Sexual Dimorphism and Hypothalamic Astrocytes: Focus on Glioprotection
Abstract
:1. Introduction
2. Sexual Dimorphism and Hormones
3. Neuron–Glial Communication, Astrocytic Functions, and Neurosteroids
4. Hypothalamic Astrocytes and Metabolic Disorders
5. Sexual Dimorphism in Neuropsychiatric and Neurological Disorders
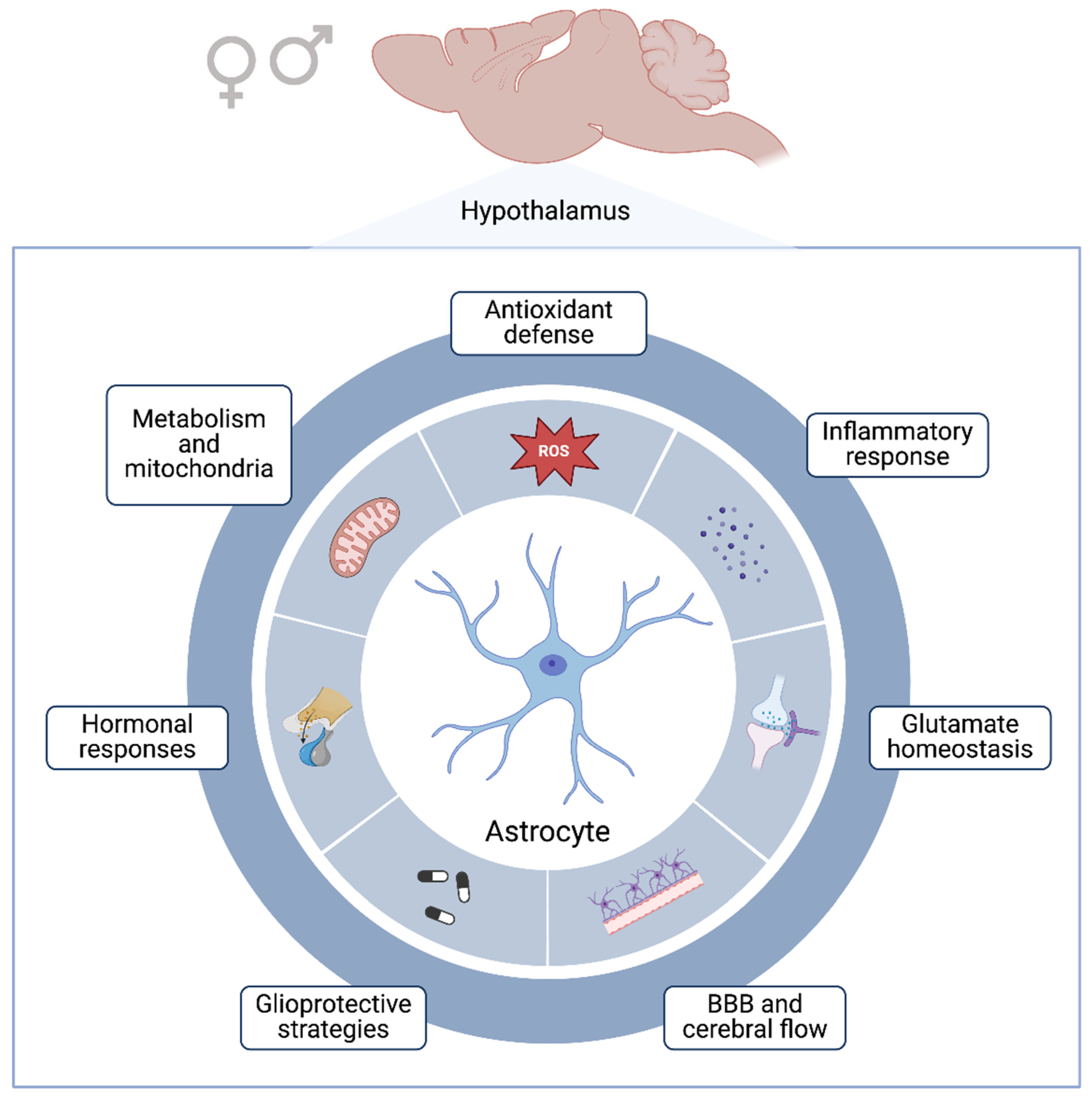
6. Glioprotective Mechanisms Associated with Hypothalamus
7. Concluding Remarks and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sovrani, V.; Bobermin, L.D.; Santos, C.L.; Brondani, M.; Gonçalves, C.-A.; Leipnitz, G.; Quincozes-Santos, A. Effects of Long-Term Resveratrol Treatment in Hypothalamic Astrocyte Cultures from Aged Rats. Mol. Cell. Biochem. 2023, 478, 1205–1216. [Google Scholar] [CrossRef]
- Fong, H.; Zheng, J.; Kurrasch, D. The Structural and Functional Complexity of the Integrative Hypothalamus. Science 2023, 382, 388–394. [Google Scholar] [CrossRef]
- Maroso, M.; Stern, P. Small and Mighty: The Hypothalamus. Science 2023, 382, 386–387. [Google Scholar] [CrossRef]
- Nampoothiri, S.; Nogueiras, R.; Schwaninger, M.; Prevot, V. Glial Cells as Integrators of Peripheral and Central Signals in the Regulation of Energy Homeostasis. Nat. Metab. 2022, 4, 813–825. [Google Scholar] [CrossRef]
- Luengo-Mateos, M.; González-Vila, A.; Vicente Dragano, N.R.; Ohinska, N.; Silveira-Loureiro, M.; González-Domínguez, M.; Estévez-Salguero, Á.; Novelle-Rodríguez, P.; López, M.; Barca-Mayo, O. Hypothalamic Astrocytic-BMAL1 Regulates Energy Homeostasis in a Sex-Dependent Manner. Cell Rep. 2023, 42, 112949. [Google Scholar] [CrossRef]
- Chowen, J.A.; Frago, L.M.; Fernández-Alfonso, M.S. Physiological and Pathophysiological Roles of Hypothalamic Astrocytes in Metabolism. J. Neuroendocrinol. 2019, 31, e12671. [Google Scholar] [CrossRef] [PubMed]
- Hatcher, K.M.; Royston, S.E.; Mahoney, M.M. Modulation of Circadian Rhythms through Estrogen Receptor Signaling. Eur. J. Neurosci. 2020, 51, 217–228. [Google Scholar] [CrossRef]
- Davis, F.C.; Darrow, J.M.; Menaker, M. Sex Differences in the Circadian Control of Hamster Wheel-Running Activity. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1983, 244, R93–R105. [Google Scholar] [CrossRef]
- Becker, J.B.; Arnold, A.P.; Berkley, K.J.; Blaustein, J.D.; Eckel, L.A.; Hampson, E.; Herman, J.P.; Marts, S.; Sadee, W.; Steiner, M.; et al. Strategies and Methods for Research on Sex Differences in Brain and Behavior. Endocrinology 2005, 146, 1650–1673. [Google Scholar] [CrossRef]
- Heberden, C. Sex Steroids and Neurogenesis. Biochem. Pharmacol. 2017, 141, 56–62. [Google Scholar] [CrossRef]
- Mong, J.A.; Glaser, E.; McCarthy, M.M. Gonadal Steroids Promote Glial Differentiation and Alter Neuronal Morphology in the Developing Hypothalamus in a Regionally Specific Manner. J. Neurosci. 1999, 19, 1464–1472. [Google Scholar] [CrossRef]
- Goodfellow, P.N.; Lovell-Badge, R. SRY and Sex Determination in Mammals. Annu. Rev. Genet. 1993, 27, 71–92. [Google Scholar] [CrossRef]
- Weisz, J.; Ward, I.L. Plasma Testosterone and Progesterone Titers of Pregnant Rats, Their Male and Female Fetuses, and Neonatal Offspring. Endocrinology 1980, 106, 306–316. [Google Scholar] [CrossRef]
- Bakker, J. The Role of Steroid Hormones in the Sexual Differentiation of the Human Brain. J. Neuroendocrinol. 2022, 34, e13050. [Google Scholar] [CrossRef]
- Singh, G.; Singh, V.; Schneider, J.S. Post-Translational Histone Modifications and Their Interaction with Sex Influence Normal Brain Development and Elaboration of Neuropsychiatric Disorders. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2019, 1865, 1968–1981. [Google Scholar] [CrossRef]
- Arambula, S.E.; McCarthy, M.M. Neuroendocrine-Immune Crosstalk Shapes Sex-Specific Brain Development. Endocrinology 2020, 161, bqaa055. [Google Scholar] [CrossRef]
- Deverman, B.E.; Patterson, P.H. Cytokines and CNS Development. Neuron 2009, 64, 61–78. [Google Scholar] [CrossRef]
- Nelson, L.H.; Lenz, K.M. The Immune System as a Novel Regulator of Sex Differences in Brain and Behavioral Development. J. Neurosci. Res. 2017, 95, 447–461. [Google Scholar] [CrossRef]
- Schwarz, J.M.; Sholar, P.W.; Bilbo, S.D. Sex Differences in Microglial Colonization of the Developing Rat Brain. J. Neurochem. 2012, 120, 948–963. [Google Scholar] [CrossRef]
