Sustainability of Impacts of Poplar Growth on Soil Organic Matter in Eutric Cambisols
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites and Soil Sampling
2.2. Determination of the Soil Carbon (C) Contents and C Stocks
2.3. Molecular-Chemical Composition of the Soil Organic Matter
2.4. Isolation of Total Lipids from Soil and Quantification by Quadrupole Time-of-Flight Mass Spectrometry
2.5. PLFA Analyses
2.6. Soil Enzyme Analyses
2.7. Statistical Analyses
3. Results
3.1. Soil Organic Matter Quantity
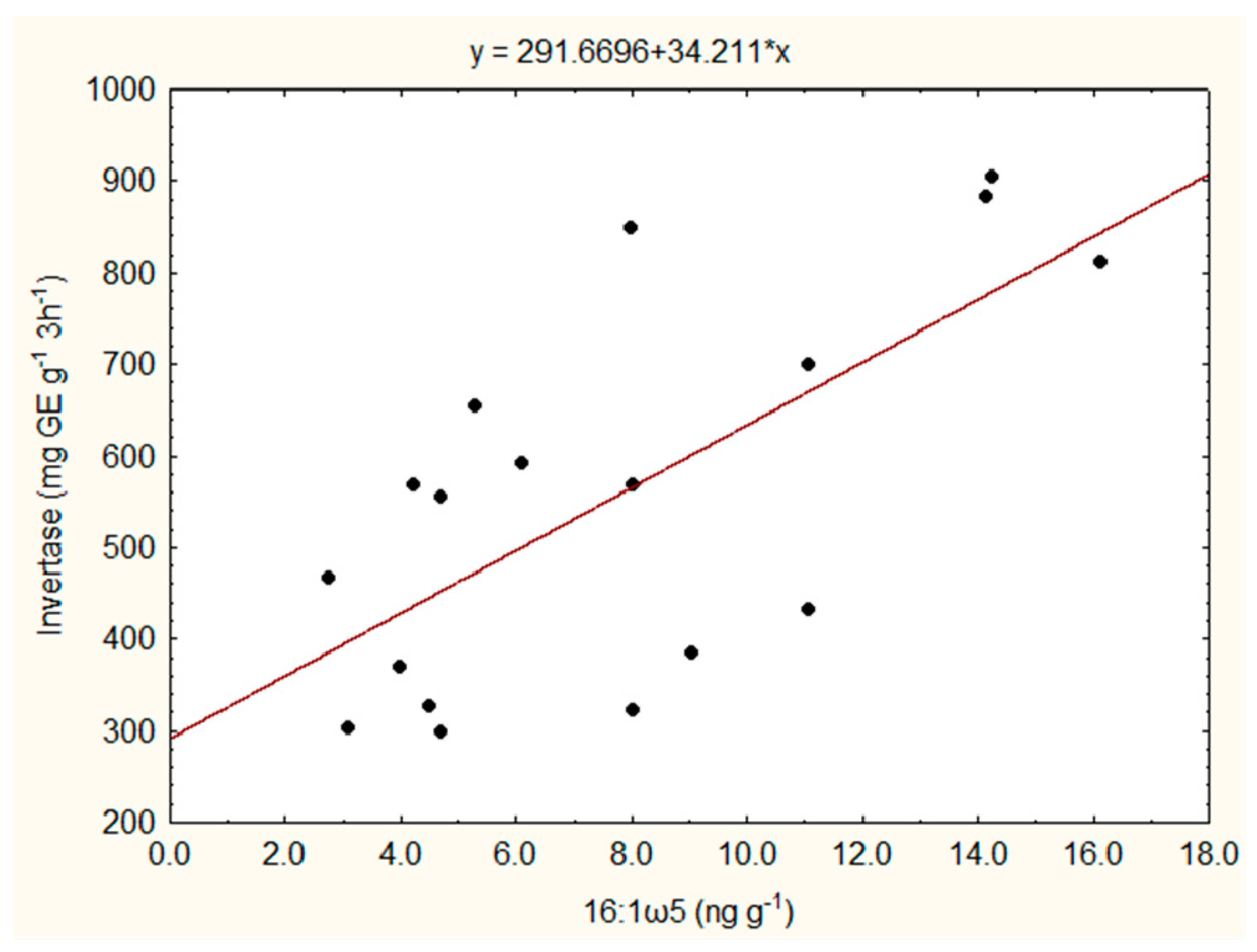
3.2. PLFA Pattern and Soil Enzyme Activities
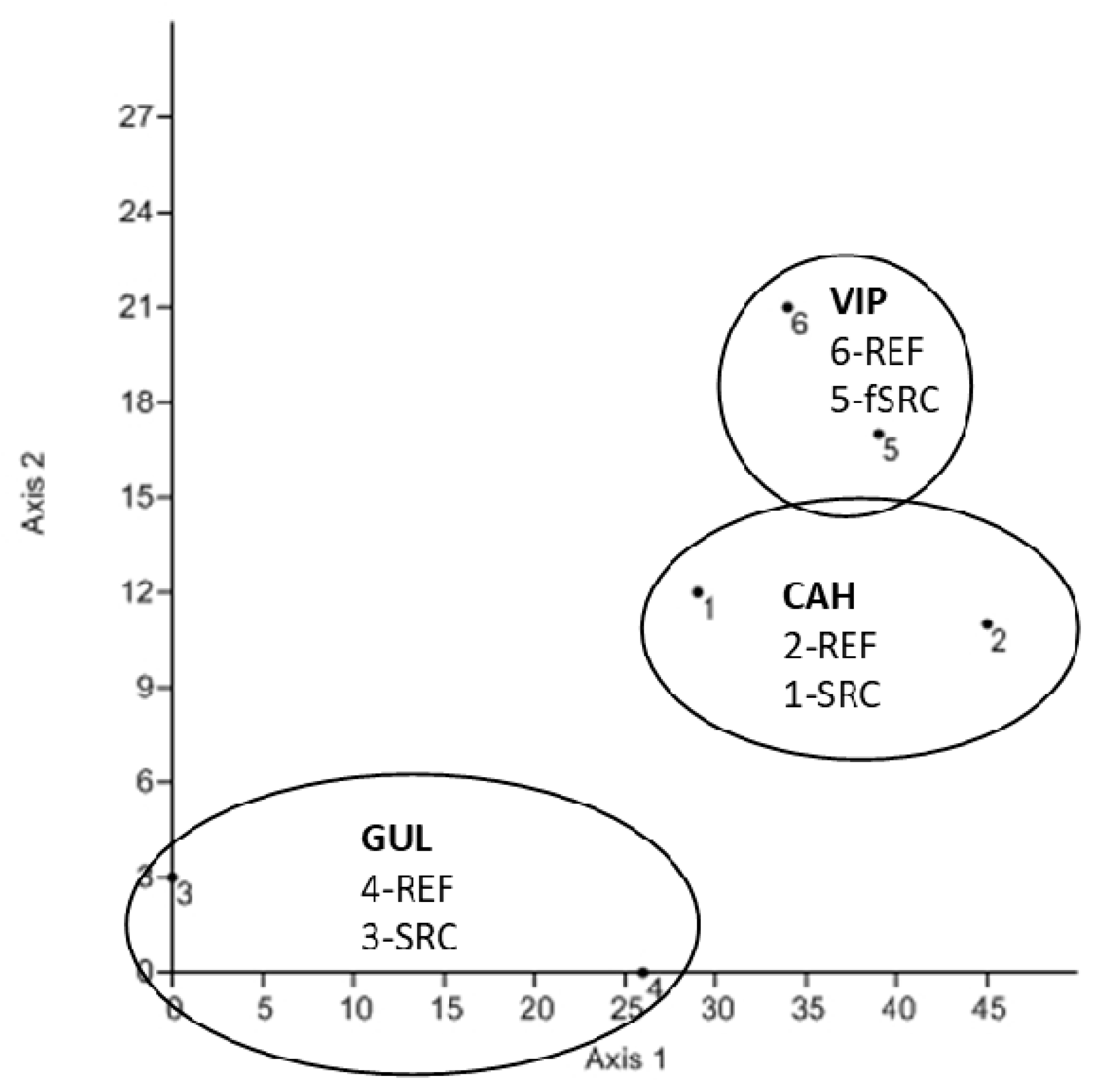
3.3. SOM Composition
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Buonocore, E.; Franzese, P.P.; Ulgiati, S. Assessing the environmental performance and sustainability of bioenergy production in Sweden: A life cycle assessment perspective. Energy 2012, 37, 69–78. [Google Scholar] [CrossRef]
- Coleman, M.D.; Isebrands, J.G.; Tolsted, D.N.; Tolbert, V.R. Comparing soil carbon of short rotation poplar plantations with agricultural crops and woodlots in North Central United States. Environ. Manag. 2004, 33, 299–308. [Google Scholar] [CrossRef]
- Jose, S.; Bardhan, S. Agroforestry for biomass production and carbon sequestration: An overview. Agrofor. Syst. 2012, 86, 105–111. [Google Scholar] [CrossRef]
- Baum, C.; Leinweber, P.; Weih, M.; Lamersdorf, N.; Dimitriou, I. Effects of short rotation coppice with willows and poplar on soil ecology. vTI Agric. For. Res. 2009, 59, 183–196. [Google Scholar]
- Kahle, P.; Möller, J.; Baum, C.; Gurgel, A. Tillage-induced changes in the distribution of soil organic matter and soil aggregate stability under a former short rotation coppice. Soil Tillage Res. 2013, 133, 49–53. [Google Scholar] [CrossRef]
