Contribution of Central and Peripheral Glial Cells in the Development and Persistence of Itch: Therapeutic Implication of Glial Modulation
Abstract
1. Introduction
2. Chronic Itch, Risk Factors, and Underlying Mechanisms
2.1. Chronic Itch Epidemiology and Etiology
2.2. Proposed Mechanisms Underlying Chronic Itch
2.3. Risk Factors for Chronic Itch
3. Glial Cells of the Nervous System
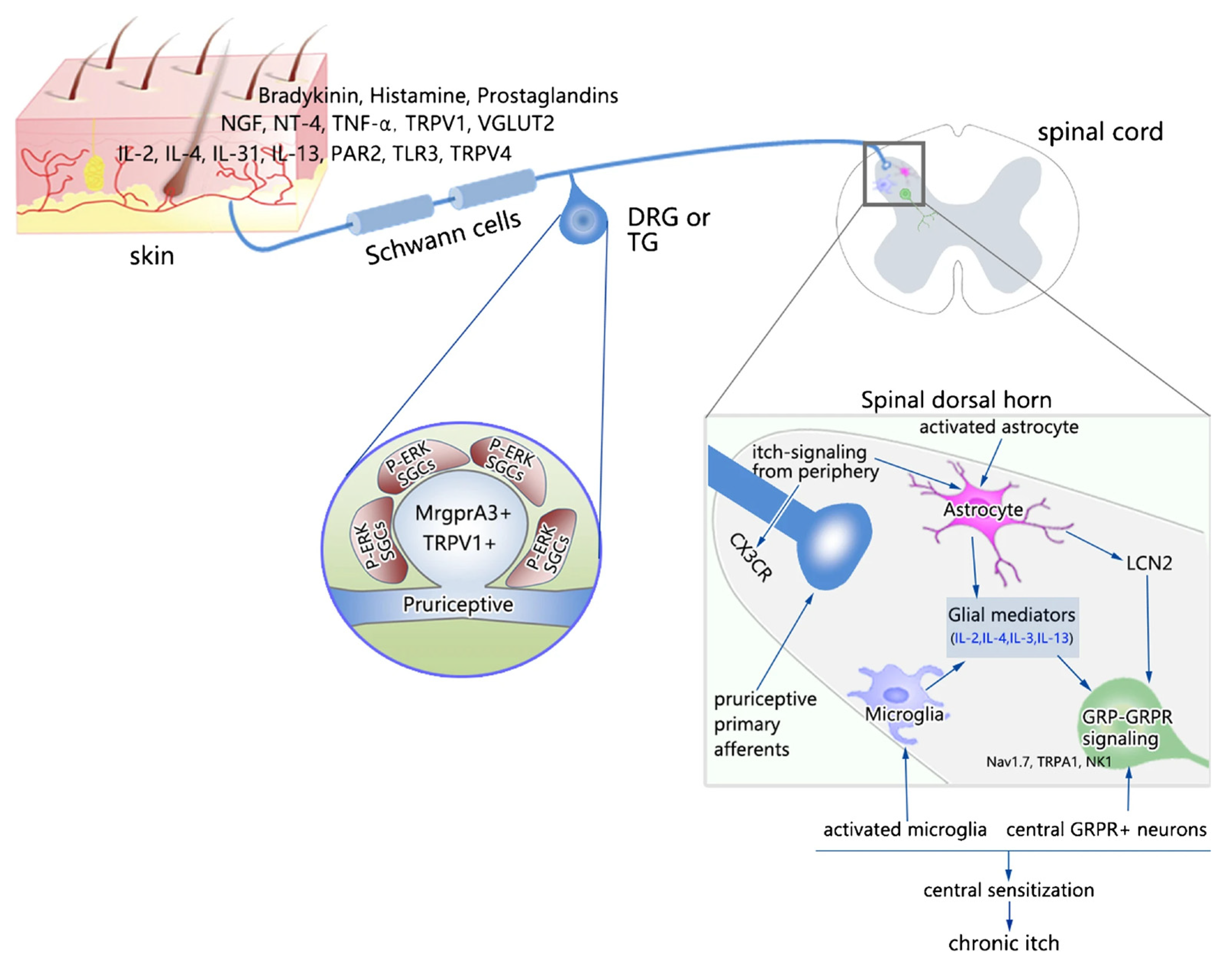
4. Glial Cells and Itch
4.1. Astrocytes in Itch
4.1.1. Activation of Spinal Astrocytes in the Itch–Scratch Cycle
4.1.2. Targeting Astrocytes in Itch
4.1.3. Clinical Implication of Targeting Astrocytes in Itch
4.2. Microglia in Itch
4.3. Satellite Glial Cells in Itch
4.3.1. SGCs in Cholestatic Itch
4.3.2. SGCs in Trigeminal Itch
4.4. Schwann Cells in Itch
5. Concluding Remarks and Future Perspectives
Funding
Conflicts of Interest
References
- Weisshaar, E. Itch: A Global Problem? Front. Med. 2021, 8, 665575. [Google Scholar] [CrossRef]
- Schmelz, M. How Do Neurons Signal Itch? Front. Med. 2021, 8, 643006. [Google Scholar] [CrossRef]
- Andersen, H.H.; Arendt-Nielsen, L.; Gazerani, P. Glial Cells are Involved in Itch Processing. Acta Derm. Venereol. 2016, 96, 723–727. [Google Scholar] [CrossRef]
- Yang, H.; Chen, W.; Zhu, R.; Wang, J.; Meng, J. Critical Players and Therapeutic Targets in Chronic Itch. Int. J. Mol. Sci. 2022, 23, 9935. [Google Scholar] [CrossRef]
- Cevikbas, F.; Lerner, E.A. Physiology and Pathophysiology of Itch. Physiol. Rev. 2020, 100, 945–982. [Google Scholar] [CrossRef]
- Shiratori-Hayashi, M.; Tsuda, M. Spinal glial cells in itch modulation. Pharmacol. Res. Perspect. 2021, 9, e00754. [Google Scholar] [CrossRef]
- Ji, R.-R. Third Special Issue on Mechanisms of Pain and Itch. Neurosci. Bull. 2022, 38, 339–341. [Google Scholar] [CrossRef]
- Ständer, S. Classification of Itch. Curr. Probl. Dermatol. 2016, 50, 1–4. [Google Scholar] [CrossRef]
- Ujiie, H.; Rosmarin, D.; Schön, M.P.; Ständer, S.; Boch, K.; Metz, M.; Maurer, M.; Thaci, D.; Schmidt, E.; Cole, C.; et al. Unmet Medical Needs in Chronic, Non-communicable Inflammatory Skin Diseases. Front. Med. 2022, 9, 875492. [Google Scholar] [CrossRef]
- Hashimoto, T.; Yosipovitch, G. Itching as a systemic disease. J. Allergy Clin. Immunol. 2019, 144, 375–380. [Google Scholar] [CrossRef]
- Kremer, A.E.; Mettang, T.; Weisshaar, E. Non-dermatological Challenges of Chronic Itch. Acta Derm. Venereol. 2020, 100, adv00025. [Google Scholar] [CrossRef]
- Warlich, B.; Fritz, F.; Osada, N.; Bruland, P.; Stumpf, A.; Schneider, G.; Dugas, M.; Pfleiderer, B.; Ständer, S. Health-Related Quality of Life in Chronic Pruritus: An Analysis Related to Disease Etiology, Clinical Skin Conditions and Itch Intensity. Dermatology 2015, 231, 253–259. [Google Scholar] [CrossRef]
