3.1. Recovery Rates of Aromatic Model Compounds
Figure 2 shows the HPLC chromatograms of aqueous solutions of three aromatic model compounds treated in the solution plasma (gap = 1.5 mm, 28 W). The peak areas of the aromatic model compounds (a, phenol; b, guaiacol; c, syringol) decrease with the plasma treatment time, indicating their decomposition by the solution plasma. In contrast, several product peaks emerge and increase in area over time. These aqueous phase products are discussed in
Section 3.2. Note that the boiling points of water, phenol, guaiacol, and syringol are 100 °C, 182 °C, 205 °C, and 262 °C, respectively, under ambient pressure [
22]. As shown in
Appendix A, the solution’s temperature was maintained below 60 °C under this discharge condition; therefore, their volatilization into the gas bag did not occur.
Figure 3 shows the recovery rates of the three aromatic model compounds over plasma treatment time under different discharge conditions based on the HPLC peak areas. The recovery rates of all aromatic model compounds decrease with treatment time, and the decrease is more pronounced with a larger electrode gap, i.e., higher discharge power. This figure also includes error bars indicating experimental variation, but the variation remained very small, demonstrating the high reproducibility of the experiments in this study.
Thus, since the discharge power and treatment time influenced the recovery rate,
Figure 4 presents a re-plotted graph in which the horizontal axis of treatment time was converted to energy input (=discharge power × treatment time). As a result, the differences due to the electrode gap nearly disappeared, demonstrating that within the setup and experimental conditions of this study, the decomposition rate of the aromatic model compounds (100—recovery rate, %) was determined by the energy input.
Figure 4 also shows that at the same energy input, the decomposition rate follows the order syringol > guaiacol > phenol. This indicates that compounds with a greater number of methoxy groups tend to decompose more easily in aqueous solution plasma. Since both hydroxyl and methoxy groups, which are substituents of these model compounds, are electron-donating groups, the electron density of the aromatic ring may influence the decomposition reaction.
3.2. Aqueous Phase Products
The HPLC chromatograms shown in
Figure 2 were obtained using a UV detector; therefore, only compounds with UV absorption could be detected. Several small product peaks are observed in the retention time range of approximately 20 to 80 min.
Figure 5 provides enlarged views of this region. The compound names and chemical structures of these products are also indicated in this figure. These products were identified by comparing their retention times with those of authentic samples. For confirmation, the chemical structures of these products were further verified by proton nuclear magnetic resonance spectrometry (
1H-NMR), as explained in
Appendix B.
From phenol, compounds hydroxylated at the ortho, para, and both positions of the phenolic hydroxyl group (catechol, hydroquinone, and hydroxyquinol, respectively) were produced, consistent with the report by Tezuka and Iwasaki [
18]. As they stated, this hydroxylation is caused by hydroxyl (·OH) radicals generated from water in aqueous solution plasma. Similarly, from guaiacol, compounds hydroxylated at the ortho and para positions (3-methoxy-catechol and methoxy-hydroquinone, respectively) were detected. In addition, catechol was produced by methoxy-to-hydroxyl replacement, while phenol was produced by demethoxylation. From syringol, a compound hydroxylated at the para position (2,6-dimethoxy-hydroquinone) was detected, along with 3-methoxy-catechol from methoxy-to-hydroxyl replacement and guaiacol formed by demethoxylation.
Figure 6 presents the quantification results of the identified aromatic products on the basis of their HPLC peak areas. The total yield of aromatic products (carbon-based) was highest for syringol, followed by guaiacol and phenol. The major products from phenol were compounds hydroxylated at the ortho or para position, with the ortho-hydroxylated products’ yield being approximately twice that of the para-hydroxylated products. This result is reasonable, as phenol has two ortho positions available for hydroxylation. For guaiacol, the major product was catechol formed by methoxy-to-hydroxyl substitution, while the formation of ortho- or para-hydroxylated products and phenol via demethoxylation was minor. Similarly, the methoxy-to-hydroxyl substitution product (3-methoxy-catechol) was the most abundant in syringol, but the para-hydroxylated product (2,6-dimethoxy-hydroquinone) was also produced in relatively large amounts. However, the formation of guaiacol via demethoxylation was rare.