- Arevalo, C.B.; Bhatti, J.S.; Chang, S.X.; Sidders, D. Land use change effects on ecosystem carbon balance: From agricultural to hybrid poplar plantation. Agric. Ecosyst. Environ. 2011, 141, 342–349. [Google Scholar] [CrossRef]
- Stauffer, M.; Leyval, C.; Brun, J.-J.; Leportier, P.; Berthelin, P. Effect of willow short rotation coppice on soil properties after three years of growth as compared to forest, grassland and arable land uses. Plant Soil 2014, 377, 423–438. [Google Scholar] [CrossRef]
- Benbi, D.K.; Brar, K.; Toor, A.S.; Singh, P.; Singh, H. Soil carbon pools under poplar-based agroforestry, rice-wheat, and maize-wheat cropping systems in semi-arid India. Nutr. Cycl. Agroecosyst. 2012, 92, 107–118. [Google Scholar] [CrossRef]
- Ferré, C.; Leip, A.; Matteucci, G.; Previtali, F.; Seufert, G. Impact of 40 years poplar cultivation on soil carbon stocks and greenhouse gas fluxes. Biogeosci. Discuss. Eur. Geosci. Union (EGU) 2005, 2, 897–931. [Google Scholar] [CrossRef]
- Jandl, G.; Baum, C.; Blumschein, A.; Leinweber, P. The impact of short rotation coppice on the concentrations of aliphatic soil lipids. Plant Soil 2012, 350, 163–177. [Google Scholar] [CrossRef]
- Ekblad, A.; Wallander, H.; Godbold, D.L.; Cruz, C.; Johnson, D.; Baldrian, P.; Björk, R.G.; Epron, D.; Kieliszewska-Rokicka, B.; Kjøller, R.; et al. The production and turnover of extramatrical mycelium of ectomycorrhizal fungi in forest soils: Role in carbon cycling. Plant Soil 2013, 366, 1–27. [Google Scholar] [CrossRef]