- VanRyzin, J.W.; Marquardt, A.E.; Argue, K.J.; Vecchiarelli, H.A.; Ashton, S.E.; Arambula, S.E.; Hill, M.N.; McCarthy, M.M. Microglial Phagocytosis of Newborn Cells Is Induced by Endocannabinoids and Sculpts Sex Differences in Juvenile Rat Social Play. Neuron 2019, 102, 435–449.e6. [Google Scholar] [CrossRef]
- Garcia-Segura, L.M.; Suarez, I.; Segovia, S.; Tranque, P.A.; Calés, J.M.; Aguilera, P.; Olmos, G.; Guillamón, A. The Distribution of Glial Fibrillary Acidic Protein in the Adult Rat Brain Is Influenced by the Neonatal Levels of Sex Steroids. Brain Res. 1988, 456, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Been, L.E.; Sheppard, P.A.S.; Galea, L.A.M.; Glasper, E.R. Hormones and Neuroplasticity: A Lifetime of Adaptive Responses. Neurosci. Biobehav. Rev. 2022, 132, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Dafny, N.; Feldman, S. Unit Responses and Convergence of Sensory Stimuli in the Hypothalamus. Brain Res. 1970, 17, 243–257. [Google Scholar] [CrossRef] [PubMed]
- Sominsky, L.; Jasoni, C.L.; Twigg, H.R.; Spencer, S.J. Hormonal and Nutritional Regulation of Postnatal Hypothalamic Development. J. Endocrinol. 2018, 237, R47–R64. [Google Scholar] [CrossRef]
- Carrer, H.F.; Cambiasso, M.J. Sexual Differentiation of the Brain: Genes, Estrogen, and Neurotrophic Factors. Cell. Mol. Neurobiol. 2002, 22, 479–500. [Google Scholar] [CrossRef]
- Silva, M.S.B.; Giacobini, P. New Insights into Anti-Müllerian Hormone Role in the Hypothalamic–Pituitary–Gonadal Axis and Neuroendocrine Development. Cell. Mol. Life Sci. 2021, 78, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Marcondes, F.K.; Bianchi, F.J.; Tanno, A.P. Determination of the Estrous Cycle Phases of Rats: Some Helpful Considerations. Braz. J. Biol. 2002, 62, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Bridges, R.S. Neuroendocrine Regulation of Maternal Behavior. Front. Neuroendocrinol. 2015, 36, 178–196. [Google Scholar] [CrossRef]
- Frederich, R.C.; Hamann, A.; Anderson, S.; Löllmann, B.; Lowell, B.B.; Flier, J.S. Leptin Levels Reflect Body Lipid Content in Mice: Evidence for Diet-Induced Resistance to Leptin Action. Nat. Med. 1995, 1, 1311–1314. [Google Scholar] [CrossRef]
- Koebele, S.V.; Bimonte-Nelson, H.A. Modeling Menopause: The Utility of Rodents in Translational Behavioral Endocrinology Research. Maturitas 2016, 87, 5–17. [Google Scholar] [CrossRef]
- Qian, J.; Morris, C.J.; Caputo, R.; Wang, W.; Garaulet, M.; Scheer, F.A.J.L. Sex Differences in the Circadian Misalignment Effects on Energy Regulation. Proc. Natl. Acad. Sci. USA 2019, 116, 23806–23812. [Google Scholar] [CrossRef] [PubMed]
- Novozhilova, M.; Mishchenko, T.; Kondakova, E.; Lavrova, T.; Gavrish, M.; Aferova, S.; Franceschi, C.; Vedunova, M. Features of Age-Related Response to Sleep Deprivation: In Vivo Experimental Studies. Aging 2021, 13, 19108–19126. [Google Scholar] [CrossRef] [PubMed]
- Wyse, A.T.; Siebert, C.; Bobermin, L.D.; Dos Santos, T.M.; Quincozes-Santos, A. Changes in Inflammatory Response, Redox Status and Na+, K+-ATPase Activity in Primary Astrocyte Cultures from Female Wistar Rats Subject to Ovariectomy. Neurotox. Res. 2020, 37, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Sumien, N.; Cunningham, J.T.; Davis, D.L.; Engelland, R.; Fadeyibi, O.; Farmer, G.E.; Mabry, S.; Mensah-Kane, P.; Trinh, O.T.P.; Vann, P.H.; et al. Neurodegenerative Disease: Roles for Sex, Hormones, and Oxidative Stress. Endocrinology 2021, 162, bqab185. [Google Scholar] [CrossRef] [PubMed]
- Razmara, A.; Duckles, S.P.; Krause, D.N.; Procaccio, V. Estrogen Suppresses Brain Mitochondrial Oxidative Stress in Female and Male Rats. Brain Res. 2007, 1176, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, R.; Cheung, A.S.; Pang, K.; Saffery, R.; Novakovic, B. Sexual Dimorphism in Innate Immunity: The Role of Sex Hormones and Epigenetics. Front. Immunol. 2021, 11, 604000. [Google Scholar] [CrossRef]
- Sohrabji, F.; Okoreeh, A.; Panta, A. Sex Hormones and Stroke: Beyond Estrogens. Horm. Behav. 2019, 111, 87–95. [Google Scholar] [CrossRef]
- Quincozes-Santos, A.; Santos, C.L.; de Souza Almeida, R.R.; da Silva, A.; Thomaz, N.K.; Costa, N.L.F.; Weber, F.B.; Schmitz, I.; Medeiros, L.S.; Medeiros, L.; et al. Gliotoxicity and Glioprotection: The Dual Role of Glial Cells. Mol. Neurobiol. 2021, 58, 6577–6592. [Google Scholar] [CrossRef] [PubMed]
