [CrossRef]
- Zhang, J.P.; Guo, L.; Chen, Q.L.; Zhang, K.Y.; Wang, T.; An, G.Z.; Zhang, X.F.; Li, H.P.; Ding, G.R. Effects and mechanisms of cold atmospheric plasma on skin wound healing of rats. Contrib. Plasma Phys. 2019, 59, 92–101. [Google Scholar] [CrossRef]
- Oliver, M.A.; Hussein, L.K.; Molina, E.A.; Keyloun, J.W.; McKnight, S.M.; Jimenez, L.M.; Moffatt, L.T.; Shupp, J.W.; Carney, B.C. Cold atmospheric plasma is bactericidal to wound-relevant pathogens and is compatible with burn wound healing. Burns 2024, 50, 1192–1212. [Google Scholar] [CrossRef] [PubMed]
- Lou, B.S.; Hsieh, J.H.; Chen, C.M.; Hou, C.W.; Wu, H.Y.; Chou, P.Y.; Lai, C.H.; Lee, J.W. Helium/Argon-Generated Cold Atmospheric Plasma Facilitates Cutaneous Wound Healing. Front. Bioeng. Biotechnol. 2020, 8, 683. [Google Scholar] [CrossRef]
- Lertpatipanpong, P.; Sillapachaiyaporn, C.; Oh, G.; Kang, Y.H.; Hwang, C.Y.; Baek, S.J. Effect of cold atmospheric microwave plasma (CAMP) on wound healing in canine keratinocytes. Front. Cell Dev. Biol. 2023, 11, 105692. [Google Scholar] [CrossRef] [PubMed]
- Shome, D.; Von Woedtke, T.; Riedel, K.; Masur, K. The HIPPO Transducer YAP and Its Targets CTGF and Cyr61 Drive a Paracrine Signalling in Cold Atmospheric Plasma-Mediated Wound Healing. Oxid. Med. Cell. Longev. 2020, 2020, 4910280. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wang, L.; Xia, C.; Yang, X.; Cao, Z.; Zheng, L.; Ko, R.; Shen, C.; Yang, C.; Cheng, C. Cold atmospheric plasma promotes different types of superficial skin erosion wounds healing. Int. Wound J. 2019, 16, 1103–1111. [Google Scholar] [CrossRef]
- Schmidt, A.; Bekeschus, S.; Jarick, K.; Hasse, S.; Von Woedtke, T.; Wende, K. Cold physical plasma modulates p53 and mitogen-activated protein kinase signaling in keratinocytes. Oxid. Med. Cell. Longev. 2019, 2019, 7017363. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Bekeschus, S.; Wende, K.; Vollmar, B.; von Woedtke, T. A cold plasma jet accelerates wound healing in a murine model of full-thickness skin wounds. Exp. Dermatol. 2017, 26, 156–162. [Google Scholar] [CrossRef]
- Ma, L.; Chen, Y.; Gong, Q.; Cheng, Z.; Ran, C.; Liu, K.; Shi, C. Cold atmospheric plasma alleviates radiation-induced skin injury by suppressing inflammation and promoting repair. Free Radic. Biol. Med. 2023, 204, 184–194. [Google Scholar] [CrossRef]
- Marches, A.; Clement, E.; Albérola, G.; Rols, M.P.; Cousty, S.; Simon, M.; Merbahi, N. Cold Atmospheric Plasma Jet Treatment Improves Human Keratinocyte Migration and Wound Closure Capacity without Causing Cellular Oxidative Stress. Int. J. Mol. Sci. 2022, 23, 10650. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, R.; Daeschlein, G.; von Woedtke, T.; Smeets, R.; Gosau, M.; Metelmann, H.R. Long-term Risk Assessment for Medical Application of Cold Atmospheric Pressure Plasma. Diagnostics 2020, 10, 210. [Google Scholar] [CrossRef] [PubMed]
