Abstract
Introduction: Acute brain injury is one of the most important causes of morbidity, mortality and disability worldwide. Time is the most important aspect of acute brain injury management. In this context, biomarkers could mitigate the limitations of neuroimaging. Neuro-biomarkers could be used both to diagnose intracranial pathology and to predict the effectiveness of treatment applications. Aim: The aim of this review is to describe the role of various and specific markers of brain damage with particular emphasis on acute brain injury and stroke. Results/discussion: The diagnostic and prognostic value of modern biomarkers remains relatively questionable, although grouping biomarkers into panels is improving their usefulness. The groups of biomarkers that will be analyzed include astrocytic, axonal, neuronal as well as extracellular biomarkers. Conclusion: Future studies will demonstrate the utility of neuro-biomarkers in the diagnosis, prognosis and therapeutic monitoring of patients with acute brain injury in the intensive care unit.
Keywords:
biomarkers; traumatic brain injury; stroke; GFAP; S100b; neurofilaments; UCHL1; extracellular vesicles; exosomes; proenkephalin A 1. Introduction
The central nervous system consists of the brain and spinal cord and is mainly composed of neurons and glial cells. Nervous tissue is characterized by a particular sensitivity to various injuries due to the reduced ability of neurons to regenerate. Brain damage is characterized by great heterogeneity, both etiological and pathophysiological [1,2]. The two main causes that will be considered are acute brain injuries and strokes. Several mechanisms, which include primary parenchymal destruction, secondary inflammatory processes and vascular damage, lead to respective clinical and imaging profiles [1,2]. Time is the most important aspect of acute brain injury management as neuronal loss, with its clinical consequences and therapeutic interventions, is strictly time dependent [3]. Therefore, it is extremely important that the diagnostic process is carried out as quickly as possible. A primary position in diagnosis is occupied by clinical tests to assess the patient’s condition, with the Glasgow Scale (GCS) as a typical example [1]. Imaging is crucial for diagnosing acute brain injuries, with Computed Tomography (CT) and Magnetic Resonance Imaging (MRI) being the primary techniques used in emergency and non-emergency settings, respectively [2]. CT is preferred during the acute phase due to its speed and ability to visualize cerebral edema, hemorrhage and large lesions [4]. However, CT has several limitations: it often underestimates the extent of lesions, shows poor correlation with patient outcomes, and cannot account for the heterogeneity of injury mechanisms, leading to varied clinical outcomes for similar lesions [5]. Additionally, imaging may not always be available when needed. These limitations are particularly problematic in cases of mild traumatic brain injury (mTBI), where there can be a long asymptomatic period with false-negative clinical tests, and imaging may fail to detect small parenchymal changes [1].
In this context, the role of biomarkers becomes apparent. In 1998, the National Institutes of Health Definitions Biomarkers Group defined a biomarker as “a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacological responses to a therapeutic intervention” [6]. By extension, a neuro-biomarker is a molecule produced and/or secreted by the cells of the nervous system whose appearance in the blood depends on the permeability of the blood-brain barrier (BBB) [7]. According to Wang et al. [8], a biomarker to be considered diagnostically ideal should have the following four characteristics: a. its levels in body fluids increase in case of brain damage, b. this increase is significant and clinically detectable, c. a clear correlation (quantitative and qualitative) with the severity of the damage should be observed and d. there should be agreement with the findings of conventional diagnostic methods (clinical and imaging). Neuro-biomarker detection in blood and cerebrospinal fluid (CSF) has been described in previous studies. As a result, neuro-biomarkers could be used not only for the diagnosis of intracranial pathology but also for monitoring the course of the disease, prognosis and monitoring the effectiveness of applied treatments. The groups of biomarkers that will be analyzed include astrocytic, axonal, neuronal as well as extracellular biomarkers. The main biomarkers of each group in terms of sensitivity, specificity and ability to differentiate between diseases will be discussed below.
The aim of the present review is to gather existing knowledge regarding neuro-biomarkers, analyzing their reliability and utility in detecting brain injury.
Classification of Brain Injury and Stroke
According to CDC and NIH, a traumatic brain injury (TBI) refers to a brain injury that is caused by an outside force. Traumatic brain injury constitutes a disruption in the normal function of the brain that can be caused by a bump, blow or jolt to the head, or penetrating head injury [9]. Brain injuries can be variously categorized into open or closed, localized or diffuse, and primary or secondary depending on the mechanism and location of the injury. Examples of localized lesions are epidural hematomas, subdural hematomas and intracerebral hemorrhage, while a typical example of diffuse lesions is axial damage. Primary injury results directly from the trauma and include acceleration and deceleration linear forces, rotational forces, compression and penetration by a projectile and, generally, it is not alterable [10]. Secondary injury develops as a result of the primary injury and includes ischemic/hypoxic damage, cerebral edema, raised intracranial pressure and even infection. In addition, they can be classified as mild, moderate and severe depending on the severity which is determined by clinical (GCS, duration of loss of consciousness and post-traumatic amnesia) and imaging criteria [11]. From a pathophysiological point of view, primary injury leads to immediate destruction of cell membranes (especially axons) affecting the flow of ions such as potassium and calcium and causing the release of excitatory neurotransmitters. In addition, the vasculature is impaired, resulting in conditions of ischemia and hypoxia. All of the above secondary lesions cause further cell destruction, cerebral edema and inflammation aggravating the clinical condition [12].
Ischemic stroke occurs when a vessel that supplies blood to the brain becomes blocked. Over time, ischemia becomes irreversible leading to disruption of transporter function, abnormal calcium ion influx and release of excitatory neurotransmitters and oxygen free radicals, blood–brain barrier disruption and ultimately neuronal destruction [13,14]. According to the TOAST classification, there are five main mechanisms/subtypes of ischemic stroke: cardioembolic, large vessel disease, small vessel disease, other determined causes and undetermined causes (or cryptogenic, which is the most common cause) [15,16].
2. Methods
This paper is a narrative review. The search was conducted on PubMed and included articles in English without time limitations. The following keywords were used: biomarkers; traumatic brain injury; stroke; GFAP; S100b; neurofilaments; UCHL1; extracellular vesicles; exosomes; proenkephalin A.
3. Results/Discussion
The groups of biomarkers that will be analyzed include astrocytic, axonal, neuronal as well as extracellular biomarkers (Figure 1).
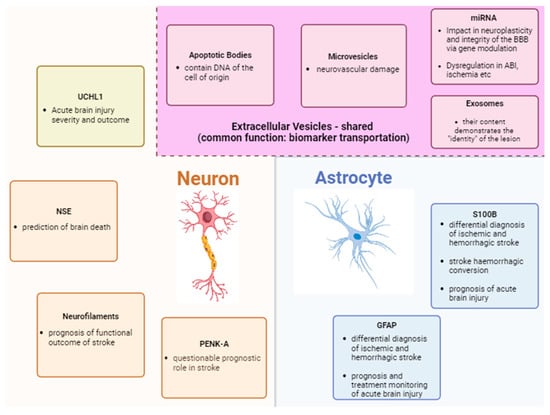
Figure 1.
Summary of biomarkers of acute brain injury and their main roles.
3.1. Astrocyte Markers
3.1.1. S-100b
S100b is a member of a family of low-molecular-weight calcium-binding proteins mainly expressed in astrocytes [17]. Their role concerns intracellular signaling and regulation of cell shape and is accomplished through enzyme modification, control of calcium metabolism and interactions with the cytoskeleton [18]. Its release from cells in both physiological conditions led to its localization in cerebrospinal fluid and plasma (with physiological concentrations of 1 to 2 µg/L and <0.15 µg/L, respectively) with a half-life of 2 h [5]. S-100b is the most extensively studied biomarker with uses in the diagnosis and prognosis mainly of traumatic brain injury (TBI) and cerebrovascular accidents [19]. However, it is important that S-100b is also produced by tissues other than the CNS (muscle, adipose, lymphatic), limiting the value of its measurements which must be interpreted critically [1].
In relation to brain trauma, S-100b levels below 0.12 μg/L on patient admission can be used prognostically for mild injury showing correlation with normal CT and leading to a reduction in cost and radiation dose in low-risk patients [20]. Furthermore, there is a positive correlation between S-100b levels and intracranial pressure [21]. Finally, it is worth noting that since 2013, S-100b has been included in the guidelines of the Scandinavian Neurotrauma Committee for the treatment of adult patients with mild trauma (Glasgow scale values—GCS: 14–15) without risk factors. Specifically, patients with S-100b levels <0.10 μg/L within 6 h of trauma may not undergo CT [22,23].
