A Three-Dimensional Assessment of Soil δ13C in a Subtropical Savanna: Implications for Vegetation Change and Soil Carbon Dynamics
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Woody Plant Encroachment Altered Spatial Patterns of Soil δ13C throughout the Soil Profile
3.2. Vegetation Changes Based on Spatial Patterns of Soil δ13C
3.3. SOC Sources Inferred from Spatial Patterns of Soil δ13C
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bailey, R.G. Ecosystem Geography: From Ecoregions to Sites; Springer: New York, NY, USA, 2009. [Google Scholar]
- Jordan, M.; Meyer, W.B.; Kates, R.W.; Clark, W.C.; Richards, J.F.; Turner, B.L.; Mathews, J.T. The Earth as Transformed by Human Action: Global and Regional Changes in the Biosphere over the Past 300 Years; Cambridge University Press: Cambridge, UK, 1993. [Google Scholar]
- Lal, R. Carbon sequestration in dryland ecosystems. Environ. Manag. 2004, 33, 528–544. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Baquerizo, M.; Maestre, F.T.; Gallardol, A.; Bowker, M.A.; Wallenstein, M.D.; Quero, J.L.; Ochoa, V.; Gozalo, B.; Garcia-Gomez, M.; Soliveres, S.; et al. Decoupling of soil nutrient cycles as a function of aridity in global drylands. Nature 2013, 502, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Poulter, B.; Frank, D.; Ciais, P.; Myneni, R.B.; Andela, N.; Bi, J.; Broquet, G.; Canadell, J.G.; Chevallier, F.; Liu, Y.Y.; et al. Contribution of semi-arid ecosystems to interannual variability of the global carbon cycle. Nature 2014, 509, 600–603. [Google Scholar] [CrossRef] [PubMed]
- Stevens, N.; Lehmann, C.E.R.; Murphy, B.P.; Durigan, G. Savanna woody encroachment is widespread across three continents. Glob. Chang. Biol. 2017, 23, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Archer, S.R.; Andersen, E.M.; Predick, K.I.; Schwinning, S.; Steidl, R.J.; Woods, S.R. Woody plant encroachment: Causes and consequences. In Rangeland Systems; Springer Nature: Cham, Switzerland, 2017; pp. 25–84. [Google Scholar] [CrossRef]
- Bond, W.J.; Midgley, G.F. Carbon dioxide and the uneasy interactions of trees and savannah grasses. Phil. Trans. R. Soc. B 2012, 367, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Devine, A.P.; McDonald, R.A.; Quaife, T.; Maclean, I.M.D. Determinants of woody encroachment and cover in African savannas. Oecologia 2017, 183, 939–951. [Google Scholar] [CrossRef]
- Kulmatiski, A.; Beard, K.H. Woody plant encroachment facilitated by increased precipitation intensity. Nat. Clim. Chang. 2013, 3, 833–837. [Google Scholar] [CrossRef]
- Morgan, J.A.; Milchunas, D.G.; LeCain, D.R.; West, M.; Mosier, A.R. Carbon dioxide enrichment alters plant community structure and accelerates shrub growth in the shortgrass steppe. Proc. Nat. Acad. Sci. USA 2007, 104, 14724–14729. [Google Scholar] [CrossRef]
- Scholes, R.J.; Archer, S.R. Tree-grass interactions in savannas. Annu. Rev. Ecol. Syst. 1997, 28, 517–544. [Google Scholar] [CrossRef]
- Stevens, N.; Erasmus, B.F.N.; Archibald, S.; Bond, W.J. Woody encroachment over 70 years in South African savannahs: Overgrazing, global change or extinction aftershock? Phil. Trans. R. Soc. B 2016, 371, 20150437. [Google Scholar] [CrossRef]
- Van Auken, O.W. Causes and consequences of woody plant encroachment into western North American grasslands. J. Environ. Manag. 2009, 90, 2931–2942. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Brandt, M.; Penuelas, J.; Guichard, F.; Tong, X.; Tian, F.; Fensholt, R. Ecosystem structural changes controlled by altered rainfall climatology in tropical savannas. Nat. Commun. 2019, 10, 671. [Google Scholar] [CrossRef] [PubMed]