- Camandola, S. Astrocytes, Emerging Stars of Energy Homeostasis. Cell Stress 2018, 2, 246–252. [Google Scholar] [CrossRef]
- Linnerbauer, M.; Wheeler, M.A.; Quintana, F.J. Astrocyte Crosstalk in CNS Inflammation. Neuron 2020, 108, 608–622. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Nedergaard, M. Physiology of Astroglia. Physiol. Rev. 2018, 98, 239–389. [Google Scholar] [CrossRef] [PubMed]
- Collignon, A.; Dion-Albert, L.; Ménard, C.; Coelho-Santos, V. Sex, Hormones and Cerebrovascular Function: From Development to Disorder. Fluids Barriers CNS 2024, 21, 2. [Google Scholar] [CrossRef] [PubMed]
- Micevych, P.; Sinchak, K. Estradiol Regulation of Progesterone Synthesis in the Brain. Mol. Cell. Endocrinol. 2008, 290, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Ubuka, T.; Tsutsui, K. Neuropeptidergic Control of Neurosteroids Biosynthesis. Front. Neuroendocrinol. 2022, 65, 100976. [Google Scholar] [CrossRef]
- Chen, C.; Kuo, J.; Wong, A.; Micevych, P. Estradiol Modulates Translocator Protein (Tspo) and Steroid Acute Regulatory Protein (StAR) via Protein Kinase A (PKA) Signaling in Hypothalamic Astrocytes. Endocrinology 2014, 155, 2976–2985. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, J.; Karolczak, M.; Krust, A.; Chambon, P.; Beyer, C. Estrogen Receptor-α Is Associated with the Plasma Membrane of Astrocytes and Coupled to the MAP/Src-Kinase Pathway. Glia 2005, 50, 270–275. [Google Scholar] [CrossRef]
- Heemers, H.V.; Tindall, D.J. Androgen Receptor (AR) Coregulators: A Diversity of Functions Converging on and Regulating the AR Transcriptional Complex. Endocr. Rev. 2007, 28, 778–808. [Google Scholar] [CrossRef] [PubMed]
- Foradori, C.D.; Weiser, M.J.; Handa, R.J. Non-Genomic Actions of Androgens. Front. Neuroendocrinol. 2008, 29, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Gore, A.C.; Oung, T.; Yung, S.; Flagg, R.A.; Woller, M.J. Neuroendocrine Mechanisms for Reproductive Senescence in the Female Rat: Gonadotropin-Releasing Hormone Neurons. Endocrine 2000, 13, 315–323. [Google Scholar] [CrossRef]
- Illarionova, N.B.; Illarionava, N.B.; Brismar, H.; Aperia, A.; Gunnarson, E. Role of Na,K-ATPase A1 and A2 Isoforms in the Support of Astrocyte Glutamate Uptake. PLoS ONE 2014, 9, e98469. [Google Scholar] [CrossRef]
- Chakraborti, A.; Gulati, K.; Banerjee, B.D.; Ray, A. Possible Involvement of Free Radicals in the Differential Neurobehavioral Responses to Stress in Male and Female Rats. Behav. Brain Res. 2007, 179, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Guevara, R.; Santandreu, F.M.; Valle, A.; Gianotti, M.; Oliver, J.; Roca, P. Sex-Dependent Differences in Aged Rat Brain Mitochondrial Function and Oxidative Stress. Free Radic. Biol. Med. 2009, 46, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Sobočanec, S.; Balog, T.; Kušić, B.; Šverko, V.; Šarić, A.; Marotti, T. Differential Response to Lipid Peroxidation in Male and Female Mice with Age: Correlation of Antioxidant Enzymes Matters. Biogerontology 2008, 9, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Liddell, J. Are Astrocytes the Predominant Cell Type for Activation of Nrf2 in Aging and Neurodegeneration? Antioxidants 2017, 6, 65. [Google Scholar] [CrossRef] [PubMed]
- Gudkov, S.V.; Burmistrov, D.E.; Kondakova, E.V.; Sarimov, R.M.; Yarkov, R.S.; Franceschi, C.; Vedunova, M.V. An Emerging Role of Astrocytes in Aging/Neuroinflammation and Gut-Brain Axis with Consequences on Sleep and Sleep Disorders. Ageing Res. Rev. 2023, 83, 101775. [Google Scholar] [CrossRef] [PubMed]
- Santos-Galindo, M.; Acaz-Fonseca, E.; Bellini, M.J.; Garcia-Segura, L.M. Sex Differences in the Inflammatory Response of Primary Astrocytes to Lipopolysaccharide. Biol. Sex Differ. 2011, 2, 7. [Google Scholar] [CrossRef] [PubMed]
- García-Cáceres, C.; Quarta, C.; Varela, L.; Gao, Y.; Gruber, T.; Legutko, B.; Jastroch, M.; Johansson, P.; Ninkovic, J.; Yi, C.-X.; et al. Astrocytic Insulin Signaling Couples Brain Glucose Uptake with Nutrient Availability. Cell 2016, 166, 867–880. [Google Scholar] [CrossRef]
- Fernandez-Galaz, M.D.C.; Naftolin, F.; Garcia-Segura, L.M. Phasic Synaptic Remodeling of the Rat Arcuate Nucleus during the Estrous Cycle Depends on Insulin-like Growth Factor-I Receptor Activation. J. Neurosci. Res. 1999, 55, 286–292. [Google Scholar] [CrossRef]
