Seasonal Salinization Decreases Spatial Heterogeneity of Sulfate Reducing Activity
Abstract
:1. Introduction
2. Materials and Methods
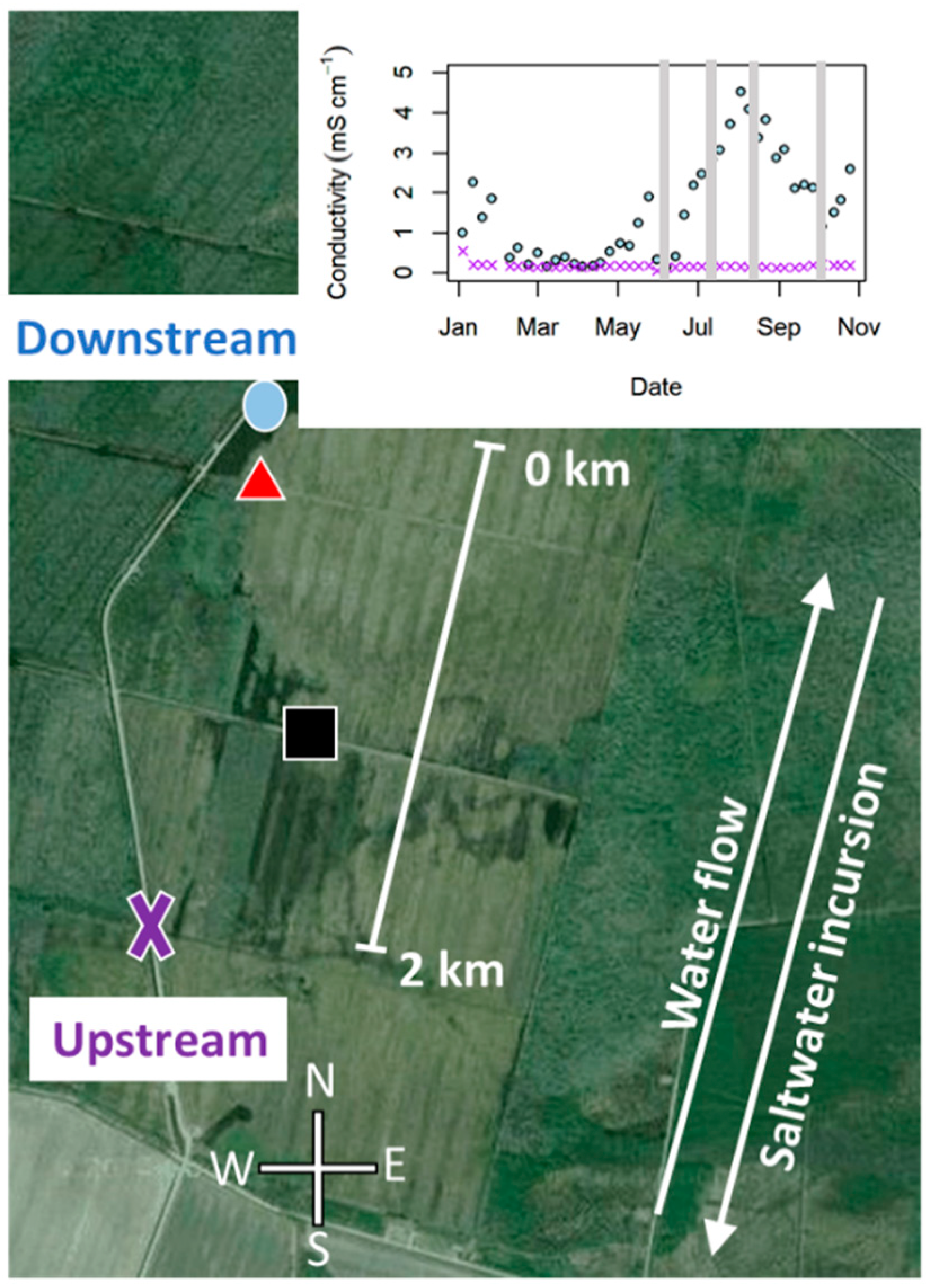
2.1. Site Description
2.2. Traditional Soil and Porewater Chemistry
2.3. Indicator of Reduction in Soils
2.4. Geostatistical Analysis
2.5. Relationships between Tranditional Chemistry and IRIS Analysis
2.6. IRIS Method Advantages and Limitations for Measuring FeS
3. Results
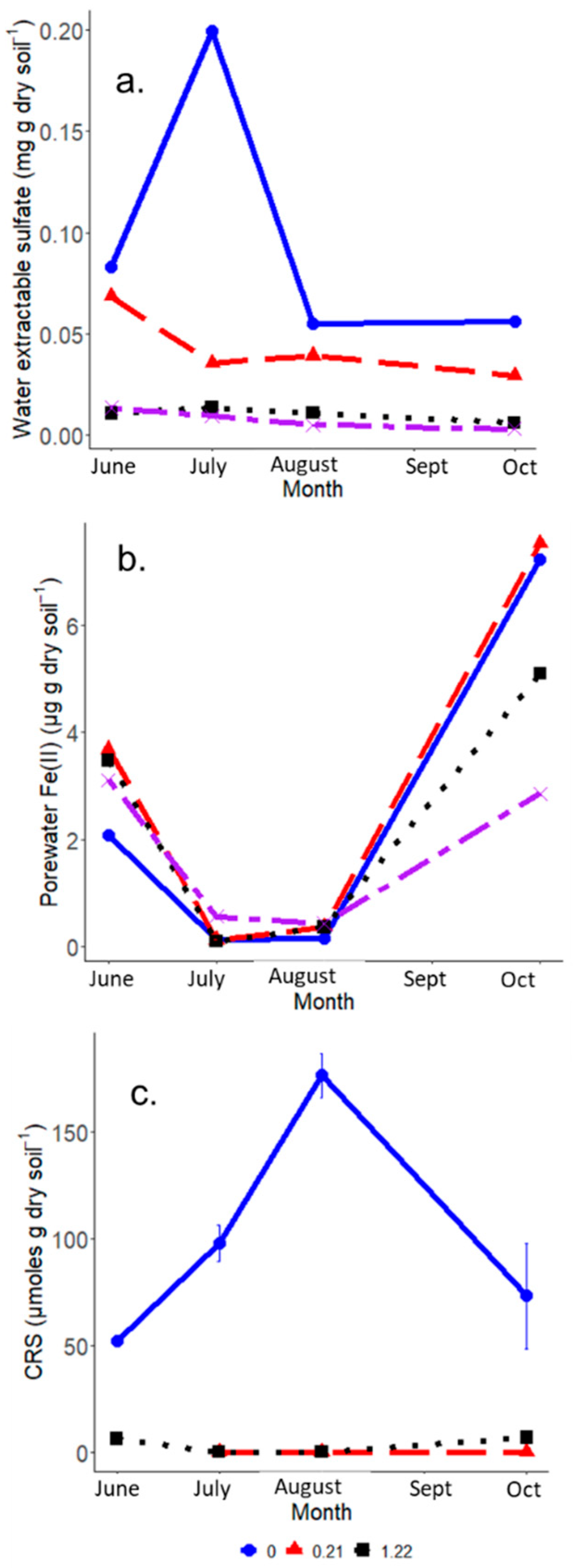
3.1. Soil and Porewater Chemistry
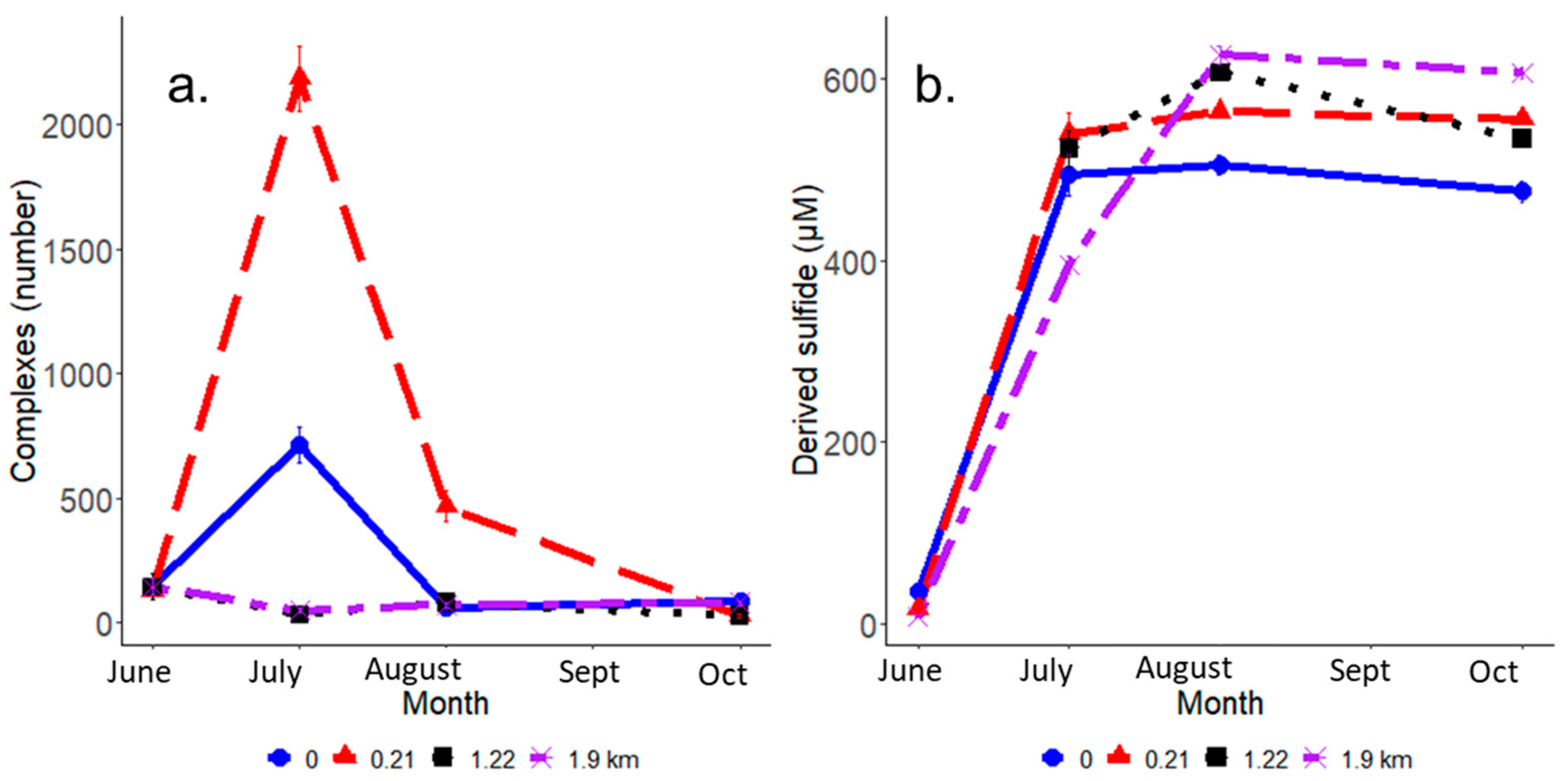
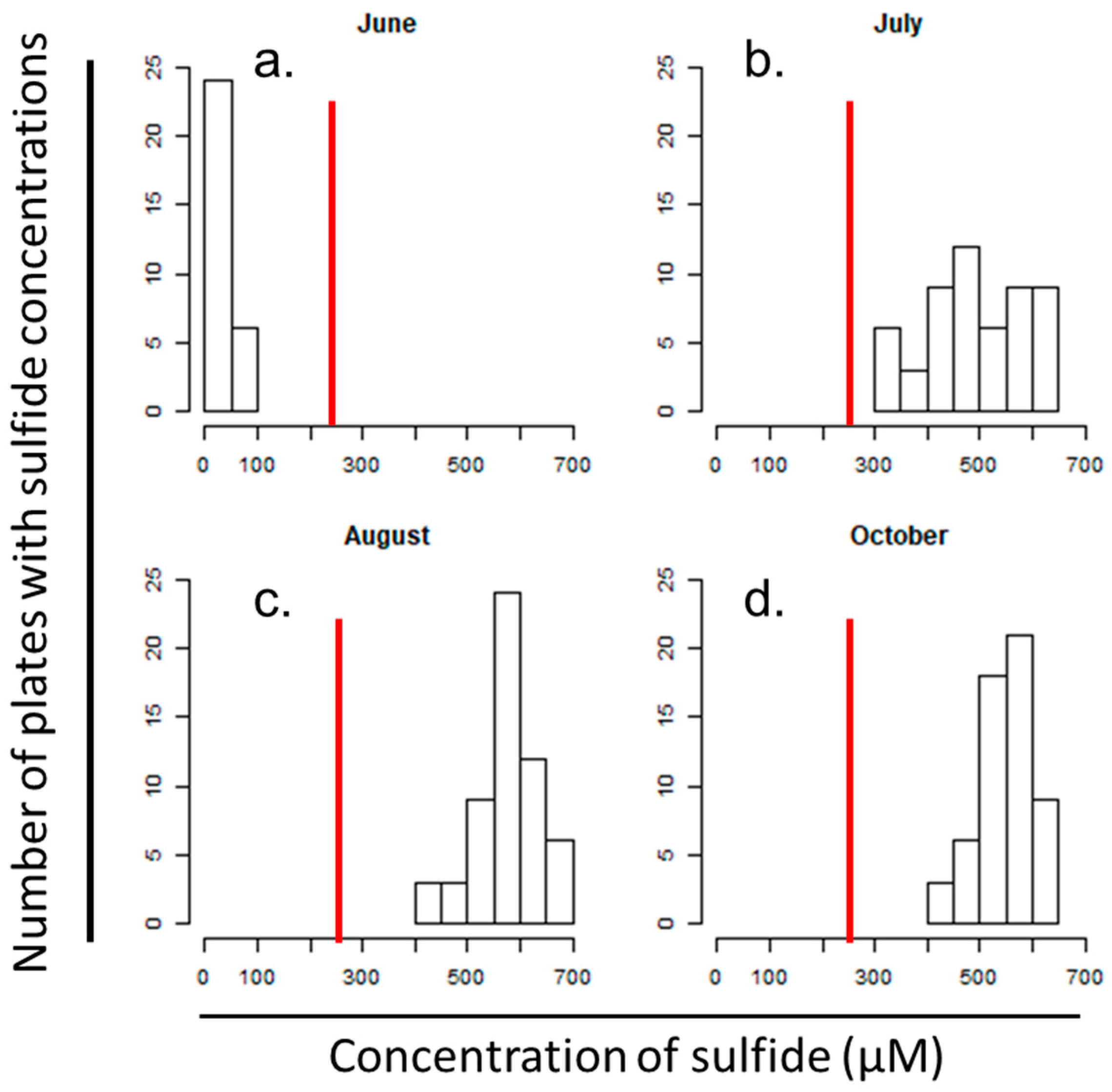
3.2. IRIS Method Indicators of Altered FeS Activity Across the Saltwater Incursion Gradient
4. Discussion
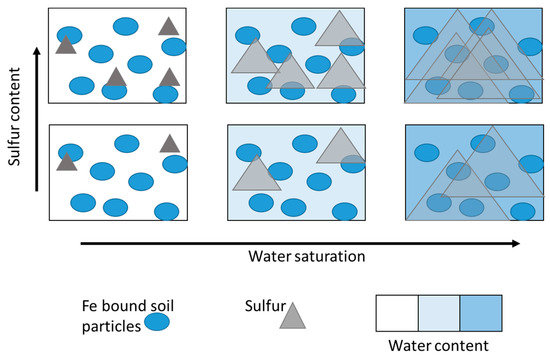
4.1. A Saltwater Reaction Front
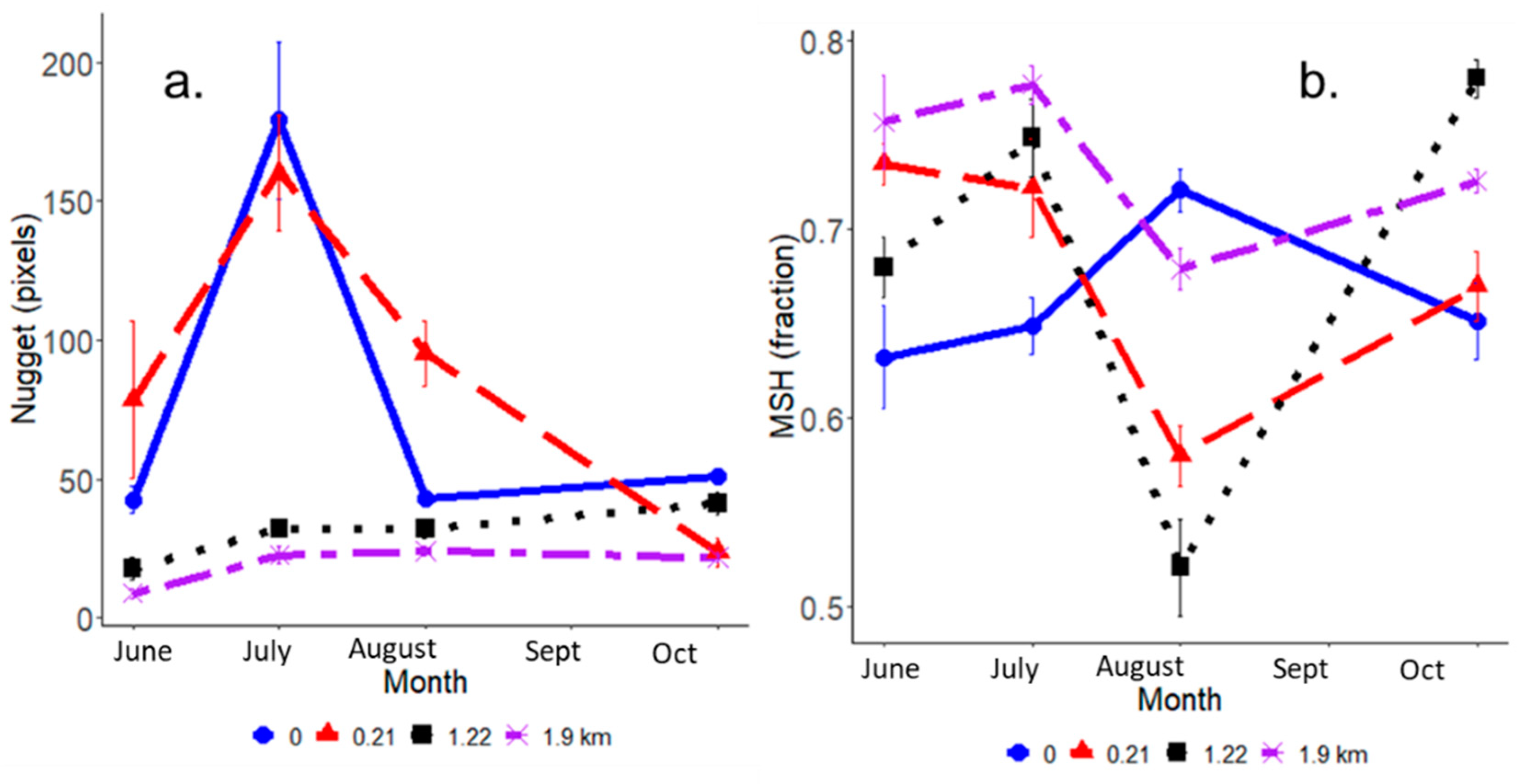
4.2. Detailed View of Heterogeneity Parameters under Heavy Salt Water Influence
4.3. IRIS Method Determination of FeS Concentrations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hines, M.M.E.M.; Knollmeyer, S.S.L.S.; Tugel, J.; Tugei, J.B.; Tugel, J. Sulfate reduction and other sedimentary biogeochemistry in a northern New England salt marsh. Limnol. Oceanogr. 1989, 34, 578–590. [Google Scholar] [CrossRef] [Green Version]
- Weston, N.B.; Dixon, R.E.; Joye, S.B. Ramifications of increased salinity in tidal freshwater sediments: Geochemistry and microbial pathways of organic matter mineralization. J. Geophys. Res. 2006, 111, 1–14. [Google Scholar] [CrossRef]
- Schoepfer, V.A.; Bernhardt, E.S.; Burgin, A.J. Iron clad wetlands: Soil iron-sulfur buffering determines coastal wetland response to salt water incursion. J. Geophys. Res. Biogeosci. 2014, 119, 2209–2219. [Google Scholar] [CrossRef] [Green Version]
