Establishment of a Simple and Versatile Evaporation Compensation Model for In Vitro Chronic Ethanol Treatment: Impact on Neuronal Viability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
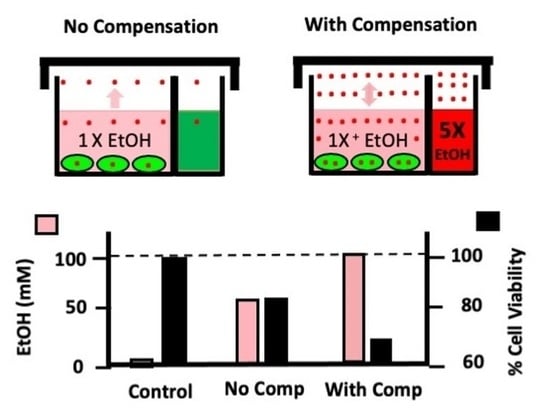
2.2. Ethanol Evaporation Compensation Model
2.3. Determination of Ethanol Concentrations
2.4. Determination of the Effect of Ethanol on Cell Viability
2.5. Statistical Analysis
3. Results
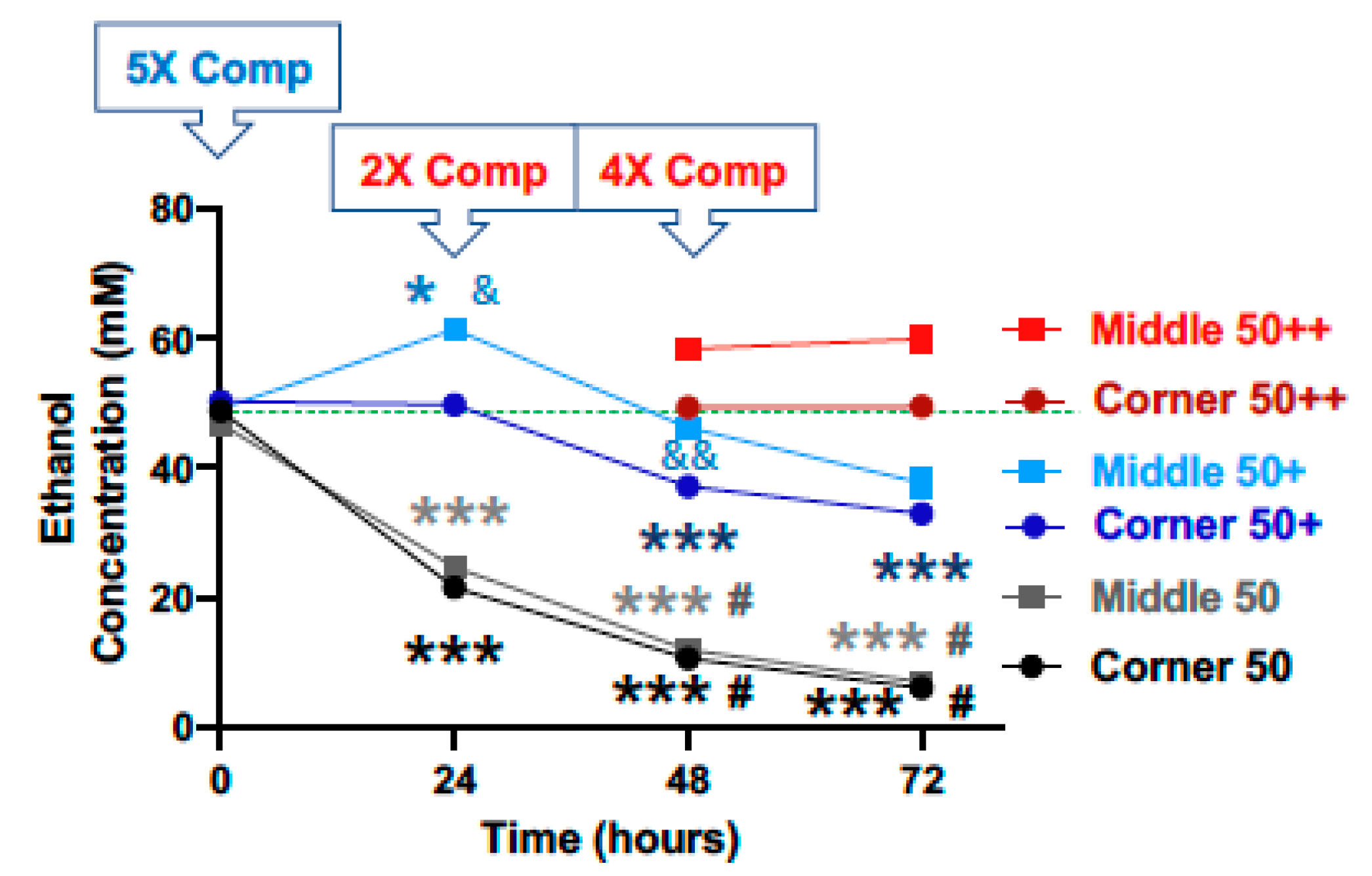
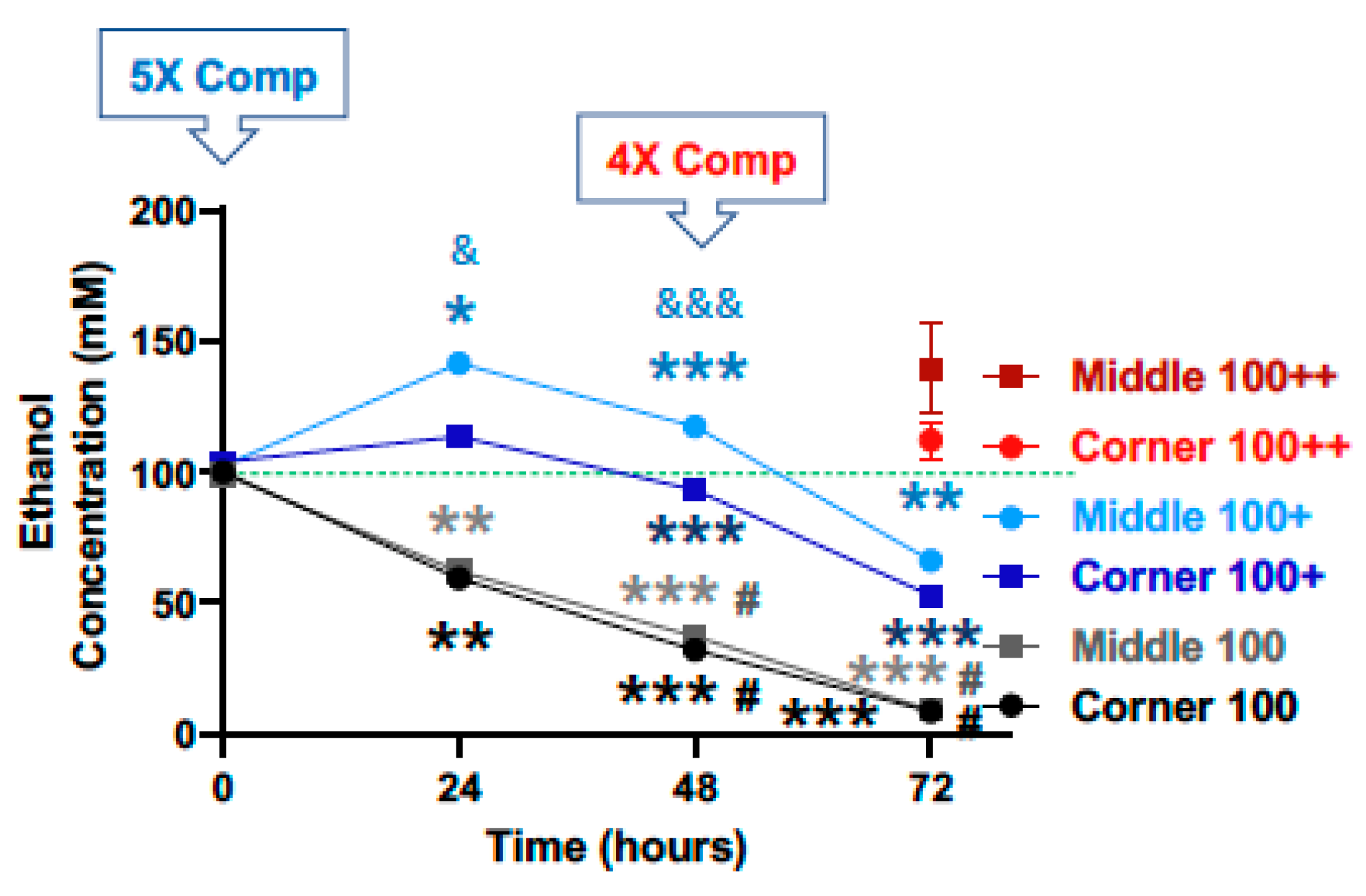
3.1. Characterization of Ethanol Evaporation and Effects of Compensation

3.2. Compensation for Evaporation Enhances the Effects of Ethanol on Cell Viability
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mokdad, A.H.; Marks, J.S.; Stroup, D.F.; Gerberding, J.L. Actual causes of death in the United States, 2000. JAMA 2004, 291, 1238–1245. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.F.; Heilig, M.; Perez, A.; Probst, C.; Rehm, J. Alcohol use disorders. Lancet 2019, 394, 781–792. [Google Scholar] [CrossRef]
- Zeilinger, K.; Freyer, N.; Damm, G.; Seehofer, D.; Knospel, F. Cell sources for in vitro human liver cell culture models. Exp. Biol. Med. 2016, 241, 1684–1698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, P.; Culver-Cochran, A.E.; Stevens, S.M., Jr.; Liu, B. Characterization of a SILAC method for proteomic analysis of primary rat microglia. Proteomics 2016, 16, 1341–1346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pickering, C.; Wicher, G.; Rosendahl, S.; Schioth, H.B.; Fex-Svenningsen, A. A low ethanol dose affects all types of cells in mixed long-term embryonic cultures of the cerebellum. Basic Clin. Pharmacol. Toxicol. 2010, 106, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Di Rocco, G.; Baldari, S.; Pani, G.; Toietta, G. Stem cells under the influence of alcohol: Effects of ethanol consumption on stem/progenitor cells. Cell Mol. Life Sci. 2019, 76, 31–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, P.; Wong, T.A.; Lokuta, K.M.; Turner, D.E.; Vujisic, K.; Liu, B. Microglia enhance manganese chloride-induced dopaminergic neurodegeneration: Role of free radical generation. Exp. Neurol. 2009, 217, 219–230. [Google Scholar] [CrossRef] [Green Version]