- Björnström, L.; Sjöberg, M. Signal Transducers and Activators of Transcription as Downstream Targets of Nongenomic Estrogen Receptor Actions. Mol. Endocrinol. 2002, 16, 2202–2214. [Google Scholar] [CrossRef]
- Childs, G.V.; Odle, A.K.; MacNicol, M.C.; MacNicol, A.M. The Importance of Leptin to Reproduction. Endocrinology 2021, 162, bqaa204. [Google Scholar] [CrossRef]
- Bélanger, M.; Allaman, I.; Magistretti, P.J. Brain Energy Metabolism: Focus on Astrocyte-Neuron Metabolic Cooperation. Cell Metab. 2011, 14, 724–738. [Google Scholar] [CrossRef] [PubMed]
- Ciofi, P.; Garret, M.; Lapirot, O.; Lafon, P.; Loyens, A.; Prévot, V.; Levine, J.E. Brain-Endocrine Interactions: A Microvascular Route in the Mediobasal Hypothalamus. Endocrinology 2009, 150, 5509–5519. [Google Scholar] [CrossRef]
- Kaczkurkin, A.N.; Raznahan, A.; Satterthwaite, T.D. Sex Differences in the Developing Brain: Insights from Multimodal Neuroimaging. Neuropsychopharmacology 2019, 44, 71–85. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, X.; Feinberg, D.; Guenther, M.; Gregori, J.; Weiner, M.W.; Schuff, N. Arterial Spin Labeling MRI Study of Age and Gender Effects on Brain Perfusion Hemodynamics. Magn. Reson. Med. 2012, 68, 912–922. [Google Scholar] [CrossRef]
- Chen, Z.; Yuhanna, I.S.; Galcheva-Gargova, Z.; Karas, R.H.; Mendelsohn, M.E.; Shaul, P.W. Estrogen Receptor α Mediates the Nongenomic Activation of Endothelial Nitric Oxide Synthase by Estrogen. J. Clin. Investig. 1999, 103, 401–406. [Google Scholar] [CrossRef]
- Yilmaz, C.; Karali, K.; Fodelianaki, G.; Gravanis, A.; Chavakis, T.; Charalampopoulos, I.; Alexaki, V.I. Neurosteroids as Regulators of Neuroinflammation. Front. Neuroendocrinol. 2019, 55, 100788. [Google Scholar] [CrossRef] [PubMed]
- Mellon, S.H.; Griffin, L.D. Neurosteroids: Biochemistry and Clinical Significance. Trends Endocrinol. Metab. 2002, 13, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Melcangi, R.C.; Garcia-Segura, L.M.; Mensah-Nyagan, A.G. Neuroactive Steroids: State of the Art and New Perspectives. Cell. Mol. Life Sci. 2008, 65, 777–797. [Google Scholar] [CrossRef]
- Mauch, D.H.; Nägler, K.; Schumacher, S.; Göritz, C.; Müller, E.C.; Otto, A.; Pfrieger, F.W. CNS Synaptogenesis Promoted by Glia-Derived Cholesterol. Science 2001, 294, 1354–1357. [Google Scholar] [CrossRef]
- Meffre, D.; Labombarda, F.; Delespierre, B.; Chastre, A.; de Nicola, A.F.; Stein, D.G.; Schumacher, M.; Guennoun, R. Distribution of Membrane Progesterone Receptor Alpha in the Male Mouse and Rat Brain and Its Regulation after Traumatic Brain Injury. Neuroscience 2013, 231, 111–124. [Google Scholar] [CrossRef]
- Lorenz, B.; Garcia-Segura, L.M.; DonCarlos, L.L. Cellular Phenotype of Androgen Receptor-Immunoreactive Nuclei in the Developing and Adult Rat Brain. J. Comp. Neurol. 2005, 492, 456–468. [Google Scholar] [CrossRef] [PubMed]
- Stone, D.J.; Song, Y.; Anderson, C.P.; Krohn, K.K.; Finch, C.E.; Rozovsky, I. Bidirectional Transcription Regulation of Glial Fibrillary Acidic Protein by Estradiol in Vivo and in Vitro. Endocrinology 1998, 139, 3202–3209. [Google Scholar] [CrossRef] [PubMed]
- Day, J.R.; Laping, N.J.; Lampert-Etchells, M.; Brown, S.A.; O’Callaghan, J.P.; McNeill, T.H.; Finch, C.E. Gonadal Steroids Regulate the Expression of Glial Fibrillary Acidic Protein in the Adult Male Rat Hippocampus. Neuroscience 1993, 55, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, M.; Pekny, M. Enriched Environment and Astrocytes in Central Nervous System Regeneration. J. Rehabil. Med. 2007, 39, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.T.; Schneider, A.; DonCarlos, L.L.; Breedlove, S.M.; Jordan, C.L. Astrocytes in the Rat Medial Amygdala Are Responsive to Adult Androgens. J. Comp. Neurol. 2012, 520, 2531–2544. [Google Scholar] [CrossRef] [PubMed]
- Borrás, C.; Sastre, J.; García-Sala, D.; Lloret, A.; Pallardó, F.V.; Viña, J. Mitochondria from Females Exhibit Higher Antioxidant Gene Expression and Lower Oxidative Damage than Males. Free Radic. Biol. Med. 2003, 34, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Morken, T.S.; Brekke, E.; Håberg, A.; Widerøe, M.; Brubakk, A.-M.; Sonnewald, U. Altered Astrocyte–Neuronal Interactions After Hypoxia-Ischemia in the Neonatal Brain in Female and Male Rats. Stroke 2014, 45, 2777–2785. [Google Scholar] [CrossRef] [PubMed]
