The Antiglioma Potential of Plant Lectins: Molecular Targets, Mechanisms, and Future Directions
Abstract
1. Gliomas
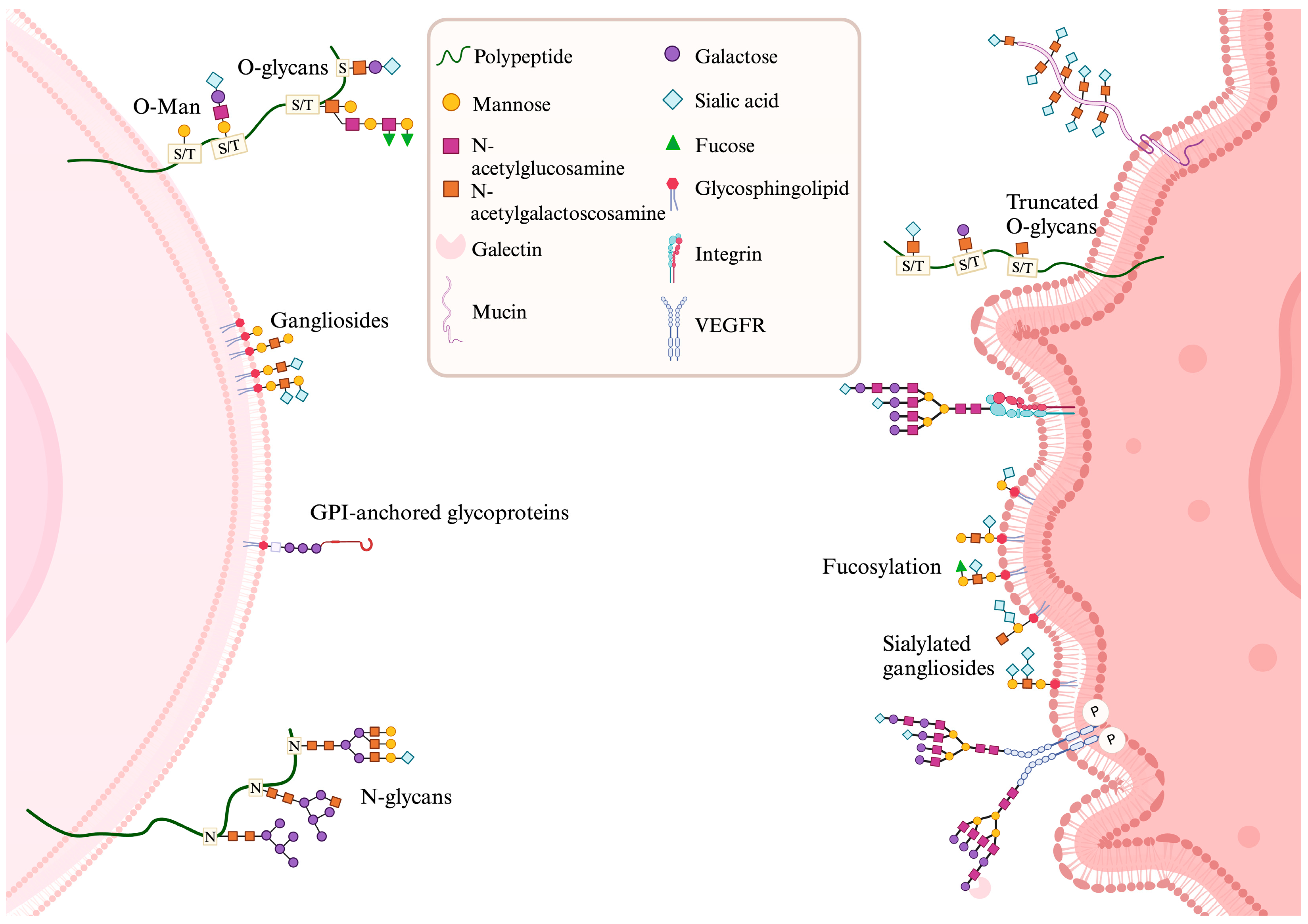
2. Glycobiology of Gliomas, Tumor Microenvironment, and Cell Death
3. Lectins
3.1. Antiglioma Lectins
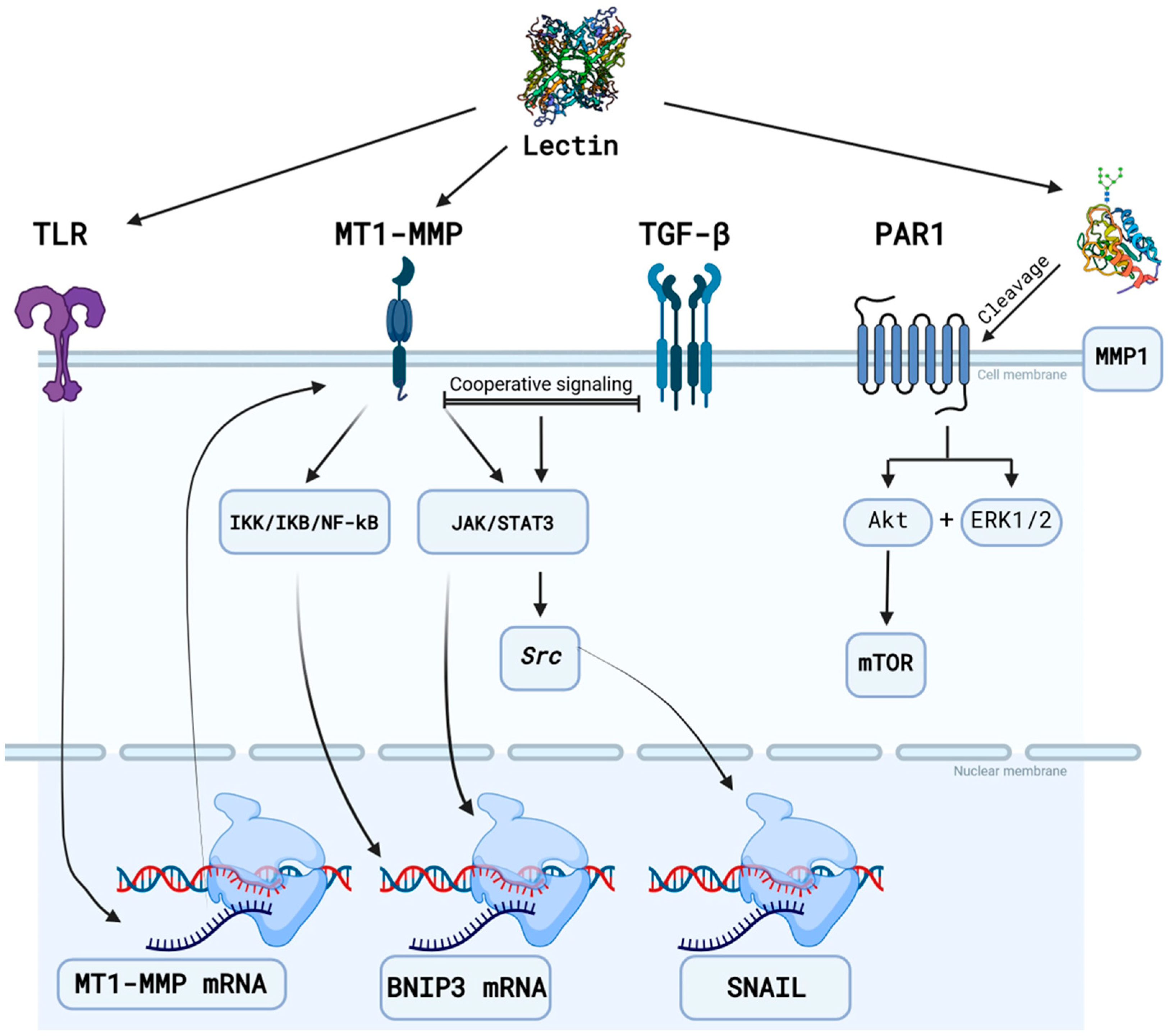
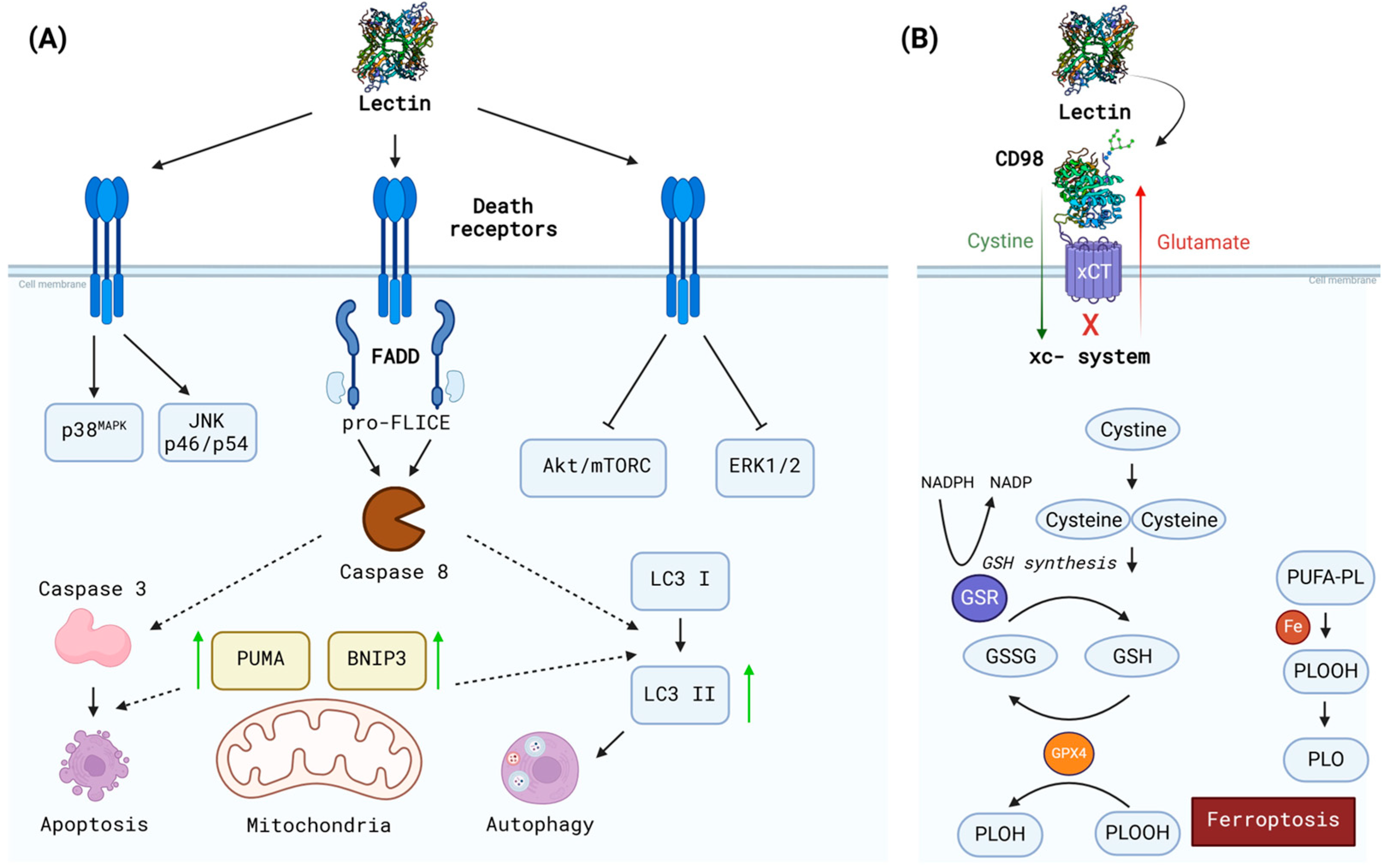
3.2. Suggested Mechanisms
3.3. Suggested Targets
3.3.1. Matrix Metalloproteinases
3.3.2. Epidermal Growth Factor Receptor (EGFR)
3.3.3. CD98hc (4F2hc)
3.3.4. AMPA Receptor (AMPA-R)
3.3.5. CD73 (Ecto-5′-nucleotidase)
3.4. Other Applications of Lectins
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A Summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Felistia, Y.; Wen, P.Y. Molecular Profiling and Targeted Therapies in Gliomas. Curr. Neurol. Neurosci. Rep. 2023, 23, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.; Huang, R.; Lan, Z.; Xiao, B.; Luo, Z. Abnormal Glycosylation in Glioma: Related Changes in Biology, Biomarkers and Targeted Therapy. Biomark. Res. 2023, 11, 54. [Google Scholar] [CrossRef]
- Sun, Y.-F.; Zhang, L.-C.; Niu, R.-Z.; Chen, L.; Xia, Q.-J.; Xiong, L.-L.; Wang, T.-H. Predictive Potentials of Glycosylation-Related Genes in Glioma Prognosis and Their Correlation with Immune Infiltration. Sci. Rep. 2024, 14, 4478. [Google Scholar] [CrossRef]
- Fuster, M.M.; Brown, J.R.; Wang, L.; Esko, J.D. A Disaccharide Precursor of Sialyl Lewis X Inhibits Metastatic Potential of Tumor Cells. Cancer Res. 2003, 63, 2775–2781. [Google Scholar]
- Cuello, H.A.; Ferreira, G.M.; Gulino, C.A.; Toledo, A.G.; Segatori, V.I.; Gabri, M.R. Terminally Sialylated and Fucosylated Complex N-Glycans Are Involved in the Malignant Behavior of High-Grade Glioma. Oncotarget 2020, 11, 4822–4835. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Zhou, X.; Wang, X. Glycosylation: Mechanisms, Biological Functions and Clinical Implications. Signal Transduct. Target. Ther. 2024, 9, 194. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, A.; Relvas-Santos, M.; Azevedo, R.; Santos, L.L.; Ferreira, J.A. Protein Glycosylation and Tumor Microenvironment Alterations Driving Cancer Hallmarks. Front. Oncol. 2019, 9, 380. [Google Scholar] [CrossRef]
- González-Tablas Pimenta, M.; Otero, Á.; Arandia Guzman, D.A.; Pascual-Argente, D.; Ruíz Martín, L.; Sousa-Casasnovas, P.; García-Martin, A.; Roa Montes de Oca, J.C.; Villaseñor-Ledezma, J.; Torres Carretero, L.; et al. Tumor Cell and Immune Cell Profiles in Primary Human Glioblastoma: Impact on Patient Outcome. Brain Pathol. 2021, 31, 365–380. [Google Scholar] [CrossRef]
- Ma, J.; Chen, C.C.; Li, M. Macrophages/microglia in the Glioblastoma Tumor Microenvironment. Int. J. Mol. Sci. 2021, 22, 5775. [Google Scholar] [CrossRef]
- Dusoswa, S.A.; Verhoeff, J.; Abels, E.; Méndez-Huergo, S.P.; Croci, D.O.; Kuijper, L.H.; de Miguel, E.; Wouters, V.M.C.J.; Best, M.G.; Rodriguez, E.; et al. Glioblastomas Exploit Truncated O-Linked Glycans for Local and Distant Immune Modulation via the Macrophage Galactose-Type Lectin. Proc. Natl. Acad. Sci. USA 2020, 117, 3693–3703. [Google Scholar] [CrossRef] [PubMed]
- Sethi, M.K.; Downs, M.; Shao, C.; Hackett, W.E.; Phillips, J.J.; Zaia, J. In-Depth Matrisome and Glycoproteomic Analysis of Human Brain Glioblastoma versus Control Tissue. Mol. Cell. Proteom. 2022, 21, 100216. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Pang, L.; Dunterman, M.; Lesniak, M.S.; Heimberger, A.B.; Chen, P. Macrophages and Microglia in Glioblastoma: Heterogeneity, Plasticity, and Therapy. J. Clin. Investig. 2023, 133, e163446. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, M.; Ji, C.; Liu, X.; Gu, B.; Dong, T. Macrophage Polarization in the Tumor Microenvironment: Emerging Roles and Therapeutic Potentials. Biomed. Pharmacother. 2024, 177, 116930. [Google Scholar] [CrossRef] [PubMed]
- Basak, U.; Sarkar, T.; Mukherjee, S.; Chakraborty, S.; Dutta, A.; Dutta, S.; Nayak, D.; Kaushik, S.; Das, T.; Sa, G. Tumor-Associated Macrophages: An Effective Player of the Tumor Microenvironment. Front. Immunol. 2023, 14, 1295257. [Google Scholar] [CrossRef]
- Taiarol, L.; Formicola, B.; Fagioli, S.; Sierri, G.; D’Aloia, A.; Kravicz, M.; Renda, A.; Viale, F.; Dal Magro, R.; Ceriani, M.; et al. The 3.0 Cell Communication: New Insights in the Usefulness of Tunneling Nanotubes for Glioblastoma Treatment. Cancers 2021, 13, 4001. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Mallela, A.N.; Shi, D.D.; Tang, L.W.; Abou-Al-Shaar, H.; Gersey, Z.C.; Zhang, X.; McBrayer, S.K.; Abdullah, K.G. Isocitrate Dehydrogenase Mutations in Gliomas: A Review of Current Understanding and Trials. Neurooncol. Adv. 2023, 5, vdad053. [Google Scholar] [CrossRef]
- Pace, A.; Scirocchi, F.; Napoletano, C.; Zizzari, I.G.; D’Angelo, L.; Santoro, A.; Nuti, M.; Rahimi, H.; Rughetti, A. Glycan-Lectin Interactions as Novel Immunosuppression Drivers in Glioblastoma. Int. J. Mol. Sci. 2022, 23, 6312. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kang, H.; Powathil, G.; Kim, H.; Trucu, D.; Lee, W.; Lawler, S.; Chaplain, M. Role of Extracellular Matrix and Microenvironment in Regulation of Tumor Growth and LAR-Mediated Invasion in Glioblastoma. PLoS ONE 2018, 13, e0204865. [Google Scholar] [CrossRef]
- Sun, X.; Turcan, S. From Laboratory Studies to Clinical Trials: Temozolomide Use in IDH-Mutant Gliomas. Cells 2021, 10, 1225. [Google Scholar] [CrossRef] [PubMed]
- Jezierzański, M.; Nafalska, N.; Stopyra, M.; Furgoł, T.; Miciak, M.; Kabut, J.; Gisterek-Grocholska, I. Temozolomide (TMZ) in the Treatment of Glioblastoma Multiforme-A Literature Review and Clinical Outcomes. Curr. Oncol. 2024, 31, 3994–4002. [Google Scholar] [CrossRef] [PubMed]