- Schneider, G.; Ständer, S.; Kahnert, S.; Pereira, M.P.; Mess, C.; Huck, V.; Agelopoulos, K.; Frank, G.; Schneider, S.W. Biological and psychosocial factors associated with the persistence of pruritus symptoms: Protocol for a prospective, exploratory observational study in Germany (individual project of the Interdisciplinary SOMACROSS Research Unit [RU 5211]). BMJ Open 2022, 12, e060811. [Google Scholar] [CrossRef]
- Pereira, M.P.; Zeidler, C.; Storck, M.; Agelopoulos, K.; Philipp-Dormston, W.G.; Zink, A.; Ständer, S. Challenges in Clinical Research and Care in Pruritus. Acta Derm. Venereol. 2020, 100, adv00028. [Google Scholar] [CrossRef] [PubMed]
- Fowler, E.; Yosipovitch, G. A New Generation of Treatments for Itch. Acta Derm. Venereol. 2020, 100, adv00027. [Google Scholar] [CrossRef] [PubMed]
- McEwen, M.W.; Fite, E.M.; Yosipovitch, G.; Patel, T. Drugs on the Horizon for Chronic Pruritus. Dermatol. Clin. 2018, 36, 335–344. [Google Scholar] [CrossRef]
- Rasband, M.N. Glial Contributions to Neural Function and Disease. Mol. Cell. Proteom. 2016, 15, 355–361. [Google Scholar] [CrossRef]
- Zuchero, J.B.; Barres, B.A. Glia in mammalian development and disease. Development 2015, 142, 3805–3809. [Google Scholar] [CrossRef]
- Wang, F.; Kim, B.S. Itch: A Paradigm of Neuroimmune Crosstalk. Immunity 2020, 52, 753–766. [Google Scholar] [CrossRef]
- Ji, R.R. Neuroimmune interactions in itch: Do chronic itch, chronic pain, and chronic cough share similar mechanisms? Pulm. Pharmacol. Ther. 2015, 35, 81–86. [Google Scholar] [CrossRef]
- Bieber, T. Atopic dermatitis: An expanding therapeutic pipeline for a complex disease. Nat. Rev. Drug Discov. 2022, 21, 21–40. [Google Scholar] [CrossRef]
- Mack, M.R.; Kim, B.S. The Itch-Scratch Cycle: A Neuroimmune Perspective. Trends Immunol. 2018, 39, 980–991. [Google Scholar] [CrossRef] [PubMed]
- Yosipovitch, G.; Rosen, J.D.; Hashimoto, T. Itch: From mechanism to (novel) therapeutic approaches. J. Allergy Clin. Immunol. 2018, 142, 1375–1390. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Kim, H.J.; Back, S.K.; Na, H.S. Common and discrete mechanisms underlying chronic pain and itch: Peripheral and central sensitization. Pflügers Arch. -Eur. J. Physiol. 2021, 473, 1603–1615. [Google Scholar] [CrossRef]
- Dong, X.; Dong, X. Peripheral and Central Mechanisms of Itch. Neuron 2018, 98, 482–494. [Google Scholar] [CrossRef]
- Jin, S.-Y.; Wang, F. Sensitization Mechanisms of Chronic Itch. Int. J. Dermatol. Venereol. 2019, 2, 211–215. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Ho, M.S.; Zorec, R.; Parpura, V. The Concept of Neuroglia. Adv. Exp. Med. Biol. 2019, 1175, 1–13. [Google Scholar] [CrossRef]
- Losada-Perez, M. Glia: From ‘just glue’ to essential players in complex nervous systems: A comparative view from flies to mammals. J. Neurogenet. 2018, 32, 78–91. [Google Scholar] [CrossRef]