- Torrens-Mas, M.; Pons, D.-G.; Sastre-Serra, J.; Oliver, J.; Roca, P. Sexual Hormones Regulate the Redox Status and Mitochondrial Function in the Brain. Pathological implications. Redox Biol. 2020, 31, 101505. [Google Scholar] [CrossRef]
- Wu, J.; Williams, D.; Walter, G.A.; Thompson, W.E.; Sidell, N. Estrogen Increases Nrf2 Activity through Activation of the PI3K Pathway in MCF-7 Breast Cancer Cells. Exp. Cell Res. 2014, 328, 351–360. [Google Scholar] [CrossRef]
- Chistyakov, D.V.; Azbukina, N.V.; Astakhova, A.A.; Goriainov, S.V.; Chistyakov, V.V.; Sergeeva, M.G. Sex-Mediated Differences in LPS Induced Alterations of TNFα, IL-10 Expression, and Prostaglandin Synthesis in Primary Astrocytes. Int. J. Mol. Sci. 2018, 19, 2793. [Google Scholar] [CrossRef]
- Jha, M.K.; Jo, M.; Kim, J.-H.; Suk, K. Microglia-Astrocyte Crosstalk: An Intimate Molecular Conversation. Neuroscientist 2019, 25, 227–240. [Google Scholar] [CrossRef]
- Lennol, M.P.; Canelles, S.; Guerra-Cantera, S.; Argente, J.; García-Segura, L.M.; de Ceballos, M.L.; Chowen, J.A.; Frago, L.M. Amyloid-Β1-40 Differentially Stimulates Proliferation, Activation of Oxidative Stress and Inflammatory Responses in Male and Female Hippocampal Astrocyte Cultures. Mech. Ageing Dev. 2021, 195, 111462. [Google Scholar] [CrossRef]
- Musatov, S.; Chen, W.; Pfaff, D.W.; Mobbs, C.V.; Yang, X.-J.; Clegg, D.J.; Kaplitt, M.G.; Ogawa, S. Silencing of Estrogen Receptor Alpha in the Ventromedial Nucleus of Hypothalamus Leads to Metabolic Syndrome. Proc. Natl. Acad. Sci. USA 2007, 104, 2501–2506. [Google Scholar] [CrossRef]
- Frye, C.A. Neurosteroids’ Effects and Mechanisms for Social, Cognitive, Emotional, and Physical Functions. Psychoneuroendocrinology 2009, 34 (Suppl. 1), S143–S161. [Google Scholar] [CrossRef]
- Jais, A.; Brüning, J.C. Hypothalamic Inflammation in Obesity and Metabolic Disease. J. Clin. Investig. 2017, 127, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Louwe, M.C.; van der Hoorn, J.W.A.; van den Berg, S.A.A.; Jukema, J.W.; Romijn, J.A.; van Dijk, K.W.; Rensen, P.C.N.; Smit, J.W.A.; Steendijk, P. Gender-Dependent Effects of High-Fat Lard Diet on Cardiac Function in C57Bl/6J Mice. Appl. Physiol. Nutr. Metab. 2012, 37, 214–224. [Google Scholar] [CrossRef]
- Morselli, E.; Criollo, A.; Rodriguez-Navas, C.; Clegg, D.J. Chronic High Fat Diet Consumption Impairs Metabolic Health of Male Mice. Inflamm. Cell Signal. 2014, 1, e561. [Google Scholar] [CrossRef] [PubMed]
- Bonvento, G.; Bolaños, J.P. Astrocyte-Neuron Metabolic Cooperation Shapes Brain Activity. Cell Metab. 2021, 33, 1546–1564. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Reichel, J.M.; Han, C.; Zuniga-Hertz, J.P.; Cai, D. Astrocytic Process Plasticity and IKKβ/NF-κB in Central Control of Blood Glucose, Blood Pressure, and Body Weight. Cell Metab. 2017, 25, 1091–1102.e4. [Google Scholar] [CrossRef]
- Wu, X.; Huang, J.; Shen, C.; Liu, Y.; He, S.; Sun, J.; Yu, B. NRF2 Deficiency Increases Obesity Susceptibility in a Mouse Menopausal Model. PLoS ONE 2020, 15, e0228559. [Google Scholar] [CrossRef]
- Hidalgo-Lanussa, O.; González Santos, J.; Barreto, G.E. Sex-Specific Vulnerabilities in Human Astrocytes Underpin the Differential Impact of Palmitic Acid. Neurobiol. Dis. 2024, 195, 106489. [Google Scholar] [CrossRef] [PubMed]
- Allison, M.B.; Myers, M.G. 20 Years of Leptin: Connecting Leptin Signaling to Biological Function. J. Endocrinol. 2014, 223, T25–T35. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A. Brain Meets Body: The Blood-Brain Barrier as an Endocrine Interface. Endocrinology 2012, 153, 4111–4119. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.L.; Roppa, P.H.A.; Truccolo, P.; Fontella, F.U.; Souza, D.O.; Bobermin, L.D.; Quincozes-Santos, A. Age-Dependent Neurochemical Remodeling of Hypothalamic Astrocytes. Mol. Neurobiol. 2018, 55, 5565–5579. [Google Scholar] [CrossRef] [PubMed]
- García-Cáceres, C.; Fuente-Martín, E.; Burgos-Ramos, E.; Granado, M.; Frago, L.M.; Barrios, V.; Horvath, T.; Argente, J.; Chowen, J.A. Differential Acute and Chronic Effects of Leptin on Hypothalamic Astrocyte Morphology and Synaptic Protein Levels. Endocrinology 2011, 152, 1809–1818. [Google Scholar] [CrossRef] [PubMed]
