Direct Deviations in Astrocyte Free Ca2+ Concentration Control Multiple Arteriole Tone States
Abstract
:1. Introduction
2. Materials and Methods
3. Results
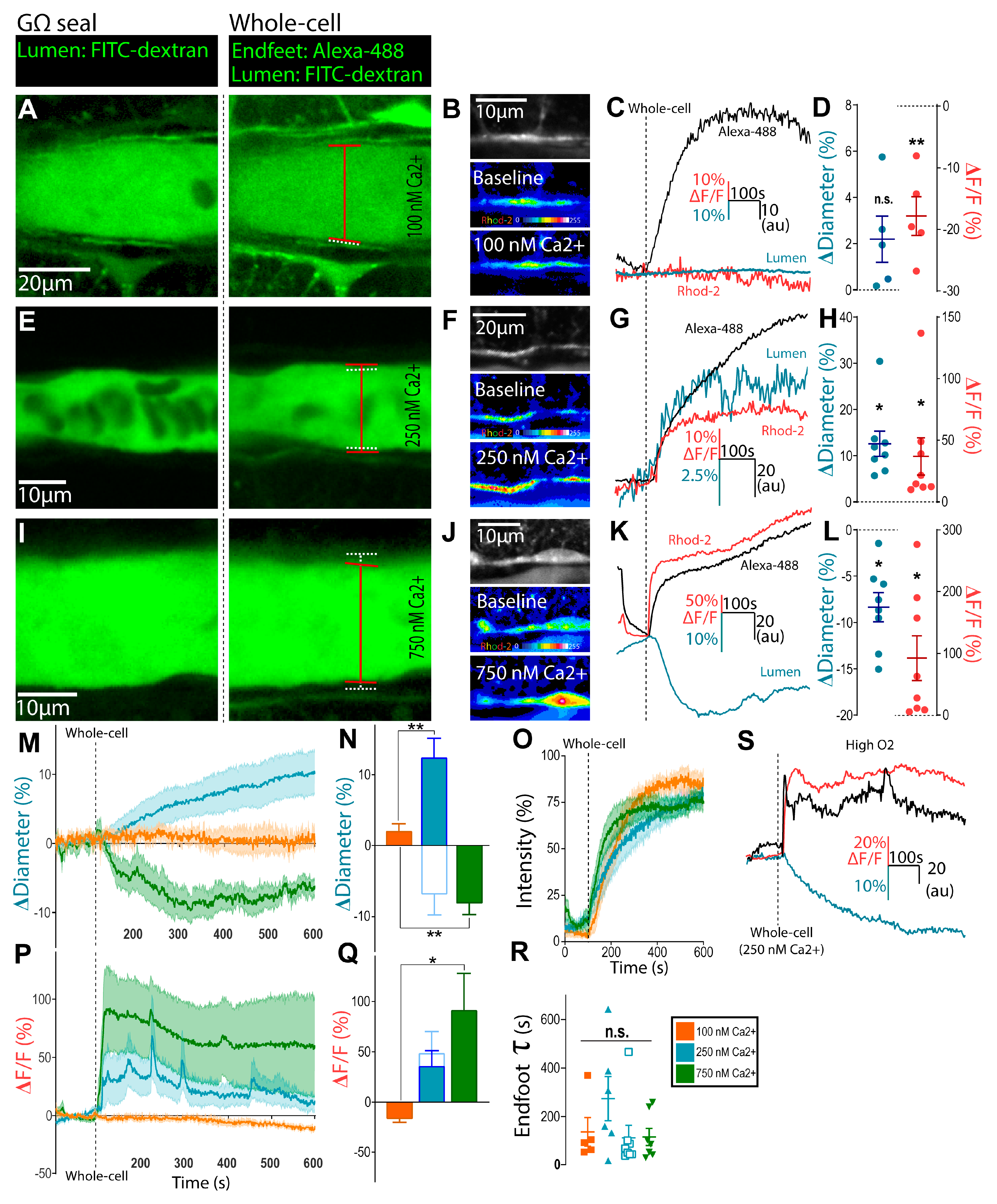
3.1. Clamping Astrocyte Ca2+ to Different Elevated Levels Elicits Opposite Arteriole Tone States
3.2. Bidirectional Changes in Arteriole Diameter Evoked by Elevations in Astrocyte Ca2+ Are Mediated by COX-1 and 20-HETE
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zonta, M.; Angulo, M.C.; Gobbo, S.; Rosengarten, B.; Hossmann, K.-A.; Pozzan, T.; Carmignoto, G. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat. Neurosci. 2003, 6, 43–50. [Google Scholar] [CrossRef]
- Mulligan, S.J.; MacVicar, B.A. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature 2004, 431, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Filosa, J.A.; Bonev, A.D.; Nelson, M.T. Calcium Dynamics in Cortical Astrocytes and Arterioles during Neurovascular Coupling. Circ. Res. 2004, 95, e73–e81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Girouard, H.; Bonev, A.D.; Hannah, R.M.; Meredith, A.; Aldrich, R.W.; Nelson, M.T. Astrocytic endfoot Ca2+ and BK channels determine both arteriolar dilation and constriction. Proc. Natl. Acad. Sci. USA 2010, 107, 3811–3816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanco, V.M.; Stern, J.E.; Filosa, J.A. Tone-dependent vascular responses to astrocyte-derived signals. Am. J. Physiol.-Heart Circ. Physiol. 2008, 294, H2855–H2863. [Google Scholar] [CrossRef] [PubMed]
- Haidey, J.N.; Peringod, G.; Institoris, A.; Gorzo, K.A.; Nicola, W.; Vandal, M.; Ito, K.; Liu, S.; Fielding, C.; Visser, F.; et al. Astrocytes regulate ultra-slow arteriole oscillations via stretch-mediated TRPV4-COX-1 feedback. Cell Rep. 2021, 36, 109405. [Google Scholar] [CrossRef]
- Gordon, G.; Choi, H.; Ellis-Davies, G.; MacVicar, B. Brain metabolic state dictates the polarity of astrocyte control over the cerebrovasculature. Nature 2008, 456, 745–749. [Google Scholar] [CrossRef] [Green Version]
- Mishra, A.; Hamid, A.; Newman, E.A. Oxygen modulation of neurovascular coupling in the retina. Proc. Natl. Acad. Sci. USA 2011, 108, 17827–17831. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.J.; Iddings, J.A.; Stern, J.E.; Blanco, V.M.; Croom, D.; Kirov, S.A.; Filosa, J.A. Astrocyte Contributions to Flow/Pressure-Evoked Parenchymal Arteriole Vasoconstriction. J. Neurosci. 2015, 35, 8245–8257. [Google Scholar] [CrossRef] [Green Version]
- Angelova, P.R.; Kasymov, V.; Christie, I.; Sheikhbahaei, S.; Turovsky, E.; Marina, N.; Korsak, A.; Zwicker, J.; Teschemacher, A.G.; Ackland, G.L.; et al. Functional Oxygen Sensitivity of Astrocytes. J. Neurosci. 2015, 35, 10460–10473. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Tabatabaei, M.; Bélanger, S.; Girouard, H.; Moeini, M.; Lu, X.; Lesage, F. Astrocytic endfoot Ca2+correlates with parenchymal vessel responses during 4-AP induced epilepsy: An in vivo two-photon lifetime microscopy study. J. Cereb. Blood Flow Metab. 2017, 39, 260–271. [Google Scholar] [CrossRef]