- Betancourt-Ángeles, M.; Peña-Eguiluz, R.; López-Callejas, R.; Domínguez-Cadena, N.A.; Mercado-Cabrera, A.; Muñoz-Infante, J.; Rodríguez-Méndez, B.G.; Valencia-Alvarado, R.; Moreno-Tapia, J.A. Treatment in the healing of burns with a cold plasma source. Int. J. Burn. Trauma 2017, 7, 142. [Google Scholar]
- Bagheri, M.; von Kohout, M.; Zoric, A.; Fuchs, P.C.; Schiefer, J.L.; Opländer, C. Can Cold Atmospheric Plasma Be Used for Infection Control in Burns? A Preclinical Evaluation. Biomedicines 2023, 11, 1239. [Google Scholar] [CrossRef]
- Winter, S.; Meyer-Lindenberg, A.; Wolf, G.; Reese, S.; Nolff, M.C. In vitro evaluation of the decontamination effect of cold atmospheric argon plasma on selected bacteria frequently encountered in small animal bite injuries. J. Microbiol. Methods 2020, 169, 105728. [Google Scholar] [CrossRef] [PubMed]
- Brehmer, F.; Haenssle, H.A.; Daeschlein, G.; Ahmed, R.; Pfeiffer, S.; Görlitz, A.; Simon, D.; Schön, M.P.; Wandke, D.; Emmert, S. Alleviation of chronic venous leg ulcers with a hand-held dielectric barrier discharge plasma generator (PlasmaDerm® VU-2010): Results of a monocentric, two-armed, open, prospective, randomized and controlled trial (NCT01415622). J. Eur. Acad. Dermatol. Venereol. 2015, 29, 148–155. [Google Scholar] [CrossRef]
- Stratmann, B.; Costea, T.C.; Nolte, C.; Hiller, J.; Schmidt, J.; Reindel, J.; Masur, K.; Motz, W.; Timm, J.; Kerner, W.; et al. Effect of Cold Atmospheric Plasma Therapy vs Standard Therapy Placebo on Wound Healing in Patients With Diabetic Foot Ulcers: A Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e2010411. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lee, H.; Lee, H.J.; Kim, G.C.; Kim, S.S.; Han, S.; Song, K. Non-thermal atmospheric pressure plasma is an excellent tool to activate proliferation in various mesoderm-derived human adult stem cells. Free Radic. Biol. Med. 2019, 134, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Klotz, L.O.; Sánchez-Ramos, C.; Prieto-Arroyo, I.; Urbánek, P.; Steinbrenner, H.; Monsalve, M. Redox regulation of FoxO transcription factors. Redox Biol. 2015, 6, 51–72. [Google Scholar] [CrossRef]
- Elsaadany, M.; Subramanian, G.; Ayan, H.; Yildirim-Ayan, E. Exogenous nitric oxide (NO) generated by NO-plasma treatment modulates osteoprogenitor cells early differentiation. J. Phys. D Appl. Phys. 2015, 48, 345401. [Google Scholar] [CrossRef]
- Bourdens, M.; Jeanson, Y.; Taurand, M.; Juin, N.; Carrière, A.; Clément, F.; Casteilla, L.; Bulteau, A.L.; Planat-Bénard, V. Short exposure to cold atmospheric plasma induces senescence in human skin fibroblasts and adipose mesenchymal stromal cells. Sci. Rep. 2019, 9, 8671. [Google Scholar] [CrossRef]
- Ermakov, A.M.; Ermakova, O.N.; Afanasyeva, V.A.; Popov, A.L. Dose-dependent effects of cold atmospheric argon plasma on the mesenchymal stem and osteosarcoma cells in vitro. Int. J. Mol. Sci. 2021, 22, 6797. [Google Scholar] [CrossRef]
- Gherardi, M.; Tonini, R.; Colombo, V. Plasma in Dentistry: Brief History and Current Status. Trends Biotechnol. 2018, 36, 583–585. [Google Scholar] [CrossRef]