- Pinto, S.; Roseberry, A.G.; Liu, H.; Diano, S.; Shanabrough, M.; Cai, X.; Friedman, J.M.; Horvath, T.L. Rapid Rewiring of Arcuate Nucleus Feeding Circuits by Leptin. Science 2004, 304, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Burguera, B.; Couce, M.E.; Curran, G.L.; Jensen, M.D.; Lloyd, R.V.; Cleary, M.P.; Poduslo, J.F. Obesity Is Associated with a Decreased Leptin Transport across the Blood-Brain Barrier in Rats. Diabetes 2000, 49, 1219–1223. [Google Scholar] [CrossRef] [PubMed]
- Thaler, J.P.; Yi, C.-X.; Schur, E.A.; Guyenet, S.J.; Hwang, B.H.; Dietrich, M.O.; Zhao, X.; Sarruf, D.A.; Izgur, V.; Maravilla, K.R.; et al. Obesity Is Associated with Hypothalamic Injury in Rodents and Humans. J. Clin. Investig. 2012, 122, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.L.; Bobermin, L.D.; Souza, D.O.; Quincozes-Santos, A. Leptin Stimulates the Release of Pro-Inflammatory Cytokines in Hypothalamic Astrocyte Cultures from Adult and Aged Rats. Metab. Brain Dis. 2018, 33, 2059–2063. [Google Scholar] [CrossRef]
- Kelly, M.J.; Qiu, J. Estrogen Signaling in Hypothalamic Circuits Controling Reproduction. Brain Res. 2010, 1364, 44–52. [Google Scholar] [CrossRef]
- Kamitakahara, A.; Bouyer, K.; Wang, C.-H.; Simerly, R. A Critical Period for the Trophic Actions of Leptin on AgRP Neurons in the Arcuate Nucleus of the Hypothalamus. J. Comp. Neurol. 2018, 526, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Young, E.A.; Lopez, J.F.; Murphy-Weinberg, V.; Watson, S.J.; Akil, H. Hormonal Evidence for Altered Responsiveness to Social Stress in Major Depression. Neuropsychopharmacology 2000, 23, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Kajantie, E.; Phillips, D.I.W. The Effects of Sex and Hormonal Status on the Physiological Response to Acute Psychosocial Stress. Psychoneuroendocrinology 2006, 31, 151–178. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-C.; Hung, C.-F.; Lin, P.-Y.; Lee, Y.; Wu, C.-C.; Hsu, S.-T.; Chen, C.-C.; Chong, M.-Y.; Lin, C.-H.; Wang, L.-J. Gender Differences in Susceptibility to Schizophrenia: Potential Implication of Neurosteroids. Psychoneuroendocrinology 2017, 84, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Manoli, D.S.; Tollkuhn, J. Gene Regulatory Mechanisms Underlying Sex Differences in Brain Development and Psychiatric Disease. Ann. N. Y. Acad. Sci. 2018, 1420, 26–45. [Google Scholar] [CrossRef] [PubMed]
- Trotman, H.D.; Holtzman, C.W.; Ryan, A.T.; Shapiro, D.I.; MacDonald, A.N.; Goulding, S.M.; Brasfield, J.L.; Walker, E.F. The Development of Psychotic Disorders in Adolescence: A Potential Role for Hormones. Horm. Behav. 2013, 64, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Patchev, V.K.; Hayashi, S.; Orikasa, C.; Almeida, O.F.X. Implications of Estrogen-Dependent Brain Organization for Gender Differences in Hypothalamo-Pituitary-Adrenal Regulation. FASEB J. 1995, 9, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Zorumski, C.F.; Paul, S.M.; Izumi, Y.; Covey, D.F.; Mennerick, S. Neurosteroids, Stress and Depression: Potential Therapeutic Opportunities. Neurosci. Biobehav. Rev. 2013, 37, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Hu, S.; Fu, X.; Zhou, H.; Yang, S.; Yang, C. Neurosteroids: A Potential Target for Neuropsychiatric Disorders. J. Steroid Biochem. Mol. Biol. 2024, 239, 106485. [Google Scholar] [CrossRef]
- Guennoun, R.; Labombarda, F.; Gonzalez Deniselle, M.C.; Liere, P.; de Nicola, A.F.; Schumacher, M. Progesterone and Allopregnanolone in the Central Nervous System: Response to Injury and Implication for Neuroprotection. J. Steroid Biochem. Mol. Biol. 2015, 146, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Schüle, C.; Nothdurfter, C.; Rupprecht, R. The Role of Allopregnanolone in Depression and Anxiety. Prog. Neurobiol. 2014, 113, 79–87. [Google Scholar] [CrossRef]
- Vallée, M. Structure-Activity Relationship Studies on Neuroactive Steroids in Memory, Alcohol and Stress-Related Functions: A Crucial Benefit from Endogenous Level Analysis. Psychopharmacology 2014, 231, 3243–3255. [Google Scholar] [CrossRef] [PubMed]
- Tizabi, Y.; Getachew, B.; Hauser, S.R.; Tsytsarev, V.; Manhães, A.C.; da Silva, V.D.A. Role of Glial Cells in Neuronal Function, Mood Disorders, and Drug Addiction. Brain Sci. 2024, 14, 558. [Google Scholar] [CrossRef] [PubMed]