- Allen, N.J.; Lyons, D.A. Glia as architects of central nervous system formation and function. Science 2018, 362, 181–185. [Google Scholar] [CrossRef]
- Sinegubov, A.; Andreeva, D.; Burzak, N.; Vasyutina, M.; Murashova, L.; Dyachuk, V. Heterogeneity and Potency of Peripheral Glial Cells in Embryonic Development and Adults. Front. Mol. Neurosci. 2022, 15, 737949. [Google Scholar] [CrossRef]
- Hanani, M.; Spray, D.C. Emerging importance of satellite glia in nervous system function and dysfunction. Nat. Rev. Neurosci. 2020, 21, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Verkhratsky, A.; Butt, A.M. Neuroglia: Definition, Classification, Evolution, Numbers, Development. In Glial Physiology and Pathophysiology; Wiley: New York, NY, USA, 2013; Chapter 3; pp. 73–104. [Google Scholar]
- Hanslik, K.L.; Marino, K.M.; Ulland, T.K. Modulation of Glial Function in Health, Aging, and Neurodegenerative Disease. Front. Cell Neurosci. 2021, 15, 718324. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, C.R.; Andriessen, A.S.; Chen, G.; Wang, K.; Jiang, C.; Maixner, W.; Ji, R.-R. Central Nervous System Targets: Glial Cell Mechanisms in Chronic Pain. Neurotherapeutics 2020, 17, 846–860. [Google Scholar] [CrossRef]
- Li, T.; Chen, X.; Zhang, C.; Zhang, Y.; Yao, W. An update on reactive astrocytes in chronic pain. J. Neuroinflamm. 2019, 16, 140. [Google Scholar] [CrossRef]
- Tsuda, M. Modulation of Pain and Itch by Spinal Glia. Neurosci. Bull. 2018, 34, 178–185. [Google Scholar] [CrossRef]
- Tsuda, M. Spinal dorsal horn astrocytes: New players in chronic itch. Allergol. Int. 2017, 66, 31–35. [Google Scholar] [CrossRef]
- Hanani, M.; Verkhratsky, A. Satellite Glial Cells and Astrocytes, a Comparative Review. Neurochem. Res. 2021, 46, 2525–2537. [Google Scholar] [CrossRef]
- Westergard, T.; Rothstein, J.D. Astrocyte Diversity: Current Insights and Future Directions. Neurochem. Res. 2020, 45, 1298–1305. [Google Scholar] [CrossRef]
- Liu, X.; Ying, J.; Wang, X.; Zheng, Q.; Zhao, T.; Yoon, S.; Yu, W.; Yang, D.; Fang, Y.; Hua, F. Astrocytes in Neural Circuits: Key Factors in Synaptic Regulation and Potential Targets for Neurodevelopmental Disorders. Front. Mol. Neurosci. 2021, 14, 729273. [Google Scholar] [CrossRef]
- Chiareli, R.A.; Carvalho, G.A.; Marques, B.L.; Mota, L.S.; Oliveira-Lima, O.C.; Gomes, R.M.; Birbrair, A.; Gomez, R.S.; Simão, F.; Klempin, F.; et al. The Role of Astrocytes in the Neurorepair Process. Front. Cell. Dev. Biol. 2021, 9, 665795. [Google Scholar] [CrossRef]
- Stephan, J.; Eitelmann, S.; Zhou, M. Approaches to Study Gap Junctional Coupling. Front. Cell. Neurosci. 2021, 15, 640406. [Google Scholar] [CrossRef] [PubMed]
- Jurga, A.M.; Paleczna, M.; Kadluczka, J.; Kuter, K.Z. Beyond the GFAP-Astrocyte Protein Markers in the Brain. Biomolecules 2021, 11, 1361. [Google Scholar] [CrossRef]
