Fungicide and Bactericide Effects on Carbon and Nitrogen Cycling in Soils: A Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Compilation
- Studies need to report soil and microbial properties for at least one biocide application and for a control.
- Studies need to report at least one of the following properties: Microbial biomass C and N, respiration rate, available NH4+, ammonification, available NO3−, nitrification, and N2O.
- Studies need to report the amount of biocide applied, and the time of measurements since application.
2.2. Statistical Analyses
3. Results
4. Discussion
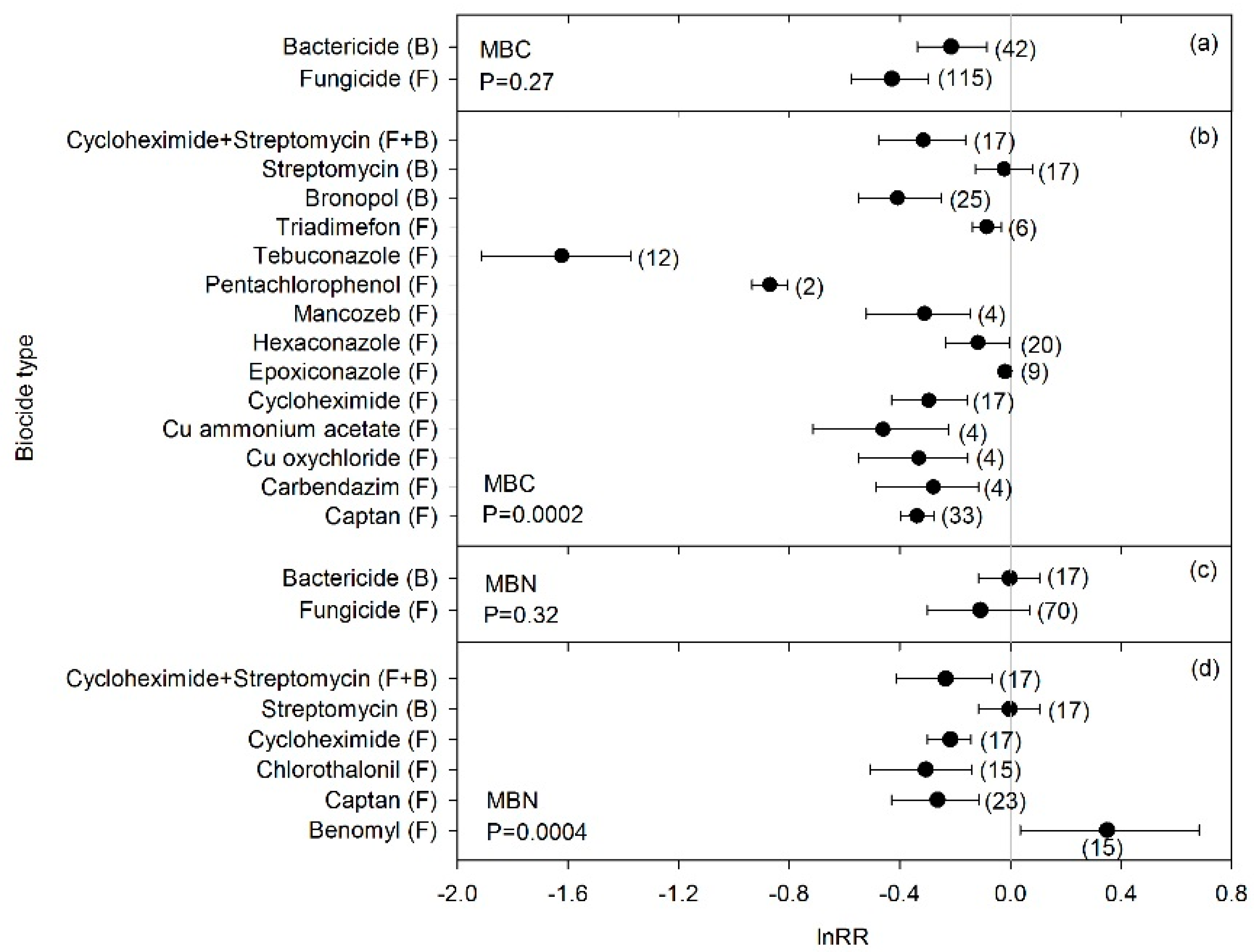
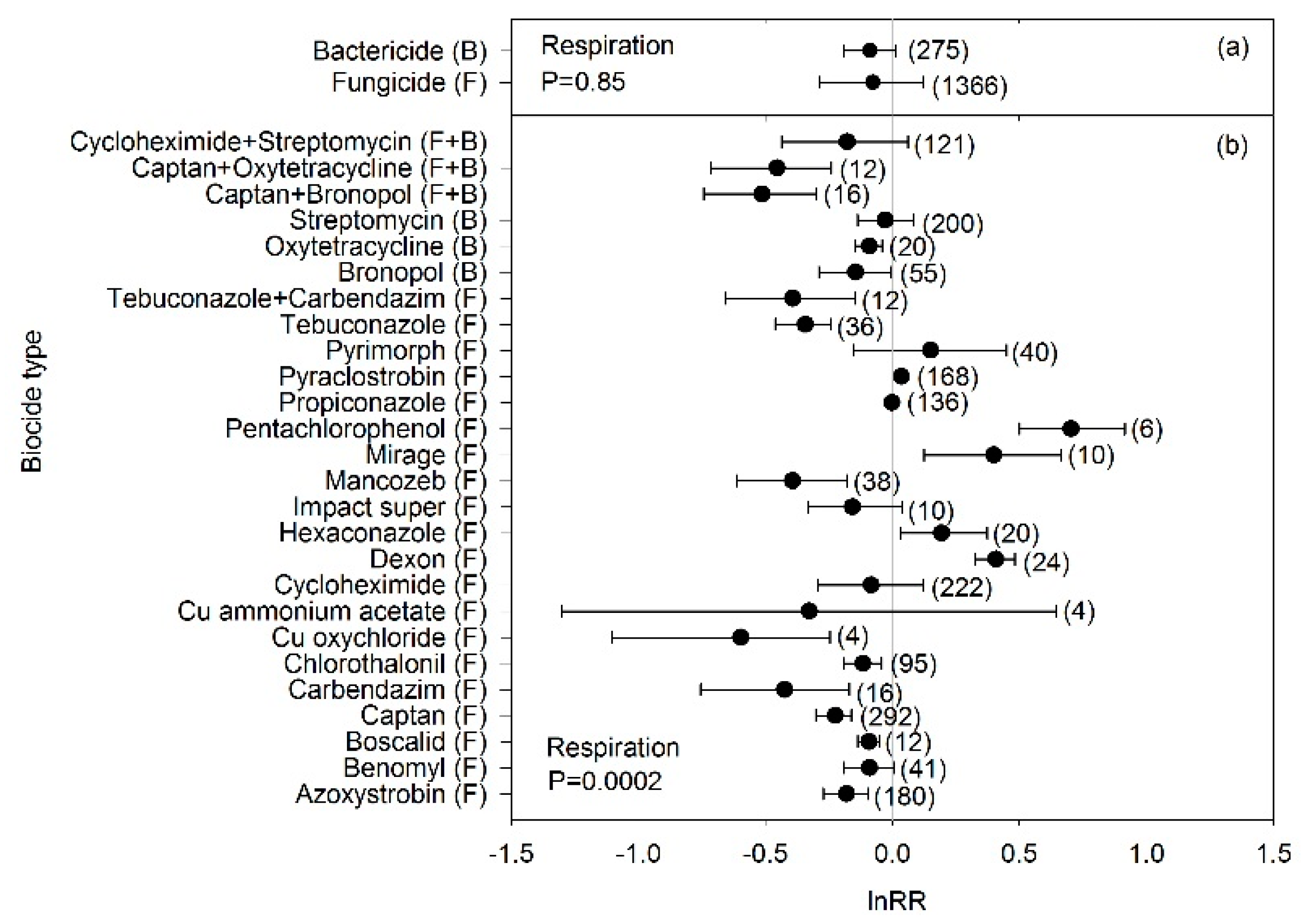
4.1. Biocide Effects on Microbial Biomass and C-Cycle Processes
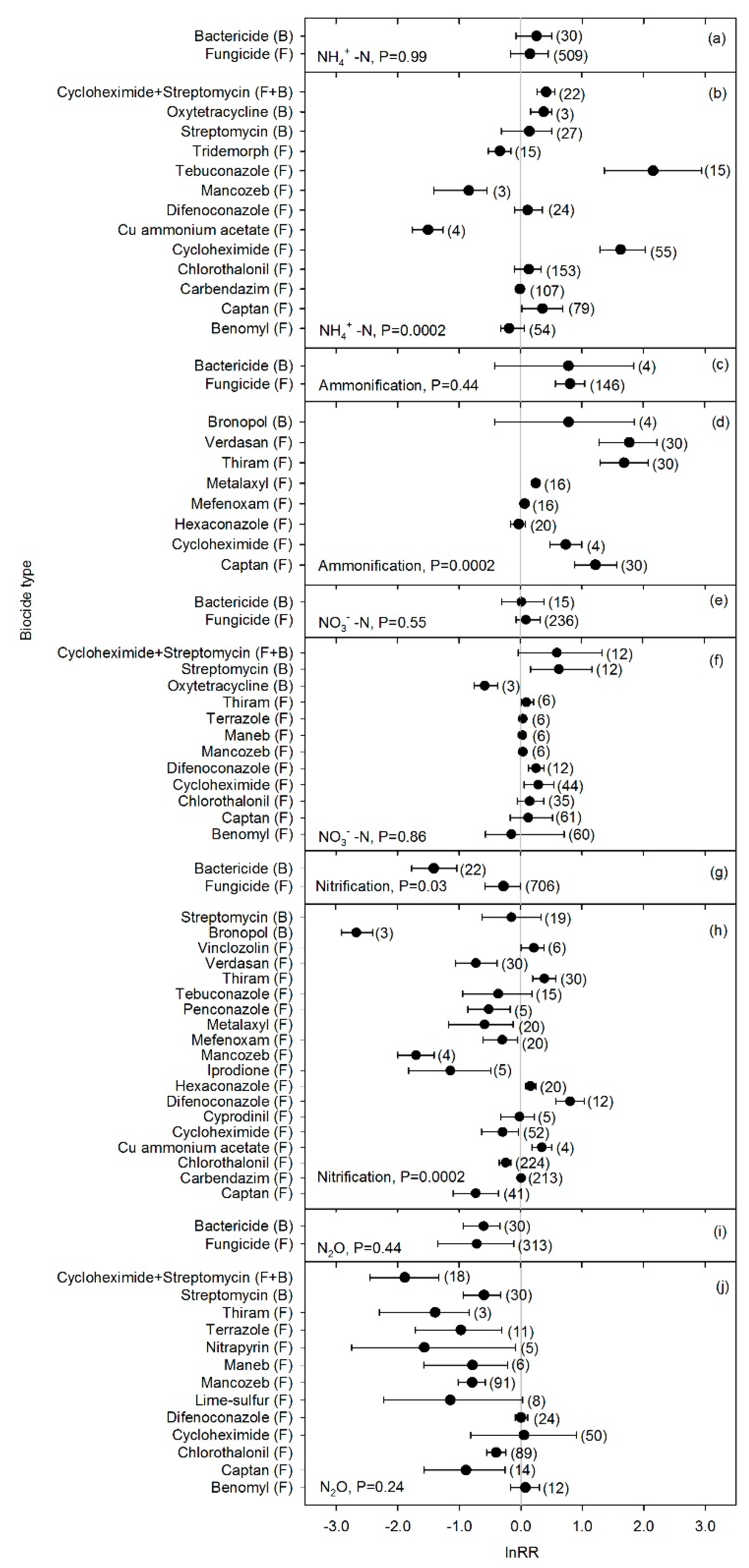
4.2. Biocide Effects on N-cycle Processes
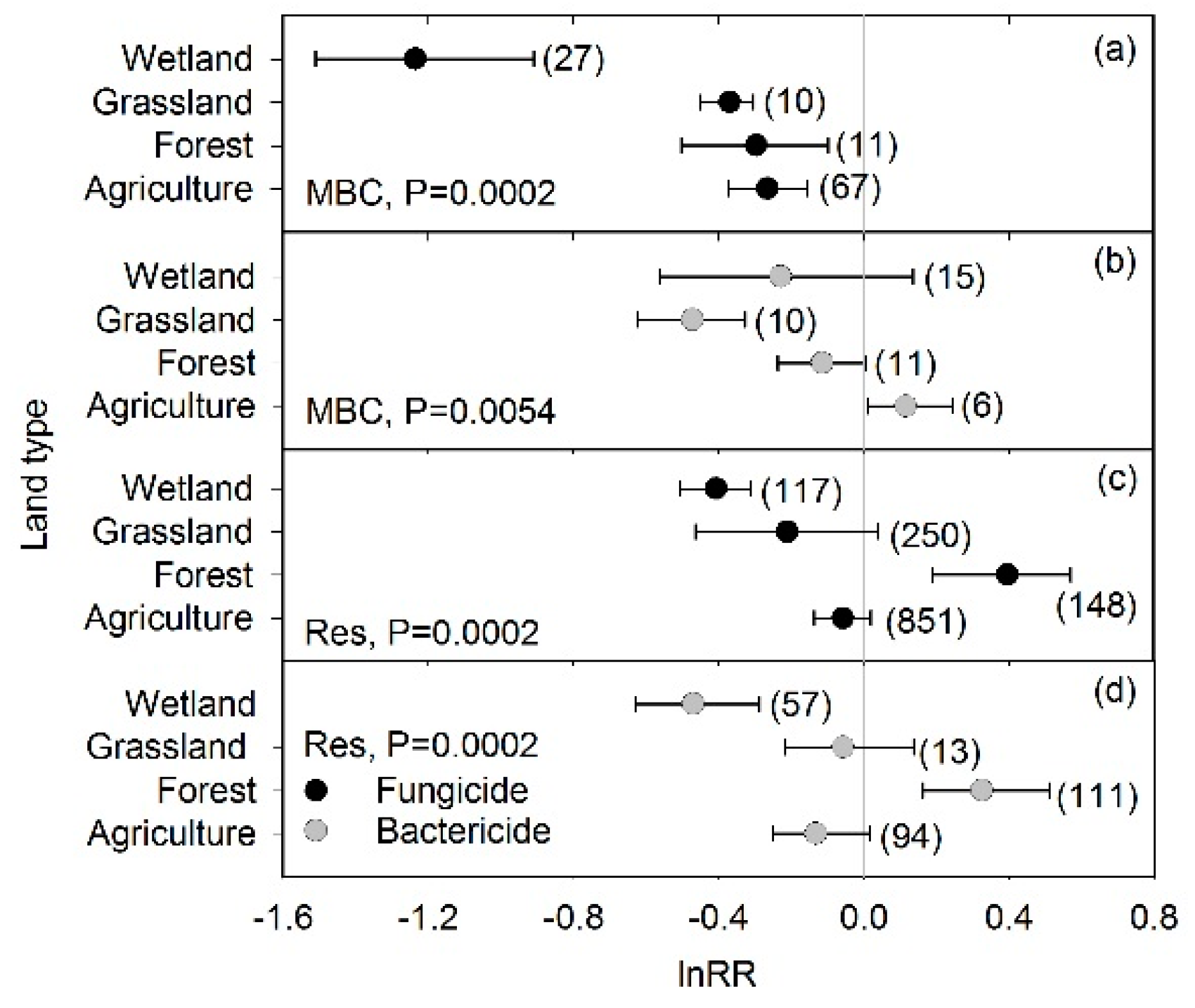
4.3. Fungicide and Bactericide Effects in Relation to Land Type
4.4. Fungicide and Bactericide Effects in Relation to Soil pH and C
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ågren, G.I. Climate change: Microbial mitigation. Nat. Geosci. 2010, 3, 303–304. [Google Scholar] [CrossRef]
- Schimel, J.P.; Bennett, J. Nitrogen mineralization: Challenges of a changing paradigm. Ecology 2004, 85, 591–602. [Google Scholar] [CrossRef]
- Prosser, J.I.; Bohannan, B.J.M.; Curtis, T.P.; Ellis, R.J.; Firestone, M.K.; Freckleton, R.P.; Green, J.L.; Green, L.E.; Killham, K.; Lennon, J.J. The role of ecological theory in microbial ecology. Nat. Rev. Microbiol. 2007, 5, 384. [Google Scholar] [CrossRef] [PubMed]
- Bååth, E.; Anderson, T.-H. Comparison of soil fungal/bacterial ratios in a pH gradient using physiological and PLFA-based techniques. Soil Biol. Biochem. 2003, 35, 955–963. [Google Scholar] [CrossRef] [Green Version]