- Chivero, E.T.; Sil, S.; Kumar, M.; Buch, S. Substance Use, Microbiome and Psychiatric Disorders. Pharmacol. Biochem. Behav. 2022, 219, 173432. [Google Scholar] [CrossRef]
- Schmitz, I.; da Silva, A.; Bobermin, L.D.; Gonçalves, C.-A.; Steiner, J.; Quincozes-Santos, A. The Janus Face of Antipsychotics in Glial Cells: Focus on Glioprotection. Exp. Biol. Med. 2023, 248, 2120–2130. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Kinnersley, B.; Wrensch, M.R.; Eckel-Passow, J.E.; Armstrong, G.; Rice, T.; Chen, Y.; Wiencke, J.K.; McCoy, L.S.; Hansen, H.M.; et al. Sex-Specific Glioma Genome-Wide Association Study Identifies New Risk Locus at 3p21.31 in Females, and Finds Sex-Differences in Risk at 8q24.21. Sci. Rep. 2018, 8, 7352. [Google Scholar] [CrossRef]
- Daswani, B.; Khan, Y. Insights into the Role of Estrogens and Androgens in Glial Tumorigenesis. J. Carcinog. 2021, 20, 10. [Google Scholar] [CrossRef]
- Kokras, N.; Dioli, C.; Paravatou, R.; Sotiropoulos, M.G.; Delis, F.; Antoniou, K.; Calogeropoulou, T.; Charalampopoulos, I.; Gravanis, A.; Dalla, C. Psychoactive Properties of BNN27, a Novel Neurosteroid Derivate, in Male and Female Rats. Psychopharmacology 2020, 237, 2435–2449. [Google Scholar] [CrossRef] [PubMed]
- Powrie, Y.S.L.; Smith, C. Central Intracrine DHEA Synthesis in Ageing-Related Neuroinflammation and Neurodegeneration: Therapeutic Potential? J. Neuroinflamm. 2018, 15, 289. [Google Scholar] [CrossRef]
- Pike, C.J. Sex and the Development of Alzheimer’s Disease. J. Neurosci. Res. 2017, 95, 671–680. [Google Scholar] [CrossRef]
- Gillies, G.E.; Murray, H.E.; Dexter, D.; McArthur, S. Sex Dimorphisms in the Neuroprotective Effects of Estrogen in an Animal Model of Parkinson’s Disease. Pharmacol. Biochem. Behav. 2004, 78, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Escartin, C.; Galea, E.; Lakatos, A.; O’Callaghan, J.P.; Petzold, G.C.; Serrano-Pozo, A.; Steinhäuser, C.; Volterra, A.; Carmignoto, G.; Agarwal, A.; et al. Reactive Astrocyte Nomenclature, Definitions, and Future Directions. Nat. Neurosci. 2021, 24, 312–325. [Google Scholar] [CrossRef] [PubMed]
- Cerciat, M.; Unkila, M.; Garcia-Segura, L.M.; Arevalo, M.-A. Selective Estrogen Receptor Modulators Decrease the Production of Interleukin-6 and Interferon-γ-inducible Protein-10 by Astrocytes Exposed to Inflammatory Challenge in Vitro. Glia 2010, 58, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Giraud, S.N.; Caron, C.M.; Pham-Dinh, D.; Kitabgi, P.; Nicot, A.B. Estradiol Inhibits Ongoing Autoimmune Neuroinflammation and NFκB-Dependent CCL2 Expression in Reactive Astrocytes. Proc. Natl. Acad. Sci. USA 2010, 107, 8416–8421. [Google Scholar] [CrossRef] [PubMed]
- Marin, R. Estrogen Receptors in Lipid Raft Signalling Complexes for Neuroprotection. Front. Biosci. 2012, E4, 1420–1433. [Google Scholar] [CrossRef]
- Rutkowsky, J.M.; Wallace, B.K.; Wise, P.M.; O’Donnell, M.E. Effects of Estradiol on Ischemic Factor-Induced Astrocyte Swelling and AQP4 Protein Abundance. Am. J. Physiol.-Cell Physiol. 2011, 301, C204–C212. [Google Scholar] [CrossRef] [PubMed]
- Habib, P.; Dang, J.; Slowik, A.; Victor, M.; Beyer, C. Hypoxia-Induced Gene Expression of Aquaporin-4, Cyclooxygenase-2 and Hypoxia-Inducible Factor 1α in Rat Cortical Astroglia Is Inhibited by 17β-Estradiol and Progesterone. Neuroendocrinology 2014, 99, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Gu, F.; Hata, R.; Toku, K.; Yang, L.; Ma, Y.; Maeda, N.; Sakanaka, M.; Tanaka, J. Testosterone Up-regulates Aquaporin-4 Expression in Cultured Astrocytes. J. Neurosci. Res. 2003, 72, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Giordano, G.; Tait, L.; Furlong, C.E.; Cole, T.B.; Kavanagh, T.J.; Costa, L.G. Gender Differences in Brain Susceptibility to Oxidative Stress Are Mediated by Levels of Paraoxonase-2 Expression. Free Radic. Biol. Med. 2013, 58, 98–108. [Google Scholar] [CrossRef]
- Dhandapani, K.M.; Brann, D.W. Neuroprotective Effects of Estrogen and Tamoxifen In Vitro: A Facilitative Role for Glia? Endocrine 2003, 21, 59–66. [Google Scholar] [CrossRef]