- Van Dijk, G.; Smolders, A.J.P.; Loeb, R.; Bout, A.; Roelofs, J.G.M.; Lamers, L.P.M. Salinization of coastal freshwater wetlands; effects of constant versus fluctuating salinity on sediment biogeochemistry. Biogeochemistry 2015, 126, 71–84. [Google Scholar] [CrossRef]
- Herbert, E.R.; Boon, P.; Burgin, A.J.A.J.; Neubauer, S.C.S.C.; Franklin, R.B.R.B.; Ardón, M.; Hopfensperger, K.N.; Lamers, L.P.M.; Gell, P.; Ardon, M.; et al. A global perspective on wetland salinization: Ecological consequences of a growing threat to freshwater wetlands. Ecosphere 2015, 6, art206. [Google Scholar] [CrossRef]
- Burton, E.D.; Bush, R.T.; Sullivan, L.A.; Hocking, R.K.; Mitchell, D.R.G.; Johnston, S.G.; Fitzpatrick, R.W.; Raven, M.; McClure, S.; Jang, L.Y. Iron-Monosulfide Oxidation in Natural Sediments: Resolving Microbially Mediated S Transformations Using XANES, Electron Microscopy, and Selective Extractions. Environ. Sci. Technol. 2009, 43, 3128–3134. [Google Scholar] [CrossRef]
- Gao, Y.; van de Velde, S.; Williams, P.N.; Baeyens, W.; Zhang, H. Two-dimensional images of dissolved sulfide and metals in anoxic sediments by a novel diffusive gradients in thin film probe and optical scanning techniques. TrAC Trends Anal. Chem. 2015, 66, 63–71. [Google Scholar] [CrossRef]
- Niazi, N.K.; Burton, E.D. Arsenic sorption to nanoparticulate mackinawite (FeS): An examination of phosphate competition. Environ. Pollut. 2016, 218, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Karimian, N.; Johnston, S.G.; Burton, E.D. Effect of cyclic redox oscillations on water quality in freshwater acid sulfate soil wetlands. Sci. Total Environ. 2017, 581–582, 314–327. [Google Scholar] [CrossRef] [PubMed]