- Banan, A.; Fields, J.Z.; Decker, H.; Zhang, Y.; Keshavarzian, A. Nitric oxide and its metabolites mediate ethanol-induced microtubule disruption and intestinal barrier dysfunction. J. Pharmacol. Exp. Ther. 2000, 294, 997–1008. [Google Scholar] [PubMed]
- Moulin, M.; Carpentier, S.; Levade, T.; Arrigo, A.P. Potential roles of membrane fluidity and ceramide in hyperthermia and alcohol stimulation of TRAIL apoptosis. Apoptosis 2007, 12, 1703–1720. [Google Scholar] [CrossRef] [PubMed]
- Dolganiuc, A.; Bakis, G.; Kodys, K.; Mandrekar, P.; Szabo, G. Acute ethanol treatment modulates Toll-like receptor-4 association with lipid rafts. Alcohol. Clin. Exp. Res. 2006, 30, 76–85. [Google Scholar] [CrossRef]
- Bor, S.; Caymaz-Bor, C.; Tobey, N.A.; Abdulnour-Nakhoul, S.; Marten, E.; Orlando, R.C. Effect of ethanol on the structure and function of rabbit esophageal epithelium. Am. J. Physiol. 1998, 274, G819–G826. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.C.; Kramer, R.E. Cytotoxicity of short-chain alcohols. Annu. Rev. Pharmacol. Toxicol. 1999, 39, 127–150. [Google Scholar] [CrossRef]
- Crews, F.T.; Waage, H.G.; Wilkie, M.B.; Lauder, J.M. Ethanol pretreatment enhances NMDA excitotoxicity in biogenic amine neurons: Protection by brain derived neurotrophic factor. Alcohol. Clin. Exp. Res. 1999, 23, 1834–1842. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.N.; Parker, G.C.; Zhao, J.; Barami, K.; Lyman, W.D. Human neural stem cells are more sensitive than astrocytes to ethanol exposure. Alcohol. Clin. Exp. Res. 2003, 27, 1310–1317. [Google Scholar] [CrossRef] [PubMed]
- Lamarche, F.; Gonthier, B.; Signorini, N.; Eysseric, H.; Barret, L. Acute exposure of cultured neurones to ethanol results in reversible DNA single-strand breaks; whereas chronic exposure causes loss of cell viability. Alcohol Alcohol. 2003, 38, 550–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pantazis, N.J.; Dohrman, D.P.; Goodlett, C.R.; Cook, R.T.; West, J.R. Vulnerability of cerebellar granule cells to alcohol-induced cell death diminishes with time in culture. Alcohol. Clin. Exp. Res. 1993, 17, 1014–1021. [Google Scholar] [CrossRef] [PubMed]