- Schultz, M.J.; Swindall, A.F.; Bellis, S.L. Regulation of the Metastatic Cell Phenotype by Sialylated Glycans. Cancer Metastasis Rev. 2012, 31, 501–518. [Google Scholar] [CrossRef]
- Estornes, Y.; Dondelinger, Y.; Weber, K.; Bruggeman, I.; Peall, A.; MacFarlane, M.; Lebecque, S.; Vandenabeele, P.; Bertrand, M.J.M. N-Glycosylation of Mouse TRAIL-R Restrains TRAIL-Induced Apoptosis. Cell Death Dis. 2018, 9, 494. [Google Scholar] [CrossRef]
- Micheau, O. Regulation of TNF-Related Apoptosis-Inducing Ligand Signaling by Glycosylation. Int. J. Mol. Sci. 2018, 19, 715. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-W.; Liu, M.-B.; Jiang, X.; Song, T.; Feng, S.-X.; Wu, J.-Y.; Deng, P.-F.; Wang, X.-Y. GALNT14 Regulates Ferroptosis and Apoptosis of Ovarian Cancer through the EGFR/mTOR Pathway. Future Oncol. 2022, 18, 149–161. [Google Scholar] [CrossRef]
- Stockwell, B.R. Ferroptosis Turns 10: Emerging Mechanisms, Physiological Functions, and Therapeutic Applications. Cell 2022, 185, 2401–2421. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wang, Y.; Li, X.; Zhou, J.; Yang, W.; Wang, X.; Jiao, S.; Zuo, W.; You, Z.; Ying, W.; et al. O-GlcNAcylation Regulates the Stability of Transferrin Receptor (TFRC) to Control the Ferroptosis in Hepatocellular Carcinoma Cells. Redox Biol. 2024, 73, 103182. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Chen, X.; Mo, S.; Zhang, Y.; Mao, X.; Chen, J.; Liu, Y.; Tong, W.-M.; Lu, Z.; Yu, S.; et al. Targeting N-Glycosylation of 4F2hc Mediated by Glycosyltransferase B3GNT3 Sensitizes Ferroptosis of Pancreatic Ductal Adenocarcinoma. Cell Death Differ. 2023, 30, 1988–2004. [Google Scholar] [CrossRef] [PubMed]
- de Souza, I.; Ramalho, M.C.C.; Guedes, C.B.; Osawa, I.Y.A.; Monteiro, L.K.S.; Gomes, L.R.; Rocha, C.R.R. Ferroptosis Modulation: Potential Therapeutic Target for Glioblastoma Treatment. Int. J. Mol. Sci. 2022, 23, 6879. [Google Scholar] [CrossRef] [PubMed]
- Teng, W.; Ling, Y.; Long, N.; Cen, W.; Zhang, H.; Jiang, L.; Liu, J.; Zhou, X.; Chu, L. Repurposing Flubendazole for Glioblastoma Ferroptosis by Affecting xCT and TFRC Proteins. J. Cell. Mol. Med. 2024, 28, e70188. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Xu, Y.; Sun, Q.; Hong, Y.; Liang, S.; Jiang, H.; Zhang, X.; Zhang, S.; Chen, Q. Targeting Ferroptosis Opens New Avenues in Gliomas. Int. J. Biol. Sci. 2024, 20, 4674–4690. [Google Scholar] [CrossRef] [PubMed]
- Debnath, J.; Gammoh, N.; Ryan, K.M. Autophagy and Autophagy-Related Pathways in Cancer. Nat. Rev. Mol. Cell Biol. 2023, 24, 560–575. [Google Scholar] [CrossRef] [PubMed]
- Noonan, J.; Zarrer, J.; Murphy, B.M. Targeting Autophagy in Glioblastoma. Crit. Rev. Oncog. 2016, 21, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wan, W. Acetylation in the Regulation of Autophagy. Autophagy 2023, 19, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Nie, H.; Saini, P.; Miyamoto, T.; Liao, L.; Zielinski, R.J.; Liu, H.; Zhou, W.; Wang, C.; Murphy, B.; Towers, M.; et al. Targeting Branched N-Glycans and Fucosylation Sensitizes Ovarian Tumors to Immune Checkpoint Blockade. Nat. Commun. 2024, 15, 2853. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.; Cabral, H. Abnormal Glycosylation of Cancer Stem Cells and Targeting Strategies. Front. Oncol. 2021, 11, 649338. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Gu, Z.; Zhong, J.; Wen, D.; Chen, G.; He, L.; Wu, J.; Gu, Z. Advances in Glycosylation-Mediated Cancer-Targeted Drug Delivery. Drug Discov. Today 2018, 23, 1126–1138. [Google Scholar] [CrossRef]
- Thomas, D.; Rathinavel, A.K.; Radhakrishnan, P. Altered glycosylation in cancer: A promising target for biomarkers and therapeutics. Biochim. Biophys. Acta Rev. Cancer 2021, 1875, 188464. [Google Scholar] [CrossRef]
- Yau, T.; Dan, X.; Ng, C.C.W.; Ng, T.B. Lectins with Potential for Anti-Cancer Therapy. Molecules 2015, 20, 3791–3810. [Google Scholar] [CrossRef]
- Cavada, B.S.; Oliveira, M.V.; Osterne, V.J.S.; Pinto-Junior, V.R.; Correia-Neto, C.; Nascimento, K.S. Lectins Applied to Diagnosis and Treatment of Prostate Cancer and Benign Hyperplasia: A Review. Int. J. Biol. Macromol. 2021, 190, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Cavada, B.S.; de Oliveira, M.V.; Osterne, V.J.S.; Pinto-Junior, V.R.; Martins, F.W.V.; Correia-Neto, C.; Pinheiro, R.F.; Leal, R.B.; Nascimento, K.S. Recent Advances in the Use of Legume Lectins for the Diagnosis and Treatment of Breast Cancer. Biochimie 2022, 208, 100–116. [Google Scholar] [CrossRef] [PubMed]
- Hopper, J.T.S.; Ambrose, S.; Grant, O.C.; Krumm, S.A.; Allison, T.M.; Degiacomi, M.T.; Tully, M.D.; Pritchard, L.K.; Ozorowski, G.; Ward, A.B.; et al. The Tetrameric Plant Lectin BanLec Neutralizes HIV through Bidentate Binding to Specific Viral Glycans. Structure 2017, 25, 773–782.e5. [Google Scholar] [CrossRef] [PubMed]
- Barre, A.; Simplicien, M.; Benoist, H.; Van Damme, E.J.M.; Rougé, P. Mannose-Specific Lectins from Marine Algae: Diverse Structural Scaffolds Associated to Common Virucidal and Anti-Cancer Properties. Mar. Drugs 2019, 17, 440. [Google Scholar] [CrossRef] [PubMed]
- Marques, G.F.O.; Osterne, V.J.S.; Almeida, L.M.; Oliveira, M.V.; Brizeno, L.A.C.; Pinto-Junior, V.R.; Santiago, M.Q.; Neco, A.H.B.; Mota, M.R.L.; Souza, L.A.G.; et al. Contribution of the Carbohydrate-Binding Ability of Vatairea Guianensis Lectin to Induce Edematogenic Activity. Biochimie 2017, 140, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Stillmark, H. Ueber Ricin, Ein Giftiges Ferment Aus Den Samen von Ricinus Comm. L. Und Einigen Anderen Euphorbiaceen: Inaugural-Dissertation; Schnakenburg: Dorpat, Estonia, 1888. [Google Scholar]
- Tsaneva, M.; Van Damme, E.J.M. 130 Years of Plant Lectin Research. Glycoconj. J. 2020, 37, 533–551. [Google Scholar] [CrossRef] [PubMed]