- Chuquet, J.; Hollender, L.; Nimchinsky, E.A. High-Resolution In Vivo Imaging of the Neurovascular Unit during Spreading Depression. J. Neurosci. 2007, 27, 4036–4044. [Google Scholar] [CrossRef]
- Rosenegger, D.G.; Tran, C.H.T.; Wamsteeker Cusulin, J.I.; Gordon, G.R. Tonic Local Brain Blood Flow Control by Astrocytes Independent of Phasic Neurovascular Coupling. J. Neurosci. 2015, 35, 13463–13474. [Google Scholar] [CrossRef] [PubMed]
- Kur, J.; Newman, E.A. Purinergic control of vascular tone in the retina. J. Physiol. 2014, 592, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Mehina, E.M.F.; Murphy-Royal, C.; Gordon, G.R. Steady-state free Ca2+ in astrocytes is decreased by experience and impacts arteriole tone. J. Neurosci. 2017, 37, 0239-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenegger, D.G.; Tran, C.H.; LeDue, J.; Zhou, N.; Gordon, G.R. A high performance, cost-effective, open-source microscope for scanning two-photon microscopy that is modular and readily adaptable. PLoS ONE 2014, 9, e110475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patton, C. Webmaxc Standard, MaxChelator; Stanford University: Stanford, CA, USA, 2009. [Google Scholar]
- Henneberger, C.; Papouin, T.; Oliet, S.H.R.; Rusakov, D.A. Long-term potentiation depends on release of d-serine from astrocytes. Nature 2010, 463, 232–236. [Google Scholar] [CrossRef]
- Parpura, V.; Haydon, P.G. Physiological astrocytic calcium levels stimulate glutamate release to modulate adjacent neurons. Proc. Natl. Acad. Sci. USA 2000, 97, 8629–8634. [Google Scholar] [CrossRef] [Green Version]
- Kuchibhotla, K.V.; Lattarulo, C.R.; Hyman, B.T.; Brian, J.; Kuchibhotla, K.V.; Lattarulo, C.R.; Hyman, B.T.; Bacskai, B. Synchronous Hyperactivity and Intercellular Calcium Waves in Astrocytes in Alzheimer Mice. Science 2009, 323, 1211–1215. [Google Scholar] [CrossRef] [Green Version]
- Zheng, K.; Bard, L.; Reynolds, J.P.; King, C.; Jensen, T.P.; Gourine, A.V.; Rusakov, D.A. Time-Resolved Imaging Reveals Heterogeneous Landscapes of Nanomolar Ca2+ in Neurons and Astroglia. Neuron 2015, 88, 277–288. [Google Scholar] [CrossRef] [Green Version]
- Fordsmann, J.C.; Ko, R.W.Y.; Choi, H.B.; Thomsen, K.; Witgen, B.M.; Mathiesen, C.; Lønstrup, M.; Piilgaard, H.; MacVicar, B.A.; Lauritzen, M. Increased 20-HETE synthesis explains reduced cerebral blood flow but not impaired neurovascular coupling after cortical spreading depression in rat cerebral cortex. J. Neurosci. 2013, 33, 2562–2570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pappas, A.C.; Koide, M.; Wellman, G.C. Purinergic signaling triggers endfoot high-amplitude Ca 2+ signals and causes inversion of neurovascular coupling after subarachnoid hemorrhage. J. Cereb. Blood Flow Metab. 2016, 36, 1901–1912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, S.; Han, G.; White, R.E. PGE2 action in human coronary artery smooth muscle: Role of potassium channels and signaling cross-talk. J. Vasc. Res. 2002, 39, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Takano, T.; Tian, G.-F.; Peng, W.; Lou, N.; Libionka, W.; Han, X.; Nedergaard, M. Astrocyte-mediated control of cerebral blood flow. Nat. Neurosci. 2006, 9, 260–267. [Google Scholar] [CrossRef]