- Vaudry, D.; Rousselle, C.; Basille, M.; Falluel-Morel, A.; Pamantung, T.F.; Fontaine, M.; Fournier, A.; Vaudry, H.; Gonzalez, B.J. Pituitary adenylate cyclase-activating polypeptide protects rat cerebellar granule neurons against ethanol-induced apoptotic cell death. Proc. Natl. Acad. Sci. USA 2002, 99, 6398–6403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawrimore, C.J.; Crews, F.T. Ethanol, TLR3, and TLR4 Agonists Have Unique Innate Immune Responses in Neuron-Like SH-SY5Y and Microglia-Like BV2. Alcohol. Clin. Exp. Res. 2017, 41, 939–954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bell-Temin, H.; Liu, B.; Zhang, P.; Stevens, S.M., Jr. Biomarkers of Drug Abuse-Induced Brain Changes: Role of Microglia in Alcohol-induced Neurotoxicity, 1st ed.; CRC Press: Boca Raton, FL, USA, 2014; p. 650. [Google Scholar] [CrossRef]
- Guergues, J.; Wohlfahrt, J.; Zhang, P.; Liu, B.; Stevens, S.M., Jr. Deep proteome profiling reveals novel pathways associated with pro-inflammatory and alcohol-induced microglial activation phenotypes. J. Proteom. 2020, 220, 103753. [Google Scholar] [CrossRef]
- Bell-Temin, H.; Zhang, P.; Chaput, D.; King, M.A.; You, M.; Liu, B.; Stevens, S.M., Jr. Quantitative proteomic characterization of ethanol-responsive pathways in rat microglial cells. J. Proteome Res. 2013, 12, 2067–2077. [Google Scholar] [CrossRef] [PubMed]
- Goeke, C.M.; Hashimoto, J.G.; Guizzetti, M.; Vitalone, A. Effects of ethanol-and choline-treated astrocytes on hippocampal neuron neurite outgrowth in vitro. Sci. Prog. 2021, 104. [Google Scholar] [CrossRef] [PubMed]