- Barger, N.N.; Archer, S.R.; Campbell, J.L.; Huang, C.; Morton, J.A.; Knapp, A.K. Woody plant proliferation in North American drylands: A synthesis of impacts on ecosystem carbon balance. J. Geophys. Res. Biogeosci. 2011, 116, G00K07. [Google Scholar] [CrossRef]
- Eldridge, D.J.; Bowker, M.A.; Maestre, F.T.; Roger, E.; Reynolds, J.F.; Whitford, W.G. Impacts of shrub encroachment on ecosystem structure and functioning: Towards a global synthesis. Ecol. Lett. 2011, 14, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.B.; Banner, J.L.; Jobbagy, E.G.; Pockman, W.T.; Wall, D.H. Ecosystem carbon loss with woody plant invasion of grasslands. Nature 2002, 418, 623–626. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Boutton, T.W.; Ben Wu, X. Soil carbon response to woody plant encroachment: Importance of spatial heterogeneity and deep soil storage. J. Ecol. 2017, 105, 1738–1749. [Google Scholar] [CrossRef]
- Pacala, S.W.; Hurtt, G.C.; Baker, D.; Peylin, P.; Houghton, R.A.; Birdsey, R.A.; Heath, L.; Sundquist, E.T.; Stallard, R.F.; Ciais, P.; et al. Consistent land- and atmosphere-based us carbon sink estimates. Science 2001, 292, 2316–2320. [Google Scholar] [CrossRef]
- Houghton, R.A.; Hackler, J.L. Changes in terrestrial carbon storage in the United States. 1: The roles of agriculture and forestry. Glob. Ecol. Biogeogr. 2000, 9, 125–144. [Google Scholar] [CrossRef]
- Climate Change Science Program. The First State of the Carbon Cycle Report (SOCCR): The North American Carbon Budget and Implications for the Global Carbon Cycle. In A Report by the U.S. Climate Change Science Program and the Subcommittee on Global Change Research; King, A.W., Dilling, L., Zimmerman, G.P., Fairman, D.M., Houghton, R.A., Marland, G., Rose, A.Z., Wilbanks, T.J., Eds.; National Oceanic and Atmospheric Administration, National Climatic Data Center: Asheville, NC, USA, 2007. [Google Scholar]
- Blaser, W.J.; Shanungu, G.K.; Edwards, P.J.; Venterink, H.O. Woody encroachment reduces nutrient limitation and promotes soil carbon sequestration. Ecol. Evol. 2014, 4, 1423–1438. [Google Scholar] [CrossRef]
- Liao, J.D.; Boutton, T.W.; Jastrow, J.D. Storage and dynamics of carbon and nitrogen in soil physical fractions following woody plant invasion of grassland. Soil Biol. Biochem. 2006, 38, 3184–3196. [Google Scholar] [CrossRef]
- Springsteen, A.; Loya, W.; Liebig, M.; Hendrickson, J. Soil carbon and nitrogen across a chronosequence of woody plant expansion in North Dakota. Plant Soil 2010, 328, 369–379. [Google Scholar] [CrossRef]
- Hughes, R.F.; Archer, S.R.; Asner, G.P.; Wessman, C.A.; McMurtry, C.; Nelson, J.; Ansley, R.J. Changes in aboveground primary production and carbon and nitrogen pools accompanying woody plant encroachment in a temperate savanna. Glob. Chang. Biol. 2006, 12, 1733–1747. [Google Scholar] [CrossRef]
- Li, H.; Shen, H.H.; Chen, L.Y.; Liu, T.Y.; Hu, H.F.; Zhao, X.; Zhou, L.H.; Zhang, P.J.; Fang, J.Y. Effects of shrub encroachment on soil organic carbon in global grasslands. Sci. Rep. 2016, 6, 28974. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, W.H.; Raikes, J.A.; Hartley, A.E.; Cross, A.E. On the spatial pattern of soil nutrients in desert ecosystems. Ecology 1996, 77, 364–374. [Google Scholar] [CrossRef]
- Boutton, T.W.; Archer, S.R.; Midwood, A.J.; Zitzer, S.F.; Bol, R. δ13C values of soil organic carbon and their use in documenting vegetation change in a subtropical savanna ecosystem. Geoderma 1998, 82, 5–41. [Google Scholar] [CrossRef]
- Dzurec, R.; Boutton, T.; Caldwell, M.; Smith, B. Carbon isotope ratios of soil organic matter and their use in assessing community composition changes in Curlew Valley, Utah. Oecologia 1985, 66, 17–24. [Google Scholar] [CrossRef]