- Gebremedhin, D.; Lange, A.R.; Narayanan, J.; Aebly, M.R.; Jacobs, E.R.; Harder, D.R. Cat cerebral arterial smooth muscle cells express cytochrome P450 4A2 enzyme and produce the vasoconstrictor 20-HETE which enhances L-type Ca2+ current. J. Physiol. 1998, 507, 771–781. [Google Scholar] [CrossRef]
- Tanaka, K.; Shibuya, I.; Kabashima, N.; Ueta, Y.; Yamashita, H. Inhibition of voltage-dependent calcium channels by prostaglandin E2 in rat melanotrophs. Endocrinology 1998, 139, 4801–4810. [Google Scholar] [CrossRef]
- Sun, W.; McConnell, E.; Pare, J.-F.; Xu, Q.; Chen, M.; Peng, W.; Lovatt, D.; Han, X.; Smith, Y.; Nedergaard, M. Glutamate-Dependent Neuroglial Calcium Signaling Differs Between Young and Adult Brain. Science 2013, 339, 197–200. [Google Scholar] [CrossRef] [Green Version]
- Bonder, D.E.; McCarthy, K.D. Astrocytic Gq-GPCR-Linked IP3R-Dependent Ca2+ Signaling Does Not Mediate Neurovascular Coupling in Mouse Visual Cortex In Vivo. J. Neurosci. 2014, 34, 13139–13150. [Google Scholar] [CrossRef]
- Bazargani, N.; Attwell, D. Astrocyte calcium signaling: The third wave. Nat. Neurosci. 2016, 19, 182–189. [Google Scholar] [CrossRef]
- Nizar, K.; Uhlirova, H.; Tian, P.; Saisan, P.A.; Cheng, Q.; Reznichenko, L.; Weldy, K.L.; Steed, T.C.; Sridhar, V.B.; MacDonald, C.L.; et al. In vivo Stimulus-Induced Vasodilation Occurs without IP3 Receptor Activation and May Precede Astrocytic Calcium Increase. J. Neurosci. 2013, 33, 8411–8422. [Google Scholar] [CrossRef]
- Schummers, J.; Yu, H.; Sur, M. Tuned responses of astrocytes and their influence on hemodynamic signals in the visual cortex. Science 2008, 320, 1638–1643. [Google Scholar] [CrossRef] [Green Version]
- Schulz, K.; Sydekum, E.; Krueppel, R.; Engelbrecht, C.J.; Schlegel, F.; Schröter, A.; Rudin, M.; Helmchen, F. Simultaneous BOLD fMRI and fiber-optic calcium recording in rat neocortex. Nat. Methods 2012, 9, 597–602. [Google Scholar] [CrossRef]
- Winship, I.R.; Plaa, N.; Murphy, T.H. Rapid Astrocyte Calcium Signals Correlate with Neuronal Activity and Onset of the Hemodynamic Response In Vivo. J. Neurosci. 2007, 27, 6268–6272. [Google Scholar] [CrossRef] [Green Version]
- Lind, B.; Brazhe, A.R.; Jessen, S.; Tan, F.C.C.; Lauritzen, M.J. Rapid stimulus-evoked astrocyte Ca 2+ elevations and hemodynamic responses in mouse somatosensory cortex in vivo. Proc. Natl. Acad. Sci. USA 2013, 110. [Google Scholar] [CrossRef] [Green Version]
