Abstract
Neurotrauma injuries are notoriously difficult to deal with both clinically as well as experimentally, as the cellular and molecular events ensuing after injury complicate the neuroinflammatory processes. Spinal cord injuries are further complicated by the formation of scars at the injury sites, which can provide a physical barrier to repair. The lack of effective clinical therapy for spinal cord injury underscores the need for experimental approaches to generate effective therapies. To repair the injury, cell transplantation offers the potential to replace lost cells and create a permissive bridge to promote neural regeneration across the injury site. Olfactory ensheathing cells (OECs), which are the glia of the olfactory nerve, stand apart from other candidate cell types due to their innate natural abilities to manage nerve injury and promote repair and regeneration. This is evidenced by their physiological role in the daily repair and maintenance of the olfactory nerve. Here, we explain their properties in relation to their physiological role and their most relevant cellular attributes, including cellular interactions, phagocytosis, migration, axonal guidance and support, and modulation of neuroinflammation. We highlight some critical drawbacks in the current approaches and identify some ways to address them.
1. Spinal Cord Injury Can Be Devastating but There Is a Promising Way Out
According to a recent survey, over 20,000 Australians are living with spinal cord injury (SCI). Globally, between 250,000 and 500,000 new cases of SCI are recorded every year. A third of SCIs result in severe injury with motor, sensory and autonomic dysfunctions. In addition to dealing a devastating blow to a person’s quality of life, and that of their families, SCI also places a huge economic burden for the country itself. The total life cost of SCIs in Australia is currently over AUD 75 billion per year [1].
There is no definitive cure for SCIs; however, cell transplantation approaches show promise in experimental settings. Olfactory ensheathing cells (OECs), which are the resident glial cells of the human olfactory nervous system, are one such candidate [2,3].
2. Olfactory Ensheathing Cells: From the Nose to the Cord
The mammalian primary olfactory system is the only part of the nervous system that regenerates every day as part of its normal process, with 1–3% of its sensory neurons turning over each day. OECs are strongly implicated for this unique ability of the primary olfactory system [4]. The OECs are located both in the olfactory bulb and the olfactory mucosa, where they form continuous tunnel-like structures through which the olfactory axons travel from the nose to the brain. Importantly, they not only offer physical support to the axons, but they are also considered the “immunocytes” of the primary olfactory system, with both phagocytic and immunomodulatory capacities, enabling them to clear the axonal debris and tweak the inflammatory profile of the olfactory mucosa in favor of nerve regrowth [4]. These characteristics have attracted significant attention to the OECs, making them potential candidates to repair other neural tracts, such as the spinal cord after an extensive injury.
Autologous OEC-based transplantation therapies have been the focus of many experiments in the past decades. Several successful outcomes have been reported in animal models (murine, canine, and feline as well as primate) [3,5,6], showing variable partial functional recovery. A human clinical trial in 2002 in Australia established the safety and feasibility of the approach [7,8]. This paved the path for several subsequent trials over the last two decades, which have resulted in functional regain, ranging from minor to significant.
3. OECs: What Makes Them Highly Suitable
Other cell-based therapies are being investigated as well, such as bone marrow mesenchymal stem cells, umbilical mesenchymal stem cells, adipose-derived mesenchymal stem cells, neural stem cells, neural progenitor cells, embryonic stem cells, and induced pluripotent stem cells [9]. Unlike other cell types, there are very few, if any, reports of OECs forming tumors within the olfactory nerve [10], which gives them a better safety profile. Thus, the option of using OECs is clinically feasible, ethically uncomplicated, relatively safe, and accessible with concurrent scientific and technological approaches.
4. OECs: Integrate, Modulate, and Manipulate
OECs are arguably the most promising candidate for a cell-based transplantation therapy for SCI due to their peculiar cellular characteristics [11].
First, OECs are already in a mature, fully differentiated state, with well-established physiological roles and cellular abilities. Within the olfactory nerve, the primary role of OECs is to provide physical guidance to the axons of olfactory nerves. To do this, OECs closely interact with fibroblasts, forming tunnel-like structures which ensheathe the axons to guide them from the olfactory mucosa to the olfactory bulb [12]. At the interface between the peripheral and central nervous systems within the olfactory bulb, the OECs interact with astrocytes. Within the spinal cord, OECs can physiologically integrate well, as the cord’s internal milieu is very similar to the OECs’ natural habitat, with the same accompanying cells: fibroblasts and astrocytes, the main cells forming the astro-fibrotic scar. With a similar environment, the OECs are able to continue their physiological role, where, instead of the olfactory axons, they interact and stabilize the axons in the spinal cord, guiding them to close the gap at the injury site.