- Victoria, R.L.; Fernandes, F.; Martinelli, L.A.; Piccolo, M.D.C.; Decamargo, P.B.; Trumbore, S. Past vegetation changes in the Brazilian pantanal arboreal–grassy savanna ecotone by using carbon isotopes in the soil organic matter. Glob. Chang. Biol. 1995, 1, 165–171. [Google Scholar] [CrossRef]
- Balesdent, J.; Girardin, C.; Mariotti, A. Site-δ13C of tree leaves and soil organic matter in a temperate forest. Ecology 1993, 74, 1713–1721. [Google Scholar] [CrossRef]
- Boutton, T.W. Stable carbon isotope ratios of organic matter and their use as indicators of vegetation and climate changes. In Mass Spectrometry of Soils; Boutton, T.W., Yamasaki, S.I., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 1996; pp. 47–82. [Google Scholar]
- Breecker, D.O.; Bergel, S.; Nadel, M.; Tremblay, M.M.; Osuna-Orozco, R.; Larson, T.E.; Sharp, Z.D. Minor stable carbon isotope fractionation between respired carbon dioxide and bulk soil organic matter during laboratory incubation of topsoil. Biogeochemistry 2015, 123, 83–98. [Google Scholar] [CrossRef]
- Ehleringer, J.R.; Buchmann, N.; Flanagan, L.B. Carbon isotope ratios in belowground carbon cycle processes. Ecol. Appl. 2000, 10, 412–422. [Google Scholar] [CrossRef]
- Lerch, T.Z.; Nunan, N.; Dignac, M.F.; Chenu, C.; Mariotti, A. Variations in microbial isotopic fractionation during soil organic matter decomposition. Biogeochemistry 2011, 106, 5–21. [Google Scholar] [CrossRef]
- Bai, E.; Boutton, T.W.; Wu, X.B.; Liu, F.; Archer, S.R. Landscape-scale vegetation dynamics inferred from spatial patterns of soil δ13C in a subtropical savanna parkland. J. Geophys. Res. Biogeosci. 2009, 114, G01019. [Google Scholar] [CrossRef]
- Biedenbender, S.H.; McClaran, M.P.; Quade, J.; Weltz, M.A. Landscape patterns of vegetation change indicated by soil carbon isotope composition. Geoderma 2004, 119, 69–83. [Google Scholar] [CrossRef]
- Freier, K.P.; Glaser, B.; Zech, W. Mathematical modeling of soil carbon turnover in natural Podocarpus forest and Eucalyptus plantation in Ethiopia using compound specific δ13C analysis. Glob. Chang. Biol. 2010, 16, 1487–1502. [Google Scholar] [CrossRef]
- Liao, J.D.; Boutton, T.W.; Jastrow, J.D. Organic matter turnover in soil physical fractions following woody plant invasion of grassland: Evidence from natural 13C and 15N. Soil Biol. Biochem. 2006, 38, 3197–3210. [Google Scholar] [CrossRef]
- Puttock, A.; Dungait, J.A.J.; Bol, R.; Dixon, E.R.; Macleod, C.J.A.; Brazier, R.E. Stable carbon isotope analysis of fluvial sediment fluxes over two contrasting C4-C3 semi-arid vegetation transitions. Rapid Commun. Mass Spectrom. 2012, 26, 2386–2392. [Google Scholar] [CrossRef]
- Krull, E.; Bray, S.; Harms, B.; Baxter, N.; Bol, R.; Farquhar, G. Development of a stable isotope index to assess decadal-scale vegetation change and application to woodlands of the Burdekin catchment, Australia. Glob. Change Biol. 2007, 13, 1455–1468. [Google Scholar] [CrossRef]
- Turnbull, L.; Brazier, R.E.; Wainwright, J.; Dixon, L.; Bol, R. Use of carbon isotope analysis to understand semi-arid erosion dynamics and long-term semi-arid land degradation. Rapid Commun. Mass Spectrom. 2008, 22, 1697–1702. [Google Scholar] [CrossRef]
- Pringle, R.M.; Doak, D.F.; Brody, A.K.; Jocque, R.; Palmer, T.M. Spatial pattern enhances ecosystem functioning in an African savanna. PloS Biol. 2010, 8, e1000377. [Google Scholar] [CrossRef]
- Ettema, C.H.; Wardle, D.A. Spatial soil ecology. Trends Ecol. Evol. 2002, 17, 177–183. [Google Scholar] [CrossRef]
- Liu, F.; Wu, X.B.; Bai, E.; Boutton, T.W.; Archer, S.R. Quantifying soil organic carbon in complex landscapes: An example of grassland undergoing encroachment of woody plants. Glob. Chang. Biol. 2011, 17, 1119–1129. [Google Scholar] [CrossRef]