- Crabbe, J.C.; Harris, R.A.; Koob, G.F. Preclinical studies of alcohol binge drinking. Ann. N. Y. Acad. Sci. 2011, 1216, 24–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russo, M.; Humes, S.T.; Figueroa, A.M.; Tagmount, A.; Zhang, P.; Loguinov, A.; Lednicky, J.A.; Sabo-Attwood, T.; Vulpe, C.D.; Liu, B. Organochlorine Pesticide Dieldrin Suppresses Cellular Interferon-Related Antiviral Gene Expression. Toxicol. Sci. 2021, 182, 260–274. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Sobh, A.; Zhang, P.; Loguinov, A.; Tagmount, A.; Vulpe, C.D.; Liu, B. Functional Pathway Identification With CRISPR/Cas9 Genome-wide Gene Disruption in Human Dopaminergic Neuronal Cells Following Chronic Treatment With Dieldrin. Toxicol. Sci. 2020, 176, 366–381. [Google Scholar] [CrossRef] [PubMed]
- Rath, M.; Tawfic, J.; Abrorkhujaeva, A.; Sowell, S.; Wu, S.; Eans, S.O.; Peris, J.; McLaughlin, J.P.; Stevens, S.M.; Liu, B. Binge ethanol consumption-associated behavioral impairments in male mice using a gelatin-based drinking-in-the dark model. Alcohol 2021, 95, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Rath, M.; Guergues, J.; Pinho, J.P.C.; Zhang, P.; Nguyen, T.G.; MacFadyen, K.A.; Peris, J.; McLaughlin, J.P.; Stevens, S.M., Jr.; Liu, B. Chronic Voluntary Binge Ethanol Consumption Causes Sex-Specific Differences in Microglial Signaling Pathways and Withdrawal-associated Behaviors in Mice. Alcohol. Clin. Exp. Res. 2020, 44, 1791–1806. [Google Scholar] [CrossRef] [PubMed]
- Dutta, G.; Barber, D.S.; Zhang, P.; Doperalski, N.J.; Liu, B. Involvement of dopaminergic neuronal cystatin C in neuronal injury-induced microglial activation and neurotoxicity. J. Neurochem. 2012, 122, 752–763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blitzer, R.D.; Gil, O.; Landau, E.M. Long-term potentiation in rat hippocampus is inhibited by low concentrations of ethanol. Brain Res. 1990, 537, 203–208. [Google Scholar] [CrossRef]
- Zhang, X.; Bhattacharyya, S.; Kusumo, H.; Goodlett, C.R.; Tobacman, J.K.; Guizzetti, M. Arylsulfatase B modulates neurite outgrowth via astrocyte chondroitin-4-sulfate: Dysregulation by ethanol. Glia 2014, 62, 259–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carson, E.J.; Pruett, S.B. Development and characterization of a binge drinking model in mice for evaluation of the immunological effects of ethanol. Alcohol. Clin. Exp. Res. 1996, 20, 132–138. [Google Scholar] [CrossRef]