- Aparecida Delben, J.; Evelin Zago, C.; Tyhovych, N.; Duarte, S.; Eduardo Vergani, C. Effect of Atmospheric-Pressure Cold Plasma on Pathogenic Oral Biofilms and In Vitro Reconstituted Oral Epithelium. PLoS ONE 2016, 11, e0155427. [Google Scholar] [CrossRef] [PubMed]
- Beni, M.S.; Han, W.; Yu, K.N. Dispersion of OH Radicals in Applications Related to Fear-Free Dentistry Using Cold Plasma. Appl. Sci. 2019, 9, 2119. [Google Scholar] [CrossRef]
- Jiang, C.; Miles, J.; Hornef, J.; Carter, C.; Adams, S. Electron densities and temperatures of an atmospheric-pressure nanosecond pulsed helium plasma jet in air. Plasma Sources Sci. Technol. 2019, 28, 085009. [Google Scholar] [CrossRef]
- Asnaashari, M.; Mehrabinia, P.; Yadegari, Z.; Hoseini, H.; Sadafi, M.; Shojaeian, S. Evaluation of Antibacterial Effects of Cold Atmospheric Plasma, Calcium Hydroxide, and Triple Antibiotic Paste on Enterococcus faecalis Biofilm in the Root Canal System: An In Vitro Study. J. Lasers Med. Sci. 2022, 13, e50. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, X.; Liu, Z.; Wang, Y.; Zou, L.; Chen, Y.; Han, Q. The effect of argon cold atmospheric plasma on the metabolism and demineralization of oral plaque biofilms. Front. Cell. Infect. Microbiol. 2023, 13, 1116021. [Google Scholar] [CrossRef]
- Dong, X.; Chen, M.; Wang, Y.; Yu, Q. A mechanistic study of plasma treatment effects on demineralized dentin surfaces for improved adhesive/dentin interface bonding. Clin. Plasma Med. 2014, 2, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Manouchehri, N.; Ghodsi, S.; Atri, F.; Sarraf, P.; Seyedi, D.; Valizadeh, S. Effect of pretreatment of root dentin surface with cold atmospheric plasma on improving the bond strength of fiber post and resin cement: In vitro study. Clin. Exp. Dent. Res. 2023, 9, 653–660. [Google Scholar] [CrossRef]
- J-Plasma—Apyx Medical. Available online: https://apyxmedical.com/jplasma/ (accessed on 19 December 2023).
- Neoplas Med GmbH. Available online: https://neoplas-med.eu/en/ (accessed on 19 December 2023).
- Electrosurgical Products—PlasmaBlade Soft Tissue Dissection Devices. Available online: https://europe.medtronic.com/xd-en/healthcare-professionals/products/general-surgery/electrosurgical/peak-plasmablade-device.html (accessed on 19 December 2023).
- FDA Approves Clinical Trial of Cold Plasma ‘Scalpel’ for Cancer Treatment. Available online: https://newatlas.com/cancer-cold-plasma-clinical-trial/61138/ (accessed on 19 December 2023).
- Wundheilung Mit Kaltem Plasma: Ein Bedeutender Schritt. 2022. Available online: https://plasmaderm.de/technologie/ (accessed on 19 December 2023).
- Home—Adtec Healthcare Limited. Available online: https://adtechealthcare.com/ (accessed on 19 December 2023).
- Plasma Surgical—PlasmaJet. Available online: https://plasmasurgical.com/plasmajet-2/ (accessed on 19 December 2023).
- PiezoBrush PZ3. Plasma Handheld for Manual Use. Available online: https://www.relyon-plasma.com/piezobrush-pz3/?lang=en (accessed on 19 December 2023).