- Borch, T.; Kretzschmar, R.; Kappler, A.; Cappellen, P.V.; Ginder-Vogel, M.; Voegelin, A.; Campbell, K. Biogeochemical Redox Processes and their Impact on Contaminant Dynamics. Environ. Sci. Technol. 2010, 44, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Burton, E.D.; Sullivan, L.A.; Bush, R.T.; Johnston, S.G.; Keene, A.F. A simple and inexpensive chromium-reducible sulfur method for acid-sulfate soils. Appl. Geochem. 2008, 23, 2759–2766. [Google Scholar] [CrossRef]
- Kefi, S.; Guttal, V.; Brock, W.A.; Carpenter, S.R.; Ellison, A.M.; Livina, V.N.; Seekell, D.A.; Scheffer, M.; Van Nes, E.H.; Dakos, V.; et al. Early warning signals of ecological transitions: Methods for spatial patterns. PLoS ONE 2014, 9, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Santner, J.; Larsen, M.; Kreuzeder, A.; Glud, R.N. Two decades of chemical imaging of solutes in sediments and soils—A review. Anal. Chim. Acta 2015, 878, 9–42. [Google Scholar] [CrossRef] [PubMed]
- Johnston, S.G.; Burton, E.D.; Aaso, T.; Tuckerman, G. Sulfur, iron and carbon cycling following hydrological restoration of acidic freshwater wetlands. Chem. Geol. 2014, 371, 9–26. [Google Scholar] [CrossRef]
- Ettema, C.H.; Wardle, D.A. Spatial soil ecology. Trends Ecol. Evol. 2002, 17, 177–183. [Google Scholar] [CrossRef]
- Bennett, W.W.; Teasdale, P.R.; Welsh, D.T.; Panther, J.G.; Jolley, D.F. Optimization of colorimetric DET technique for the in situ, two-dimensional measurement of iron(II) distributions in sediment porewaters. Talanta 2012, 88, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Stockdale, A.; Davison, W.; Zhang, H. Micro-scale biogeochemical heterogeneity in sediments: A review of available technology and observed evidence. Earth-Sci. Rev. 2009, 92, 81–97. [Google Scholar] [CrossRef] [Green Version]