- De Coninck, T.; Van Damme, E.J.M. Review: The Multiple Roles of Plant Lectins. Plant Sci. 2021, 313, 111096. [Google Scholar] [CrossRef]
- Cavada, B.S.; Osterne, V.J.S.; Oliveira, M.V.; Pinto-Junior, V.R.; Silva, M.T.L.; Bari, A.U.; Lima, L.D.; Lossio, C.F.; Nascimento, K.S. Reviewing Mimosoideae Lectins: A Group of under Explored Legume Lectins. Int. J. Biol. Macromol. 2020, 154, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Cavada, B.S.; Pinto-Junior, V.R.; Osterne, V.J.S.; Oliveira, M.V.; Lossio, C.F.; Silva, M.T.L.; Bari, A.U.; Lima, L.D.; Souza-Filho, C.H.D.; Nascimento, K.S. Comprehensive Review on Caelsalpinioideae Lectins: From Purification to Biological Activities. Int. J. Biol. Macromol. 2020, 162, 333–348. [Google Scholar] [CrossRef]
- Cavada, B.S.; Pinto-Junior, V.R.; Oliveira, M.V.; Osterne, V.J.S.; Lossio, C.F.; Nascimento, K.S. A Review of Vicieae Lectins Studies: End of the Book or a Story in the Writing? Int. J. Biol. Macromol. 2021, 181, 1104–1123. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, K.S.; Silva, M.T.L.; Oliveira, M.V.; Lossio, C.F.; Pinto-Junior, V.R.; Osterne, V.J.S.; Cavada, B.S. Dalbergieae Lectins: A Review of Lectins from Species of a Primitive Papilionoideae (leguminous) Tribe. Int. J. Biol. Macromol. 2020, 144, 509–526. [Google Scholar] [CrossRef] [PubMed]
- Cavada, B.S.; Pinto-Junior, V.R.; Osterne, V.J.S.; Nascimento, K.S. ConA-Like Lectins: High Similarity Proteins as Models to Study Structure/Biological Activities Relationships. Int. J. Mol. Sci. 2018, 20, 30. [Google Scholar] [CrossRef] [PubMed]
- Musthafa, S.A.; Muthu, K.; Vijayakumar, S.; George, S.J.; Murali, S.; Govindaraj, J.; Munuswamy-Ramanujam, G. Lectin Isolated from Abelmoschus Esculentus Induces Caspase Mediated Apoptosis in Human U87 Glioblastoma Cell Lines and Modulates the Expression of Circadian Clock Genes. Toxicon 2021, 202, 98–109. [Google Scholar] [CrossRef]
- Cavada, B.S.; Silva, M.T.L.; Osterne, V.J.S.; Pinto-Junior, V.R.; Nascimento, A.P.M.; Wolin, I.A.V.; Heinrich, I.A.; Nobre, C.A.S.; Moreira, C.G.; Lossio, C.F.; et al. Canavalia bonariensis Lectin: Molecular Bases of Glycoconjugates Interaction and Antiglioma Potential. Int. J. Biol. Macromol. 2018, 106, 369–378. [Google Scholar] [CrossRef]
- Wolin, I.A.V.; Heinrich, I.A.; Nascimento, A.P.M.; Welter, P.G.; Sosa, L.D.V.; De Paul, A.L.; Zanotto-Filho, A.; Nedel, C.B.; Lima, L.D.; Osterne, V.J.S.; et al. ConBr Lectin Modulates MAPKs and Akt Pathways and Triggers Autophagic Glioma Cell Death by a Mechanism Dependent upon Caspase-8 Activation. Biochimie 2021, 180, 186–204. [Google Scholar] [CrossRef] [PubMed]
- Pratt, J.; Annabi, B. Induction of Autophagy Biomarker BNIP3 Requires a JAK2/STAT3 and MT1-MMP Signaling Interplay in Concanavalin-A-Activated U87 Glioblastoma Cells. Cell. Signal. 2014, 26, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Sina, A.; Proulx-Bonneau, S.; Roy, A.; Poliquin, L.; Cao, J.; Annabi, B. The Lectin Concanavalin-A Signals MT1-MMP Catalytic Independent Induction of COX-2 through an IKKgamma/NF-kappaB-Dependent Pathway. J. Cell Commun. Signal. 2010, 4, 31–38. [Google Scholar] [CrossRef]
- Nascimento, A.P.M.; Knaut, J.L.; Rieger, D.K.; Wolin, I.A.V.; Heinrich, I.A.; Mann, J.; Juarez, A.V.; Sosa, L.D.V.; De Paul, A.L.; Moreira, C.G.; et al. Anti-Glioma Properties of DVL, a Lectin Purified from Dioclea violacea. Int. J. Biol. Macromol. 2018, 120, 566–577. [Google Scholar] [CrossRef]
- Nascimento, A.P.M.; Wolin, I.A.V.; Welter, P.G.; Heinrich, I.A.; Zanotto-Filho, A.; Osterne, V.J.S.; Lossio, C.F.; Silva, M.T.L.; Nascimento, K.S.; Cavada, B.S.; et al. Lectin from Dioclea violacea Induces Autophagy in U87 Glioma Cells. Int. J. Biol. Macromol. 2019, 134, 660–672. [Google Scholar] [CrossRef]
- Leal, R.B.; Mann, J.; Pinto-Junior, V.R.; Oliveira, M.V.; Osterne, V.J.S.; Wolin, I.A.V.; Nascimento, A.P.M.; Welter, P.G.; Ferreira, V.M.S.; Silva, A.A.; et al. Structural Prediction and Characterization of Canavalia grandiflora (ConGF) Lectin Complexed with MMP1: Unveiling the Antiglioma Potential of Legume Lectins. Molecules 2022, 27, 89. [Google Scholar] [CrossRef] [PubMed]
- Osterne, V.J.S.; Silva-Filho, J.C.; Santiago, M.Q.; Pinto-Junior, V.R.; Almeida, A.C.; Barreto, A.A.G.C.; Wolin, I.A.V.; Nascimento, A.P.M.; Amorim, R.M.F.; Rocha, B.A.M.; et al. Structural Characterization of a Lectin from Canavalia virosa Seeds with Inflammatory and Cytotoxic Activities. Int. J. Biol. Macromol. 2017, 94, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Yamazaki, K.; Yamori, T.; Endo, T. Inhibition of Proliferation and Induction of Differentiation of Glioma Cells with Datura Stramonium Agglutinin. Br. J. Cancer 2002, 87, 918–923. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Endo, T. Both Cell-Surface Carbohydrates and Protein Tyrosine Phosphatase Are Involved in the Differentiation of Astrocytes In Vitro. Glia 2000, 32, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, K.S.; Santiago, M.Q.; Pinto-Junior, V.R.; Osterne, V.J.S.; Martins, F.W.V.; Nascimento, A.P.M.; Wolin, I.A.V.; Heinrich, I.A.; Martins, M.G.Q.; Silva, M.T.L.; et al. Structural Analysis of Dioclea lasiocarpa Lectin: A C6 Cells Apoptosis-Inducing Protein. Int. J. Biochem. Cell Biol. 2017, 92, 79–89. [Google Scholar] [CrossRef]