- Waring, B.G.; Averill, C.; Hawkes, C.V. Differences in fungal and bacterial physiology alter soil carbon and nitrogen cycling: Insights from meta-analysis and theoretical models. Ecol. Lett. 2013, 16, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Godbold, D.L.; Hoosbeek, M.R.; Lukac, M.; Cotrufo, M.F.; Janssens, I.A.; Ceulemans, R.; Polle, A.; Velthorst, E.J.; Scarascia-Mugnozza, G.; De Angelis, P.; et al. Mycorrhizal hyphal turnover as a dominant process for carbon input into soil organic matter. Plant Soil 2006, 281, 15–24. [Google Scholar] [CrossRef]
- Wallander, H.; Nilsson, L.O.; Hagerberg, D.; Bååth, E. Estimation of the biomass and production of external mycelium of ectomycorrhizal fungi in the field. New Phytol. 2001, 151, 753–760. [Google Scholar] [CrossRef]
- Yannikos, N.; Leinweber, P.; Helgason, B.L.; Baum, C.; Walley, F.L.; Van Rees, K.C.J. Impact of Populus trees on the composition of organic matter and the soil microbial community in Orthic Gray Luvisols in Saskatchewan (Canada). Soil Biol. Biochem. 2014, 70, 5–11. [Google Scholar] [CrossRef]
- Purton, K.; Pennock, D.; Leinweber, P.; Walley, F. Will changes in climate and land use affect soil organic matter composition? Evidence from an ecotonal climosequence. Geoderma 2015, 253–254, 4860–4867. [Google Scholar] [CrossRef]
- Li, N.; Xu, Y.-Z.; Han, X.-Z.; He, H.-B.; Zhang, X.-D.; Zhang, B. Fungi contribute more than bacteria to soil organic matter through necromass accumulation under different agricultural practices during the early pedogenesis of a Mollisol. Eur. J. Soil Biol. 2015, 67, 51–58. [Google Scholar] [CrossRef]
- Zelles, L. Fatty acid patterns of phospholipids and lipopolysaccharides in the characterization of microbial communities in soil: A review. Biol. Fertil. Soils 1999, 29, 111–129. [Google Scholar] [CrossRef]