- Loescher, H.; Ayres, E.; Duffy, P.; Luo, H.Y.; Brunke, M. Spatial variation in soil properties among North American ecosystems and guidelines for sampling designs. PLoS ONE 2014, 9, e83216. [Google Scholar] [CrossRef] [PubMed]
- Biggs, T.H.; Quade, J.; Webb, R.H. δ13C values of soil organic matter in semiarid grassland with mesquite (Prosopis) encroachment in southeastern Arizona. Geoderma 2002, 110, 109–130. [Google Scholar] [CrossRef]
- van Kessel, C.; Farrell, R.E.; Pennock, D.J. Carbon-13 and nitrogen-15 natural-abundance in crop residues and soil organic-matter. Soil Sci. Soc. Am. J. 1994, 58, 382–389. [Google Scholar] [CrossRef]
- Zhou, Y.; Boutton, T.W.; Wu, X.B. Woody plant encroachment amplifies spatial heterogeneity of soil phosphorus to considerable depth. Ecology 2018, 99, 136–147. [Google Scholar] [CrossRef]
- Archer, S. Tree-grass dynamics in a Prosopis-thornscrub savanna parkland: Reconstructing the past and predicting the future. Ecoscience 1995, 2, 83–99. [Google Scholar] [CrossRef]
- Archer, S.; Scifres, C.; Bassham, C.; Maggio, R. Autogenic succession in a subtropical savanna: Conversion of grassland to thorn woodland. Ecol. Monogr. 1988, 58, 111–127. [Google Scholar] [CrossRef]
- Zhou, Y.; Boutton, T.W.; Wu, X.B.; Yang, C.H. Spatial heterogeneity of subsurface soil texture drives landscape-scale patterns of woody patches in a subtropical savanna. Landsc. Ecol. 2017, 32, 915–929. [Google Scholar] [CrossRef]
- Zhou, Y.; Watts, S.E.; Boutton, T.W.; Archer, S.R. Root density distribution and biomass allocation of co-occurring woody plants on contrasting soils in a subtropical savanna parkland. Plant Soil 2019, 438, 263–279. [Google Scholar] [CrossRef]
- U.S.D.A. Soil Conservation Service. Soil Survey of Jim Wells County, Texas; Soil Conservation Service: Washington, DC, USA, 1979.
- Loomis, L.E. Influence of Heterogeneous Subsoil Development on Vegetation Patterns in a Subtropical Savanna Parkland, Texas. Ph.D. Dissertation, Texas A&M University, College Station, TX, USA, 1989. [Google Scholar]
- Whittaker, R.; Gilbert, L.; Connell, J. Analysis of a two-phase pattern in a mesquite grassland, Texas. J. Ecol. 1979, 935–952. [Google Scholar] [CrossRef]
- Bai, E.; Boutton, T.W.; Liu, F.; Wu, X.B.; Archer, S.R. Spatial patterns of soil δ13C reveal grassland-to-woodland successional processes. Org. Geochem. 2012, 42, 1512–1518. [Google Scholar] [CrossRef]
- McCulley, R.L.; Archer, S.R.; Boutton, T.W.; Hons, F.M.; Zuberer, D.A. Soil respiration and nutrient cycling in wooded communities developing in grassland. Ecology 2004, 85, 2804–2817. [Google Scholar] [CrossRef]
- Zhou, Y.; Boutton, T.W.; Wu, X.B. Soil phosphorus does not keep pace with soil carbon and nitrogen accumulation following woody encroachment. Glob. Chang. Biol. 2018, 24, 1992–2007. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.G.; Gardner, R.H.; O’Neill, R.V. Landscape Ecology in Theory and Practice; Springer: New York, NY, USA, 2001. [Google Scholar] [CrossRef]
- Boutton, T.W.; Liao, J.D.; Filley, T.; Archer, S.R. Belowground carbon storage and dynamics accompanying woody plant encroachment in a subtropical savanna. In Soil Carbon Sequestration and the Greenhouse Effect, 2nd ed.; Lal, R., Follett, R., Eds.; Soil Science Society of America: Madison, WI, USA, 2009; pp. 181–205. [Google Scholar] [CrossRef]
- Harris, D.; Horwath, W.R.; van Kessel, C. Acid fumigation of soils to remove carbonates prior to total organic carbon or 13C isotopic analysis. Soil Sci. Soc. Am. J. 2001, 65, 1853–1856. [Google Scholar] [CrossRef]