- MacVicar, B.A.; Newman, E.A. Astrocyte regulation of blood flow in the brain. Cold Spring Harb. Perspect Biol. 2015, 7, a020388. [Google Scholar] [CrossRef] [PubMed]
- Allen, N.J.; Eroglu, C. Cell Biology of Astrocyte-Synapse Interactions. Neuron 2017, 96, 697–708. [Google Scholar] [CrossRef]
- Linnerbauer, M.; Wheeler, M.A.; Quintana, F.J. Astrocyte Crosstalk in CNS Inflammation. Neuron 2020, 108, 608–622. [Google Scholar] [CrossRef]
- Stephenson, J.; Nutma, E.; van der Valk, P.; Amor, S. Inflammation in CNS neurodegenerative diseases. Immunology 2018, 154, 204–219. [Google Scholar] [CrossRef]
- Mithaiwala, M.N.; Santana-Coelho, D.; Porter, G.A.; O’Connor, J.C. Neuroinflammation and the Kynurenine Pathway in CNS Disease: Molecular Mechanisms and Therapeutic Implications. Cells 2021, 10, 1548. [Google Scholar] [CrossRef]
- Lu, H.-J.; Gao, Y.-J. Astrocytes in Chronic Pain: Cellular and Molecular Mechanisms. Neurosci. Bull. 2022. [CrossRef]
- Ji, R.R.; Donnelly, C.R.; Nedergaard, M. Astrocytes in chronic pain and itch. Nat. Rev. Neurosci. 2019, 20, 667–685. [Google Scholar] [CrossRef]
- Green, D.; Dong, X. Supporting itch: A new role for astrocytes in chronic itch. Nat. Med. 2015, 21, 841–842. [Google Scholar] [CrossRef]
- Liu, T.; Han, Q.; Chen, G.; Huang, Y.; Zhao, L.X.; Berta, T.; Gao, Y.J.; Ji, R.R. Toll-like receptor 4 contributes to chronic itch, alloknesis, and spinal astrocyte activation in male mice. Pain 2016, 157, 806–817. [Google Scholar] [CrossRef]
- Ottevanger, R.; van Beugen, S.; Evers, A.W.M.; Willemze, R.; Vermeer, M.H.; Quint, K.D. Itch in patients with cutaneous T-cell lymphoma as a quality of life indicator. JAAD Int. 2022, 9, 57–64. [Google Scholar] [CrossRef]
- Shiratori-Hayashi, M.; Koga, K.; Tozaki-Saitoh, H.; Kohro, Y.; Toyonaga, H.; Yamaguchi, C.; Hasegawa, A.; Nakahara, T.; Hachisuka, J.; Akira, S.; et al. STAT3-dependent reactive astrogliosis in the spinal dorsal horn underlies chronic itch. Nat. Med. 2015, 21, 927–931. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Cao, T.; Jin, L.; Li, B.; Fang, H.; Zhang, J.; Zhang, Y.; Hu, J.; Wang, G. Increased Lipocalin-2 Contributes to the Pathogenesis of Psoriasis by Modulating Neutrophil Chemotaxis and Cytokine Secretion. J. Investig. Dermatol. 2016, 136, 1418–1428. [Google Scholar] [CrossRef] [PubMed]
- Koga, K.; Yamagata, R.; Kohno, K.; Yamane, T.; Shiratori-Hayashi, M.; Kohro, Y.; Tozaki-Saitoh, H.; Tsuda, M. Sensitization of spinal itch transmission neurons in a mouse model of chronic itch requires an astrocytic factor. J. Allergy Clin. Immunol. 2020, 145, 183–191.e110. [Google Scholar] [CrossRef]