- Nixon, K.; Crews, F.T. Binge ethanol exposure decreases neurogenesis in adult rat hippocampus. J. Neurochem. 2002, 83, 1087–1093. [Google Scholar] [CrossRef] [PubMed]
- Bertola, A.; Mathews, S.; Ki, S.H.; Wang, H.; Gao, B. Mouse model of chronic and binge ethanol feeding (the NIAAA model). Nat. Protoc. 2013, 8, 627–637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilcox, M.V.; Cuzon Carlson, V.C.; Sherazee, N.; Sprow, G.M.; Bock, R.; Thiele, T.E.; Lovinger, D.M.; Alvarez, V.A. Repeated binge-like ethanol drinking alters ethanol drinking patterns and depresses striatal GABAergic transmission. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2014, 39, 579–594. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zharikova, A.; Vaughan, C.H.; Bastian, J.; Zandy, S.; Esperon, L.; Axman, E.; Rowland, N.E.; Peris, J. Intermittent high-dose ethanol exposures increase motivation for operant ethanol self-administration: Possible neurochemical mechanism. Brain Res. 2010, 1310, 142–153. [Google Scholar] [CrossRef] [Green Version]
- Bonthius, D.J.; West, J.R. Alcohol-induced neuronal loss in developing rats: Increased brain damage with binge exposure. Alcohol. Clin. Exp. Res. 1990, 14, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Eysseric, H.; Gonthier, B.; Soubeyran, A.; Bessard, G.; Saxod, R.; Barret, L. There is not simple method to maintain a constant ethanol concentration in long-term cell culture: Keys to a solution applied to the survey of astrocytic ethanol absorption. Alcohol 1997, 14, 111–115. [Google Scholar] [CrossRef]
- Rodriguez, F.D.; Simonsson, P.; Alling, C. A method for maintaining constant ethanol concentrations in cell culture media. Alcohol Alcohol. 1992, 27, 309–313. [Google Scholar]
- Borgs, P.; Way, D.L.; Witte, M.H.; Witte, C.L. Effective stabilization of ethanol levels in multiple-well tissue culture plates. Alcohol 1993, 10, 31–35. [Google Scholar] [CrossRef]
- Kim, W.; Jeong, H.S.; Kim, S.C.; Choi, C.H.; Lee, K.H. Chronic Alcohol Exposure of Cells Using Controlled Alcohol-Releasing Capillaries. Cells 2021, 10, 1120. [Google Scholar] [CrossRef] [PubMed]