- Bostrom, B.; Comstedt, D.; Ekblad, A. Isotope fractionation and 13C enrichment in soil profiles during the decomposition of soil organic matter. Oecologia 2007, 153, 89–98. [Google Scholar] [CrossRef]
- Wynn, J.G.; Bird, M.I. C4-derived soil organic carbon decomposes faster than its C3 counterpart in mixed C3/C4 soils. Glob. Chang. Biol. 2007, 13, 2206–2217. [Google Scholar] [CrossRef]
- Ampleman, M.D.; Crawford, K.M.; Fike, D.A. Differential soil organic carbon storage at forb- and grass-dominated plant communities, 33 years after tallgrass prairie restoration. Plant Soil 2014, 374, 899–913. [Google Scholar] [CrossRef]
- Saiz, G.; Bird, M.; Wurster, C.; Quesada, C.A.; Ascough, P.; Domingues, T.; Schrodt, F.; Schwarz, M.; Feldpausch, T.R.; Veenendaal, E.; et al. The influence of C3 and C4 vegetation on soil organic matter dynamics in contrasting semi-natural tropical ecosystems. Biogeosciences 2015, 12, 5041–5059. [Google Scholar] [CrossRef]
- Littell, R.C.; Milliken, G.A.; Stroup, W.W.; Wolfinger, R.D.; Oliver, S. SAS for Mixed Models; SAS Institute: Cary, NC, USA, 2006. [Google Scholar]
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing: Vienna, Austria. Available online: https://www.R-project.org/ (accessed on 12 November 2019).
- Throop, H.L.; Archer, S.R. Shrub (Prosopis velutina) encroachment in a semidesert grassland: Spatial-temporal changes in soil organic carbon and nitrogen pools. Glob. Chang. Biol. 2008, 14, 2420–2431. [Google Scholar] [CrossRef]
- Rasse, D.P.; Rumpel, C.; Dignac, M.F. Is soil carbon mostly root carbon? Mechanisms for a specific stabilisation. Plant Soil 2005, 269, 341–356. [Google Scholar] [CrossRef]
- Rumpel, C.; Kogel-Knabner, I. Deep soil organic matter-a key but poorly understood component of terrestrial C cycle. Plant Soil 2011, 338, 143–158. [Google Scholar] [CrossRef]
- Schmidt, M.W.I.; Torn, M.S.; Abiven, S.; Dittmar, T.; Guggenberger, G.; Janssens, I.A.; Kleber, M.; Kogel-Knabner, I.; Lehmann, J.; Manning, D.A.C.; et al. Persistence of soil organic matter as an ecosystem property. Nature 2011, 478, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.B.; Canadell, J.; Ehleringer, J.R.; Mooney, H.A.; Sala, O.E.; Schulze, E.D. A global analysis of root distributions for terrestrial biomes. Oecologia 1996, 108, 389–411. [Google Scholar] [CrossRef] [PubMed]
- Schenk, H.J.; Jackson, R.B. Rooting depths, lateral root spreads and below-ground/above-ground allometries of plants in water-limited ecosystems. J. Ecol. 2002, 90, 480–494. [Google Scholar] [CrossRef]
- Stoker, R.L. An Object-Oriented, Spatially-Explicit Simulation Model of Vegetation Dynamics in a South Texas Savanna. Ph.D. Thesis, Texas A&M University, College Station, TX, USA, 1998. [Google Scholar]
- Archer, S.; Smeins, F. Ecosystem-level processes. In Grazing Management: An Ecological Perspective; Heitschmidt, R.K., Stuth, J.W., Eds.; Timber Press: Portland, OR, USA, 1991; pp. 109–140. [Google Scholar]
- Augustine, D.J.; Derner, J.D.; Milchunas, D.; Blumenthal, D.; Porensky, L.M. Grazing moderates increases in C3 grass abundance over seven decades across a soil texture gradient in shortgrass steppe. J. Veg. Sci. 2017, 28, 562–572. [Google Scholar] [CrossRef]
- Wang, Y.; Amundson, R.; Trumbore, S. Radiocarbon dating of soil organic matter. Quat. Res. 1996, 45, 282–288. [Google Scholar] [CrossRef]
- Polley, H.W.; Mayeux, H.S.; Johnson, H.B.; Tischler, C.R. Viewpoint: Atmospheric CO2, soil water, and shrub/grass ratios on rangelands. J. Range Manag. 1997, 50, 278–284. [Google Scholar] [CrossRef]
- NOAA National Centers for Environmental Information. Climate at a Glance: County Mapping. 2019. Available online: https://www.ncdc.noaa.gov/cag/county/mapping (accessed on 14 October 2019).