- Du, L.; Hu, X.; Yang, W.; Yasheng, H.; Liu, S.; Zhang, W.; Zhou, Y.; Cui, W.; Zhu, J.; Qiao, Z.; et al. Spinal IL-33/ST2 signaling mediates chronic itch in mice through the astrocytic JAK2-STAT3 cascade. Glia 2019, 67, 1680–1693. [Google Scholar] [CrossRef]
- Fairlie-Clarke, K.; Barbour, M.; Wilson, C.; Hridi, S.U.; Allan, D.; Jiang, H.R. Expression and Function of IL-33/ST2 Axis in the Central Nervous System Under Normal and Diseased Conditions. Front. Immunol. 2018, 9, 2596. [Google Scholar] [CrossRef]
- Sakai, K.; Akiyama, T. New insights into the mechanisms behind mechanical itch. Exp. Dermatol. 2020, 29, 680–686. [Google Scholar] [CrossRef]
- Hill, R.Z.; Loud, M.C.; Dubin, A.E.; Peet, B.; Patapoutian, A. PIEZO1 transduces mechanical itch in mice. Nature 2022, 607, 104–110. [Google Scholar] [CrossRef]
- Shiratori-Hayashi, M.; Tsuda, M. Role of reactive astrocytes in the spinal dorsal horn under chronic itch conditions. J. Pharmacol. Sci. 2020, 144, 147–150. [Google Scholar] [CrossRef]
- Jing, P.B.; Cao, D.L.; Li, S.S.; Zhu, M.; Bai, X.Q.; Wu, X.B.; Gao, Y.J. Chemokine Receptor CXCR3 in the Spinal Cord Contributes to Chronic Itch in Mice. Neurosci. Bull. 2018, 34, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Ji, R.R. New insights into the mechanisms of itch: Are pain and itch controlled by distinct mechanisms? Pflugers Arch. 2013, 465, 1671–1685. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, T.; Kuwaki, T.; Kashiwadani, H. Hypothalamic orexinergic neurons modulate pain and itch in an opposite way: Pain relief and itch exacerbation. J. Physiol. Sci. 2022, 72, 21. [Google Scholar] [CrossRef] [PubMed]
- Hiraga, S.I.; Itokazu, T.; Nishibe, M.; Yamashita, T. Neuroplasticity related to chronic pain and its modulation by microglia. Inflamm. Regen. 2022, 42, 15. [Google Scholar] [CrossRef] [PubMed]
- Pottorf, T.S.; Rotterman, T.M.; McCallum, W.M.; Haley-Johnson, Z.A.; Alvarez, F.J. The Role of Microglia in Neuroinflammation of the Spinal Cord after Peripheral Nerve Injury. Cells 2022, 11, 2083. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, M. Microglia-Mediated Regulation of Neuropathic Pain: Molecular and Cellular Mechanisms. Biol. Pharm. Bull. 2019, 42, 1959–1968. [Google Scholar] [CrossRef]
- Xu, Z.; Qin, Z.; Zhang, J.; Wang, Y. Microglia-mediated chronic psoriatic itch induced by imiquimod. Mol. Pain 2020, 16, 1744806920934998. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, J.; Hu, R.; Sun, Y.; Ma, Y.; Chen, Z.; Jiang, H. Microglia are involved in pruritus induced by DNFB via the CX3CR1/p38 MAPK pathway. Cell Physiol. Biochem. 2015, 35, 1023–1033. [Google Scholar] [CrossRef]
- Silva, R.; Malcangio, M. Fractalkine/CX(3)CR(1) Pathway in Neuropathic Pain: An Update. Front. Pain Res. 2021, 2, 684684. [Google Scholar] [CrossRef]
- Liu, T.; Xu, Z.Z.; Park, C.K.; Berta, T.; Ji, R.R. Toll-like receptor 7 mediates pruritus. Nat. Neurosci. 2010, 13, 1460–1462. [Google Scholar] [CrossRef]