- Gavahian, M.; Khaneghah, A.M. Cold plasma as a tool for the elimination of food contaminants: Recent advances and future trends. Crit. Rev. Food Sci. Nutr. 2020, 60, 1581–1592. [Google Scholar] [CrossRef] [PubMed]
- Makhneva, E.; Barillas, L.; Farka, Z.; Pastucha, M.; Skládal, P.; Weltmann, K.D.; Fricke, K. Functional Plasma Polymerized Surfaces for Biosensing. ACS Appl. Mater. Interfaces 2020, 12, 17100–17112. [Google Scholar] [CrossRef] [PubMed]
- Saman, N.M.; Ahmad, M.H.; Buntat, Z. Application of Cold Plasma in Nanofillers Surface Modification for Enhancement of Insulation Characteristics of Polymer Nanocomposites: A Review. IEEE Access 2021, 9, 80906–80930. [Google Scholar] [CrossRef]
- Tucker, B.S.; Aliakbarshirazi, S.; Vijayan, V.M.; Thukkaram, M.; De Geyter, N.; Thomas, V. Nonthermal plasma processing for nanostructured biomaterials and tissue engineering scaffolds: A mini review. Curr. Opin. Biomed. Eng. 2021, 17, 100259. [Google Scholar] [CrossRef]
- Ercan, U.K.; Özdemir, G.D.; Özdemir, M.A.; Güren, O. Plasma medicine: The era of artificial intelligence. Plasma Process. Polym. 2023, 20, e2300066. [Google Scholar] [CrossRef]

| Issue | Current Status | Improvement Suggestion | Ref. |
|---|---|---|---|
| Specificity | Conventional methods used in oncology can be inefficient and uncomfortable for patients. Some tumours are difficult to reach or even untreatable. CAP therapy displays promising results in cancer treatment. In general, the treatment leads to deceleration or even reduction in the tumour that stays out of reach for traditional therapies. | Achieving a tumour-specific targeted therapy via CAP would create a far more accurate and convenient treatment method for patients, thus resulting in better prognosis. The researchers should concentrate on revealing deeper layers of anti-tumour mechanism and its connection with cancer-targeted treatment. | [36] |
| Mechanism | The precise mechanisms underlying the CAP-induced anti-cancer effects are not perfectly clear. The results can vary significantly between tumour types or even between individual cell lines. | Further research to confirm specific molecular mechanisms behind CAP’s anti-tumour actions is mandatory to optimize treatment and improve its efficacy. | [37] |
| Standardization | Various plasma devices, delivery methods, treatment parameters across studies increase the difficulty in comparing results or drawing valid conclusions. | Defining parameters such as plasma device specifications, treatment duration, power input, gas composition, and distance from the target may overcome the challenges associated with variability in plasma medicine and create a helpful guideline for further research. | [38] |
| Penetration | Tumours are often found in locations which are not easily accessible. CAP’s ability to penetrate deep-seated tumours is limited. It is challenging to ensure enhanced availability while maintaining therapeutic efficacy. | A wide range of indirect CAP delivery methods known as PAM or PTL create a possibility to inject liquid displaying therapeutic plasma properties. It allows us to reach the designated area without compromising efficacy of treatment. | [39] |
| Tumour Heterogeneity | Cancer is an example of a heterogeneous disease. There are multiple types and subtypes of tumour that may exhibit various responses to CAP treatment. | An endeavour to enhanced CAP’s effectiveness against specific tumour type should be an inspiration for researchers. It could be achieved through adopting a more personalized form of treatment by targeting biomarkers characteristic for a certain tumour. | [40] |
| Immune Response | It is possible to modulate the immune system via CAP therapy, adjusting the responses to enhance its anti-tumour effects. However, the complexity of such processes makes it challenging to selects and optimize any treatment protocols. | Advancing the research in the matter of the modulation of CAP-induced immune responses and their impact on the tumour microenvironment is required to identify and clarify the underlying mechanisms. | [41] |
| Combination Therapies | CAP anti-tumour therapy by itself displays promising results and emerges as a novel treatment method. Even though CAP often surpasses the conventional methods, they should not be disregarded. Traditional treatment modalities such as chemotherapy, radiotherapy, or immunotherapy may display promising synergistic effects when combined with assets of CAP. | Careful evaluation is required to determine optimal protocols, strategies and sequence of treatments. Further investigation of this synergistic approach could potentially enhance penetration depth, therapy efficacy and improve overall prognosis for patients. | [42] |