Regarding stroke, it has been shown that S100b increases more in hemorrhagic than in ischemic stroke, while the increase in ischemic stroke is slower [24]. This is probably explained by the reactive gliosis around the ischemic area which limits the release of S-100b into the blood [5]. However, its levels alone do not seem to have significant diagnostic power, so it is mainly used for differential diagnosis between hemorrhagic and ischemic strokes [25]. In addition, it shows a high negative predictive value in the hemorrhagic conversion of ischemic stroke [26] as well as a positive correlation both with the volume of the ischemic area [27] and with the National Institutes of Health Stroke Score (NIHSS) according to respective studies [28]. Despite the above, the delayed increase in S-100b seems to limit its use in the acute phase of stroke diagnosis.
3.1.2. GFAP
Glial fibrillary acidic protein (GFAP) is a structural protein of the astrocyte cytoskeleton with a key role in the support of astrocytic fibers and intracellular signaling [29]. Increased GFAP expression is characteristic of astrocytic injury with subsequent disruption of the blood–brain barrier leading to its release into the blood [30].
By extension, activation of astrocytes by brain trauma and ischemia leads to reactive gliosis and increased GFAP release [5]. High levels of serum GFAP, after admission of patients, were associated with a worse prognosis of traumatic brain injury and with lower values of the Glasgow Scale [31]. At the same time, in prospective studies, it was shown that GFAP itself (as well as its breakdown products in the plasma) can separate the non-injured (control group) from the patients with mild traumatic injury and GCS 15, showing a good correlation with the imaging findings of computed tomography [32,33]. Finally, in a prospective study of patients with severe traumatic brain injury, elevated serum GFAP levels on admission showed a gradual decline until cessation of therapeutic hypothermia with slow rewarming [34]. GFAP levels were significantly higher in patients who died or had an unfavorable outcome [34]. Therefore, assessment of GFAP levels can be used to monitor the effectiveness of various brain injury treatments.
Similarly to strokes, the increase in GFAP in ischemic stroke is slow and progressive with peak values between days 2 and 4 in contrast to the faster increase in hemorrhagic stroke where its levels are significantly higher [35]. The above feature makes GFAP a potential differential diagnosis biomarker between hemorrhagic and ischemic stroke [36].
3.2. Neuroaxonal Markers
3.2.1. Neurofilaments—NF
Neurofibrils are structural protein components of the cytoskeleton of neurons and include light-, medium- and heavy-chain neurofibrils (NF-L, NF-M, NF-H, respectively) as well as α-internexin [37]. The function of neurofibrils consists in the structural support, maintenance and growth of axons while α-internexin appears to be additionally related to axon maturation and CNS embryogenesis [38].
Brain injuries disrupt the ionic balance of the axon, causing a massive influx of calcium which activates calcium-dependent proteases and other enzymes and leads to the proteolytic breakdown of neurofibrils and their release into the cerebrospinal fluid and blood [39]. Thus, patients with traumatic brain injury show increased levels of neurofibrils. Furthermore, elevated NF-H levels have shown a negative correlation with the Glasgow Scale (higher NF-H values were associated with lower GCS values) and a positive correlation with the extent of brain damage on CT, 3-month mortality and poor prognosis of traumatic spinal cord injury after 1 year [40,41].
Additionally, with regard to stroke, NF-H and NF-L levels were found to be elevated in patients with subarachnoid hemorrhage while serum levels of NF-L after ischemic stroke remained elevated after a 3-month period [42]. Even in a prospective study of Chinese patients with ischemic stroke [43], NF-L concentrations were significantly increased in patients with progressively increasing stroke severity and functional impairment (from mild to moderate to severe damage), while for each increase in NF-L by 1 quartile, the risk of poor outcome increased by 236% and 102%. NF-L is thus emerging as an important biomarker for monitoring the severity and functional outcome of ischemic stroke.
3.2.2. Proenkephalin A (PENK-A)
Proenkephalin A is a precursor substance of enkephalins which belong to the endogenous opioids with main actions as neurotransmitters in pain sensation pathways [5]. A prospective study with 189 patients (2012) with ischemic stroke showed that the levels of PENK-A were significantly increased in those with stroke compared to transient ischemic attack, and furthermore, its levels were correlated with the severity of the stroke with infarct extent on computed tomography as well as with three-month outcome in terms of mortality and recurrence [44]. On the other hand, a more recent prospective study (2020) with 320 patients with stroke reported that PENK-A was not associated with the functional outcome of the patients or with mortality within three months [45]. Therefore, more studies are needed to determine the role of PENK-A as a prognostic marker in CVD.
3.3. Neuronic Biomarkers
3.3.1. NSE
The NSE molecule (neuron-specific enolase) is a glycolytic enzyme of the cytoplasm of nerve and neuroendocrine cells. Upon damage and destruction of these cells, NSE is released into the extracellular environment, and through its measurement, neural damage can be indirectly evaluated [46]. Its increase in serum is observed in just 12 h after ABI, with its levels gradually returning to normal over a period of hours or even days [1]. Its correlation with the severity of the damage is very strong: in particular, in moderate and severe ABI, prolonged high levels of the enzyme have been found, while a second increase in the amount in the serum is an indication of a severe outcome [47,48]. A clear increase in NSE levels is therefore observed in hypoxic episodes [49], as well as in cases of brain death [50]. The above findings are also consistent with the results of traditional methods, which strengthens the reliability of the biomarker [48]. It is worth noting that a significant drop in its levels is observed after treatment with memantine, along with an improvement in the GCS scale [51]. Its greatest utility concerns the prediction of brain death [52]. Important disadvantages of NSE are its minimal plasma levels, high rates of protein binding and rapid metabolism [53], characteristics that make it difficult to detect [54]. Also, a negative element is its high concentrations in erythrocytes, but also in various cancer cells, and the consequent increase in serum in cases of hemolysis or neuroblastoma [47].
3.3.2. UCHL1
The UCHL1 molecule (Ubiquitin C-Terminal Hydrolase-L1) is located in the cytoplasm of neurons and participates in ubiquitin-mediated cellular regulation [55,56]. Ubiquitin is a protein molecule whose main function is the degradation of misfolded and dysfunctional proteins [57], while it also plays a role in autophagy, transmembrane transport and DNA repair [58]. UCH-L1 has a deubiquitinase (DUB) role and indirectly regulates cellular processes by maintaining stable levels of free ubiquitin in the cell [59]. Its direct action in reversing ubiquitination of molecules is debated [60]. In the brain, it constitutes up to 5% of the total proteins [61], while it is found in significantly smaller amounts in the gonads [59] and in some tumors [62]. The high concentration of UCH-L1 in the brain makes it a useful biomarker in cases of acute brain injury [63]. In ABI, damaged neurons release their cytoplasmic components, including UCHL1, into the interstitial fluid, and then into the CSF [64,65]. UCHL1 enters the circulation via the exposed BBB, usually within 24 h [66]. It provides information about the severity of the damage [67], as in moderate and severe OET, a large increase in serum occurs due to the extensive destruction of AEF [68,69,70]. Also, the rapid increase (within 6 h) in serum and CSF demonstrates a survival expectancy of up to 3 months [66]. Levels of UCH-L1 (as well as GFAP) are a strong predictor of lesion outcome [63]. Conventional methods of ABI diagnosis (GCS, CT) usually confirm the findings [71,72]. Its indications are limited for long-term lesions (>6 months) [67]. Also, it is an unstable marker, as its levels fluctuate according to the integrity of the BBB [73,74,75,76] and has only an indirect correlation with pathophysiology [2]. Finally, it is not specific for the CNS, since it is also expressed in the PNS, endocrine, smooth muscle and some cancer cells [77,78].
3.4. Extracellular Vesicles
Extracellular vesicles (EVs) are membranous bodies, which, moving through body fluids, contribute to intercellular communication, especially in brain cells [79]. They consist of an outer lipid bilayer, which encloses and protects marker molecules, such as proteins, nucleic acids or metabolites. These markers are specific and characterize the cell from which each vesicle was created [1]. They are also detected in healthy people, but in ABI, an increase is observed, both in their concentration in fluids and in their load in indicators [71]. Their isolation becomes a simpler process compared to the rest of the biomarkers. It can be carried out either from body fluids (serum, CSF, saliva) or more easily from cell cultures [80,81].
Extracellular vesicles generally have a protective role in the progression of brain damage, and their function is largely determined by their cells of origin. Thus, oligodendrocytes produce EVs with antioxidant enzymes inside them [82], while microglial EVs contribute to neurogenesis and have anti-inflammatory and anti-autophagic activity, guided by the miR-124 molecule inside them [2].
Their main characteristic is the ability to penetrate the BBB intact [83,84], providing the possibility for non-invasive assessment of brain damage [2]. The combination of markers that each vesicle contains reveals the cell of origin and offers a prism for understanding the pathology, but also for predicting the course of the damage. The combined assessment of multiple vesicle species contributes to the identification of the various endophenotypes of ABI [2].