- Robertson, D.; Welsh, D.T.; Teasdale, P.R. Investigating biogenic heterogeneity in coastal sediments with two-dimensional measurements of iron(II) and sulfide. Environ. Chem. 2009, 6, 60–69. [Google Scholar] [CrossRef]
- Viollier, E.; Rabouille, C.; Apitz, S.E.; Breuer, E.; Chaillou, G.; Dedieu, K.; Furukawa, Y.; Grenz, C.; Hall, P.; Janssen, F.; et al. Benthic biogeochemistry: State of the art technologies and guidelines for the future of in situ survey. J. Exp. Mar. Biol. Ecol. 2003, 285–286, 5–31. [Google Scholar] [CrossRef]
- Gao, Y.; Leermakers, M.; Elskens, M.; Billon, G.; Ouddane, B.; Fischer, J.C.; Baeyens, W. High resolution profiles of thallium, manganese and iron assessed by DET and DGT techniques in riverine sediment pore waters. Sci. Total Environ. 2007, 373, 526–533. [Google Scholar] [CrossRef]
- Vandenhove, H.; Antunes, K.; Wannijn, J.; Duquène, L.; Van Hees, M. Method of diffusive gradients in thin films (DGT) compared with other soil testing methods to predict uranium phytoavailability. Sci. Total Environ. 2007, 373, 542–555. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Wang, S.; Jiao, L.; Wu, F. The simultaneous measurement of phosphorus, sulfide, and trace metals by ferrihydrite/AgI/Chelex-100 DGT (diffusive gradients in thin films) Probe at sediment/water interface (SWI) and remobilization assessment. Water Air Soil Pollut. 2014, 225. [Google Scholar] [CrossRef]