| Clinical transition | Despite promising results in the preclinical environment, the translation of CAP therapy from the laboratory to clinical practice is hindered by several challenges, such as safety regulations, scalability, and cost-effectiveness. | It is important to conduct well-designed clinical trials, thus reassuring the safety, convenience and efficacy of CAP therapy in the matter of cancer treatment. | [43] |
| Treatment | Tumour Growth Inhibition | Prevention of Metastasis | Cell Viability |
|---|---|---|---|
| CAP | Inhibition of tumor growth in preclinical studies across various cancer types, including breast, lung, prostate, and melanoma. CAP treatment induces apoptosis, causes cell cycle arrest, leading to a selective targeting of cancer cells [100,101,102,103,115,120]. | Preventing metastasis by targeting cancer cells’ migratory and invasive properties. through inhibition of the migration and invasion of cancer cells by modulating signalling pathways involved in metastasis [126]. | CAP induced selective cancer cell death while sparing healthy cells, reducing overall cell viability within tumors through, apoptosis, disrupt cellular functions, and modulate signalling pathways [127]. |
| Conventional | Chemotherapy unselectively targets rapidly dividing cells, often causes significant side effects [128]. Radiotherapy reduces tumour sizes damaging surrounding healthy tissues [129]. Surgery is effective for removing localized tumors without addressing metastatic disease [130]. | Chemotherapy or targeted therapy may prevent metastasis by targeting circulating tumor cells and micrometastases. Their effectiveness strongly depends on the cancer type as well as stage. Unfortunately, the treatments impact healthy cells surrounding tumours [128]. | Reduced cancer cell viability through apoptosis, DNA damage, or inhibiting cell proliferation. However, conventional therapies also affect healthy cells, resulting in side effects, i.e., neurophathy [128]. |
| Treatment Target | CAP Device | Effect |
|---|---|---|
| Glioblastoma (U373MG) | * DIT 120 prototype [68] | Membrane permeabilization, mitochondrial membrane depolarization and caspase-independent cell death [68]. |
| Amyloid β | * Pulsed radio-frequency cold atmospheric plasma jet [158] | Oxidation of methionine in amyloid B (Met35) slows down the progression of Alzheimer’s disease [158]. |
| Astrocyte | * PetriPlas+ [159] | Selective wound healing without inducing a gliotic inflammatory reaction [159]. |
| Sciatic nerve | * Ar/coaxial-DBD plasma [160] | CAP treatment resulted in more dense Schwann cells and a well-established continuity of nerve fibres, restoring neuronal structure and leading to nerve recovery [160]. |
| Treatment Target | CAP Device | Effect |
|---|---|---|
| Canine keratinocyte (CPEK) [171] Human keratinocyte (HaCaT) [175] (N/TERT-1) [175] | Bio Stimulation Microwave Plasma v1.0 (Ion Medical Inc., Gyeonggi-do, South Korea) [170] He/plasma Jet device (PlasmaKin, Stryker Corporation, Kalamazoo, Michigan, USA) [176] | Increase in both cell lines’ migration [170]. Accelerate wound closure in vitro by improving keratinocyte migration [176]. |
| Chronic skin radiation injury | kINPen MED (Neoplas Tools GmbH, Greifswald, Germany) [175] | Enhanced proliferation, migration and cellular antioxidant stress and promote DNA damage repair through regulated nuclear translocation of NRF2 [175]. |
| CO2 laser skin damage | kINPen MED (Neoplas Tools GmbH, Greifswald, Germany) [177] | No adverse effects of CAP were displayed [177]. |
| Burn wound | * He/plasma needle [178] Plasma One (PlasmaOne Medical, Düsseldorf, Germany) [179] | Reduced urticarial and feeling of pain, followed by re-epithelization [178]. Reduced microbial load (Pseudomonas aeruginosa) and inhibition of biofilm formation [179]. |
| Traumatic wound | PlasmaDerm VU-2010 (Cinogy GmbH, Duderstadt, Germany) [172] | Reduced inflammation [172]. |
| Dog bite wound | kINPen VET (Neoplas Tools GmbH, Greifswald, Germany) [180] | Antibacterial action [180]. |
| Chronic venous leg ulcers [102] Diabetic Foot Ulcers [103] | PlasmaDerm VU-2010 (Cinogy GmbH, Duderstadt, Germany) [181] kINPen Med (Neoplas Tools GmbH, Greifswald, Germany) [182] | Quicker healing, and reduction in microbial burden [181]. Reduction in wound size, clinical infection, and microbial load compared with treatment start [182]. |
| Pyoderma gangrenosum | PlasmaDerm VU-2010 (Cinogy GmbH, Duderstadt, Germany) [172] | Wound repair, drying [172]. |
| Treatment Target | CAP Device | Effect |
|---|---|---|