- Leal, R.B.; Pinto-Junior, V.R.; Osterne, V.J.S.; Wolin, I.A.V.; Nascimento, A.P.M.; Neco, A.H.B.; Araripe, D.A.; Welter, P.G.; Neto, C.C.; Correia, J.L.A.; et al. Crystal Structure of DlyL, a Mannose-Specific Lectin from Dioclea lasiophylla Mart. Ex Benth Seeds That Display Cytotoxic Effects against C6 Glioma Cells. Int. J. Biol. Macromol. 2018, 114, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, K.S.; Andrade, M.L.L.; Silva, I.B.; Domingues, D.L.; Chicas, L.S.; Silva, M.T.L.; Bringel, P.H.S.F.; Marques, G.F.O.; Martins, M.G.Q.; Lóssio, C.F.; et al. Heterologous Production of α-Chain of Dioclea sclerocarpa Lectin: Enhancing the Biological Effects of a Wild-Type Lectin. Int. J. Biol. Macromol. 2020, 156, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Varmes Fernandes, A.; Viana Ramos, M.; Maria Vasconcelos, I.; Cristina Oliveira Monteiro Moreira, A.; Bruno Moreno, F.; Odair Pereira, J.; Francisco de Carvalho Goncalves, J. Purification and Characterization of a Lectin of the Swartzieae Legume Taxa. Protein Pept. Lett. 2012, 19, 1082–1088. [Google Scholar] [CrossRef]
- Kar, F.; Kacar, S.; Hacioglu, C.; Kanbak, G.; Sahinturk, V. Concanavalin A Induces Apoptosis in a Dose-Dependent Manner by Modulating Thiol/disulfide Homeostasis in C6 Glioblastoma Cells. J. Biochem. Mol. Toxicol. 2021, 35, e22742. [Google Scholar] [CrossRef] [PubMed]
- Giakoumettis, D.; Kritis, A.; Foroglou, N. C6 Cell Line: The Gold Standard in Glioma Research. Hippokratia 2018, 22, 105–112. [Google Scholar] [PubMed]
- Costa Nunes, F.; Silva, L.B.; Winter, E.; Silva, A.H.; de Melo, L.J.; Rode, M.; Martins, M.A.P.; Zanatta, N.; Feitosa, S.C.; Bonacorso, H.G.; et al. Tacrine Derivatives Stimulate Human Glioma SF295 Cell Death and Alter Important Proteins Related to Disease Development: An Old Drug for New Targets. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 1527–1536. [Google Scholar] [CrossRef]
- Yu, M.; Sato, H.; Seiki, M.; Thompson, E.W. Complex Regulation of Membrane-Type Matrix Metalloproteinase Expression and Matrix Metalloproteinase-2 Activation by Concanavalin A in MDA-MB-231 Human Breast Cancer Cells. Cancer Res. 1995, 55, 3272–3277. [Google Scholar]
- Gingras, D.; Pagé, M.; Annabi, B.; Béliveau, R. Rapid Activation of Matrix Metalloproteinase-2 by Glioma Cells Occurs through a Posttranslational MT1-MMP-Dependent Mechanism. Biochim. Biophys. Acta 2000, 1497, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Pratt, J.; Roy, R.; Annabi, B. Concanavalin-A-Induced Autophagy Biomarkers Requires Membrane Type-1 Matrix Metalloproteinase Intracellular Signaling in Glioblastoma Cells. Glycobiology 2012, 22, 1245–1255. [Google Scholar] [CrossRef]
- Huldani, H.; Rashid, A.I.; Turaev, K.N.; Opulencia, M.J.C.; Abdelbasset, W.K.; Bokov, D.O.; Mustafa, Y.F.; Al-Gazally, M.E.; Hammid, A.T.; Kadhim, M.M.; et al. Concanavalin A as a Promising Lectin-Based Anti-Cancer Agent: The Molecular Mechanisms and Therapeutic Potential. Cell Commun. Signal. 2022, 20, 167. [Google Scholar] [CrossRef]
- Annabi, B.; Laflamme, C.; Sina, A.; Lachambre, M.-P.; Béliveau, R. A MT1-MMP/NF-kappaB Signaling Axis as a Checkpoint Controller of COX-2 Expression in CD133+ U87 Glioblastoma Cells. J. Neuroinflamm. 2009, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Djediai, S.; Gonzalez Suarez, N.; El Cheikh-Hussein, L.; Rodriguez Torres, S.; Gresseau, L.; Dhayne, S.; Joly-Lopez, Z.; Annabi, B. MT1-MMP Cooperates with TGF-β Receptor-Mediated Signaling to Trigger SNAIL and Induce Epithelial-to-Mesenchymal-like Transition in U87 Glioblastoma Cells. Int. J. Mol. Sci. 2021, 22, 13006. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.K.; Lee, D.-H.; Lee, E.C.; Oh, J.S. Importance of Autophagy Regulation in Glioblastoma with Temozolomide Resistance. Cells 2024, 13, 1332. [Google Scholar] [CrossRef] [PubMed]
- Ravikumar, B.; Sarkar, S.; Davies, J.E.; Futter, M.; Garcia-Arencibia, M.; Green-Thompson, Z.W.; Jimenez-Sanchez, M.; Korolchuk, V.I.; Lichtenberg, M.; Luo, S.; et al. Regulation of Mammalian Autophagy in Physiology and Pathophysiology. Physiol. Rev. 2010, 90, 1383–1435. [Google Scholar] [CrossRef] [PubMed]
- Dikic, I.; Elazar, Z. Mechanism and Medical Implications of Mammalian Autophagy. Nat. Rev. Mol. Cell Biol. 2018, 19, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Ma, Y.; Zhang, Y.; Yin, X. Therapeutic Potential of ASK1 Activators in Cancer Treatment: Current Insights and Future Directions. Biomed. Pharmacother. 2024, 178, 117214. [Google Scholar] [CrossRef]
- Pinto-Junior, V.R.; Seeger, R.L.; Souza-Filho, C.H.D.; França, A.P.; Sartori, N.; Oliveira, M.V.; Osterne, V.J.S.; Nascimento, K.S.; Leal, R.B.; Cavada, B.S. Xc- System as a Possible Target for ConBr Lectin Interaction in Glioma Cells. Neuroglia 2024, 5, 202–222. [Google Scholar] [CrossRef]
- Hagemann, C.; Anacker, J.; Ernestus, R.-I.; Vince, G.H. A Complete Compilation of Matrix Metalloproteinase Expression in Human Malignant Gliomas. World J. Clin. Oncol. 2012, 3, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Boon, L.; Ugarte-Berzal, E.; Vandooren, J.; Opdenakker, G. Glycosylation of Matrix Metalloproteases and Tissue Inhibitors: Present State, Challenges and Opportunities. Biochem. J. 2016, 473, 1471–1482. [Google Scholar] [CrossRef] [PubMed]
- Saarinen, J.; Welgus, H.G.; Flizar, C.A.; Kalkkinen, N.; Helin, J. N-Glycan Structures of Matrix Metalloproteinase-1 Derived from Human Fibroblasts and from HT-1080 Fibrosarcoma Cells. Eur. J. Biochem. 1999, 259, 829–840. [Google Scholar] [CrossRef]
- Wilhelm, S.M.; Eisen, A.Z.; Teter, M.; Clark, S.D.; Kronberger, A.; Goldberg, G. Human Fibroblast Collagenase: Glycosylation and Tissue-Specific Levels of Enzyme Synthesis. Proc. Natl. Acad. Sci. USA 1986, 83, 3756–3760. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.M.; Collier, I.E.; Kronberger, A.; Eisen, A.Z.; Marmer, B.L.; Grant, G.A.; Bauer, E.A.; Goldberg, G.I. Human Skin Fibroblast Stromelysin: Structure, Glycosylation, Substrate Specificity, and Differential Expression in Normal and Tumorigenic Cells. Proc. Natl. Acad. Sci. USA 1987, 84, 6725–6729. [Google Scholar] [CrossRef] [PubMed]