Additionally, it has been shown that OECs actively contribute to inflammatory modulation in the primary olfactory system, secreting TNF-a, CXCL1, CXCL2 and IL1-RA, which is similar to the immune cells present in the CNS. Thus, the OECs are a major cell type involved in the regulation of inflammation and regeneration within the primary olfactory nerve, and these characteristics can be applied to the repair of spinal cord injury [4].
OECs can also manipulate the surrounding extracellular matrix by releasing tropic factors (nerve growth factor, brain-derived neurotrophic factor, glial-cell-line-derived neurotrophic factor) and ECM proteins (laminin, fibronectin, and collagen IV) [4], which can further aid repair mechanisms.
5. Olfactory Ensheathing Cells: Phagocytic and Migratory Abilities
The OECs’ innate ability to migrate and phagocytose add further value to their favorable therapeutic profile for treating SCI [2,13].
OECs are inherently migratory cells, and therefore are able to quickly reach where they are needed after injury in the primary olfactory system. Their ability to exhibit the same after transplantation into the SCI site has been tested and confirmed. Interestingly, OECs prefer cellular contact and therefore are able to migrate in bundles to form “bridges” across the injury site to better guide the regrowing axons. Furthermore, OECs are the main phagocytic cells in the primary olfactory system: their role is to clear the environment from any debris that would inhibit the regrowth of the axons and raise the inflammation [4]. Similarly, following their transplantation into the injury site, OECs can migrate in the surrounding environment and phagocytose any debris of myelin and necrotic axons, promoting neuronal regrowth after transplantation [4,6]. The OECs’ post-transplantation roles are depicted in Figure 1.
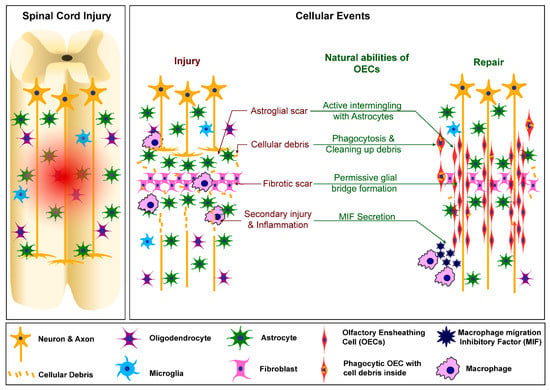
Figure 1.
Cellular events following spinal cord injury and how OECs can modify them favourably to induce neural repair.
6. Cellular Programming and Reprograming: Integrating Futuristic Technologies
Being glial cells, OECs naturally interact with other glial cells and neurons. The recent advances in genetic modifications, transgenics and our understanding of developmental cell fate open a new avenue to enhance the functions, utility, and efficacy of the OECs for nerve repair. Research has been conducted to genetically improve the therapeutic potential of OECs by overexpressing prostacyclin synthetase [14], Nogo receptors and Chondroitinase ABC enzyme [15], for example. Additionally, recent development of techniques into the cell fate specifications of fibroblasts [16] and glial cell reprogramming [17] provide novel ways to purify OECs in culture and improve the cell yield, as well as enhancing the transplantation outcomes using OECs. These potential advances open up the possibility of transplanting genetically modified OECs to target different stages of injury to combat inhibitory molecules and to introduce new cell types to replace lost cells.
7. The Downsides That Need to Be Addressed
Even though an OEC-based transplantation therapy seems a promising option and has shown some efficacy, the outcomes are not yet consistently reproducible. Some trials have reported patients who have had no improvement at all and there have even been some catastrophic outcomes [6,18]. This inconsistency is mainly due to OECs being a heterogenous cell population [4], which is notorious to purify [6].