A correlation has also been established between specific EVs and the triggering of the PERK signaling pathway, which appears to be involved in the unfolded protein response (UPR). This response occurs when the body detects high levels of misfolded proteins (as in ABI) [85]. It consists in the degradation of these proteins and the generalized reduction of translation levels [86], and it seems that EVs contribute to the initiation of this response [1].
These perspectives are subject to certain limitations. To date, there are several gaps in knowledge regarding endophenotypes, packaging of markers into EVs, their release and their association with ABI sequelae such as epilepsy and sleep and mood disorders [2].
There are three main classes of extracellular vesicles: apoptotic bodies, microvesicles and exosomes [87]. The latter have the most diagnostic perspectives and will be analyzed more extensively (Figure 2).
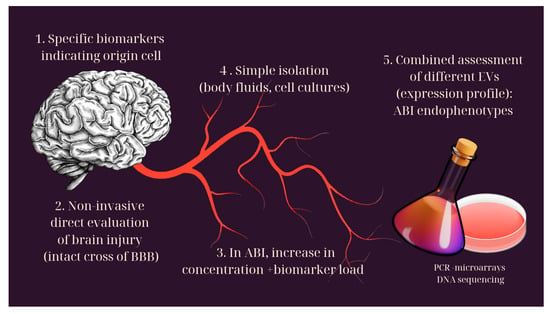
Figure 2.
Extracellular vesicles and their role in the diagnosis of brain injury.
3.4.1. Apoptotic Bodies
Apoptotic bodies have the largest size (50–5000 nm) and are the only EVs that require the death of the cell of origin for their formation. During apoptosis, high hydrostatic pressure results in the detachment of membranes from the cytoskeleton. The result of this process is apoptotic bodies, which contain DNA of the dead cell [88,89].
3.4.2. Microvesicles
Microvesicles or microparticles (MVs/MPs) are medium in size (100–1000 nm). They are generated by cell membrane protrusions of the cells of origin [88], which are mediated by enzymes and calcium [90]. They contain lipid microdomains, composed of cholesterol, phospholipids and various receptors [91]. In severe ABI, there is an increase in microvesicles in body fluids, which contain neural cell antigens [92,93,94]. However, the increase in their levels could also be the result of cerebral vascular damage, originating from leukocytes, platelets or endothelial cells [92,95]. From experiments on mice, the participation of microvesicles in neuroinflammatory processes, in the activation of microglial cells and also in immune responses was apparent [96].
3.4.3. Exosomes
Exosomes are small (30–120 nm) cup-shaped intracellular vesicles, created by the intraluminal projections of the cell membrane. The so-called intracellular polycystic bodies that result, if they remain intracellular, are called endosomes, while after their exocytosis, they turn into exosomes [97,98]. Their production probably contributes to the elimination of unnecessary cellular products [99,100]. The evaluation of their content is of great diagnostic value, as their load of biomarkers demonstrates the pathological “identity” of the lesion [101]. The different species of RNA (m-/ncRNA) provide information about the cell of origin of the exosome [102]. They also probably indicate failure in the attempt to treat the brain injury [1]. The protein load increases five times in ABI cases [71]. Increased GFAP/NFL levels are seen in diffuse lesions (specifically NFL in recurrent mild ABI events) [102]. A UCHL1/occludin combination is detected in acute lesions [2], while UCHL1, in particular, is associated with reduced survival [102]. The presence of tau/amyloid signals chronic or recurrent lesions [103,104] and potential for neurodegeneration and neuropsychiatric symptomatology [103,105,106]. IL10 is associated with behavioral disorders [2], while ASC, NLRP1, caspases and complement are associated with neuroinflammation [107]. The above findings become detectable thanks to PCR, microarray and sequencing techniques [108].
3.4.4. miRNAs
Another form of extracellular biomarkers with diagnostic potential are miRNAs, non-coding RNA molecules involved in the post-transcriptional regulation of gene expression. They seem to have an active role in neuroplasticity, in the birth of neural networks and in brain development in general [109,110,111]. Dysregulation of miRNAs levels has been observed in many pathological conditions, such as ischemic and neurodegenerative conditions, as well as in cases of ABI. This dysregulation was found to lead to impaired cognition, memory and learning [112], while the severity of the disorders was related to the expression profile of miRNAs.
These molecules can be detected in body fluids, with the help of microarrays. In cases of ABI, an increase in miR-765 (within 24 h), miR-502 and miR-425-5p has been found [113]. As an example of an miRNA profile regarding a specific condition, in saliva from children with post-concussive symptoms, an increase in let-7a-3p, miR-133a-5p, miR-320c-1 and miR-769-5p was observed [114]. Moreover, an increase in miR-629, let-7b-5p and miR-320c-1 was associated with symptoms of headache, fatigue and memory disorders after ABI [115].
Some miRNA molecules provide more specific information. miR-320c appears to play a role in neuroplasticity, and its detection in CSF provides a time frame for ABI symptoms [114,116,117]. miR-92a shows an acute increase in serum, while in severe ABI, it is also detected in CSF. Its characteristic increase was found in ischemic episodes [116,118,119,120]. Finally, miR-30 is increased in severe ABI in serum [within hours–days], CSF [within weeks], and saliva [112,113,116,118]. An anti-inflammatory action, as well as its contribution to maintaining the integrity of the BBB, is likely [121,122,123].
It should be pointed out, nevertheless, that the great heterogeneity of miRNA expression in the population is an important limitation of using such profiles in the diagnosis of ABI.
In addition to their diagnostic value, EVs can, through their effect on target cells, also contribute therapeutically to nerve tissue regeneration and recovery [124,125]. This effect is achieved through connection with receptors, fusion with the cell membrane and their endocytosis. A precursor of EV treatment is the use of stem cells [40]. The reported rejection of these cells by the body and associated tumorigenesis limits their use [126,127]. The discovery of EVs embodies all the benefits of the above treatment, combined with the positives of an acellular method [128]. Mesenchymal stem cells (MSCs), cells with obvious regenerative properties and the ability to grow outside the body (ex vivo), were chosen as EV export cells [129,130,131]. These EVs stimulate the expression of antiapoptotic cells (BCL2) [132], anti-inflammatory (CD206, arginase1) and neurotrophic (NGF) factors [133]. They also inhibit pro-apoptotic (BAX) and pro-inflammatory (TNFa, IL1b, iNOS) factors [132]. The end result is the limitation of inflammation and gliosis [132,134,135,136], neural death, cognitive, motor and behavioral disorders, but also the promotion of neuroplasticity [131,137].
In addition to their purely therapeutic effect, EVs can also be used as means of transport to the CNS, exploiting their ability to pass through the AEF [138]. In this way, CNS concentrations of neurotrophic factors such as BDNF [139], as well as anti-inflammatory/antioxidants such as CEP [2], can be increased.
Limitations to these techniques are again the heterogeneity and complexity of the mechanisms governing ABI, and the discrepancy between animal and human specimens [2].
Lastly, it should be pointed out that the action of the EV is not always beneficial for the organization. It has been found, for example, in glioblastoma that tumor cell proliferation and remodeling of the tumor vascular microenvironment are partially mediated by EVs [140]. It has also been established that they have a pathological component in neuroinflammation, as well as in the production of pathological proteins such as β-amyloid, with subsequent neurodegeneration [2]. Their contribution is direct, both in the pathology and in the recovery of the organism.
4. Conclusions
The importance of neuro-biomarkers in diagnosis, prognosis and therapeutic decision making as well as some critical processes for the course of acute brain damage have been clearly demonstrated. Although there are individual biomarkers with significant diagnostic and prognostic value, their value has been shown to multiply when used in panel-based combinations [24]. The prospect of a therapeutic use of certain molecules has also been emphasized. Since cases of acute brain injury constitute a large proportion of hospitalizations in intensive care units, any progress in the diagnostic and therapeutic approach will have a clear positive impact on this special category of patients as well.
However, the use of biomarkers is governed by several limitations: they require sensitive and often unavailable analytical tools. Furthermore, the inability for some of them to pass through an intact BBB is a major problem., Also, their levels may be affected by CSF flow disturbances in a brain injury [1]. Finally, the great heterogeneity in the population makes it difficult to use biomarkers, even in combinations, to highlight damage and predict the recovery process in each patient [2].
The diagnostic value of modern biomarkers is questionable, so it is yet unclear if they could replace traditional methods. Therefore, at present, their use remains experimental and adjunctive [2]. Additional studies are needed so that they can be integrated into the multifaceted treatment and monitoring of patients in the intensive care unit environment.