- Neubauer, S.C.; Anderson, I.C.; Neikirk, B.B. Nitrogen cycling and ecosystem exchanges in a Virginia tidal freshwater marsh. Estuaries 2005, 28, 909–922. [Google Scholar] [CrossRef]
- Rabenhorst, M.C.; Megonigal, J.P.; Keller, J. Synthetic Iron Oxides for Documenting Sulfide in Marsh Pore Water. Soil Sci. Soc. Am. J. 2010, 74, 1383. [Google Scholar] [CrossRef]
- Rabenhorst, M.C. A System for Making and Deploying Oxide-Coated Plastic Films for Environmental Assessment of Soils. Soil Sci. Soc. Am. J. 2018, 82, 1301. [Google Scholar] [CrossRef]
- Rabenhorst, M.C.; Burch, S.N. Synthetic Iron Oxides as an Indicator of Reduction in Soils (IRIS). Soil Sci. Soc. Am. J. 2006, 70, 1227. [Google Scholar] [CrossRef]
- Rabenhorst, M.C. Visual Assessment of IRIS Tubes in Field Testing for Soil Reduction. Wetlands 2010, 30, 847–852. [Google Scholar] [CrossRef]
- Vaness, B.M.; Wilson, S.D.; Macdougall, A.S. Decreased root heterogeneity and increased root length following grassland invasion. Funct. Ecol. 2014, 28, 1266–1273. [Google Scholar] [CrossRef] [Green Version]
- Zuo, X.A.; Zhao, X.Y.; Zhao, H.L.; Guo, Y.R.; Zhang, T.H.; Cui, J.Y. Spatial pattern and heterogeneity of soil organic carbon and nitrogen in sand dunes related to vegetation change and geomorphic position in Horqin Sandy Land, Northern China. Environ. Monit. Asses. 2010, 164, 29–42. [Google Scholar] [CrossRef]
- Lin, Y.; Hong, M.; Han, G.; Zhao, M.; Bai, Y.; Chang, S.X. Grazing intensity affected spatial patterns of vegetation and soil fertility in a desert steppe. Agric. Ecosyst. Environ. 2010, 138, 282–292. [Google Scholar] [CrossRef]
- Schlesinger, W.H.; Raikes, J.A.; Hartley, A.E.; Cross, A.F. On the Spatial Pattern of Soil Nutrients in Desert Ecosystems. Ecology 1996, 77, 364–374. [Google Scholar] [CrossRef]