- Madzharova, E.; Kastl, P.; Sabino, F.; Auf dem Keller, U. Post-Translational Modification-Dependent Activity of Matrix Metalloproteinases. Int. J. Mol. Sci. 2019, 20, 3077. [Google Scholar] [CrossRef]
- Takeshita, M.; Kuno, A.; Suzuki, K.; Matsuda, A.; Shimazaki, H.; Nakagawa, T.; Otomo, Y.; Kabe, Y.; Suematsu, M.; Narimatsu, H.; et al. Alteration of Matrix Metalloproteinase-3 O-Glycan Structure as a Biomarker for Disease Activity of Rheumatoid Arthritis. Arthritis Res. Ther. 2016, 18, 112. [Google Scholar] [CrossRef]
- Yagi, F.; Miyamoto, M.; Abe, T.; Minami, Y.; Tadera, K.; Goldstein, I.J. Purification and Carbohydrate-Binding Specificity of Agrocybe Cylindracea Lectin. Glycoconj. J. 1997, 14, 281–288. [Google Scholar] [CrossRef]
- Kuno, A.; Kato, Y.; Matsuda, A.; Kaneko, M.K.; Ito, H.; Amano, K.; Chiba, Y.; Narimatsu, H.; Hirabayashi, J. Focused Differential Glycan Analysis with the Platform Antibody-Assisted Lectin Profiling for Glycan-Related Biomarker Verification. Mol. Cell. Proteom. 2009, 8, 99–108. [Google Scholar] [CrossRef]
- Matsuda, A.; Kuno, A.; Nakagawa, T.; Ikehara, Y.; Irimura, T.; Yamamoto, M.; Nakanuma, Y.; Miyoshi, E.; Nakamori, S.; Nakanishi, H.; et al. Lectin Microarray-Based Sero-Biomarker Verification Targeting Aberrant O-Linked Glycosylation on Mucin 1. Anal. Chem. 2015, 87, 7274–7281. [Google Scholar] [CrossRef]
- Mattu, T.S.; Royle, L.; Langridge, J.; Wormald, M.R.; Van den Steen, P.E.; Van Damme, J.; Opdenakker, G.; Harvey, D.J.; Dwek, R.A.; Rudd, P.M. O-Glycan Analysis of Natural Human Neutrophil Gelatinase B Using a Combination of Normal Phase-HPLC and Online Tandem Mass Spectrometry: Implications for the Domain Organization of the Enzyme. Biochemistry 2000, 39, 15695–15704. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, G.; Van den Steen, P.E.; Cohen, S.R.; Grossmann, J.G.; Frenkel, J.; Sertchook, R.; Slack, N.; Strange, R.W.; Opdenakker, G.; Sagi, I. Insights into the Structure and Domain Flexibility of Full-Length pro-Matrix Metalloproteinase-9/gelatinase B. Structure 2007, 15, 1227–1236. [Google Scholar] [CrossRef] [PubMed]
- Vandooren, J.; Knoops, S.; Aldinucci Buzzo, J.L.; Boon, L.; Martens, E.; Opdenakker, G.; Kolaczkowska, E. Differential Inhibition of Activity, Activation and Gene Expression of MMP-9 in THP-1 Cells by Azithromycin and Minocycline versus Bortezomib: A Comparative Study. PLoS ONE 2017, 12, e0174853. [Google Scholar] [CrossRef] [PubMed]
- Duellman, T.; Burnett, J.; Yang, J. Functional Roles of N-Linked Glycosylation of Human Matrix Metalloproteinase 9. Traffic 2015, 16, 1108–1126. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, T.; Liu, S.; Yoshida, D.; Teramoto, A. The Expression of Matrix Metalloproteinase-2 and -9 in Human Gliomas of Different Pathological Grades. Brain Tumor Pathol. 2003, 20, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Knäuper, V.; López-Otin, C.; Smith, B.; Knight, G.; Murphy, G. Biochemical Characterization of Human Collagenase-3. J. Biol. Chem. 1996, 271, 1544–1550. [Google Scholar] [CrossRef] [PubMed]
- Phang, W.-M.; Tan, A.-A.; Gopinath, S.C.B.; Hashim, O.H.; Kiew, L.V.; Chen, Y. Secretion of N- and O-Linked Glycoproteins from 4T1 Murine Mammary Carcinoma Cells. Int. J. Med. Sci. 2016, 13, 330–339. [Google Scholar] [CrossRef]
- Wu, Y.I.; Munshi, H.G.; Sen, R.; Snipas, S.J.; Salvesen, G.S.; Fridman, R.; Stack, M.S. Glycosylation Broadens the Substrate Profile of Membrane Type 1 Matrix Metalloproteinase. J. Biol. Chem. 2004, 279, 8278–8289. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.I.; Munshi, H.G.; Snipas, S.J.; Salvesen, G.S.; Fridman, R.; Stack, M.S. Activation-Coupled Membrane-Type 1 Matrix Metalloproteinase Membrane Trafficking. Biochem. J. 2007, 407, 171–177. [Google Scholar] [CrossRef]
- Kinoshita, T.; Sato, H.; Okada, A.; Ohuchi, E.; Imai, K.; Okada, Y.; Seiki, M. TIMP-2 Promotes Activation of Progelatinase A by Membrane-Type 1 Matrix Metalloproteinase Immobilized on Agarose Beads. J. Biol. Chem. 1998, 273, 16098–16103. [Google Scholar] [CrossRef]
- Hatanpaa, K.J.; Burma, S.; Zhao, D.; Habib, A.A. Epidermal Growth Factor Receptor in Glioma: Signal Transduction, Neuropathology, Imaging, and Radioresistance. Neoplasia 2010, 12, 675–684. [Google Scholar] [CrossRef]
- Zhen, Y.; Caprioli, R.M.; Staros, J.V. Characterization of Glycosylation Sites of the Epidermal Growth Factor Receptor. Biochemistry 2003, 42, 5478–5492. [Google Scholar] [CrossRef] [PubMed]
- Rao, T.C.; Beggs, R.R.; Ankenbauer, K.E.; Hwang, J.; Ma, V.P.-Y.; Salaita, K.; Bellis, S.L.; Mattheyses, A.L. ST6Gal-I-Mediated Sialylation of the Epidermal Growth Factor Receptor Modulates Cell Mechanics and Enhances Invasion. J. Biol. Chem. 2022, 298, 101726. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-C.; Yen, H.-Y.; Chen, C.-Y.; Chen, C.-H.; Cheng, P.-F.; Juan, Y.-H.; Chen, C.-H.; Khoo, K.-H.; Yu, C.-J.; Yang, P.-C.; et al. Sialylation and Fucosylation of Epidermal Growth Factor Receptor Suppress Its Dimerization and Activation in Lung Cancer Cells. Proc. Natl. Acad. Sci. USA 2011, 108, 11332–11337. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.-C.; Chien, P.-H.; Wu, H.-Y.; Chen, S.-T.; Juan, H.-F.; Lou, P.-J.; Huang, M.-C. C1GALT1 Predicts Poor Prognosis and Is a Potential Therapeutic Target in Head and Neck Cancer. Oncogene 2018, 37, 5780–5793. [Google Scholar] [CrossRef] [PubMed]
- Stateva, S.R.; Villalobo, A. O-GlcNAcylation of the Human Epidermal Growth Factor Receptor. Org. Biomol. Chem. 2015, 13, 8196–8204. [Google Scholar] [CrossRef]