Author Contributions
Conceptualization, K.B. and N.L.; writing—original draft preparation, K.B., N.L.; writing—review and editing, A.A. and B.F.; supervision, A.A. and B.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ghaith, H.S.; Nawar, A.A.; Gabra, M.D.; Abdelrahman, M.E.; Nafady, M.H.; Bahbah, E.I.; Ebada, M.A.; Ashraf, G.M.; Negida, A.; Barreto, G.E. A Literature Review of Traumatic Brain Injury Biomarkers. Mol. Neurobiol. 2022, 59, 4141–4158. [Google Scholar] [CrossRef] [PubMed]
- Beard, K.; Meaney, D.F.; Issadore, D. Clinical Applications of Extracellular Vesicles in the Diagnosis and Treatment of Traumatic Brain Injury. J. Neurotrauma 2020, 37, 2045–2056. [Google Scholar] [CrossRef] [PubMed]
- Risitano, A.; Toni, D. Time is brain: Timing of revascularization of brain arteries in stroke. Eur. Heart J. Suppl. 2020, 22, L155–L159. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, K.L.H.; Czosnyka, M.; Jalloh, I.; Newcombe, V.F.J.; Helmy, A.; Shannon, R.J.; Budohoski, K.P.; Kolias, A.G.; Kirkpatrick, P.J.; Carpenter, T.A.; et al. Systemic, local, and imaging biomarkers of brain injury: More needed, and better use of those already established? Front. Neurol. 2015, 6, 26. [Google Scholar] [CrossRef]
- Mrozek, S.; Dumurgier, J.; Citerio, G.; Mebazaa, A.; Geeraerts, T. Biomarkers and acute brain injuries: Interest and limits. Crit. Care 2014, 18, 220. [Google Scholar] [CrossRef]
- Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 2001, 69, 89–95. [Google Scholar] [CrossRef]
- Chmielewska, N.; Szyndler, J.; Makowska, K.; Wojtyna, D.; Maciejak, P.; Płaźnik, A. Looking for novel, brain-derived, peripheral biomarkers of neurological disorders. Neurol. Neurochir. Pol. 2018, 52, 318–325. [Google Scholar] [CrossRef]
- Wang, K.K.; Yang, Z.; Zhu, T.; Shi, Y.; Rubenstein, R.; Tyndall, J.A.; Manley, G.T. An update on diagnostic and prognostic biomarkers for traumatic brain injury. Expert. Rev. Mol. Diagn. 2018, 18, 165–180. [Google Scholar] [CrossRef]
- E. National Academies of Sciences, H. and M. Division, B. on H. C. Services, and C. on the R. of the D. of V. A. E. for T. B. Injury. Definitions of Traumatic Brain Injury. in Evaluation of the Disability Determination Process for Traumatic Brain Injury in Veterans, National Academies Press (US). 2019. Available online: https://www.ncbi.nlm.nih.gov/books/NBK542588/ (accessed on 8 September 2024).
- Mckee, A.C.; Daneshvar, D.H. The neuropathology of traumatic brain injury. Handb. Clin. Neurol. 2015, 127, 45–66. [Google Scholar] [CrossRef]
- Capizzi, A.; Woo, J.; Verduzco-Gutierrez, M. Traumatic Brain Injury: An Overview of Epidemiology, Pathophysiology, and Medical Management. Med. Clin. North. Am. 2020, 104, 213–238. [Google Scholar] [CrossRef]
- Dixon, K.J. Pathophysiology of Traumatic Brain Injury. Phys. Med. Rehabil. Clin. N. Am. 2017, 28, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Feske, S.K. Ischemic Stroke. Am. J. Med. 2021, 134, 1457–1464. [Google Scholar] [CrossRef] [PubMed]
- Kuriakose, D.; Xiao, Z. Pathophysiology and Treatment of Stroke: Present Status and Future Perspectives. Int. J. Mol. Sci. 2020, 21, 7609. [Google Scholar] [CrossRef] [PubMed]
- TOAST Classification of Stroke|STROKE MANUAL. Available online: https://www.stroke-manual.com/toast-stroke-classification/ (accessed on 8 September 2024).
- Schöberl, F.; Ringleb, P.A.; Wakili, R.; Poli, S.; Wollenweber, F.A.; Kellert, L. Juvenile Stroke. Dtsch. Arztebl. Int. 2017, 114, 527–534. [Google Scholar] [CrossRef]
- Michetti, F.; Clementi, M.E.; Di Liddo, R.; Valeriani, F.; Ria, F.; Rende, M.; Di Sante, G.; Spica, V.R. The S100B Protein: A Multifaceted Pathogenic Factor More Than a Biomarker. Int. J. Mol. Sci. 2023, 24, 9605. [Google Scholar] [CrossRef]
- Donato, R.; R cannon, B.; Sorci, G.; Riuzzi, F.; Hsu, K.; JWeber, D.; LGeczy, C. Functions of S100 proteins. Curr. Mol. Med. 2013, 13, 24–57. [Google Scholar] [CrossRef]
- Thelin, E.P.; Nelson, D.W.; Bellander, B.-M. A review of the clinical utility of serum S100B protein levels in the assessment of traumatic brain injury. Acta Neurochir 2017, 159, 209–225. [Google Scholar] [CrossRef]
- Zongo, D.; Ribéreau-Gayon, R.; Masson, F.; Laborey, M.; Contrand, B.; Salmi, L.R.; Montaudon, D.; Beaudeux, J.L.; Meurin, A.; Dousset, V.; et al. S100-B protein as a screening tool for the early assessment of minor head injury. Ann. Emerg. Med. 2012, 59, 209–218. [Google Scholar] [CrossRef]
- Pfortmueller, C.A.; Drexel, C.; Krähenmann-Müller, S.; Leichtle, A.B.; Fiedler, G.M.; Lindner, G.; Exadaktylos, A.K. S-100 B Concentrations Are a Predictor of Decreased Survival in Patients with Major Trauma, Independently of Head Injury. PLoS ONE 2016, 11, e0152822. [Google Scholar] [CrossRef]
- Undén, J.; Ingebrigtsen, T.; Romner, B.; Scandinavian Neurotrauma Committee (SNC). Scandinavian guidelines for initial management of minimal, mild and moderate head injuries in adults: An evidence and consensus-based update. BMC Med. 2013, 11, 50. [Google Scholar] [CrossRef]
- Ananthaharan, A.; Kravdal, G.; Straume-Naesheim, T.M. Utility and effectiveness of the Scandinavian guidelines to exclude computerized tomography scanning in mild traumatic brain injury—A prospective cohort study. BMC Emerg. Med. 2018, 18, 44. [Google Scholar] [CrossRef] [PubMed]
- di Biase, L.; Bonura, A.; Pecoraro, P.M.; Carbone, S.P.; Di Lazzaro, V. Unlocking the Potential of Stroke Blood Biomarkers: Early Diagnosis, Ischemic vs. Haemorrhagic Differentiation and Haemorrhagic Transformation Risk: A Comprehensive Review. Int. J. Mol. Sci. 2023, 24, 11545. [Google Scholar] [CrossRef] [PubMed]
- González-García, S.; González-Quevedo, A.; Peña-Sánchez, M.; Menéndez-Saínz, C.; Fernández-Carriera, R.; Arteche-Prior, M.; Pando-Cabrera, A.; Fernández-Concepción, O. Serum neuron-specific enolase and S100 calcium binding protein B biomarker levels do not improve diagnosis of acute stroke. J. R. Coll. Physicians Edinb. 2012, 42, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Kazmierski, R.; Michalak, S.; Wencel-Warot, A.; Nowinski, W.L. Serum tight-junction proteins predict hemorrhagic transformation in ischemic stroke patients. Neurology 2012, 79, 1677–1685. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, O.; Wardlaw, J.; Whiteley, W.N. Correlation of levels of neuronal and glial markers with radiological measures of infarct volume in ischaemic stroke: A systematic review. Cerebrovasc. Dis. 2012, 33, 47–54. [Google Scholar] [CrossRef]
- Jauch, E.C.; Lindsell, C.; Broderick, J.; Fagan, S.C.; Tilley, B.C.; Levine, S.R. Association of serial biochemical markers with acute ischemic stroke: The National Institute of Neurological Disorders and Stroke recombinant tissue plasminogen activator Stroke Study. Stroke 2006, 37, 2508–2513. [Google Scholar] [CrossRef]
- Barmpagiannos, K.; Theotokis, P.; Petratos, S.; Pagnin, M.; Einstein, O.; Kesidou, E.; Boziki, M.; Artemiadis, A.; Bakirtzis, C.; Grigoriadis, N. The Diversity of Astrocyte Activation during Multiple Sclerosis: Potential Cellular Targets for Novel Disease Modifying Therapeutics. Healthcare 2023, 11, 1585. [Google Scholar] [CrossRef]