On the basis of these results, the reactions of the lignin aromatic units are summarized in
Figure 7. The phenolic hydroxyl group in the aromatic model compounds is an electron-donating group, resulting in relatively high electron density at the ortho and para positions (marked with * in
Figure 7). As shown in
Figure 7a, hydroxylation is expected to selectively occur at the ortho and para positions through the attack of electron-deficient ·OH radicals at these sites. For phenol, this hydroxylation was the dominant reaction. As shown in
Figure 7b, methoxy-to-hydroxyl replacement appears to proceed via the same mechanism as hydroxylation. In guaiacol and syringol, which contain methoxy groups, this reaction was predominant. The preference for methoxy-to-hydroxyl replacement over hydroxylation remains unclear. In
Figure 4, the decomposition rate follows the order syringol > guaiacol > phenol, which may be attributed to the increasing number of electron-donating groups, leading to higher electron density in the aromatic ring and greater reactivity with ·OH radicals.
In contrast, as shown in
Figure 7c, demethoxylation, or methoxy-to-hydrogen replacement, was observed but rare. This suggests that the reactions of the aromatic model compounds primarily occur in the liquid phase. The formation of ·OH and ·H radicals from water molecules occurs in the plasma region (gas phase) [
23]. However, in the liquid phase, ·H radicals immediately react with other species and disappear (e.g., ·H + H
2O → H
2 + ·OH). On the other hand, ·OH radicals can be present even in the liquid phase [
24]. For example, an ·OH radical can react with another one, producing H
2O
2, which is stable in the liquid phase and can return to ·OH radicals. Thus, ·OH radicals primarily contribute to reactions in the liquid phase. Since the lignin aromatic model compounds have higher boiling points than water, they predominantly remain in the liquid phase, where they react with ·OH radicals, while reactions with ·H radicals, as shown in
Figure 7c, appear to be infrequent.
On the other hand, a single peak distinct from the aromatic products is observed from each aromatic model compound at a retention time of approximately 5 min in the HPLC chromatograms shown in
Figure 2. This peak appears to contain multiple products, as such an early retention time indicates minimal retention in the column. Since the column used in this study tends to elute compounds with higher polarity and lower molecular weight more quickly, this peak appears to be a mixture of such compounds. Therefore, GC/MS analysis was conducted for the separation and identification of these compounds.
Figure 8 shows the GC/MS total ion chromatograms of the products from the aromatic model compounds. The samples were analyzed after freeze-drying and TMS derivatization. This figure also presents the names and structures of the compounds identified on the basis of the match of mass fragments and retention times with those of authentic samples. From phenol, guaiacol, and syringol, dicarboxylic acids such as oxalic, malonic, and succinic acids were detected, along with hydroxylated dicarboxylic acids including tartronic, malic, and tartaric acids, as well as glycolic acid as a monocarboxylic acid.
Figure 9 presents the quantification results of these organic acids based on GC/MS peak areas. For phenol and guaiacol, the peaks of succinic acid and catechol overlapped; therefore, quantification was based solely on mass fragments derived from succinic acid. From all aromatic models, oxalic acid was the predominant organic acid product. The total yield and composition of organic acids showed no significant differences among the aromatic model compounds.
In the GC/MS analysis, some of the products may have partially volatilized during the freeze-drying process. Therefore, 80 mL aliquots from each of the 100 mL aromatic model solutions after plasma treatment were directly subjected to potentiometric titration, yielding pH curves as shown in
Figure 10. The consumption of 0.05 M KOH (titration factor: 0.906) at the neutralization points was 11.1, 10.2, and 12.1 mL for the phenol, guaiacol, and syringol solutions, respectively, corresponding to H⁺ concentrations of 6.3, 5.8, and 6.9 mmol/L. On the other hand, the H⁺ concentrations calculated on the basis of the GC/MS analysis in
Figure 9 were 5.5, 5.5, and 5.7 mmol/L for the phenol, guaiacol, and syringol solutions, respectively. Since the H⁺ concentrations determined by GC/MS and titration were in close agreement, the loss of organic acids during freeze-drying was minimal, confirming the reliability of the quantification results in
Figure 9.