- Fierer, N.; Strickland, M.S.; Liptzin, D.; Bradford, M.A.; Cleveland, C.C. Global patterns in belowground communities. Ecol. Lett. 2009, 12, 1238–1249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bardgett, R.D.; Freeman, C.; Ostle, N.J. Microbial contributions to climate change through carbon cycle feedbacks. ISME J. 2008, 2, 805–814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailey, V.L.; Smith, J.L.; Bolton, H., Jr. Fungal-to-bacterial ratios in soils investigated for enhanced C sequestration. Soil Biol. Biochem. 2002, 34, 997–1007. [Google Scholar] [CrossRef]
- Milne, R.M.; Haynes, R.J. Soil organic matter, microbial properties, and aggregate stability under annual and perennial pastures. Biol. Fertil. Soils 2004, 39, 172–178. [Google Scholar] [CrossRef]
- Liang, C.; Balser, T.C. Microbial production of recalcitrant organic matter in global soils: Implications for productivity and climate policy. Nat. Rev. Microbiol. 2011, 9, 75. [Google Scholar] [CrossRef]
- Schimel, J.; Schaeffer, S.M. Microbial control over carbon cycling in soil. Front. Microbiol. 2012, 3, 348. [Google Scholar] [CrossRef]
- Schurig, C.; Smittenberg, R.H.; Berger, J.; Kraft, F.; Woche, S.K.; Goebel, M.-O.; Heipieper, H.J.; Miltner, A.; Kaestner, M. Microbial cell-envelope fragments and the formation of soil organic matter: A case study from a glacier forefield. Biogeochemistry 2013, 113, 595–612. [Google Scholar] [CrossRef]
- De Gryze, S.; Six, J.; Brits, C.; Merckx, R. A quantification of short-term macroaggregate dynamics: Influences of wheat residue input and texture. Soil Biol. Biochem. 2005, 37, 55–66. [Google Scholar] [CrossRef]
- Six, J.; Frey, S.D.; Thiet, R.K.; Batten, K.M. Bacterial and fungal contributions to carbon sequestration in agroecosystems. Soil Sci. Soc. Am. J. 2006, 70, 555–569. [Google Scholar] [CrossRef]
- Zechmeister-Boltenstern, S.; Keiblinger, K.M.; Mooshammer, M.; Peñuelas, J.; Richter, A.; Sardans, J.; Wanek, W. The application of ecological stoichiometry to plant–microbial–soil organic matter transformations. Ecol. Monogr. 2015, 85, 133–155. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.; Wang, C.; Zheng, M.; Jiang, L.; Luo, Y. Patterns and mechanisms of responses by soil microbial communities to nitrogen addition. Soil Biol. Biochem. 2017, 115, 433–441. [Google Scholar] [CrossRef]