- Soderquist, A.M.; Carpenter, G. Glycosylation of the Epidermal Growth Factor Receptor in A-431 Cells. The Contribution of Carbohydrate to Receptor Function. J. Biol. Chem. 1984, 259, 12586–12594. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.Y.; Benguría, A.; Kafert, S.; André, S.; Gabius, H.J.; Villalobo, A. Differential Response of the Epidermal Growth Factor Receptor Tyrosine Kinase Activity to Several Plant and Mammalian Lectins. Mol. Cell. Biochem. 1995, 142, 117–124. [Google Scholar] [CrossRef]
- Rebbaa, A.; Yamamoto, H.; Moskal, J.R.; Bremer, E.G. Binding of Erythroagglutinating Phytohemagglutinin Lectin from Phaseolus Vulgaris to the Epidermal Growth Factor Receptor Inhibits Receptor Function in the Human Glioma Cell Line, U373 MG. J. Neurochem. 1996, 67, 2265–2272. [Google Scholar] [CrossRef]
- Arriagada, C.; Cavieres, V.A.; Luchsinger, C.; González, A.E.; Muñoz, V.C.; Cancino, J.; Burgos, P.V.; Mardones, G.A. GOLPH3 Regulates EGFR in T98G Glioblastoma Cells by Modulating Its Glycosylation and Ubiquitylation. Int. J. Mol. Sci. 2020, 21, 8880. [Google Scholar] [CrossRef] [PubMed]
- Sechi, S.; Frappaolo, A.; Belloni, G.; Colotti, G.; Giansanti, M.G. The Multiple Cellular Functions of the Oncoprotein Golgi Phosphoprotein 3. Oncotarget 2015, 6, 3493–3506. [Google Scholar] [CrossRef]
- Nawashiro, H.; Otani, N.; Shinomiya, N.; Fukui, S.; Nomura, N.; Yano, A.; Shima, K.; Matsuo, H.; Kanai, Y. The Role of CD98 in Astrocytic Neoplasms. Hum. Cell 2002, 15, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Digomann, D.; Kurth, I.; Tyutyunnykova, A.; Chen, O.; Löck, S.; Gorodetska, I.; Peitzsch, C.; Skvortsova, I.-I.; Negro, G.; Aschenbrenner, B.; et al. The CD98 Heavy Chain Is a Marker and Regulator of Head and Neck Squamous Cell Carcinoma Radiosensitivity. Clin. Cancer Res. 2019, 25, 3152–3163. [Google Scholar] [CrossRef] [PubMed]
- Digomann, D.; Linge, A.; Dubrovska, A. SLC3A2/CD98hc, Autophagy and Tumor Radioresistance: A Link Confirmed. Autophagy 2019, 15, 1850–1851. [Google Scholar] [CrossRef]
- Console, L.; Scalise, M.; Salerno, S.; Scanga, R.; Giudice, D.; De Bartolo, L.; Tonazzi, A.; Indiveri, C. N-Glycosylation Is Crucial for Trafficking and Stability of SLC3A2 (CD98). Sci. Rep. 2022, 12, 14570. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.L.; Deme, J.C.; Kolokouris, D.; Kuteyi, G.; Biggin, P.C.; Lea, S.M.; Newstead, S. Molecular Basis for Redox Control by the Human Cystine/glutamate Antiporter System Xc. Nat. Commun. 2021, 12, 7147. [Google Scholar] [CrossRef]
- Zhang, C.; Shafaq-Zadah, M.; Pawling, J.; Ng, D.W.J.; Hesketh, G.G.; Dransart, E.; Pacholczyk, K.; Longo, J.; Gingras, A.-C.; Penn, L.Z.; et al. SLC3A2 N-Glycosylation and Alternate Evolutionary Trajectories for Amino Acid Metabolism. bioRxiv 2022. [Google Scholar] [CrossRef]
- Mukaro, V.R.; Bylund, J.; Hodge, G.; Holmes, M.; Jersmann, H.; Reynolds, P.N.; Hodge, S. Lectins Offer New Perspectives in the Development of Macrophage-Targeted Therapies for COPD/emphysema. PLoS ONE 2013, 8, e56147. [Google Scholar] [CrossRef]
- Cappoli, N.; Jenkinson, M.D.; Dello Russo, C.; Dickens, D. LAT1, a Novel Pharmacological Target for the Treatment of Glioblastoma. Biochem. Pharmacol. 2022, 201, 115103. [Google Scholar] [CrossRef]
- Anagnostakis, F.; Kokkorakis, M.; Markouli, M.; Piperi, C. Impact of Solute Carrier Transporters in Glioma Pathology: A Comprehensive Review. Int. J. Mol. Sci. 2023, 24, 9393. [Google Scholar] [CrossRef] [PubMed]
- McDonald, C.A.; Yang, J.Y.; Marathe, V.; Yen, T.-Y.; Macher, B.A. Combining Results from Lectin Affinity Chromatography and Glycocapture Approaches Substantially Improves the Coverage of the Glycoproteome. Mol. Cell. Proteom. 2009, 8, 287–301. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, K.; Ikeda, S.; Yaga, M.; Watanabe, K.; Urakawa, R.; Iehara, A.; Iwai, M.; Hashiguchi, S.; Morimoto, S.; Fujiki, F.; et al. Selective Targeting of Multiple Myeloma Cells with a Monoclonal Antibody Recognizing the Ubiquitous Protein CD98 Heavy Chain. Sci. Transl. Med. 2022, 14, eaax7706. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.A.; Gurd, J.W.; Bissoon, N.; Tricaud, N.; Molnar, E.; Zamze, S.E.; Dwek, R.A.; McIlhinney, R.A.; Wing, D.R. Identification of Lectin-Purified Neural Glycoproteins, GPs 180, 116, and 110, with NMDA and AMPA Receptor Subunits: Conservation of Glycosylation at the Synapse. J. Neurochem. 1998, 70, 2594–2605. [Google Scholar] [CrossRef]
- Hemelikova, K.; Kolcheva, M.; Skrenkova, K.; Kaniakova, M.; Horak, M. Lectins Modulate the Functional Properties of GluN1/GluN3-Containing NMDA Receptors. Neuropharmacology 2019, 157, 107671. [Google Scholar] [CrossRef]
- Jang, S.; Yu, J.-Y.; Ahn, J.-H.; Oh, S. Modulation of NMDA Receptor Subunits Expression by Concanavalin A. Neurochem. Res. 2016, 41, 1887–1898. [Google Scholar] [CrossRef] [PubMed]
- Tucholski, J.; Pinner, A.L.; Simmons, M.S.; Meador-Woodruff, J.H. Evolutionarily Conserved Pattern of AMPA Receptor Subunit Glycosylation in Mammalian Frontal Cortex. PLoS ONE 2014, 9, e94255. [Google Scholar] [CrossRef] [PubMed]
- Gangwar, S.P.; Yelshanskaya, M.V.; Nadezhdin, K.D.; Yen, L.Y.; Newton, T.P.; Aktolun, M.; Kurnikova, M.G.; Sobolevsky, A.I. Kainate Receptor Channel Opening and Gating Mechanism. Nature 2024, 630, 762–768. [Google Scholar] [CrossRef] [PubMed]
- Biegański, M.; Szeliga, M. Disrupted Glutamate Homeostasis as a Target for Glioma Therapy. Pharmacol. Rep. 2024, 76, 1305–1317. [Google Scholar] [CrossRef] [PubMed]
- Kumaria, A.; Ashkan, K. Novel Therapeutic Strategies in Glioma Targeting Glutamatergic Neurotransmission. Brain Res. 2023, 1818, 148515. [Google Scholar] [CrossRef]