- Frostegård, A.; Bååth, E. The use of phospholipid fatty acid analysis to estimate bacterial and fungal biomass in soil. Biol. Fertil. Soils 1996, 22, 59–65. [Google Scholar] [CrossRef]
- Olsson, P.A. Signature fatty acids provide tools for determination of distribution and interactions of mycorrhizal fungi in soil. FEMS Microbiol. Ecol. 1999, 29, 303–310. [Google Scholar] [CrossRef]
- Frostegård, A.; Petersen, S.O.; Bååth, E.; Nielsen, T.H. Dynamics of a microbial community associated with manure hot spots as revealed by phospholipid fatty acid analyses. Appl. Environ. Microbiol. 1997, 63, 2224–2231. [Google Scholar]
- White, D.C.; Meadows, P.; Eglinton, G.; Coleman, M.L. In situ measurement of microbial biomass, community structure and nutritional status. Philos. Trans. R. Soc. Math. Phys. Eng. Sci. 1993, 344, 59–67. [Google Scholar]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014: International Soil Classification System for Naming Soils and Creating Legends for Soil Maps. Update 2015. World Soil Resour. Rep. 2015, 106, 191. [Google Scholar]
- Landgraf, D.; Böcker, L.; Oldenburg, C. Landwirte als Energieholz-Produzenten. Afz-Der Wald. Allg. Forst-Z. Für Waldwirtsch. Und Umweltvorsorge 2007, 14, 751–753. (In German). Available online: https://www.openagrar.de/receive/zimport_mods_00002435 (accessed on 1 February 2018).
- Boelcke, B.; Kahle, P. Leistung schnellwachsender Baumarten im Kurzumtrieb auf landwirtschaftlichen Flächen im Nordosten Deutschlands und erste Auswirkungen auf die Bodeneigenschaften. Die Holzzucht 2000, 53, 5–10. (In German). Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&ved=2ahUKEwj_zvbK7_vhAhWDOnAKHXdjB74QFjAAegQIARAC&url=http%3A%2F%2Fwww.landwirtschaft-mv.de%2Fserviceassistent%2Fdownload%3Fid%3D1578927&usg=AOvVaw0Zq0PiP-VxjTuh-kd7G_8E; (accessed on 1 February 2018).