- Lecrux, C.; Toussay, X.; Kocharyan, A.; Fernandes, P.; Neupane, S.; Le, M.; Plaisier, F.; Shmuel, A.; Cauli, B.; Hamel, E.; et al. Pyramidal Neurons Are “ Neurogenic Hubs ” in the Neurovascular Coupling Response to Whisker Stimulation. J. Neurosci. 2011, 31, 9836–9847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, A.; Reynolds, J.P.; Chen, Y.; Gourine, A.V.; Rusakov, D.A.; Attwell, D. Astrocytes mediate neurovascular signaling to capillary pericytes but not to arterioles. Nat. Neurosci. 2016, 19, 1619–1627. [Google Scholar] [CrossRef] [Green Version]
- Howarth, C.; Sutherland, B.A.; Choi, H.B.; Martin, C.; Lind, B.L.; Khennouf, L.; LeDue, J.M.; Pakan, J.M.P.; Ko, R.W.Y.; Ellis-Davies, G.; et al. A Critical Role for Astrocytes in Hypercapnic Vasodilation in Brain. J. Neurosci. 2017, 37, 2403–2414. [Google Scholar] [CrossRef] [PubMed]
- Howarth, C. The contribution of astrocytes to the regulation of cerebral blood flow. Front. Neurosci. 2014, 8, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, A.; O’Farrell, F.M.; Reynell, C.; Hamilton, N.B.; Hall, C.N.; Attwell, D. Imaging pericytes and capillary diameter in brain slices and isolated retinae. Nat. Protoc. 2014, 9, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Niwa, K.; Haensel, C.; Ross, M.E.; Iadecola, C. Cyclooxygenase-1 participates in selected vasodilator responses of the cerebral circulation. Circ. Res. 2001, 88, 600–608. [Google Scholar] [CrossRef] [Green Version]
- Lacroix, A.; Toussay, X.; Anenberg, E.; Lecrux, C.; Ferreiro, N.; Burgess, S.A.; Hillman, E.M.C.; Tegeder, I.; Murphy, T.H.; Hamel, E.; et al. COX-2-Derived Prostaglandin E2 Produced by Pyramidal Neurons Contributes to Neurovascular Coupling in the Rodent Cerebral Cortex. J. Neurosci. 2015, 35, 11791–11810. [Google Scholar] [CrossRef] [PubMed]
- Peters, O.; Schipke, C.G.; Hashimoto, Y.; Kettenmann, H. Different Mechanisms Promote Astrocyte Ca2+ Waves and Spreading Depression in the Mouse Neocortex. J. Neurosci. 2003, 23, 9888–9896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seidel, J.L.; Escartin, C.; Ayata, C.; Bonvento, G.; Shuttleworth, C.W. Multifaceted roles for astrocytes in spreading depolarization. Glia 2016, 64, 5–20. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haidey, J.N.; Gordon, G.R. Direct Deviations in Astrocyte Free Ca2+ Concentration Control Multiple Arteriole Tone States. Neuroglia 2021, 2, 48-56. https://doi.org/10.3390/neuroglia2010006
Haidey JN, Gordon GR. Direct Deviations in Astrocyte Free Ca2+ Concentration Control Multiple Arteriole Tone States. Neuroglia. 2021; 2(1):48-56. https://doi.org/10.3390/neuroglia2010006
Chicago/Turabian StyleHaidey, Jordan N., and Grant R. Gordon. 2021. "Direct Deviations in Astrocyte Free Ca2+ Concentration Control Multiple Arteriole Tone States" Neuroglia 2, no. 1: 48-56. https://doi.org/10.3390/neuroglia2010006
APA StyleHaidey, J. N., & Gordon, G. R. (2021). Direct Deviations in Astrocyte Free Ca2+ Concentration Control Multiple Arteriole Tone States. Neuroglia, 2(1), 48-56. https://doi.org/10.3390/neuroglia2010006