- Lladó, S.; López-Mondéjar, R.; Baldrian, P. Forest soil bacteria: Diversity, involvement in ecosystem processes, and response to global change. Microbiol. Mol. Biol. Rev. 2017, 81, e00063-16. [Google Scholar] [CrossRef] [PubMed]
- Strickland, M.S.; Rousk, J. Considering fungal: Bacterial dominance in soils–methods, controls, and ecosystem implications. Soil Biol. Biochem. 2010, 42, 1385–1395. [Google Scholar] [CrossRef]
- Rousk, J.; Bååth, E. Growth of saprotrophic fungi and bacteria in soil. FEMS Microbiol. Ecol. 2011, 78, 17–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Högberg, M.N.; Högberg, P.; Myrold, D.D. Is microbial community composition in boreal forest soils determined by pH, C-to-N ratio, the trees, or all three? Oecologia 2007, 150, 590–601. [Google Scholar] [CrossRef] [PubMed]
- Firestone, M.K.; Davidson, E.A. Microbiological basis of NO and N2O production and consumption in soils. In Exchange of Trace Gases between Terrestrial Ecosystems and the Atmosphere; Andreae, M.O., Schimel, D.S., Eds.; John Wiley and Sons: New York, NY, USA, 1989; Volume 47, pp. 7–21. [Google Scholar]
- Boyle, S.A.; Yarwood, R.R.; Bottomley, P.J.; Myrold, D.D. Bacterial and fungal contributions to soil nitrogen cycling under Douglas fir and red alder at two sites in Oregon. Soil Biol. Biochem. 2008, 40, 443–451. [Google Scholar] [CrossRef]
- Hart, S.C.; Binkley, D.; Perry, D.A. Influence of red alder on soil nitrogen transformations in two conifer forests of contrasting productivity. Soil Biol. Biochem. 1997, 29, 1111–1123. [Google Scholar] [CrossRef]
- Merrick, M.J.; Edwards, R.A. Nitrogen control in bacteria. Microbiol. Rev. 1995, 59, 604–622. [Google Scholar]
- Marzluf, G.A. Genetic regulation of nitrogen metabolism in the fungi. Microbiol Mol. Biol. Rev. 1997, 61, 17–32. [Google Scholar]