- Chen, G.; Luo, X.; Qadri, M.Y.; Berta, T.; Ji, R.R. Sex-Dependent Glial Signaling in Pathological Pain: Distinct Roles of Spinal Microglia and Astrocytes. Neurosci. Bull. 2018, 34, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Gwak, Y.S.; Hulsebosch, C.E.; Leem, J.W. Neuronal-Glial Interactions Maintain Chronic Neuropathic Pain after Spinal Cord Injury. Neural. Plast 2017, 2017, 2480689. [Google Scholar] [CrossRef]
- Garland, E.F.; Hartnell, I.J.; Boche, D. Microglia and Astrocyte Function and Communication: What Do We Know in Humans? Front. Neurosci. 2022, 16, 824888. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zhang, Y.-Q.; Qadri, Y.J.; Serhan, C.N.; Ji, R.-R. Microglia in Pain: Detrimental and Protective Roles in Pathogenesis and Resolution of Pain. Neuron 2018, 100, 1292–1311. [Google Scholar] [CrossRef] [PubMed]
- Wittekindt, M.; Kaddatz, H.; Joost, S.; Staffeld, A.; Bitar, Y.; Kipp, M.; Frintrop, L. Different Methods for Evaluating Microglial Activation Using Anti-Ionized Calcium-Binding Adaptor Protein-1 Immunohistochemistry in the Cuprizone Model. Cells 2022, 11, 1723. [Google Scholar] [CrossRef] [PubMed]
- Hanani, M. Satellite glial cells in sensory ganglia: From form to function. Brain Res. Brain Res. Rev. 2005, 48, 457–476. [Google Scholar] [CrossRef]
- Hanani, M. How Is Peripheral Injury Signaled to Satellite Glial Cells in Sensory Ganglia? Cells 2022, 11, 512. [Google Scholar] [CrossRef]
- Gazerani, P. Satellite Glial Cells in Pain Research: A Targeted Viewpoint of Potential and Future Directions. Front. Pain Res. 2021, 2, 646068. [Google Scholar] [CrossRef]
- Andreeva, D.; Murashova, L.; Burzak, N.; Dyachuk, V. Satellite Glial Cells: Morphology, functional heterogeneity, and role in pain. Front. Cell Neurosci. 2022, 16, 1019449. [Google Scholar] [CrossRef]
- Cohen, M.; Feldman-Goriachnik, R.; Hanani, M. Satellite Glial Cells and Neurons in Trigeminal Ganglia Are Altered in an Itch Model in Mice. Cells 2022, 11, 886. [Google Scholar] [CrossRef]
- Costa, F.A.L.; Neto, F.L.M. Satellite glial cells in sensory ganglia: Its role in pain. Braz. J. Anesthesiol. Engl. Ed. 2015, 65, 73–81. [Google Scholar] [CrossRef]
- Mitterreiter, J.G.; Ouwendijk, W.J.D.; van Velzen, M.; van Nierop, G.P.; Osterhaus, A.; Verjans, G. Satellite glial cells in human trigeminal ganglia have a broad expression of functional Toll-like receptors. Eur. J. Immunol. 2017, 47, 1181–1187. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.M.; Itson-Zoske, B.; Cai, Y.; Qiu, C.; Pan, B.; Stucky, C.L.; Hogan, Q.H.; Yu, H. Satellite glial cells in sensory ganglia express functional transient receptor potential ankyrin 1 that is sensitized in neuropathic and inflammatory pain. Mol. Pain 2020, 16, 1744806920925425. [Google Scholar] [CrossRef]