- Ardón, M.; Morse, J.L.; Colman, B.P.; Bernhardt, E.S. Drought-induced saltwater incursion leads to increased wetland nitrogen export. Glob. Chang. Biol. 2013, 19, 2976–2985. [Google Scholar] [CrossRef]
- Ardón, M.; Montanari, S.; Morse, J.L.; Doyle, M.W.; Bernhardt, E.S. Phosphorus export from a restored wetland ecosystem in response to natural and experimental hydrologic fluctuations. J. Geophys. Res. 2010, 115. [Google Scholar] [CrossRef] [Green Version]
- Morse, J.L.; Ardón, M.; Bernhardt, E.S. Greenhouse gas fluxes in southeastern U.S. coastal plain wetlands under contrasting land uses. Ecol. Appl. 2012, 22, 264–280. [Google Scholar] [CrossRef] [Green Version]
- Helton, A.M.; Bernhardt, E.S.; Fedders, A. Biogeochemical regime shifts in coastal landscapes: The contrasting effects of saltwater incursion and agricultural pollution on greenhouse gas emissions from a freshwater wetland. Biogeochemistry 2014, 120, 133–147. [Google Scholar] [CrossRef]
- Ardón, M.; Helton, A.M.; Scheuerell, M.D.; Bernhardt, E.S. Fertilizer legacies meet saltwater incursion: Challenges and constraints for coastal plain wetland restoration. Elem. Sci. Anth. 2017, 5, 41. [Google Scholar] [CrossRef]
- Ardón, M.; Morse, J.L.; Doyle, M.W.; Bernhardt, E.S. The Water Quality Consequences of Restoring Wetland Hydrology to a Large Agricultural Watershed in the Southeastern Coastal Plain. Ecosystems 2010, 13, 1060–1078. [Google Scholar] [CrossRef]
- Neubauer, S.C.; Givler, K.; Valentine, S.S.; Megonigal, J.P. Seasonal Patterns and Plant-Mediated Controls of Subsurface Wetland Biogeochemistry. Ecology 2005, 86, 3334–3344. [Google Scholar] [CrossRef]