- Escartin, C.; Galea, E.; Lakatos, A.; O’callaghan, J.P.; Petzold, G.C.; Serrano-Pozo, A.; Steinhäuser, C.; Volterra, A.; Carmignoto, G.; Agarwal, A.; et al. Reactive astrocyte nomenclature, definitions, and future directions. Nat. Neurosci. 2021, 24, 312–325. [Google Scholar] [CrossRef]
- Mondello, S.; Papa, L.; Buki, A.; Bullock, M.R.; Czeiter, E.; Tortella, F.C.; Wang, K.K.; Hayes, R.L. Neuronal and glial markers are differently associated with computed tomography findings and outcome in patients with severe traumatic brain injury: A case control study. Crit. Care 2011, 15, R156. [Google Scholar] [CrossRef]
- Gill, J.; Latour, L.; Diaz-Arrastia, R.; Motamedi, V.; Turtzo, C.; Shahim, P.; Mondello, S.; DeVoto, C.; Veras, E.; Hanlon, D.; et al. Glial fibrillary acidic protein elevations relate to neuroimaging abnormalities after mild TBI. Neurology 2018, 91, e1385–e1389. [Google Scholar] [CrossRef]
- Papa, L.; Lewis, L.M.; Falk, J.L.; Zhang, Z.; Silvestri, S.; Giordano, P.; Brophy, G.M.; Demery, J.A.; Dixit, N.K.; Ferguson, I.; et al. Elevated levels of serum glial fibrillary acidic protein breakdown products in mild and moderate traumatic brain injury are associated with intracranial lesions and neurosurgical intervention. Ann. Emerg. Med. 2012, 59, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Gao, G.; Feng, J.; Jin, Y.; Wang, C.; Mao, Q.; Jiang, J. Glial fibrillary acidic protein as a biomarker in severe traumatic brain injury patients: A prospective cohort study. Crit. Care 2015, 19, 362. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, R.; Warrier, A.R.; Sreenivas, V.; Bali, P.; Sisodia, P.; Gupta, A.; Singh, N.; Srivastava, M.P.; Prasad, K. Role of Blood Biomarkers in Differentiating Ischemic Stroke and Intracerebral Hemorrhage. Neurol. India 2020, 68, 824–829. [Google Scholar] [CrossRef] [PubMed]
- Foerch, C.; Curdt, I.; Yan, B.; Dvorak, F.; Hermans, M.; Berkefeld, J.; Raabe, A.; Neumann-Haefelin, T.; Steinmetz, H.; Sitzer, M. Serum glial fibrillary acidic protein as a biomarker for intracerebral haemorrhage in patients with acute stroke. J. Neurol. Neurosurg. Psychiatry 2006, 77, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Lambertsen, K.L.; Soares, C.B.; Gaist, D.; Nielsen, H.H. Neurofilaments: The C-Reactive Protein of Neurology. Brain Sci. 2020, 10, 56. [Google Scholar] [CrossRef]
- Mak, G.; Menon, S.; Lu, J.-Q. Neurofilaments in neurologic disorders and beyond. J. Neurol. Sci. 2022, 441, 120380. [Google Scholar] [CrossRef]
- Yuan, A.; Rao, M.V.; Nixon, R.A. Neurofilaments and Neurofilament Proteins in Health and Disease. Cold Spring Harb. Perspect. Biol. 2017, 9, a018309. [Google Scholar] [CrossRef]
- Shahim, P.; Gren, M.; Liman, V.; Andreasson, U.; Norgren, N.; Tegner, Y.; Mattsson, N.; Andreasen, N.; Öst, M.; Zetterberg, H.; et al. Serum neurofilament light protein predicts clinical outcome in traumatic brain injury. Sci. Rep. 2016, 6, 36791. [Google Scholar] [CrossRef]
- Stukas, S.; Cooper, J.; Gill, J.; Fallah, N.; Skinnider, M.A.; Belanger, L.; Ritchie, L.; Tsang, A.; Dong, K.; Streijger, F.; et al. Association of CSF and Serum Neurofilament Light and Glial Fibrillary Acidic Protein, Injury Severity, and Outcome in Spinal Cord Injury. Neurology 2023, 100, e1221–e1233. [Google Scholar] [CrossRef]
- Wang, P.; Fan, J.; Yuan, L.; Nan, Y.; Nan, S. Serum Neurofilament Light Predicts Severity and Prognosis in Patients with Ischemic Stroke. Neurotox. Res. 2020, 37, 987–995. [Google Scholar] [CrossRef]
- Doehner, W.; von Haehling, S.; Suhr, J.; Ebner, N.; Schuster, A.; Nagel, E.; Melms, A.; Wurster, T.; Stellos, K.; Gawaz, M.; et al. Elevated plasma levels of neuropeptide proenkephalin a predict mortality and functional outcome in ischemic stroke. J. Am. Coll. Cardiol. 2012, 60, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Gruber, P.; Fluri, F.; Schweizer, J.; Luft, A.; Müller, B.; Christ-Crain, M.; Katan, M. Proenkephalin A Adds No Incremental Prognostic Value After Acute Ischemic Stroke. Clin. Appl. Thromb. Hemost. 2020, 26, 1076029619895318. [Google Scholar] [CrossRef] [PubMed]
- Traenka, C.; Disanto, G.; Seiffge, D.J.; Gensicke, H.; Hert, L.; Grond-Ginsbach, C.; Peters, N.; Regeniter, A.; Kloss, M.; De Marchis, G.M.; et al. Serum Neurofilament Light Chain Levels Are Associated with Clinical Characteristics and Outcome in Patients with Cervical Artery Dissection. Cerebrovasc. Dis. 2015, 40, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Mozaffari, K.; Dejam, D.; Duong, C.; Ding, K.; French, A.; Ng, E.; Preet, K.; Franks, A.; Kwan, I.; Phillips, H.W.; et al. Systematic Review of Serum Biomarkers in Traumatic Brain Injury. Cureus 2021, 13, e17056. [Google Scholar] [CrossRef] [PubMed]
- Bishop, P.; Rocca, D.; Henley, J.M. Ubiquitin C-terminal hydrolase L1 (UCH-L1): Structure, distribution and roles in brain function and dysfunction. Biochem. J. 2016, 473, 2453–2462. [Google Scholar] [CrossRef]
- Reed, S.L.; Escayg, A. Extracellular vesicles in the treatment of neurological disorders. Neurobiol. Dis. 2021, 157, 105445. [Google Scholar] [CrossRef]
- Azar, S.; Hasan, A.; Younes, R.; Najdi, F.; Baki, L.; Ghazale, H.; Kobeissy, F.H.; Zibara, K.; Mondello, S. Biofuid proteomics and biomarkers in traumatic brain injury. Methods Mol. Biol. 2017, 1598, 45–63. [Google Scholar] [CrossRef]
- Lorente, L. Biomarkers associated with the outcome of traumatic brain injury patients. Brain Sci. 2017, 7, 142. [Google Scholar] [CrossRef]
- Cheng, F.; Yuan, Q.; Yang, J.; Wang, W.; Liu, H. The prognostic value of serum neuron-specifc enolase in traumatic brain injury: Systematic review and meta-analysis. PLoS ONE 2014, 9, e106680. [Google Scholar] [CrossRef]
- Mercier, E.; Boutin, A.; Shemilt, M.; Lauzier, F.; Zarychanski, R.; Fergusson, D.A.; Moore, L.; McIntyre, L.A.; Archambault, P.; Légaré, F.; et al. Predictive value of neuron-specifc enolase for prognosis in patients with moderate or severe traumatic brain injury: A systematic review and meta-analysis. CMAJ Open 2016, 4, E371–E382. [Google Scholar] [CrossRef]
- Gong, B.; Leznik, E. The role of ubiquitin C-terminal hydrolase L1 in neurodegenerative disorders. Drug News Perspect. 2007, 20, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Mondello, S.; Linnet, A.; Buki, A.; Robicsek, S.; Gabrielli, A.; Tepas, J.; Papa, L.; Brophy, G.M.; Tortella, F.; Hayes, R.L.; et al. Clinical utility of serum levels of ubiquitin C-terminal hydrolase as a biomarker for severe traumatic brain injury. Neurosurgery 2012, 70, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yu, C.; Sun, Y.; Li, Y. Serum ubiquitin C-terminal hydrolase L1 as a biomarker for traumatic brain injury: A systematic review and meta-analysis. Am. J. Emerg. Med. 2015, 33, 1191–1196. [Google Scholar] [CrossRef] [PubMed]