- Chen, J.; Stark, J.M. Plant species effects and carbon and nitrogen cycling in a sagebrush–crested wheatgrass soil. Soil Biol. Biochem. 2000, 32, 47–57. [Google Scholar] [CrossRef]
- Ponge, J.F. Humus forms in terrestrial ecosystems: A framework to biodiversity. Soil Biol. Biochem. 2003, 35, 935–945. [Google Scholar] [CrossRef]
- De Vries, F.T.; Van Groenigen, J.W.; Hoffland, E.; Bloem, J. Nitrogen losses from two grassland soils with different fungal biomass. Soil Biol. Biochem. 2011, 43, 997–1005. [Google Scholar] [CrossRef]
- Pennanen, T.; Liski, J.; Bååth, E.; Kitunen, V.; Uotila, J.; Westman, C.J.; Fritze, H. Structure of the microbial communities in coniferous forest soils in relation to site fertility and stand development stage. Microbiol. Ecol. 1999, 38, 168–179. [Google Scholar] [CrossRef]
- Leckie, S.E.; Prescott, C.E.; Grayston, S.J.; Neufeld, J.D.; Mohn, W.W. Characterization of humus microbial communities in adjacent forest types that differ in nitrogen availability. Microbiol. Ecol. 2004, 48, 29–40. [Google Scholar] [CrossRef]
- Rousk, J.; Demoling, L.A.; Bahr, A.; Bååth, E. Examining the fungal and bacterial niche overlap using selective inhibitors in soil. FEMS Microbiol. Ecol. 2008, 63, 350–358. [Google Scholar] [CrossRef] [Green Version]
- McManus, P.S.; Stockwell, V.O.; Sundin, G.W.; Jones, A.L. Antibiotic use in plant agriculture. Annu. Rev. Phytopathol. 2002, 40, 443–465. [Google Scholar] [CrossRef]
- Piotrowska-Seget, Z.; Engel, R.; Nowak, E.; Kozdrój, J. Successive soil treatment with captan or oxytetracycline affects non-target microorganisms. World J. Microbiol. Biotechnol. 2008, 24, 2843. [Google Scholar] [CrossRef]
- Milenkovski, S.; Bååth, E.; Lindgren, P.E.; Berglund, O. Toxicity of fungicides to natural bacterial communities in wetland water and sediment measured using leucine incorporation and potential denitrification. Ecotoxicology 2010, 19, 285–294. [Google Scholar] [CrossRef]