- Adickes, E.D.; Mollner, T.J.; Lockwood, S.K. Closed chamber system for delivery of ethanol to cell cultures. Alcohol Alcohol. 1988, 23, 377–381. [Google Scholar] [CrossRef] [Green Version]
- Xicoy, H.; Wieringa, B.; Martens, G.J. The SH-SY5Y cell line in Parkinson’s disease research: A systematic review. Mol. Neurodegener. 2017, 12, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, J.; Miller, M.W. Basic fibroblast growth factor- and platelet-derived growth factor-mediated cell proliferation in B104 neuroblastoma cells: Effect of ethanol on cell cycle kinetics. Brain Res. 1997, 770, 139–150. [Google Scholar] [CrossRef]
- Ramlochansingh, C.; Taylor, R.E.; Tizabi, Y. Toxic effects of low alcohol and nicotine combinations in SH-SY5Y cells are apoptotically mediated. Neurotox. Res. 2011, 20, 263–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rath, M.; Figueroa, A.M.; Zhang, P.; Stevens, S.M., Jr.; Liu, B. Establishment of a Simple and Versatile Evaporation Compensation Model for In Vitro Chronic Ethanol Treatment: Impact on Neuronal Viability. Neuroglia 2022, 3, 61-72. https://doi.org/10.3390/neuroglia3020004
Rath M, Figueroa AM, Zhang P, Stevens SM Jr., Liu B. Establishment of a Simple and Versatile Evaporation Compensation Model for In Vitro Chronic Ethanol Treatment: Impact on Neuronal Viability. Neuroglia. 2022; 3(2):61-72. https://doi.org/10.3390/neuroglia3020004
Chicago/Turabian StyleRath, Meera, Ariana M. Figueroa, Ping Zhang, Stanley M. Stevens, Jr., and Bin Liu. 2022. "Establishment of a Simple and Versatile Evaporation Compensation Model for In Vitro Chronic Ethanol Treatment: Impact on Neuronal Viability" Neuroglia 3, no. 2: 61-72. https://doi.org/10.3390/neuroglia3020004
APA StyleRath, M., Figueroa, A. M., Zhang, P., Stevens, S. M., Jr., & Liu, B. (2022). Establishment of a Simple and Versatile Evaporation Compensation Model for In Vitro Chronic Ethanol Treatment: Impact on Neuronal Viability. Neuroglia, 3(2), 61-72. https://doi.org/10.3390/neuroglia3020004