- Fang, X.X.; Wang, H.; Song, H.L.; Wang, J.; Zhang, Z.J. Neuroinflammation Involved in Diabetes-Related Pain and Itch. Front. Pharmacol. 2022, 13, 921612. [Google Scholar] [CrossRef] [PubMed]
- Robering, J.W.; Gebhardt, L.; Wolf, K.; Kühn, H.; Kremer, A.E.; Fischer, M.J.M. Lysophosphatidic acid activates satellite glia cells and Schwann cells. Glia 2019, 67, 999–1012. [Google Scholar] [CrossRef]
- Vander Does, A.; Levy, C.; Yosipovitch, G. Cholestatic Itch: Our Current Understanding of Pathophysiology and Treatments. Am. J. Clin. Dermatol. 2022, 23, 647–659. [Google Scholar] [CrossRef]
- Patel, S.P.; Vasavda, C.; Ho, B.; Meixiong, J.; Dong, X.; Kwatra, S.G. Cholestatic pruritus: Emerging mechanisms and therapeutics. J. Am. Acad. Dermatol. 2019, 81, 1371–1378. [Google Scholar] [CrossRef]
- Yu, H.; Zhao, T.; Liu, S.; Wu, Q.; Johnson, O.; Wu, Z.; Zhuang, Z.; Shi, Y.; Peng, L.; He, R.; et al. MRGPRX4 is a bile acid receptor for human cholestatic itch. Elife 2019, 8, e48431. [Google Scholar] [CrossRef]
- Alemi, F.; Kwon, E.; Poole, D.P.; Lieu, T.; Lyo, V.; Cattaruzza, F.; Cevikbas, F.; Steinhoff, M.; Nassini, R.; Materazzi, S.; et al. The TGR5 receptor mediates bile acid-induced itch and analgesia. J. Clin. Investig. 2013, 123, 1513–1530. [Google Scholar] [CrossRef]
- Bray, E.R.; Chéret, J.; Yosipovitch, G.; Paus, R. Schwann cells as underestimated, major players in human skin physiology and pathology. Exp. Dermatol. 2020, 29, 93–101. [Google Scholar] [CrossRef]
- Langedijk, J.; Beuers, U.H.; Oude Elferink, R.P.J. Cholestasis-Associated Pruritus and Its Pruritogens. Front. Med. 2021, 8, 639674. [Google Scholar] [CrossRef] [PubMed]
- Maglie, R.; Souza Monteiro de Araujo, D.; Antiga, E.; Geppetti, P.; Nassini, R.; De Logu, F. The Role of TRPA1 in Skin Physiology and Pathology. Int. J. Mol. Sci. 2021, 22, 3065. [Google Scholar] [CrossRef] [PubMed]
- Koivisto, A.-P.; Belvisi, M.G.; Gaudet, R.; Szallasi, A. Advances in TRP channel drug discovery: From target validation to clinical studies. Nat. Rev. Drug Discov. 2022, 21, 41–59. [Google Scholar] [CrossRef]
- Kahremany, S.; Hofmann, L.; Gruzman, A.; Cohen, G. Advances in Understanding the Initial Steps of Pruritoceptive Itch: How the Itch Hits the Switch. Int. J. Mol. Sci. 2020, 21, 4883. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Stieger, K.C.; Kozai, T.D.Y. Challenges and opportunities of advanced gliomodulation technologies for excitation-inhibition balance of brain networks. Curr. Opin. Biotechnol. 2021, 72, 112–120. [Google Scholar] [CrossRef]
- Peeters, L.M.; Missault, S.; Keliris, A.J.; Keliris, G.A. Combining designer receptors exclusively activated by designer drugs and neuroimaging in experimental models: A powerful approach towards neurotheranostic applications. Br. J. Pharmacol. 2020, 177, 992–1002. [Google Scholar] [CrossRef]