- Chen, S.-K.; Edwards, C.A.; Subler, S. Effects of the fungicides benomyl, captan and chlorothalonil on soil microbial activity and nitrogen dynamics in laboratory incubations. Soil Biol. Biochem. 2001, 33, 1971–1980. [Google Scholar] [CrossRef]
- Bailey, V.L.; Smith, J.L.; Bolton, H. Novel antibiotics as inhibitors for the selective respiratory inhibition method of measuring fungal:bacterial ratios in soil. Biol. Fertil. Soils 2003, 38, 154–160. [Google Scholar] [CrossRef]
- Rousk, J.; Demoling, L.A.; Bååth, E. Contrasting short-term antibiotic effects on respiration and bacterial growth compromises the validity of the selective respiratory inhibition technique to distinguish fungi and bacteria. Microb. Ecol. 2009, 58, 75–85. [Google Scholar] [CrossRef]
- Wang, C.; Wang, F.; Zhang, Q.; Liang, W. Individual and combined effects of tebuconazole and carbendazim on soil microbial activity. Eur. J. Soil Biol. 2016, 72, 6–13. [Google Scholar] [CrossRef]
- Ju, C.; Xu, J.; Wu, X.; Dong, F.; Liu, X.; Tian, C.; Zheng, Y. Effects of hexaconazole application on soil microbes community and nitrogen transformations in paddy soils. Sci. Total Environ. 2017, 609, 655–663. [Google Scholar] [CrossRef]
- Kalia, A.; Gosal, S.K. Effect of pesticide application on soil microorganisms. Arch. Agron. Soil Sci. 2011, 57, 569–596. [Google Scholar] [CrossRef]
- Hedges, L.V.; Gurevitch, J.; Curtis, P.S. The meta-analysis of response ratios in experimental ecology. Ecology 1999, 80, 1150–1156. [Google Scholar] [CrossRef]
- Rosenberg, M.S.; Adams, D.C.; Gurevitch, J. MetaWin: Statistical Software for Meta-Analysis; Sinauer Associates: Sunderland, MA, USA, 2000. [Google Scholar]
- Dijkstra, F.A.; Adams, M.A. Fire eases imbalances of nitrogen and phosphorus in woody plants. Ecosystems 2015, 18, 769–779. [Google Scholar] [CrossRef]
- Hedges, L.V.; Olkin, I. Statistical Methods for Meta-Analysis; Academic Press, Inc.: Orlando, FL, USA, 1985. [Google Scholar]