- Mondello, S.; Thelin, E.P.; Shaw, G.; Salzet, M.; Visalli, C.; Cizkova, D.; Kobeissy, F.; Buki, A. Extracellular vesicles: Pathogenetic, diagnostic and therapeutic value in traumatic brain injury. Expert. Rev. Proteomics 2018, 15, 451–461. [Google Scholar] [CrossRef]
- Liu, M.C.; Akinyi, L.; Scharf, D.; Mo, J.; Larner, S.F.; Muller, U.; Oli, M.W.; Zheng, W.; Kobeissy, F.; Papa, L.; et al. Ubiquitin C-terminal hydrolase-L1 as a biomarker for ischemic and traumatic brain injury in rats. Eur. J. Neurosci. 2010, 31, 722–732. [Google Scholar] [CrossRef]
- Papa, L.; Akinyi, L.; Liu, M.C.; Pineda, J.A.; Tepas, J.J., III; Oli, M.W.; Zheng, W.; Robinson, G.; Robicsek, S.A.; Gabrielli, A.; et al. Ubiquitin C-terminal hydrolase is a novel biomarker in humans for severe traumatic brain injury. Crit. Care Med. 2010, 38, 138–144. [Google Scholar] [CrossRef]
- Mondello, S.; Muller, U.; Jeromin, A.; Streeter, J.; Hayes, R.L.; Wang, K.K. Blood-based diagnostics of traumatic brain injuries. Expert. Rev. Mol. Diagn. 2011, 11, 65–78. [Google Scholar] [CrossRef]
- Karttunen, J.; Heiskanen, M.; Lipponen, A.; Poulsen, D.; Pitkanen, A. Extracellular vesicles as diagnostics and therapeutics for structural epilepsies. Int. J. Mol. Sci. 2019, 20, 1259. [Google Scholar] [CrossRef]
- Borges, F.T.; Reis, L.A.; Schor, N. Extracellular vesicles: Structure, function, and potential clinical uses in renal diseases. Braz. J. Med. Biol. Res. 2013, 46, 824–830. [Google Scholar] [CrossRef]
- Wickman, G.; Julian, L.; Olson, M.F. Olson MF How apoptotic cells aid in the removal of their own cold dead bodies. Cell Death Difer. 2012, 19, 735–742. [Google Scholar] [CrossRef]
- Todorova, D.; Simoncini, S.; Lacroix, R.; Sabatier, F.; DignatGeorge, F. Extracellular vesicles in angiogenesis. Circ. Res. 2017, 120, 1658–1673. [Google Scholar] [CrossRef] [PubMed]
- Yuana, Y.; Levels, J.; Grootemaat, A.; Sturk, A.; Nieuwland, R. Co-isolation of extracellular vesicles and high-density lipoproteins using density gradient ultracentrifugation. J. Extracell. Vesicles 2014, 3, 23262. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Ouyang, Y.; Wang, Z.; Zhang, R.; Huang, P.H.; Chen, C.; Li, H.; Li, P.; Quinn, D.; Dao, M.; et al. Isolation of exosomes from whole blood by integrating acoustics and microfuidics. Proc. Natl. Acad. Sci. USA 2017, 114, 10584–10589. [Google Scholar] [CrossRef] [PubMed]
- Frühbeis, C.; Fröhlich, D.; Kuo, W.P.; Amphornrat, J.; Thilemann, S.; Saab, A.S.; Kirchhoff, F.; Möbius, W.; Goebbels, S.; Nave, K.A.; et al. Neurotransmittertriggered transfer of exosomes mediates oligodendrocyte-neuron communication. PLoS Biol. 2013, 11, e1001604. [Google Scholar] [CrossRef]
- Scheper, W.; Hoozemans, J.J. The unfolded protein response in neurodegenerative diseases: A neuropathological perspective. Acta Neuropathol. 2015, 130, 315–331. [Google Scholar] [CrossRef]
- Brady, R.D.; Bird, S.; Sun, M.; Yamakawa, G.R.; Major, B.P.; Mychasiuk, R.; O’Brien, T.J.; McDonald, S.J.; Shultz, S.R. Activation of the protein kinase R-like endoplasmic reticulum kinase (PERK) pathway of the unfolded protein response after experimental traumatic brain injury and treatment with a PERK inhibitor. Neurotrauma Rep. 2021, 2, 330–342. [Google Scholar] [CrossRef]
- Ferreira, J.V.; Rosa Soares, A.; Ramalho, J.S.; Ribeiro-Rodrigues, T.; Máximo, C.; Zuzarte, M.; Girão, H.; Pereira, P. Exosomes and STUB1/CHIP cooperate to maintain intracellular proteostasis. PLoS ONE 2019, 14, e0223790. [Google Scholar] [CrossRef]
- Shi, M.; Sheng, L.; Stewart, T.; Zabetian, C.P.; Zhang, J. New windows into the brain: Central nervous system-derived extracellular vesicles in blood. Prog. Neurobiol. 2019, 175, 96–106. [Google Scholar] [CrossRef]
- Taylor, D.D.; Gercel-Taylor, C. Exosome platform for diagnosis and monitoring of traumatic brain injury. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2014, 369, 20130503. [Google Scholar] [CrossRef]
- Mondello, S.; Guedes, V.A.; Lai, C.; Czeiter, E.; Amrein, K.; Kobeissy, F.; Mechref, Y.; Jeromin, A.; Mithani, S.; Martin, C.; et al. Circulating brain injury exosomal proteins following moderate-to-severe traumatic brain injury: Temporal profle, outcome prediction and therapy implications. Cells 2020, 9, 977. [Google Scholar] [CrossRef]
- Kumar, A.; Stoica, B.A.; Loane, D.J.; Yang, M.; Abulwerdi, G.; Khan, N.; Kumar, A.; Thom, S.R.; Faden, A.I. Microglial-derived microparticles mediate neuroinfammation after traumatic brain injury. J. Neuroinfammation 2017, 14, 47. [Google Scholar] [CrossRef] [PubMed]
- Nekludov, M.; Bellander, B.M.; Gryth, D.; Wallen, H.; Mobarrez, F. Brain-derived microparticles in patients with severe isolated TBI. Brain Inj. 2017, 31, 1856–1862. [Google Scholar] [CrossRef] [PubMed]
- Nekludov, M.; Mobarrez, F.; Gryth, D.; Bellander, B.M.; Wallen, H. Formation of microparticles in the injured brain of patients with severe isolated traumatic brain injury. J. Neurotrauma 2014, 31, 1927–1933. [Google Scholar] [CrossRef] [PubMed]
- Andrews, A.M.; Lutton, E.M.; Merkel, S.F.; Razmpour, R.; Ramirez, S.H. Mechanical injury induces brain endothelial-derived microvesicle release: Implications for cerebral vascular injury during traumatic brain injury. Front. Cell Neurosci. 2016, 10, 43. [Google Scholar] [CrossRef]
- Morel, N.; Morel, O.; Petit, L.; Hugel, B.; Cochard, J.F.; Freyssinet, J.M.; Sztark, F.; Dabadie, P. Generation of procoagulant microparticles in cerebrospinal fuid and peripheral blood after traumatic brain injury. J. Trauma 2008, 64, 698–704. [Google Scholar] [CrossRef]
- Frühbeis, C.; Fröhlich, D.; Kuo, W.P.; Krämer-Albers, E.M. Extracellular vesicles as mediators of neuron-glia communication. Front. Cell Neurosci. 2013, 7, 182. [Google Scholar] [CrossRef]
- Yang, Y.; Boza-Serrano, A.; Dunning, C.J.; Clausen, B.H.; Lambertsen, K.L.; Deierborg, T. Inflammation leads to distinct populations of extracellular vesicles from microglia. J. Neuroinflammation 2018, 15, 168. [Google Scholar] [CrossRef]
- Chen, C.C.; Liu, L.; Ma, F.; Wong, C.W.; Guo, X.E.; Chacko, J.V.; Farhoodi, H.P.; Zhang, S.X.; Zimak, J.; Ségaliny, A.; et al. Elucidation of exosome migration across the blood-brain barrier model in vitro. Cell Mol. Bioeng. 2016, 9, 509–529. [Google Scholar] [CrossRef]
- Ni, H.; Yang, S.; Siaw-Debrah, F.; Hu, J.; Wu, K.; He, Z.; Yang, J.; Pan, S.; Lin, X.; Ye, H.; et al. Exosomes derived from bone mesenchymal stem cells ameliorate early inflammatory responses following traumatic brain injury. Front. Neurosci. 2019, 13, 14. [Google Scholar] [CrossRef]
- Yuan, D.; Zhao, Y.; Banks, W.A.; Bullock, K.M.; Haney, M.; Batrakova, E.; Kabanov, A.V. Macrophage exosomes as natural nanocarriers for protein delivery to inflamed brain. Biomaterials 2017, 142, 1–12. [Google Scholar] [CrossRef]