| Etiology of Chronic Itch | Condition | Potential Mechanisms |
|---|---|---|
| Dermatologic | Atopic dermatitis, psoriasis | Skin barrier interruption, neuroinflammation, immune system involvement |
| Neurologic | Neuropathic itch | Central sensitization, neuronal innervation, and signaling |
| Systemic | Cholestatic itch | Itch-provoking substances in the enterohepatic cycle, bile acids, and other secreted pruritogens during or as a result of metabolism processes |
| Psychologic | Psychogenic itch | Anxiety, depression, psychological and emotional signaling, opioids, acetylcholine, and dopamine involvement |
| Category | Example |
|---|---|
| Predisposing factors | Sociodemographic factors (age, gender, socioeconomic status, and migration status) |
| Psychological factors (personality traits, childhood experiences and life stressors) | |
| Biomedical factors (genetics, epigenetics, environmental factors, microbial, immunological, and endocrinological factors) | |
| Triggering factors | External and internal factors (stress, anxiety, depression, living environment (temperature and humidity), sleep, and diet |
| Maintaining and/or aggravating factors | Psychological factors (cognitive-perceptual and emotional factors such as illness perception and catastrophizing; behavioral responses (avoidance, deconditioning)) |
| Biomedical factors (disease factors (neuronal and immunological factors); treatment factors (side effects and treatment effects)) |
| Location | Type of Glia |
|---|---|
| Central nervous system (CNS) | Macroglia (Astroglia, Oligodendroglia, and NG2-glia) |
| Microglia | |
| Peripheral nervous system (PNS) | Schwann cells (SCs) |
| Satellite Glial Cells (SGCs) | |
| Olfactory ensheathing cells | |
| Enteric glia |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gazerani, P. Contribution of Central and Peripheral Glial Cells in the Development and Persistence of Itch: Therapeutic Implication of Glial Modulation. Neuroglia 2023, 4, 15-27. https://doi.org/10.3390/neuroglia4010002
Gazerani P. Contribution of Central and Peripheral Glial Cells in the Development and Persistence of Itch: Therapeutic Implication of Glial Modulation. Neuroglia. 2023; 4(1):15-27. https://doi.org/10.3390/neuroglia4010002
Chicago/Turabian StyleGazerani, Parisa. 2023. "Contribution of Central and Peripheral Glial Cells in the Development and Persistence of Itch: Therapeutic Implication of Glial Modulation" Neuroglia 4, no. 1: 15-27. https://doi.org/10.3390/neuroglia4010002
APA StyleGazerani, P. (2023). Contribution of Central and Peripheral Glial Cells in the Development and Persistence of Itch: Therapeutic Implication of Glial Modulation. Neuroglia, 4(1), 15-27. https://doi.org/10.3390/neuroglia4010002