- Venkataramani, V.; Tanev, D.I.; Strahle, C.; Studier-Fischer, A.; Fankhauser, L.; Kessler, T.; Körber, C.; Kardorff, M.; Ratliff, M.; Xie, R.; et al. Glutamatergic Synaptic Input to Glioma Cells Drives Brain Tumour Progression. Nature 2019, 573, 532–538. [Google Scholar] [CrossRef]
- Venkatesh, H.S.; Morishita, W.; Geraghty, A.C.; Silverbush, D.; Gillespie, S.M.; Arzt, M.; Tam, L.T.; Espenel, C.; Ponnuswami, A.; Ni, L.; et al. Electrical and Synaptic Integration of Glioma into Neural Circuits. Nature 2019, 573, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.R.; Barron, T.; Hui, A.; Spitzer, A.; Yalçin, B.; Ivec, A.E.; Geraghty, A.C.; Hartmann, G.G.; Arzt, M.; Gillespie, S.M.; et al. Glioma Synapses Recruit Mechanisms of Adaptive Plasticity. Nature 2023, 623, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Morise, J.; Yamamoto, S.; Midorikawa, R.; Takamiya, K.; Nonaka, M.; Takematsu, H.; Oka, S. Distinct Cell Surface Expression Patterns of N-Glycosylation Site Mutants of AMPA-Type Glutamate Receptor under the Homo-Oligomeric Expression Conditions. Int. J. Mol. Sci. 2020, 21, 5101. [Google Scholar] [CrossRef] [PubMed]
- Everts, I.; Villmann, C.; Hollmann, M. N-Glycosylation Is Not a Prerequisite for Glutamate Receptor Function but Is Essential for Lectin Modulation. Mol. Pharmacol. 1997, 52, 861–873. [Google Scholar] [CrossRef] [PubMed]
- Tucholski, J.; Simmons, M.S.; Pinner, A.L.; Haroutunian, V.; McCullumsmith, R.E.; Meador-Woodruff, J.H. Abnormal N-Linked Glycosylation of Cortical AMPA Receptor Subunits in Schizophrenia. Schizophr. Res. 2013, 146, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Kandel, M.B.; Yamamoto, S.; Midorikawa, R.; Morise, J.; Wakazono, Y.; Oka, S.; Takamiya, K. N-Glycosylation of the AMPA-Type Glutamate Receptor Regulates Cell Surface Expression and Tetramer Formation Affecting Channel Function. J. Neurochem. 2018, 147, 730–747. [Google Scholar] [CrossRef] [PubMed]
- Morita, I.; Kakuda, S.; Takeuchi, Y.; Itoh, S.; Kawasaki, N.; Kizuka, Y.; Kawasaki, T.; Oka, S. HNK-1 Glyco-Epitope Regulates the Stability of the Glutamate Receptor Subunit GluR2 on the Neuronal Cell Surface. J. Biol. Chem. 2009, 284, 30209–30217. [Google Scholar] [CrossRef]
- Morise, J.; Takematsu, H.; Oka, S. The Role of Human Natural Killer-1 (HNK-1) Carbohydrate in Neuronal Plasticity and Disease. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 2455–2461. [Google Scholar] [CrossRef] [PubMed]
- Hong, I.; Kim, J.; Hainmueller, T.; Kim, D.W.; Keijser, J.; Johnson, R.C.; Park, S.H.; Limjunyawong, N.; Yang, Z.; Cheon, D.; et al. Calcium-Permeable AMPA Receptors Govern PV Neuron Feature Selectivity. Nature 2024, 635, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Tsuzuki, K.; Ishiuchi, S.; Ozawa, S. Serum-Dependence of AMPA Receptor-Mediated Proliferation in Glioma Cells. Pathol. Int. 2006, 56, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Corsi, L.; Mescola, A.; Alessandrini, A. Glutamate Receptors and Glioblastoma Multiforme: An Old “Route” for New Perspectives. Int. J. Mol. Sci. 2019, 20, 1796. [Google Scholar] [CrossRef]
- de Groot, J.F.; Piao, Y.; Lu, L.; Fuller, G.N.; Yung, W.K.A. Knockdown of GluR1 Expression by RNA Interference Inhibits Glioma Proliferation. J. Neurooncol. 2008, 88, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Piao, Y.; Lu, L.; de Groot, J. AMPA Receptors Promote Perivascular Glioma Invasion via beta1 Integrin-Dependent Adhesion to the Extracellular Matrix. Neuro-Oncology 2009, 11, 260–273. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Li, Y.-L.; Wang, H.-Y.; Zhu, M.; Guo, D.; Wang, G.-L.; Gao, Y.-T.; Yang, Z.; Li, T.; Yang, C.-Y.; et al. Propofol Inhibits Invasion and Proliferation of C6 Glioma Cells by Regulating the Ca2+ Permeable AMPA Receptor-System Xc- Pathway. Toxicol. In Vitro 2017, 44, 57–65. [Google Scholar] [CrossRef]
- Ramaswamy, P.; Dalavaikodihalli Nanjaiah, N.; Prasad, C.; Goswami, K. Transcriptional Modulation of Calcium-Permeable AMPA Receptor Subunits in Glioblastoma by MEK-ERK1/2 Inhibitors and Their Role in Invasion. Cell Biol. Int. 2020, 44, 830–837. [Google Scholar] [CrossRef]
- Russi, M.A.; Vandresen-Filho, S.; Rieger, D.K.; Costa, A.P.; Lopes, M.W.; Cunha, R.M.S.; Teixeira, E.H.; Nascimento, K.S.; Cavada, B.S.; Tasca, C.I.; et al. ConBr, a Lectin from Canavalia brasiliensis Seeds, Protects against Quinolinic Acid-Induced Seizures in Mice. Neurochem. Res. 2012, 37, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Goswami, S.; Walle, T.; Cornish, A.E.; Basu, S.; Anandhan, S.; Fernandez, I.; Vence, L.; Blando, J.; Zhao, H.; Yadav, S.S.; et al. Immune Profiling of Human Tumors Identifies CD73 as a Combinatorial Target in Glioblastoma. Nat. Med. 2020, 26, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Heuts, D.P.H.M.; Weissenborn, M.J.; Olkhov, R.V.; Shaw, A.M.; Gummadova, J.; Levy, C.; Scrutton, N.S. Crystal Structure of a Soluble Form of Human CD73 with Ecto-5′-Nucleotidase Activity. Chembiochem 2012, 13, 2384–2391. [Google Scholar] [CrossRef]
- Burgemeister, R.; Danescu, I.; Gutensohn, W. Glycosylation and Processing of Carbohydrate Side Chains of Ecto-5′-Nucleotidase in Cultured Human Chorionic Cells. Biol. Chem. Hoppe Seyler 1990, 371, 355–361. [Google Scholar] [CrossRef]
- Zimmermann, H. 5′-Nucleotidase: Molecular Structure and Functional Aspects. Biochem. J. 1992, 285 Pt 2, 345–365. [Google Scholar] [CrossRef] [PubMed]
- Sadej, R.; Spychala, J.; Skladanowski, A.C. Ecto-5′-Nucleotidase (eN, CD73) Is Coexpressed with Metastasis Promoting Antigens in Human Melanoma Cells. Nucleosides Nucleotides Nucleic Acids 2006, 25, 1119–1123. [Google Scholar] [CrossRef] [PubMed]