- Steinbeiss, S.; Beßler, H.; Engels, C.; Temperton, V.M.; Buchmann, N.; Roscher, C.; Kreutziger, Y.; Baade, J.; Habekost, M.; Gleixner, G. Plant diversity positively affects short-term soil carbon storage in experimental grasslands. Glob. Chang. Biol. 2008, 14, 2937–2949. [Google Scholar] [CrossRef]
- Schulten, H.-R.; Leinweber, P. Thermal stability and composition of mineral-bound organic matter in density fractions of soil. Eur. J. Soil Sci. 1999, 50, 237–248. [Google Scholar] [CrossRef]
- Leinweber, P.; Jandl, G.; Eckhardt, K.-U.; Schlichting, A.; Hofmann, D.; Schulten, H.-R. Analytical pyrolysis and soft-ionization mass spectrometry. In Biophysico-chemical Processes Involving Natural Nonliving Organic Matter in Environmental Systems; Senesi, N., Xing, B., Huang, P.M., Eds.; John Wiley & Sons, Inc.: New York, NY, USA, 2009; pp. 539–588. [Google Scholar] [CrossRef]
- Kruse, J.; Abraham, M.; Amelung, W.; Baum, C.; Bol, R.; Kühn, O.; Lewandowski, H.; Niederberger, J.; Oelmann, Y.; Rüger, C.; et al. Innovative methods in soil phosphorus research: A review. J. Plant. Nutr. Soil Sci. 2015, 178, 43–88. [Google Scholar] [CrossRef]
- Gasulla, F.; Dorp, K.; Dombrink, I.; Zähringer, U.; Gisch, N.; Dörmann, P.; Bartels, D. The role of lipid metabolism in the acquisition of desiccation tolerance in Craterostigma plantagineum: A comparative approach. Plant J. 2013, 75, 726–741. [Google Scholar] [CrossRef]
- Welti, R.; Li, W.; Li, M.; Sang, Y.; Biesiada, H.; Zhou, H.-E.; Rajashekar, C.B.; Williams, T.D.; Wang, X. Profiling membrane lipids in plant stress responses. Role of phospholipase Da in freezing-induced lipid changes in Arabidopsis. J. Biol. Chem. 2002, 277, 31994–32002. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method for total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Zelles, L.; Bai, Q.Y. Fractionation of fatty acids derived from soil lipids by solid phase extraction and their quantitative analysis by GC-MS. Soil Biol. Biochem. 1993, 25, 495–507. [Google Scholar] [CrossRef]
- Gattinger, A.; Günthner, A.; Schloter, M.; Munch, J.C. Characterization of Archaea in soils by polar lipid analysis. Acta Biotechnol. 2003, 23, 21–28. [Google Scholar] [CrossRef]
- Ibekwe, A.M.; Kennedy, A.C. Phospholipid fatty acid profiles and carbon utilization patterns for analysis of microbial community structure under field and greenhouse conditions. FEMS Microbiol. Ecol. 1998, 26, 151–163. [Google Scholar] [CrossRef]
- Schinner, F.; von Mersi, W. Xylanase-, CM-cellulase- and invertase activity in soil: An improved method. Soil Biol. Biochem. 1990, 22, 511–515. [Google Scholar] [CrossRef]