- Diaz-Arrastia, R.; Wang, K.K.; Papa, L.; Sorani, M.D.; Yue, J.K.; Puccio, A.M.; McMahon, P.J.; Inoue, T.; Yuh, E.L.; Lingsma, H.F.; et al. Acute biomarkers of traumatic brain injury: Relationship between plasma levels of ubiquitin C-terminal hydrolase-L1 and glial fibrillary acidic protein. J. Neurotrauma 2014, 31, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Okonkwo, D.O.; Yue, J.K.; Puccio, A.M.; Panczykowski, D.M.; Inoue, T.; McMahon, P.J.; Sorani, M.D.; Yuh, E.L.; Lingsma, H.F.; Maas, A.I.; et al. GFAP-BDP as an acute diagnostic marker in traumatic brain injury: Results from the prospective transforming research and clinical knowledge in traumatic brain injury study. J. Neurotrauma 2013, 30, 1490–1497. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, K.K. Glial fibrillary acidic protein: From intermediate filament assembly and gliosis to neurobiomarker. Trends Neurosci. 2015, 38, 364–374. [Google Scholar] [CrossRef]
- Chodobski, A.; Zink, B.J.; Szmydynger-Chodobska, J. Blood–brain barrier pathophysiology in traumatic brain injury. Transl. Stroke Res. 2011, 2, 492–516. [Google Scholar] [CrossRef] [PubMed]
- Burda, J.E.; Sofroniew, M.V. Reactive gliosis and the multicellular response to CNS damage and disease. Neuron 2014, 81, 229–248. [Google Scholar] [CrossRef]
- Vaz, R.; Sarmento, A.; Borges, N.; Cruz, C.; Azevedo, I. Ultrastructural study of brain microvessels in patients with traumatic cerebral contusions. Acta Neurochir. 1997, 139, 215–220. [Google Scholar] [CrossRef]
- Schwarzmaier, S.M.; Kim, S.W.; Trabold, R.; Plesnila, N. Temporal profile of thrombogenesis in the cerebral microcirculation after traumatic brain injury in mice. J. Neurotrauma 2010, 27, 121–130. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, C.; Fang, S.; Ou, R.; Li, W.; Xu, Y. UCH-LI acts as a novel prognostic biomarker in gastric cardiac adenocarcinoma. Int. J. Clin. Exp. Pathol. 2015, 8, 13957–13967. [Google Scholar]
- Bedekovics, T.; Hussain, S.; Feldman, A.L.; Galardy, P.J. UCH-L1 is induced in germinal center B-cells and identifies patients with aggressive germinal center diffuse large B-cell lymphoma. Blood 2015, 127, 1564–1574. [Google Scholar] [CrossRef]
- Zetterberg, H.; Smith, D.H.; Blennow, K. Biomarkers of mild traumatic brain injury in cerebrospinal fluid and blood. Nat. Rev. Neurol. 2013, 9, 201–210. [Google Scholar] [CrossRef]
- Dadas, A.; Washington, J.; Diaz-Arrastia, R.; Janigro, D. Biomarkers in traumatic brain injury (TBI): A review. Neuropsychiatr. Dis. Treat. 2018, 14, 2989–3000. [Google Scholar] [CrossRef] [PubMed]
- Del Conde, I.; Shrimpton, C.N.; Thiagarajan, P.; López, J.A. Tissue-factor-bearing microvesicles arise from lipid rafts and fuse with activated platelets to initiate coagulation. Blood 2005, 106, 1604–1611. [Google Scholar] [CrossRef] [PubMed]
- Denzer, K.; Kleijmeer, M.J.; Heijnen, H.F.; Stoorvogel, W.; Geuze, H.J. Exosome: From internal vesicle of the multivesicular body to intercellular signaling device. J. Cell Sci. 2000, 113 Pt 19, 3365–3374. [Google Scholar] [CrossRef]
- Van Niel, G.; Raposo, G.; Candalh, C.; Boussac, M.; Hershberg, R.; Cerf–Bensussan, N.; Heyman, M. Intestinal epithelial cells secrete exosome-like vesicles. Gastroenterology 2001, 121, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Gill, J.; Mustapic, M.; Diaz-Arrastia, R.; Lange, R.; Gulyani, S.; Diehl, T.; Motamedi, V.; Osier, N.; Stern, R.A.; Kapogiannis, D. Higher exosomal tau, amyloid-beta 42 and IL-10 are associated with mild TBIs and chronic symptoms in military personnel. Brain Inj. 2018, 32, 1359–1366. [Google Scholar] [CrossRef] [PubMed]
- Kenney, K.; Qu, B.-X.; Lai, C.; Devoto, C.; Motamedi, V.; Walker, W.C.; Levin, H.S.; Nolen, T.; Wilde, E.A.; Diaz-Arrastia, R.; et al. Higher exosomal phosphorylated tau and total tau among veterans with combat-related repetitive chronic mild traumatic brain injury. Brain Inj. 2018, 32, 1276–1284. [Google Scholar] [CrossRef]
- Goetzl, E.J.; Elahi, F.M.; Mustapic, M.; Kapogiannis, D.; Pryhoda, M.; Gilmore, A.; Gorgens, K.A.; Davidson, B.; Granholm, A.; Ledreux, A. Altered levels of plasma neuron-derived exosomes and their cargo proteins characterize acute and chronic mild traumatic brain injury. FASEB J. 2019, 33, 5082–5088. [Google Scholar] [CrossRef]
- Goetzl, E.J.; Mustapic, M.; Kapogiannis, D.; Eitan, E.; Lobach, I.V.; Goetzl, L.; Schwartz, J.B.; Miller, B.L. Cargo proteins of plasma astrocyte-derived exosomes in Alzheimer’s disease. FASEB J. 2016, 30, 3853–3859. [Google Scholar] [CrossRef]
- de RiveroVaccari, J.P.; Brand, F., 3rd; Adamczak, S.; Lee, S.W.; Perez-Barcena, J.; Wang, M.Y.; Bullock, M.R.; Dietrich, W.D.; Keane, R.W. Exosome-mediated inflammasome signaling 2054 BEARD ET AL. after central nervous system injury. J. Neurochem 2016, 136 (Suppl. 1), 39–48. [Google Scholar]
- Margulies, S.; Anderson, G.; Atif, F.; Badaut, J.; Clark, R.; Empey, P.; Guseva, M.; Hoane, M.; Huh, J.; Pauly, J.; et al. Combination therapies for traumatic brain injury: Retrospective considerations. J. Neurotrauma 2016, 33, 101–112. [Google Scholar] [CrossRef]
- Diaz-Arrastia, R.; Kochanek, P.M.; Bergold, P.; Kenney, K.; Marx, C.E.; Grimes, C.J.B.; Loh, L.Y.; Adam, L.G.E.; Oskvig, D.; Curley, K.C.; et al. Pharmacotherapy of traumatic brain injury: State of the science and the road forward: Report of the Department of Defense Neurotrauma Pharmacology Workgroup. J. Neurotrauma 2014, 31, 135–158. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, A.; Lu, D.; Chopp, M. Intravenous administration of marrow stromal cells (MSCs) increases the expression of growth factors in rat brain after traumatic brain injury. J. Neurotrauma 2004, 21, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Muller, W.A. Getting leukocytes to the site of inflammation. Vet. Pathol. 2013, 50, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Herberts, C.A.; Kwa, M.S.; Hermsen, H.P. Risk factors in the development of stem cell therapy. J. Transl. Med. 2011, 9, 29. [Google Scholar] [CrossRef] [PubMed]
- Volarevic, V.; Markovic, B.S.; Gazdic, M.; Volarevic, A.; Jovicic, N.; Arsenijevic, N.; Armstrong, L.; Djonov, V.; Lako, M.; Stojkovic, M. Ethical and Safety Issues of Stem Cell-Based Therapy. Int. J. Med. Sci. 2018, 15, 36–45. [Google Scholar] [CrossRef]
- Rani, S.; Ryan, A.E.; Griffin, M.D.; Ritter, T. Mesenchymal Stem Cell-derived Extracellular Vesicles: Toward Cell-free Therapeutic Applications. Mol. Ther. 2015, 23, 812–823. [Google Scholar] [CrossRef]
- Doeppner, T.R.; Herz, J.; Görgens, A.; Schlechter, J.; Ludwig, A.-K.; Radtke, S.; de Miroschedji, K.; Horn, P.A.; Giebel, B.; Hermann, D.M. Extracellular Vesicles Improve Post-Stroke Neuroregeneration and Prevent Postischemic Immunosuppression. Stem Cells Transl. Med. 2015, 4, 1131–1143. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Li, Y.; Cui, Y.; Yang, J.J.; Zhang, Z.G.; Chopp, M. Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J. Cereb. Blood Flow Metab. 2013, 33, 1711–1715. [Google Scholar] [PubMed]