- Cardinale, B.J.; Srivastava, D.S.; Duffy, J.E.; Wright, J.P.; Downing, A.L.; Sankaran, M.; Jouseau, C. Effects of biodiversity on the functioning of trophic groups and ecosystems. Nature 2006, 443, 989–992. [Google Scholar] [CrossRef]
- Van Groenigen, K.J.; Osenberg, C.W.; Hungate, B.A. Increased soil emissions of potent greenhouse gases under increased atmospheric CO2. Nature 2011, 475, 214–216. [Google Scholar] [CrossRef]
- Strickland, T.C.; Potter, T.L.; Joo, H. Tebuconazole dissipation and metabolism in Tifton loamy sand during laboratory incubation. Pest Manag. Sci. 2004, 60, 703–709. [Google Scholar] [CrossRef]
- Muñoz-Leoz, B.; Ruiz-Romera, E.; Antigüedad, I.; Garbisu, C. Tebuconazole application decreases soil microbial biomass and activity. Soil Biol. Biochem. 2011, 43, 2176–2183. [Google Scholar] [CrossRef]
- Chen, S.-K.; Edwards, C.A.; Subler, S. A microcosm approach for evaluating the effects of the fungicides benomyl and captan on soil ecological processes and plant growth. Appl. Soil Ecol. 2001, 18, 69–82. [Google Scholar] [CrossRef]
- Castaldi, S.; Smith, K.A. Effect of cycloheximide on N2O and NO3− production in a forest and an agricultural soil. Biol. Fertil. Soils 1998, 27, 27–34. [Google Scholar] [CrossRef]
- Isobe, K.; Koba, K.; Otsuka, S.; Senoo, K. Nitrification and nitrifying microbial communities in forest soils. J. For. Res. 2011, 16, 351–362. [Google Scholar] [CrossRef]
- Zhu, T.; Meng, T.; Zhang, J.; Zhong, W.; Müller, C.; Cai, Z. Fungi-dominant heterotrophic nitrification in a subtropical forest soil of China. J. Soils Sediments 2015, 15, 705–709. [Google Scholar] [CrossRef]
- Kampschreur, M.J.; Temmink, H.; Kleerebezemm, R.; Jetten, M.S.M.; van Loosdrecht, M.C.M. Nitrous oxide emission during wastewater treatment. Water Res. 2009, 43, 4093–4103. [Google Scholar] [CrossRef]
- Shen, J.P.; Xu, Z.; He, J.Z. Frontiers in the microbial processes of ammonia oxidation in soils and sediments. J. Soils Sediments 2014, 14, 1023–1029. [Google Scholar] [CrossRef] [Green Version]