- Geiger, O.; López-Lara, I.M.; Sohlenkamp, C. Phosphatidylcholine biosynthesis and function in bacteria. Biochim. Biophys. Acta 2013, 1831, 503–513. [Google Scholar] [CrossRef]
- Neidleman, S.I. Occurrence and Response to Environmental Stresses in Nonmammalian Organisms. In Phospholipids Handbook; Cevc, G., Ed.; Marcel Dekker: New York, NY, USA, 1993; Chapter 2. [Google Scholar]
- Al Afas, N.; Marron, N.; Zavalloni, C.; Ceulemans, R. Growth and production of a short-rotation coppice culture of poplar-IV: Fine root characteristics of five poplar clones. Biomass Bioenergy 2008, 32, 494–502. [Google Scholar] [CrossRef]
- Six, J.; Elliot, E.T.; Paustian, K. Soil macroaggregate turnover and microaggregate formation: A mechanism for C sequestration under no-tillage agriculture. Soil Biol. Biochem. 2000, 32, 2099–2103. [Google Scholar] [CrossRef]
- DeGryze, S.; Six, J.; Paustian, K.; Morris, J.S.; Paul, E.A.; Merck, R. Soil organic carbon pool changes following land-use conversion. Glob. Chang. Biol. 2004, 10, 1120–1132. [Google Scholar] [CrossRef]
- Jandl, G.; Acksel, A.; Baum, C.; Leinweber, P. Indicators for soil organic matter quality under perennial crops in Central Sweden. Soil Tillage Res. 2015, 148, 74–84. [Google Scholar] [CrossRef]
- Waldrop, M.P.; Firestone, M.K. Response of microbial community composition and function to soil climate change. Microb. Ecol. 2006, 52, 716–724. [Google Scholar] [CrossRef]
- Sun, H.; Koal, P.; Liu, D.; Gerl, G.; Schroll, R.; Gattinger, A.; Joergensen, R.G.; Munch, J.C. Soil microbial community and microbial residues respond positively to minimum tillage under organic farming in Southern Germany. Appl. Soil Ecol. 2016, 108, 16–24. [Google Scholar] [CrossRef]
- Talbot, J.M.; Allison, S.D.; Treseder, K.K. Decomposer in disguise: Mycorrhizal fungi as regulators of soil C dynamics in ecosystems under global change. Funct. Ecol. 2008, 22, 955–963. [Google Scholar] [CrossRef]
- Cairney, J.W.G. Extramatrical mycelia of ectomycorrhizal fungi as moderators of carbon dynamics in forest soil. Soil Biol. Biochem. 2012, 47, 198–208. [Google Scholar] [CrossRef]
- Fernandez, C.W.; Kennedy, P.G. Revisiting the “Gadgil effect”: Do interguild fungal interactions control carbon cycling in forest soils? New Phytol. 2016, 209, 1382–1389. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, X.; Li, G.; Qin, P. Interactions between arbuscular mycorrhizal fungi and phosphate-solubilizing fungus (Mortierella sp.) and their effects on Kostelelzkya virginica growth and enzyme activities of rhizosphere and bulk soils at different salinities. Biol. Fertil. Soils 2011, 47, 543–554. [Google Scholar] [CrossRef]