The dicarboxylic acids detected in this study are known to be major products formed during the oxidative degradation of lignin. For example, in the oxidative degradation of guaiacol using H
2O
2 with chalcopyrite as a catalyst [
25], catechol and hydroquinone are initially produced, followed by their oxidation via ortho-quinone and para-quinone intermediates, respectively, leading to oxidative ring cleavage and the formation of dicarboxylic acids such as malonic, succinic, and malic acids. These reactions are all mediated by ·OH radicals. There is also a report that dicarboxylic acids were formed through oxidative cleavage during the decomposition of benzoquinone (para-quinone) in aqueous solution plasma [
26]. In the solution plasma treatment of this study, the formation of ortho- and para-hydroxyl compounds and dicarboxylic acids, which are known to be produced during the oxidative degradation of lignin, was confirmed from phenol, guaiacol, and syringol. Therefore, it is inferred that these aromatic model compounds decomposed through pathways largely similar to the previously reported oxidative degradation of lignin [
25], driven by the action of ·OH radicals generated in aqueous solution plasma.
3.3. Gaseous Phase Products
Figure 11 shows the quantitative results (mL) of the gases produced during the solution plasma treatment of aromatic model compounds (gap = 1.5 mm, 28 W, 60 min), analyzed by micro-GC. The results are presented by generated volume, but the yield (C-mol%) based on the supplied aromatic model compound is discussed in the next subsection. The main gaseous products were H
2, CO, and CO
2, with a small amount of CH
4 also being detected, while C
2 and C
3 gases were barely detected. Note that H
2 can also be generated from water. In the blank test without aromatic model compounds, approximately 45 mL of H
2 was produced under the same treatment conditions. Therefore, although approximately 60 mL of H
2 was generated from each aromatic model compound, about 45 mL is considered to originate from water.
The formation of CO and CO
2 is considered to occur via dicarboxylic acids rather than directly from aromatic compounds. CO can be generated through decarbonylation, as shown in
Figure 12a, while CO
2 can be produced via ·OH radical-induced decarboxylation, as illustrated in
Figure 12b. To further investigate this process, we conducted an aqueous solution plasma treatment of formic acid, the simplest monocarboxylic acid, and confirmed the formation of both CO and CO
2. Although the specific molecular mechanism remains unclear, this result indicates that CO and CO
2 can be formed from carboxylic acids. H
2 can be generated during the progression of decarboxylation and decarbonylation.
On the other hand, CH
4 can be generated through demethylation at the methoxy group, as shown in
Figure 13a. In this process, catechol is formed from guaiacol. In
Section 3.2, the formation of catechol from guaiacol was attributed to methoxy-to-hydroxyl substitution; however, it can also occur through demethylation. Nevertheless, demethylation is considered a minor or negligible reaction. This is because despite the number of methoxy groups being zero in phenol, one in guaiacol, and two in syringol, the CH
4 yield from syringol was the lowest. Therefore, CH
4 is more likely derived from the carbon backbone of dicarboxylic acids rather than from methoxy groups, while catechol formation primarily occurs via methoxy-to-hydroxyl substitution. When methoxy-to-hydroxyl substitution takes place, the detached methoxyl (·OCH
3) radical is expected to decompose into H
2 and CO rather than into CH
4, as illustrated in
Figure 13b.
3.4. Decomposition Behavior of Aromatic Model Compounds
Figure 14 shows the product yields, including unreacted feedstocks, from phenol, guaiacol, and syringol after aqueous solution plasma treatment (gap = 1.5 mm, 28 W, 60 min). These yields are expressed as carbon yield based on the initial feedstock, and the total should be 100 C-mol% if all products are accounted for. Notably, as the yields are carbon-based, H
2 is not included in this figure.