- Maeda, K.; Spor, A.; Edel-Hermann, V.; Heraud, C.; Breuil, M.-C.; Bizouard, F.; Toyoda, S.; Yoshida, N.; Steinberg, C.; Philippot, L. N2O production, a widespread trait in fungi. Sci. Rep. 2015, 5, 19697–19704. [Google Scholar] [CrossRef] [PubMed]
- Marusenko, Y.; Huber, D.P.; Hall, S.J. Fungi mediate nitrous oxide production but not ammonia oxidation in aridland soils of the southwestern US. Soil Biol. Biochem. 2013, 63, 24–36. [Google Scholar] [CrossRef]
- Muñoz-Leoz, B.; Garbisu, C.; Charcosset, J.-Y.; Sánchez-Pérez, J.M.; Antigüedad, I.; Ruiz-Romera, E. Non-target effects of three formulated pesticides on microbially-mediated processes in a clay-loam soil. Sci. Total Environ. 2013, 449, 345–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ananyeva, N.D.; Ivashchenko, K.V.; Stolnikova, E.V.; Stepanov, A.L.; Kudeyarov, V.N. Specific features of determination of the net production of nitrous oxide by soils. Eurasian Soil Sci. 2015, 48, 608–619. [Google Scholar] [CrossRef]
- Ma, S.; Shan, J.; Yan, X. N2O emissions dominated by fungi in an intensively managed vegetable field converted from wheat–rice rotation. Appl. Soil Ecol. 2017, 116, 23–29. [Google Scholar] [CrossRef]
- Johansson, J.F.; Paul, L.R.; Finlay, R.D. Microbial interactions in the mycorrhizosphere and their significance for sustainable agriculture. FEMS Microbiol. Ecol. 2004, 48, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kayranli, B.; Scholz, M.; Mustafa, A.; Hedmark, Å. Carbon storage and fluxes within freshwater wetlands: A critical review. Wetlands 2010, 30, 111–124. [Google Scholar] [CrossRef]
- Reddy, K.R.; DeLaune, R.D. Biogeochemistry of Wetlands: Science and Applications, 1st ed.; CRC press, Taylor and Francis Group: Boca Raton, FL, USA, 2008; p. 800. [Google Scholar]