| Tooth canal disinfection | * Nano-pulsed He/plasma jet [191] * He/O2 plasma jet [192] | Reduced bacterial infection [191]. Effective inhibition of bacterial load growth [192]. |
| Dental biofilm reduction | kINPen Med (Neoplas Tools GmbH, Greifswald, Germany) [189] * Ar/DBD device [193] | Antimicrobial activity, regeneration of oral epithelium [189]. CAP had an antibacterial ability toward biofilms stronger than ultraviolet under the same tested conditions [193]. |
| Optimization of dental structures | * Ar/Plasma brush [194] He/PlasmaJet (PlasmaTreat GmbH, Steinhagen, Germany) [195] | Improvement of connections, and improved adherence to dentin [194]. Plays a significant role in improving the bond strength of fibre post and root canal dentin [195]. |
| JPlasma (Bovie Medical Corporation, Clearwater, FL, USA) | It is an advanced energy modality which combines the unique properties of helium plasma with a proprietary RF waveform. Helium plasma focuses RF energy for greater control of tissue effect, enabling a high level of precision and virtually eliminating unintended tissue trauma. These properties may allow surgeons to use this energy on and around sensitive structures [196]. |
| kINPen (Neoplas Tools GmbH, Greifswald, Germany) | It is considered to be the world’s first plasma jet tool using argon as a carrier gas. It provides both precise and consistent treatment combined with a gentle and effective wound treatment therapy, excluding any kind of side effects or developing resistance up to the plausible closure of the wound [197]. |
| PlasmaBlade (Medtronic, Minneapolis, MN, USA) | It is a representation of advancement in radiofrequency (RF) technology. It is composed of two main elements: a soft tissue dissection device called PlasmaBlade and the PULSAR Generator. The Generator provides pulsed plasma RF energy to the PlasmaBlade making it easier to use. The device combines traditional electrosurgery-like precision and bleeding control excluding the extensive collateral tissue damage. PEAK plasma blade from Medtronic [198]. |
| Cold Plasma System and Scalpel (Plasma Surgical, Roswell, NM, USA) | Composed of a CAP generator connected with a pen-like electrosurgical scalpel. The device sprays a blue-coloured plasma jet at the tip of the scalpel. Cancer cell exposure using this device CAP treatment oscillating between two and seven minutes is proven to be effectively cytotoxic to these cells without inducing any damage to regular cell lines [199]. |
| PlasmaDerm (Cinogy GmbH, Duderstadt, Germany) | This technology is officially recognized, effective, effective and safe. It creates a tissue-friendly plasma with a temperature similar to body temperature. This makes it perfect for germ release of microbially contaminated skin and wounds. The simultaneous deep stimulation of the skin or wound surface increases microcirculation, resulting in appropriate oxygen and nutrient delivery [200]. |
| SteriPlas (Adtec Healthcare London Greater London United Kingdom) | One of the topical antibacterial, cold plasma medical devices with proven accelerated healing, high antibacterial efficacy, and a greater advantage over antibiotic-resistant microorganisms. Often used for wound treatment, dermatological conditions, and surgical site infections [201]. |
| PlasmaJet (Bovie Medical Corporation, Clearwater, FL, USA) | It is considered to be a safe, effective, and easy-to-use CAP system. It was designed to utilize cold plasma technology for cutting and blood coagulation while performing surgery. It provides more precise cutting while maintaining reduced thermal damage compared to traditional electrosurgical devices [202]. |
| PiezoBrush PZ3 (Relyon Plasma GmbH, Regensburg, Germany) | A compact plasma handheld device with a maximum power consumption of 18 W can be used to generate cold active plasma at a low temperature. Often applied in microbiology, microfluidics, food technology, medicine, and dental technology [203]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stańczyk, B.; Wiśniewski, M. The Promising Potential of Cold Atmospheric Plasma Therapies. Plasma 2024, 7, 465-497. https://doi.org/10.3390/plasma7020025
Stańczyk B, Wiśniewski M. The Promising Potential of Cold Atmospheric Plasma Therapies. Plasma. 2024; 7(2):465-497. https://doi.org/10.3390/plasma7020025
Chicago/Turabian StyleStańczyk, Beata, and Marek Wiśniewski. 2024. "The Promising Potential of Cold Atmospheric Plasma Therapies" Plasma 7, no. 2: 465-497. https://doi.org/10.3390/plasma7020025
APA StyleStańczyk, B., & Wiśniewski, M. (2024). The Promising Potential of Cold Atmospheric Plasma Therapies. Plasma, 7(2), 465-497. https://doi.org/10.3390/plasma7020025