- Alcedo, K.P.; Guerrero, A.; Basrur, V.; Fu, D.; Richardson, M.L.; McLane, J.S.; Tsou, C.-C.; Nesvizhskii, A.I.; Welling, T.H.; Lebrilla, C.B.; et al. Tumor-Selective Altered Glycosylation and Functional Attenuation of CD73 in Human Hepatocellular Carcinoma. Hepatol. Commun. 2019, 3, 1400–1414. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.E.; Yeom, J.; Kim, Y.; Lee, H.J.; Han, K.-C.; Lee, S.-T.; Lee, C.; Lee, J.E. Identification of Plasma Membrane Glycoproteins Specific to Human Glioblastoma Multiforme Cells Using Lectin Arrays and LC-MS/MS. Proteomics 2018, 18, 1700302. [Google Scholar] [CrossRef]
- Tucker-Burden, C.; Chappa, P.; Krishnamoorthy, M.; Gerwe, B.A.; Scharer, C.D.; Heimburg-Molinaro, J.; Harris, W.; Usta, S.N.; Eilertson, C.D.; Hadjipanayis, C.G.; et al. Lectins Identify Glycan Biomarkers on Glioblastoma-Derived Cancer Stem Cells. Stem Cells Dev. 2012, 21, 2374–2386. [Google Scholar] [CrossRef] [PubMed]
- Putthisen, S.; Silsirivanit, A.; Panawan, O.; Niibori-Nambu, A.; Nishiyama-Ikeda, Y.; Ma-In, P.; Luang, S.; Ohta, K.; Muisuk, K.; Wongkham, S.; et al. Targeting alpha2,3-Sialylated Glycan in Glioma Stem-like Cells by Maackia Amurensis Lectin-II: A Promising Strategy for Glioma Treatment. Exp. Cell Res. 2022, 410, 112949. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.C.; Kochi, N.; Tani, E.; Kaba, K.; Matsumoto, T.; Shindo, H. Lectin Histochemistry of Human Gliomas. Acta Neuropathol. 1989, 79, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Figols, J.; Madrid, J.F.; Cervós-Navarro, J. Lectins as Differentiation Markers of Human Gliomas. Histol. Histopathol. 1991, 6, 79–85. [Google Scholar] [PubMed]

| Glioma Type | Subgroup |
|---|---|
| (1) Diffuse gliomas, characterized by their infiltrative growth and the presence of specific genetic alterations | Adult-type diffuse glioblastoma, isocitrate dehydrogenase (IDH)-wildtype (CNS WHO grade 4): Characterized by mutations such as telomerase reverse transcriptase (TERT) promoter mutations, EGFR amplification, and/or +7/−10 chromosome (RG1) changes. Adult-type diffuse astrocytoma, IDH-mutant (grades 2, 3, or 4): Defined by the presence of an IDH mutation and the absence of 1p/19q co-deletion. Grading depends on histological and molecular features, including CDKN2A/B homozygous deletion for grade 4. Pediatric-type diffuse high-grade gliomas: Includes entities like diffuse midline glioma, H3 K27-altered and diffuse hemispheric glioma, and H3 G34-mutant. Pediatric-type diffuse low-grade gliomas: Characterized by alterations such as MYB or MYBL1 rearrangements. |
| (2) Circumscribed gliomas, tumors are typically more localized and have distinct histopathological and molecular features | Pilocytic astrocytoma (grade 1): Frequently associated with mitogen-activated protein kinase (MAPK) pathway alterations (e.g., KIAA1549-BRAF fusion). Pleomorphic xanthoastrocytoma (grade 2): Often with BRAF V600E mutations. |
| (3) Gliomas with a mixed molecular profile | Oligodendroglioma, IDH-mutant, 1p/19q co-deleted (grades 2 or 3): Defined by the presence of both IDH mutation and 1p/19q co-deletion, which distinguish it from astrocytic gliomas. |
| (4) Other glioma subtypes | Ependymomas: Now classified based on anatomic location and molecular markers (e.g., RELA Fusion-positive ependymoma). Gliomas with histone mutations: Highlighting specific subtypes with poor prognosis, such as H3 K27M-mutant gliomas. |
| Specie | Lectin | Specificity | Cell Line | Concentration (μg/mL) | Effects |
|---|---|---|---|---|---|
| Abelmoschus esculentus (L.) Moench | AEL | Galactosides | U87 | 21 |
|
| Canavalia bonariensis Lindl. | CaBo | Glucose Mannose | C6 | 100 |
|
| Canavalia brasiliensis Mart. ex Benth. | ConBr | Glucose Mannose | C6, U87, and GBM-1 | 30–50 |
|
| Canavalia ensiformis (L.) DC. | ConA | Glucose Mannose | C6 and U87 | 30 |
|
| Canavalia grandiflora Benth. | ConGF | Glucose Mannose | C6 | 30, 50, and 100 |
|
| Canavalia virosa (Roxb.) Wight & Arn. | ConV | Glucose Mannose | C6 | 30–100 |
|
| Datura stramonium (L.) | DSA | N-acetyllactosamine | C6 | 1 # | |
| Dioclea lasiocarpa Mart. ex Benth. | DLL | Glucose/mannose | C6 | 100 |
|
| Dioclea lasiophylla Mart. ex Benth. | DlyL | Glucose/mannose | C6 | 30–100 |
|
| Dioclea sclerocarpa Ducke | DSL and rDSL * | Glucose/mannose | C6 and U87 | 100 |
|
| Dioclea violacea Mart. ex Benth. | DVL | Glucose/mannose | C6 and U87 | 30–100 |
|
| Swartzia laevicarpa Amshoff | SLL | Galactosides | SF-295 | >100 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leal, R.B.; Pinto-Junior, V.R.; Oliveira, M.V.; Osterne, V.J.S.; Sartori, N.; Santos, A.C.d.; Garcez, R.C.; Nascimento, K.S.; Cavada, B.S. The Antiglioma Potential of Plant Lectins: Molecular Targets, Mechanisms, and Future Directions. Neuroglia 2025, 6, 5. https://doi.org/10.3390/neuroglia6010005
Leal RB, Pinto-Junior VR, Oliveira MV, Osterne VJS, Sartori N, Santos ACd, Garcez RC, Nascimento KS, Cavada BS. The Antiglioma Potential of Plant Lectins: Molecular Targets, Mechanisms, and Future Directions. Neuroglia. 2025; 6(1):5. https://doi.org/10.3390/neuroglia6010005
Chicago/Turabian StyleLeal, Rodrigo Bainy, Vanir Reis Pinto-Junior, Messias Vital Oliveira, Vinicius Jose Silva Osterne, Nicole Sartori, Ana Carolina dos Santos, Ricardo Castilho Garcez, Kyria Santiago Nascimento, and Benildo Sousa Cavada. 2025. "The Antiglioma Potential of Plant Lectins: Molecular Targets, Mechanisms, and Future Directions" Neuroglia 6, no. 1: 5. https://doi.org/10.3390/neuroglia6010005
APA StyleLeal, R. B., Pinto-Junior, V. R., Oliveira, M. V., Osterne, V. J. S., Sartori, N., Santos, A. C. d., Garcez, R. C., Nascimento, K. S., & Cavada, B. S. (2025). The Antiglioma Potential of Plant Lectins: Molecular Targets, Mechanisms, and Future Directions. Neuroglia, 6(1), 5. https://doi.org/10.3390/neuroglia6010005