- Nehls, U.; Grunze, N.; Willmann, M.; Reich, M.; Küster, H. Sugar for my honey: Carbohydrate partitioning in ectomycorrhizal symbiosis. Phytochemistry 2007, 68, 82–91. [Google Scholar] [CrossRef]
- Kalbitz, K.; Schwesig, D.; Schmerwitz, J.; Kaiser, K.; Haumaier, L.; Glaser, B.; Ellerbrock, R.; Leinweber, P. Changes in properties of soil-derived dissolved organic matter induced by biodegradation. Soil Biol. Biochem. 2003, 35, 1129–1142. [Google Scholar] [CrossRef]
- Berlin, M.; Balakshin, M.; Gilkes, N.; Kadla, J.; Maximenko, V. Inhibition of cellulase, xylanase and ß-glucosidase activities by softwood lignin preparations. J. Biotechnol. 2006, 125, 198–209. [Google Scholar] [CrossRef]
- Rodíguez-Kábana, R. The effects of crop rotation and fertilization on soil xylanase activity in a soil of the southeastern United States. Plant Soil 1982, 6, 237–247. [Google Scholar] [CrossRef]
- Kahle, P.; Baum, C.; Boelcke, B.; Kohl, J.; Ulrich, R. Vertical distribution of soil properties under short-rotation forestry in Northern Germany. J. Plant Nutr. Soil Sci. 2010, 173, 737–746. [Google Scholar] [CrossRef]
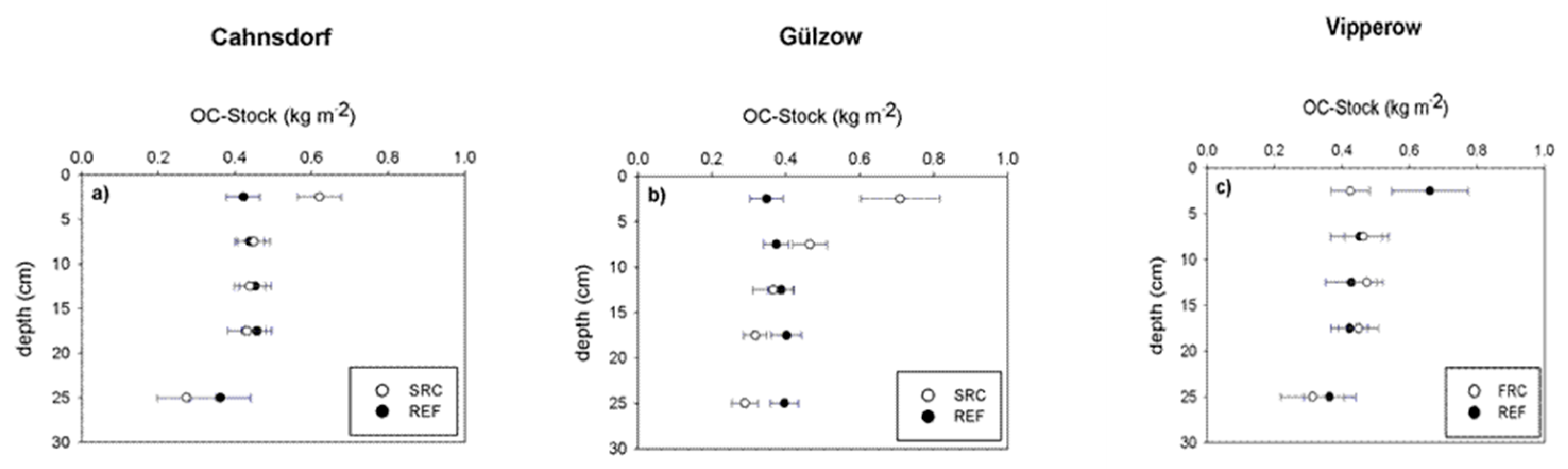


| Site with Abbreviation | Coordinates | Treatment | Use as SRC, Poplar Clone | Corg (mg g−1) | Nt (mg g−1) | C-to-N |
|---|---|---|---|---|---|---|
| Cahnsdorf (CAH) | 51°51′ N, 13°45′ E | SRC | since 2006 (4 years) Max | 12.6b (±1.5) | 1.2b (±0.1) | 10.9a (±0.5) |
| REF | 8.3a (±0.6) | 0.9a (±0.1) | 9.9a (±0.5) | |||
| Gülzow (GUL) | 53°48′ N, 12°3′ E | SRC | since 1993, (17 years) Max | 15.6b (±2.3) | 1.3b (±0.2) | 12.6b (±0.8) |
| REF | 7.1a (±0.5) | 0.8a (±0.1) | 9.4a (±0.6) | |||
| Vipperow (VIP) | 53°19′ N, 12°41′ E | fSRC | 1993–2007, (14 years) Max | 8.1a (±0.8) | 0.9a (±0.1) | 9.0a (±0.7) |
| REF | 15.6b (±3.8) | 1.5b (±0.3) | 10.7b (±0.5) |
| Factor | Soil Depth | ||||
|---|---|---|---|---|---|
| 0–5 cm | 5–10 cm | 10–15 cm | 15–20 cm | 20–30 cm | |
| Intercept | 5006.879 | 8844.388 | 8885.768 | 8654.424 | 3760.469 |
| Site | 176.012 ** | 459.629 ** | 337.368 ** | 261.747 ** | 132.831 ** |
| Management | 65.876 ** | 53.423 ** | 56.577 ** | 67.079 ** | 72.177 ** |
| Site × management | 7.904 ** | 91.025 ** | 54.491 ** | 32.147 ** | 51.359 ** |
| Site | Treatment | PLFA 18:2ω6,9 (ng g−1) | PLFA 16:1ω5 (ng g−1) | PLFA Total Bacterial (ng g−1) | Ratio Bacterial/Fungal PLFAs | Invertase (mg GE g−1 3h−1) | Xylanase (mg GE g−1 24 h−1) |
|---|---|---|---|---|---|---|---|
| Cahnsdorf | SRC | 17.7b (±0.4) | 13.1b (±1.3) | 178.8b (±25.4) | 10.1b (±1.6) | 829b (±79) | 885b (±30) |