- Fossing, H.; Jørgensen, B.B. Measurement of bacterial sulfate reduction in sediments: Evaluation of a single-step chromium reduction method. Biogeochemistry 1989, 8, 205–222. [Google Scholar] [CrossRef]
- Allen, H.E.; Fu, G.; Deng, B. Analysis of acid volatile sulfide (AVS) and simultaneously extracted metals (SEM) for the estimation of potential toxicity in aquatic sediments. Environ. Toxicol. Chem. 1993, 12, 1441–1453. [Google Scholar] [CrossRef]
- Jenkinson, B.J.; Franzmeier, D.P. Development and Evaluation of Iron-Coated Tubes that Indicate Reduction in Soils. Soil Sci. Soc. Am. J. 2006, 70, 183. [Google Scholar] [CrossRef]
- Rasband, W.S. ImageJ; U. S. National Institutes of Health: Bethesda, MD, USA, 1997.
- Pebesma, E. Spatio-temporal geostatistics using gstat. Available online: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.432.106&rep=rep1&type=pdf (accessed on 31 March 2019).
- Pebesma, E.; Bivand, R.S.S. Classes and Methods for Spatial Data: The sp Package. R News 5 (2). 2005, pp. 1–21. Available online: https://cran.r-project.org/doc/Rnews/ (accessed on 31 March 2019).
- Bivand, R.S.; Pebesma, E.J.; Gomez-Rubio, V. Applied Spatial Data Analysis with R, 2nd ed.; Springer: New York, NY, USA, 2013. [Google Scholar]
- Wickham, H. The Split-Apply-Combine Strategy for Data Analysis. J. Stat. Softw. 2011, 40, 1–29. [Google Scholar] [CrossRef]
- Redd, A. Dostats: Compute Statistics Helper Functions. 2015, pp. 1–13. Available online: https://github.com/halpo/dostats (accessed on 31 March 2019).
- Sonderegger, D. SiZer: SiZer: Significant Zero Crossings. 2015, pp. 1–15. Available online: https://github.com/dereksonderegger/SiZer (accessed on 31 March 2019).