OECs can be obtained from either the olfactory bulb or the lamina propria of the olfactory mucosa. The latter can be easily collected through a safe, minimally invasive biopsy procedure, making them the preferred candidates for clinical purposes [6], whereas obtaining them from the olfactory bulb requires invasive surgery into the cranial cavity. These two different sub-populations of OECs (bulb and mucosa) inhabit different anatomical areas and based on their different roles in their environments, they express different behaviors both in vitro and in vivo [4]. Importantly, OECs also co-exist with other cell types, which can be present within preparations of cells from the biopsies. Thus, the cell culture expanded from the biopsies are typically not a pure OEC population [19]. Currently, it is difficult, if not impossible, to purify these cells to a 100% pure population since no reliable specific marker has been identified yet [4,19]. However, there are ways to mitigate this and even use this to our advantage with cutting edge novel technologies, such as 3D cultures.
Interestingly, two schools of thought exist regarding their optimal purity: some pursue ways to obtain purest possible OEC population, whilst others subscribe to a multicellular environment approach [6]. The latter posits that OECs exist with other cell types and work synergistically with them, and hence, OECs might need the accompanying cells to optimally perform their tasks [20]. The best combination of cells still needs to be determined.
8. Conclusions
In physiological conditions OECs are responsible for supporting and guiding the axons of olfactory nerves. OECs, being glial cells, not only offer physical guidance, but they also modulate their environment favorably through the secretion of a range of factors. Being the main phagocytic cells, they also maintain the inflammatory balance. Upon transplantation into a spinal cord injury site, OECs replicate the same processes to modulate the local environment and aid the repair of neural connections in the injured area by forming permissive glial bridges [6].
Despite the highly promising prospects, further improvements can be made to enhance the reproducibility and therapeutic efficacy of OECs [3,4,19]. Improving cell survival and integration post-transplantation, and augmenting and enhancing the cellular properties of migration, phagocytosis and axonal repair, as well as optimizing the purification process are some of best ways to do so.
To conclude, OECs are a unique cell type which may lead to the establishment of a clinically safe therapy for SCI. Hopefully, scientists working in this field can overcome the present issues soon and open the way to future OEC transplantation clinical trials that will improve outcomes for people living with spinal cord injury.
Author Contributions
Writing—original draft preparation F.O. and R.R.; writing—review and editing J.S.J.; supervision, J.S.J.; funding acquisition, J.S.J. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Motor Accident Insurance Commission, the Clem Jones Foundation, and the Perry Cross Spinal Research Foundation.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Spinal Cure Australia. Spinal Cord Injury in Australia: The Case for Investing in New Treatments; Spinal Cure Australia: Sydney, NSW, Australia, 2020. [Google Scholar]
- Ekberg, J.A.; Amaya, D.; Mackay-Sim, A.; St John, J.A. The migration of olfactory ensheathing cells during development and regeneration. Neurosignals 2012, 20, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Gilmour, A.D.; Reshamwala, R.; Wright, A.A.; Ekberg, J.A.K.; St John, J.A. Optimizing Olfactory Ensheathing Cell Transplantation for Spinal Cord Injury Repair. J. Neurotrauma 2020, 37, 817–829. [Google Scholar] [CrossRef] [PubMed]
- Delarue, Q.; Guérout, N. Transplantation of Olfactory Ensheathing Cells: Properties and Therapeutic Effects after Transplantation into the Lesioned Nervous System. Neuroglia 2022, 3, 1–22. [Google Scholar] [CrossRef]