| REF | 12.1a (±4.2) | 5.0a (±1.9) | 78.5a (±23.3) | 6.8a (±0.9) | 535a (±41) | 796a (±64) | |
| Gülzow | SRC | 15.3b (±1.3) | 12.7b (±2.1) | 157.6b (±23.5) | 10.2b (±0.6) | 560a (±155) | 295a (±41) |
| REF | 13.1a (±1.4) | 6.4a (±0.9) | 98.2a (±17.1) | 7.4a (±0.7) | 699b (±94) | 631b (±55) | |
| Vipperow | fSRC | 11.3a (±1.6) | 4.1a (±0.6) | 98.6a (±8.8) | 8.8a (±1.1) | 310a (±11) | 491a (±55) |
| REF | 17.1b (±2.3) | 7.0b (±1.9) | 152.2b (±25.5) | 8.9a (±0.1) | 360b (±26) | 557b (±105) |
| Site Andvariant | TII | Carbohydrates | Phenols, Ligninmonomers | Lignin Dimers | Lipids, Alkanes, Alkenes, Fatty Acids, n-alkyl Esters | Alkylaromatics | N-containingcompounds | Sterols | Peptides | Suberins | Fatty Acids n-C16 to n-C34 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cahnsdorf SRC | 29.7b (±5.0) | 4.4a (±0.1) | 8.8a (±0.3) | 4.2a (±0.3) | 10.1b (±0.6) | 10.6a (±0.5) | 6.8a (±0.3) | 2.1b (±0.2) | 2.0a (±0.1) | 0.2b (±0.0) | 2.7b (±0.2) |
| REF | 20.8a (±0.9) | 4.6b (±0.0) | 8.8a (±0.1) | 5.0b (±0.1) | 9.6a (±0.0) | 11.2b (±0.1) | 6.7a (±0.1) | 1.6a (±0.0) | 1.9a (±0.0) | 0.1a (±0.0) | 2.1a (±0.1) |
| Gülzow SRC | 11.4b (±3.1) | 6.8a (±0.1) | 13.2a (±0.3) | 3.9b (±0.2) | 7.1b (±0.3) | 12.2a (±0.3) | 7.9a (±0.2) | 1.0b (±0.1) | 2.6a (±0.1) | 0.0a (±0.0) | 0.9b (±0.1) |
| REF | 5.0a (±0.9) | 7.8b (±0.0) | 15.2b (±0.1) | 3.0a (±0.1) | 5.2a (±0.1) | 13.2b (±0.1) | 8.8b (±0.1) | 0.0a (±0.0) | 3.0b (±0.0) | 0.0a (±0.0) | 0.2a (±0.0) |
| Vipperow fSRC | 22.0b (±1.5) | 5.2a (±0.1) | 8.7a (±0.0) | 5.2b (±0.1) | 9.7b (±0.2) | 10.9a (±0.1) | 6.8a (±0.1) | 2.0b (±0.1) | 2.1a (±0.0) | 0.1a (±0.0) | 2.1a (±0.1) |
| REF | 17.1a (±1.5) | 5.7b (±0.1) | 8.9b (±0.1) | 3.4a (±0.0) | 9.3a (±0.1) | 10.8a (±0.1) | 7.2b (±0.1) | 1.6a (±0.0) | 2.2a (±0.1) | 0.1a (±0.0) | 2.2a (±0.0) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baum, C.; Barth, M.; Henkel, K.; Siebers, M.; Eckhardt, K.-U.; Hilke, I.; Gleixner, G.; Leinweber, P. Sustainability of Impacts of Poplar Growth on Soil Organic Matter in Eutric Cambisols. Soil Syst. 2019, 3, 32. https://doi.org/10.3390/soilsystems3020032
Baum C, Barth M, Henkel K, Siebers M, Eckhardt K-U, Hilke I, Gleixner G, Leinweber P. Sustainability of Impacts of Poplar Growth on Soil Organic Matter in Eutric Cambisols. Soil Systems. 2019; 3(2):32. https://doi.org/10.3390/soilsystems3020032
Chicago/Turabian StyleBaum, Christel, Martin Barth, Kathrin Henkel, Meike Siebers, Kai-Uwe Eckhardt, Ines Hilke, Gerd Gleixner, and Peter Leinweber. 2019. "Sustainability of Impacts of Poplar Growth on Soil Organic Matter in Eutric Cambisols" Soil Systems 3, no. 2: 32. https://doi.org/10.3390/soilsystems3020032