- Baldrian, P. Forest microbiome: Diversity, complexity and dynamics. FEMS Microbiol. Rev. 2017, 41, 109–130. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Chan, Y.; Bugler-Lacap, D.C.; Bhatnagar, A.; Bhatnagar, M.; Pointing, S.B. Microbial diversity in soil, sand dune and rock substrates of the Thar Monsoon Desert, India. Indian J. Microbiol. 2016, 56, 35–45. [Google Scholar] [CrossRef]
- Crenshaw, C.L.; Lauber, C.; Sinsabaugh, R.L.; Stavely, L.K. Fungal control of nitrous oxide production in semiarid grassland. Biogeochemistry 2008, 87, 17–27. [Google Scholar] [CrossRef]
- McLain, J.E.T.; Martens, D.A. N2O production by heterotrophic N transformations in a semiarid soil. Appl. Soil Ecol. 2006, 32, 253–263. [Google Scholar] [CrossRef]
- Rousk, J.; Brookes, P.C.; Bååth, E. Contrasting soil pH effects on fungal and bacterial growth suggest functional redundancy in carbon mineralization. Appl. Environ. Microbiol. 2009, 75, 1589–1596. [Google Scholar] [CrossRef] [PubMed]
- Rousk, J.; Bååth, E.; Brookes, P.C.; Lauber, C.L.; Lozupone, C.; Caporaso, J.G.; Knight, R.; Fierer, N. Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J. 2010, 4, 1340–1351. [Google Scholar] [CrossRef] [PubMed] [Green Version]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullah, M.R.; Dijkstra, F.A. Fungicide and Bactericide Effects on Carbon and Nitrogen Cycling in Soils: A Meta-Analysis. Soil Syst. 2019, 3, 23. https://doi.org/10.3390/soilsystems3020023
Ullah MR, Dijkstra FA. Fungicide and Bactericide Effects on Carbon and Nitrogen Cycling in Soils: A Meta-Analysis. Soil Systems. 2019; 3(2):23. https://doi.org/10.3390/soilsystems3020023
Chicago/Turabian StyleUllah, Mohammad Rahmat, and Feike A. Dijkstra. 2019. "Fungicide and Bactericide Effects on Carbon and Nitrogen Cycling in Soils: A Meta-Analysis" Soil Systems 3, no. 2: 23. https://doi.org/10.3390/soilsystems3020023
APA StyleUllah, M. R., & Dijkstra, F. A. (2019). Fungicide and Bactericide Effects on Carbon and Nitrogen Cycling in Soils: A Meta-Analysis. Soil Systems, 3(2), 23. https://doi.org/10.3390/soilsystems3020023