- Martin-Jiménez, C.; Gaitán-Vaca, D.M.; Areiza, N.; Echeverria, V.; Ashraf, G.M.; González, J.; Sahebkar, A.; Garcia-Segura, L.M.; Barreto, G.E. Astrocytes Mediate Protective Actions of Estrogenic Compounds after Traumatic Brain Injury. Neuroendocrinology 2019, 108, 142–160. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.E.; Lathe, R. The Promiscuous Estrogen Receptor: Evolution of Physiological Estrogens and Response to Phytochemicals and Endocrine Disruptors. J. Steroid Biochem. Mol. Biol. 2018, 184, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wang, Q.; Cheng, X.; Li, X.; Li, N.; Liu, T.; Li, J.; Yang, Q.; Dong, R.; Zhang, Y.; et al. Inhibitive Effect of Resveratrol on the Inflammation in Cultured Astrocytes and Microglia Induced by Aβ1–42. Neuroscience 2018, 379, 390–404. [Google Scholar] [CrossRef] [PubMed]
- Bobermin, L.D.; Sesterheim, P.; da Costa, D.S.; Rezena, E.; Schmitz, I.; da Silva, A.; de Moraes, A.D.M.; Souza, D.O.; Wyse, A.T.; Leipnitz, G.; et al. Simvastatin Differentially Modulates Glial Functions in Cultured Cortical and Hypothalamic Astrocytes Derived from Interferon α/β Receptor Knockout Mice. Neurochem. Res. 2024, 49, 732–743. [Google Scholar] [CrossRef] [PubMed]
- Bobermin, L.D.; da Costa, D.S.; de Moraes, A.D.M.; da Silva, V.F.; de Oliveira, G.T.; Sesterheim, P.; Tramontina, A.C.; Basso, L.A.; Leipnitz, G.; Quincozes-Santos, A.; et al. Effect of Metformin in Hypothalamic Astrocytes from an Immunocompromised Mice Model. Biochimie 2024, 223, S0300908424000804. [Google Scholar] [CrossRef]
- Sekar, A.P.; Nurmala, S.; Matsuura, E.; Tan, X.W.; Rahmasari, R.; Sauriasari, R. Estrogen Receptor Is Required for Metformin-Induced Apoptosis in Breast Cancer Cells Under Hyperglycemic Conditions. Breast Cancer 2024, 18, 11782234241240173. [Google Scholar] [CrossRef]
- Menze, E.T.; Ezzat, H.; Shawky, S.; Sami, M.; Selim, E.H.; Ahmed, S.; Maged, N.; Nadeem, N.; Eldash, S.; Michel, H.E. Simvastatin Mitigates Depressive-like Behavior in Ovariectomized Rats: Possible Role of NLRP3 Inflammasome and Estrogen Receptors’ Modulation. Int. Immunopharmacol. 2021, 95, 107582. [Google Scholar] [CrossRef]
| Experimental Model/Subjects | Main Findings | References |
|---|---|---|
| Hypothalamic tissue | Astrocytes from females and males are morphologically different | [11] |
| Long-term sleep deprivation in mice | Weight loss and cellular adaptive processes to increase stress resistance in young but not in adult female mice | [30] |
| Cultured astrocytes from adult female ovariectomized rats | Decreased Na+, K+-ATPase activity Increased pro-inflammatory cytokine release | [31] |
| MRI in human brain | Higher cerebral flow in females | [42] |
| Brain tissue and cultured astrocytes | Expression of receptors for estrogen, progesterone and testosterone in astrocytes Steroid hormones modulate GFAP expression in astrocytes | [43,44,45,46] [47,48] |
| Rodent brain | Female brains present lower oxidative damage and increased antioxidant capacity | [49,50,51,52] |
| Neonatal hypoxia-ischemia | Astrocytes from female rats show an enhanced mitochondrial metabolism, but male rats have higher recovery | [53] |
| Primary astrocyte cultures | Female and male cells show different responses to an inflammatory stimulus β-amyloid peptides induce an increased inflammatory response in females compared with males | [54,55] [56] |
| Rat hypothalamic tissue | Hypothalamic plasticity is prominent in female brain | [57,58] |
| Human astrocytes | Cells from males showed increased reactive oxygen species production in response to palmitic acid | [59] |
| Leptin deficient (ob/ob) mice | Female animals are more responsive to the restorative effects of leptin on hypothalamus than male | [60] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomaz, N.K.; Bobermin, L.D.; Quincozes-Santos, A. Sexual Dimorphism and Hypothalamic Astrocytes: Focus on Glioprotection. Neuroglia 2024, 5, 274-288. https://doi.org/10.3390/neuroglia5030019
Thomaz NK, Bobermin LD, Quincozes-Santos A. Sexual Dimorphism and Hypothalamic Astrocytes: Focus on Glioprotection. Neuroglia. 2024; 5(3):274-288. https://doi.org/10.3390/neuroglia5030019
Chicago/Turabian StyleThomaz, Natalie K., Larissa Daniele Bobermin, and André Quincozes-Santos. 2024. "Sexual Dimorphism and Hypothalamic Astrocytes: Focus on Glioprotection" Neuroglia 5, no. 3: 274-288. https://doi.org/10.3390/neuroglia5030019