- Lane, D.R.; BassiriRad, H. Diminishing Spatial Heterogeneity in Soil Organic Matter across a Prairie Restoration Chronosequence. Restor. Ecol. 2005, 13, 403–412. [Google Scholar] [CrossRef]
- Paul, S.; Kusel, K.; Alewell, C. Reduction processes in forest wetlands: Tracking down heterogeneity of source/sink functions with a combination of methods. Soil Biol. Biochem. 2006, 38, 1028–1039. [Google Scholar] [CrossRef]
- Rickard, D.; Morse, J.W. Acid volatile sulfide (AVS). Mar. Chem. 2005, 97, 141–197. [Google Scholar] [CrossRef]
- Burton, E.D.; Bush, R.T.; Sullivan, L.A. Sedimentary iron geochemistry in acidic waterways associated with coastal lowland acid sulfate soils. Geochim. Cosmochim. Acta 2006, 70, 5455–5468. [Google Scholar] [CrossRef]
- Orr, C.H.; Predick, K.I.; Stanley, E.H.; Rogers, K.L. Spatial autocorrelation of denitrification in a restored and a natural floodplain. Wetlands 2014, 34, 89–100. [Google Scholar] [CrossRef]
- Harms, T.K.; Wentz, E.A.; Grimm, N.B. Spatial heterogeneity of denitrification in semi-arid floodplains. Ecosystems 2009, 12, 129–143. [Google Scholar] [CrossRef]
- Ekschmitt, K.; Kandeler, E.; Poll, C.; Brune, A.; Buscot, F.; Friedrich, M.; Gleixner, G.; Hartmann, A.; Kästner, M.; Marhan, S.; et al. Soil-carbon preservation through habitat constraints and biological limitations on decomposer activity. J. Plant Nutr. Soil Sci. 2008, 171, 27–35. [Google Scholar] [CrossRef]
- Kraal, P.; Burton, E.D.; Bush, R.T. Iron monosulfide accumulation and pyrite formation in eutrophic estuarine sediments. Geochim. Cosmochim. Acta 2013, 122, 75–88. [Google Scholar] [CrossRef]
- Burton, E.D.; Bush, R.; Sullivan, L. Fractionation and extractability of sulfur, iron and trace elements in sulfidic sediments. Chemosphere 2006, 64, 1421–1428. [Google Scholar] [CrossRef]
- Kallmeyer, J.; Ferdelman, T.G.; Weber, A.; Fossing, H.; Jørgensen, B.B. A cold chromium distillation procedure for radiolabeled sulfide applied to sulfate reduction measurements: Sulfate reduction cold Cr-II method. Limnol. Oceanog. Methods 2004, 2, 171–180. [Google Scholar] [CrossRef]
- Concostrina-Zubiri, L.; Huber-Sannwald, E.; Martínez, I.; Flores Flores, J.L.; Escudero, A. Biological soil crusts greatly contribute to small-scale soil heterogeneity along a grazing gradient. Soil Biol. Biochem. 2013, 64, 28–36. [Google Scholar] [CrossRef]
- Jackson, R.B.; Caldwell, M. Geostatistical patterns of soil heterogeneity around individual perennial plants. J. Ecol. 1993, 81, 683–692. [Google Scholar] [CrossRef]
- Burt, T.P.; Matchett, L.S.; Goulding, K.W.T.; Webster, C.P.; Haycock, N.E. Denitrification in riparian buffer zones: The role of floodplain hydrology. Hydrol. Process. 1999, 13, 1451–1463. [Google Scholar] [CrossRef]
- Burt, T.P.; Pinay, G. Linking hydrology and biogeochemistry in complex landscapes. Progr. Phys. Geogr. 2005, 29, 297–316. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schoepfer, V.A.; Burgin, A.J.; Loecke, T.D.; Helton, A.M. Seasonal Salinization Decreases Spatial Heterogeneity of Sulfate Reducing Activity. Soil Syst. 2019, 3, 25. https://doi.org/10.3390/soilsystems3020025
Schoepfer VA, Burgin AJ, Loecke TD, Helton AM. Seasonal Salinization Decreases Spatial Heterogeneity of Sulfate Reducing Activity. Soil Systems. 2019; 3(2):25. https://doi.org/10.3390/soilsystems3020025
Chicago/Turabian StyleSchoepfer, Valerie A., Amy J. Burgin, Terry D. Loecke, and Ashley M. Helton. 2019. "Seasonal Salinization Decreases Spatial Heterogeneity of Sulfate Reducing Activity" Soil Systems 3, no. 2: 25. https://doi.org/10.3390/soilsystems3020025
APA StyleSchoepfer, V. A., Burgin, A. J., Loecke, T. D., & Helton, A. M. (2019). Seasonal Salinization Decreases Spatial Heterogeneity of Sulfate Reducing Activity. Soil Systems, 3(2), 25. https://doi.org/10.3390/soilsystems3020025