- Zhang, Y.; Chopp, M.; Meng, Y.; Katakowski, M.; Xin, H.; Mahmood, A.; Xiong, Y. Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury. J. Neurosurg. 2015, 122, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Corps, K.N.; Roth, T.L.; McGavern, D.B. Inflammation and neuroprotection in traumatic brain injury. JAMA Neurol. 2015, 72, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Dashnaw, M.L.; Petraglia, A.L.; Bailes, J.E. An overview of the basic science of concussion and subconcussion: Where we are and where we are going. Neurosurg Focus. 2012, 33, E5–E9. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Nishida, H.; An, S.Y.; Shetty, A.K.; Bartosh, T.J.; Prockop, D.J. Chromatographically isolated CD63+CD81+ extracellular vesicles from mesenchymal stromal cells rescue cognitive impairments after TBAMELII. Proc. Natl. Acad. Sci. USA 2016, 113, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.K.; Passaro, A.P.; Latchoumane, C.-F.; Spellicy, S.E.; Bowler, M.; Goeden, M.; Martin, W.J.; Holmes, P.V.; Stice, S.L.; Karumbaiah, L. Extracellular Vesicles Mediate Neuroprotection and Functional Recovery after Traumatic Brain Injury. J. Neurotrauma. 2020, 37, 1358–1369. [Google Scholar] [CrossRef] [PubMed]
- Skog, J.; Würdinger, T.; Van Rijn, S.; Meijer, D.H.; Gainche, L.; Curry, W.T., Jr.; Carter, B.S.; Krichevsky, A.M.; Breakefield, X.O. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 2008, 10, 1470–1476. [Google Scholar] [CrossRef] [PubMed]
- Komander, D.; Rape, M. The ubiquitin code. Annu. Rev. Biochem. 2012, 81, 203–229. [Google Scholar] [CrossRef]
- Grabbe, C.; Husnjak, K.; Dikic, I. The spatial and temporal organization of ubiquitin networks. Nat. Rev. Mol. Cell Biol. 2011, 12, 295–307. [Google Scholar] [CrossRef]
- Day, I.N.; Thompson, R.J. UCHL1 (PGP 9.5): Neuronal biomarker and ubiquitin system protein. Prog. Neurobiol. 2010, 90, 327–362. [Google Scholar]
- Tezel, E.; Hibi, K.; Nagasaka, T.; Nakao, A. PGP9.5 as a prognostic factor in pancreatic cancer. Clin. Cancer Res. 2000, 6, 4764–4767. [Google Scholar]
- Takala, R.S.; Posti, J.P.; Runtti, H.; Newcombe, V.F.; Outtrim, J.; Katila, A.J.; Frantzen, J.; Ala-Seppala, H.; Kyllonen, A.; Maanpaa, H.R.; et al. GFAP and UCH-L1 as outcome predictors in traumatic brain injury. World Neurosurg. 2016, 87, 8–20. [Google Scholar] [CrossRef]
- Das, C.; Hoang, Q.Q.; Kreinbring, C.A.; Luchansky, S.J.; Meray, R.K.; Ray, S.S.; Lansbury, P.T.; Ringe, D.; Petsko, G.A. Structural basis for conformational plasticity of the Parkinson’s disease-associated ubiquitin hydrolase UCH-L1. Proc. Natl. Acad. Sci. USA 2006, 103, 4675–4680. [Google Scholar] [CrossRef]
- Böhmer, A.E.; Oses, J.P.; Schmidt, A.P.; Perón, C.S.; Krebs, C.L.; Oppitz, P.P.; D’Avila, T.T.; Souza, D.O.; Portela, L.V.; Stefani, M.A. Neuron-specific enolase, S100B, and glial fibrillary acidic protein levels as outcome predictors in patients with severe traumatic brain injury. Neurosurgery 2011, 68, 1624–1630. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, M.; Nayeb-Aghaei, H.; Kouchek, M.; Miri, M.M.; Goharani, R.; Amoozandeh, A.; Salamat, S.A.; Sistanizad, M. Effect of memantine on serum levels of neuron-specific enolase and on the Glasgow Coma Scale in patients with moderate traumatic brain injury. J. Clin. Pharmacol. 2018, 58, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Yan, E.B.; Satgunaseelan, L.; Paul, E.; Bye, N.; Nguyen, P.; Agyapomaa, D.; Kossmann, T.; Rosenfeld, J.V.; Morganti-Kossmann, M.C. Post-traumatic hypoxia is associated with prolonged cerebral cytokine production, higher serum biomarker levels, and poor outcome in patients with severe traumatic brain injury. J. Neurotrauma 2014, 31, 618–629. [Google Scholar] [CrossRef] [PubMed]
- Redell, J.B.; Moore, A.N.; Ward, N.H.; Hergenroeder, G.W.; Dash, P.K. Human traumatic brain injury alters plasma microRNA levels. J. Neurotrauma 2010, 27, 2147–2156. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, V.; Ragusa, M.; Davies, D.; Su, Z.; Hazeldine, J.; Lazzarino, G.; Hill, L.J.; Crombie, N.; Foster, M.; Purrello, M.; et al. MicroRNAs as Novel Biomarkers for the Diagnosis and Prognosis of Mild and Severe Traumatic Brain Injury. J. Neurotrauma. 2017, 34, 1948–1956. [Google Scholar] [CrossRef]
- Bhomia, M.; Balakathiresan, N.S.; Wang, K.K.; Papa, L.; Maheshwari, R.K. A Panel of Serum MiRNA Biomarkers for the Diagnosis of Severe to Mild Traumatic Brain Injury in Humans. Sci. Rep. 2016, 6, 28148. [Google Scholar] [CrossRef]
- Hicks, S.D.; Johnson, J.; Carney, M.C.; Bramley, H.; Olympia, R.P.; Loeffert, A.C.; Thomas, N.J. Overlapping MicroRNA Expression in Saliva and Cerebrospinal Fluid Accurately Identifies Pediatric Traumatic Brain Injury. J. Neurotrauma 2018, 35, 64–72. [Google Scholar] [CrossRef]
- Chen, W.; Qin, C. General hallmarks of microRNAs in brain evolution and development. RNA Biol. 2015, 12, 701–708. [Google Scholar] [CrossRef]
- Fiore, R.; Schratt, G. MicroRNAs in vertebrate synapse development. ScientificWorldJournal. 2007, 7, 167–177. [Google Scholar] [CrossRef]
- Corbin, R.; Olsson-Carter, K.; Slack, F. The role of microRNAs in synaptic development and function. BMB Rep. 2009, 42, 131–135. [Google Scholar] [CrossRef]
- Wang, W.; Kwon, E.J.; Tsai, L.-H. MicroRNAs in learning, memory, and neurological diseases. Learn Mem. 2012, 19, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.J.; Loeffert, A.C.; Stokes, J.; Olympia, R.P.; Bramley, H.; Hicks, S.D. Association of Salivary MicroRNA Changes with Prolonged Concussion Symptoms. JAMA Pediatr. 2018, 172, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Taheri, S.; Tanriverdi, F.; Zararsiz, G.; Elbuken, G.; Ulutabanca, H.; Karaca, Z.; Selcuklu, A.; Unluhizarci, K.; Tanriverdi, K.; Kelestimur, F. Circulating MicroRNAs as Potential Biomarkers for Traumatic Brain Injury-Induced Hypopituitarism. J. Neurotrauma 2016, 33, 1818–1825. [Google Scholar] [CrossRef] [PubMed]
- Bonauer, A.; Carmona, G.; Iwasaki, M.; Mione, M.; Koyanagi, M.; Fischer, A.; Burchfield, J.; Fox, H.; Doebele, C.; Ohtani, K.; et al. MicroRNA-92a controls angiogenesis and functional recovery of ischemic tissues in mice. Science 2009, 324, 1710–1713. [Google Scholar] [CrossRef] [PubMed]
- Doebele, C.; Bonauer, A.; Fischer, A.; Scholz, A.; Reiss, Y.; Urbich, C.; Hofmann, W.K.; Zeiher, A.M.; Dimmeler, S. Members of the micro-RNA-17-92 cluster exhibit a cell-intrinsic antiangiogenic function in endothelial cells. Blood 2010, 115, 4944–4950. [Google Scholar] [CrossRef]
- Joglekar, M.V.; Patil, D.; Joglekar, V.M.; Rao, G.; Reddy, N.D.; Mitnala, S.; Shouche, Y.; Hardikar, A. The miR-30 family microR-NAs confer epithelial phenotype to human pancreatic cells. Islets 2009, 1, 137–147. [Google Scholar] [CrossRef]
- Cano, A.; Pérez-Moreno, M.A.; Rodrigo, I.; Locascio, A.; Blanco, M.J.; Del Barrio, M.G.; Portillo, F.; Nieto, M.A. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat. Cell Biol. 2000, 2, 76–83. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, B.P. TNF-alpha/NF-kappaB/Snail pathway in cancer cell migration and invasion. Br. J. Cancer 2010, 102, 639–644. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).