- Ahuja, C.S.; Mothe, A.; Khazaei, M.; Badhiwala, J.H.; Gilbert, E.A.; van der Kooy, D.; Morshead, C.M.; Tator, C.; Fehlings, M.G. The leading edge: Emerging neuroprotective and neuroregenerative cell-based therapies for spinal cord injury. Stem Cells Transl. Med. 2020, 9, 1509–1530. [Google Scholar] [CrossRef] [PubMed]
- Reshamwala, R.; Shah, M.; Belt, L.; Ekberg, J.A.K.; St John, J.A. Reliable cell purification and determination of cell purity: Crucial aspects of olfactory ensheathing cell transplantation for spinal cord repair. Neural Regen. Res. 2020, 15, 2016–2026. [Google Scholar] [CrossRef] [PubMed]
- Mackay-Sim, A.; Feron, F.; Cochrane, J.; Bassingthwaighte, L.; Bayliss, C.; Davies, W.; Fronek, P.; Gray, C.; Kerr, G.; Licina, P.; et al. Autologous olfactory ensheathing cell transplantation in human paraplegia: A 3-year clinical trial. Brain 2008, 131 Pt 9, 2376–2386. [Google Scholar] [CrossRef] [PubMed]
- Féron, F.; Perry, C.; Cochrane, J.; Licina, P.; Nowitzke, A.; Urquhart, S.; Geraghty, T.; Mackay-Sim, A. Autologous olfactory ensheathing cell transplantation in human spinal cord injury. Brain 2005, 128, 2951–2960. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Fu, C.; Xiong, F.; He, C.; Wei, Q. Stem Cell Therapy for Spinal Cord Injury. Cell Transpl. 2021, 30. [Google Scholar] [CrossRef] [PubMed]
- Murtaza, M.; Chacko, A.; Delbaz, A.; Reshamwala, R.; Rayfield, A.; McMonagle, B.; St John, J.A.; Ekberg, J.A.K. Why are olfactory ensheathing cell tumors so rare? Cancer Cell Int. 2019, 19, 260. [Google Scholar] [CrossRef] [PubMed]
- Ekberg, J.A.; St John, J.A. Crucial roles for olfactory ensheathing cells and olfactory mucosal cells in the repair of damaged neural tracts. Anat. Rec. 2014, 297, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Raisman, G.; Li, Y. Repair of neural pathways by olfactory ensheathing cells. Nat. Rev. Neurosci. 2007, 8, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Nazareth, L.; Lineburg, K.E.; Chuah, M.I.; Tello Velasquez, J.; Chehrehasa, F.; St John, J.A.; Ekberg, J.A. Olfactory ensheathing cells are the main phagocytic cells that remove axon debris during early development of the olfactory system. J. Comp. Neurol. 2015, 523, 479–494. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.J.; Huang, C.T.; Huang, Y.S.; Weng, C.F.; Shyue, S.K.; Huang, M.C.; Liou, D.Y.; Lin, Y.R.; Cheng, C.H.; Kuo, H.S.; et al. Improving the regenerative potential of olfactory ensheathing cells by overexpressing prostacyclin synthetase and its application in spinal cord repair. J. Biomed. Sci. 2017, 24, 34. [Google Scholar] [CrossRef] [PubMed]
- Reginensi, D.; Carulla, P.; Nocentini, S.; Seira, O.; Serra-Picamal, X.; Torres-Espin, A.; Matamoros-Angles, A.; Gavin, R.; Moreno-Flores, M.T.; Wandosell, F.; et al. Increased migration of olfactory ensheathing cells secreting the Nogo receptor ectodomain over inhibitory substrates and lesioned spinal cord. Cell Mol. Life Sci. 2015, 72, 2719–2737. [Google Scholar] [CrossRef] [PubMed]
- Au-Shrigley, S.; Au-Pircs, K.; Au-Barker, R.A.; Au-Parmar, M.; Au-Drouin-Ouellet, J. Simple Generation of a High Yield Culture of Induced Neurons from Human Adult Skin Fibroblasts. JoVE 2018, 132, e56904. [Google Scholar] [CrossRef]
- Nolbrant, S.; Giacomoni, J.; Hoban, D.B.; Bruzelius, A.; Birtele, M.; Chandler-Militello, D.; Pereira, M.; Ottosson, D.R.; Goldman, S.A.; Parmar, M. Direct Reprogramming of Human Fetal- and Stem Cell-Derived Glial Progenitor Cells into Midbrain Dopaminergic Neurons. Stem Cell Rep. 2020, 15, 869–882. [Google Scholar] [CrossRef] [PubMed]
- Woodworth, C.F.; Jenkins, G.; Barron, J.; Hache, N. Intramedullary cervical spinal mass after stem cell transplantation using an olfactory mucosal cell autograft. CMAJ 2019, 191, E761–E764. [Google Scholar] [CrossRef] [PubMed]
- Yao, R.; Murtaza, M.; Velasquez, J.T.; Todorovic, M.; Rayfield, A.; Ekberg, J.; Barton, M.; St John, J. Olfactory Ensheathing Cells for Spinal Cord Injury: Sniffing Out the Issues. Cell Transpl. 2018, 27, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Rigby, M.J.; Gomez, T.M.; Puglielli, L. Glial Cell-Axonal Growth Cone Interactions in Neurodevelopment and Regeneration. Front. Neurosci. 2020, 14, 203. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).