The yield of unreacted feedstock after solution plasma treatment was approximately 38 C-mol% for phenol, 34 C-mol% for guaiacol, and 19 C-mol% for syringol, indicating that syringol underwent the most extensive decomposition. As previously discussed, this is likely due to the greater number of electron-donating groups in syringol. On the other hand, the total yield of aromatic products, organic acids, and gases was approximately 20 C-mol% from phenol, 22 C-mol% from guaiacol, and 27 C-mol% from syringol. Even when the unreacted feedstock is included, the total accounted for only approximately 58 C-mol% for phenol, 56 C-mol% for guaiacol, and 46 C-mol% for syringol, indicating that a significant fraction was not detected within the scope of this study.
To investigate this undetected fraction, the gas bag was rinsed with water and methanol, and the resulting liquid was analyzed by HPLC and GC/MS; however, no peaks corresponding to unreacted feedstock or products were observed. Additionally, no solid products or phase-separated substances were observed in the aqueous solution after the solution plasma treatment. Furthermore, hexane extraction of the aqueous solution followed by GC/MS analysis also did not reveal any detectable compounds. Although the undetected fraction remains an issue for further investigation, the identified products in this study reliably reflect the major reactions occurring in aqueous solution plasma. Nevertheless, the presence of this undetected fraction implies that additional, yet-unidentified reaction pathways may also be involved.
In this paper, the product yields are presented for the case with an electrode gap of 1.5 mm (average discharge power: 28 W). However, similar product distributions were observed at electrode gaps of 0.5 mm (16 W) and 1.0 mm (21 W), indicating that the discharge power had little effect on the distribution of products and affected only the decomposition rate, as shown in
Figure 3.
A characteristic feature of aqueous solution plasma treatment of the aromatic model compounds is the relatively low gas fraction among the products. In contrast, plasma treatment of high-volatility compounds such as methanol, ethanol, and 1-propanol predominantly yields gases such as H
2, CO, and CO
2, with only trace amounts of liquid phase products [
12,
13]. This suggests that gasification primarily occurs in the plasma region (gas phase). Since high-volatility compounds readily evaporate into the plasma region, gasification proceeds rapidly. In the present study, however, the intermediate products, including aromatic compounds and dicarboxylic acids, have high boiling points. For example, catechol boils at 246 °C, malic acid at 306 °C (at atmospheric pressure), and oxalic acid at 200 °C (at 0.1 Torr) [
22]. These intermediates are less volatile and tend to remain in the aqueous phase, where they are protected from gasification.
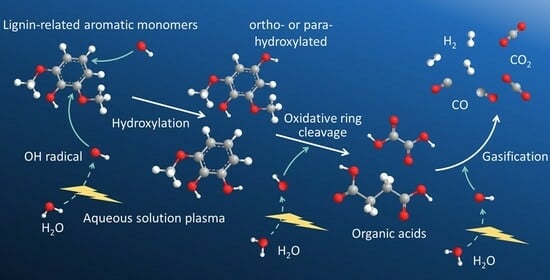
Figure 15 summarizes the decomposition reactions and major products identified in this study. Although this scheme represents the case of phenol, similar pathways are applicable to guaiacol and syringol. First, phenol undergoes hydroxylation at the ortho and para positions, forming the corresponding ortho- and para-hydroxy derivatives. These intermediates are further oxidized to ortho- and para-quinones, which subsequently undergo oxidative cleavage, leading to the ring-opening of the aromatic structure and the formation of dicarboxylic acids. Furthermore, C=C double bonds undergo oxidative cleavage, breaking down into dicarboxylic acids with a lower molecular weight. All these oxidative degradation processes are considered to be mediated by ·OH radicals derived from water. Finally, decarbonylation and decarboxylation occur at the carboxyl groups, leading to the formation of gases, primarily H
2, CO, and CO
2. However, ortho- and para-quinones were not detected in the aqueous solution by HPLC or GC/MS analysis, suggesting that they are relatively unstable and decompose readily.