- Venter, Z.S.; Cramer, M.D.; Hawkins, H.J. Drivers of woody plant encroachment over Africa. Nat. Commun. 2018, 9, 2272. [Google Scholar] [CrossRef]
- Osborne, C.P.; Charles-Dominique, T.; Stevens, N.; Bond, W.J.; Midgley, G.; Lehmann, C.E.R. Human impacts in African savannas are mediated by plant functional traits. New Phytol. 2018, 220, 10–24. [Google Scholar] [CrossRef]
- Creamer, C.A.; Filley, T.R.; Boutton, T.W. Long-term incubations of size and density separated soil fractions to inform soil organic carbon decay dynamics. Soil Biol. Biochem. 2013, 57, 496–503. [Google Scholar] [CrossRef]
- Filley, T.R.; Boutton, T.W.; Liao, J.D.; Jastrow, J.D.; Gamblin, D.E. Chemical changes to nonaggregated particulate soil organic matter following grassland-to-woodland transition in a subtropical savanna. J. Geophys. Res. Biogeosci. 2008, 113, G03009. [Google Scholar] [CrossRef]
- Kantola, I.B. Biogeochemistry of Woody Plant Invasion: Phosphorus Cycling and Microbial Community Composition. Ph.D. Thesis, Texas A&M University, College Station, TX, USA, 2012. [Google Scholar]
- Hibbard, K.A.; Schimel, D.S.; Archer, S.; Ojima, D.S.; Parton, W. Grassland to woodland transitions: Integrating changes in landscape structure and biogeochemistry. Ecol. Appl. 2003, 13, 911–926. [Google Scholar] [CrossRef]
- Liao, J.D.; Boutton, T.W. Soil microbial biomass response to woody plant invasion of grassland. Soil Biol. Biochem. 2008, 40, 1207–1216. [Google Scholar] [CrossRef]
- Creamer, C.A.; Filley, T.R.; Boutton, T.W.; Rowe, H. Grassland to woodland transitions: Dynamic response of microbial community structure and carbon use patterns. J. Geophys. Res. Biogeosci. 2016, 121, 1675–1688. [Google Scholar] [CrossRef]
- Hollister, E.B.; Schadt, C.W.; Palumbo, A.V.; Ansley, R.J.; Boutton, T.W. Structural and functional diversity of soil bacterial and fungal communities following woody plant encroachment in the southern Great Plains. Soil Biol. Biochem. 2010, 42, 1816–1824. [Google Scholar] [CrossRef]
- Biederman, L.; Boutton, T.W. Spatial variation in biodiversity and trophic structure of soil nematode communities in a subtropical savanna parkland: Responses to woody plant encroachment. Appl. Soil Ecol. 2010, 46, 168–176. [Google Scholar] [CrossRef]
- Luvuno, L.; Biggs, R.; Stevens, N.; Esler, K. Woody encroachment as a social-ecological regime shift. Sustainability 2018, 10, 2221. [Google Scholar] [CrossRef]
- Wilcox, B.P.; Birt, A.; Archer, S.R.; Fuhlendorf, S.D.; Kreuter, U.P.; Sorice, M.G.; Van Leeuwen, W.J.D.; Zou, C.B. Viewing woody plant encroachment through a social-ecological lens. BioScience 2018, 68, 691–705. [Google Scholar] [CrossRef]
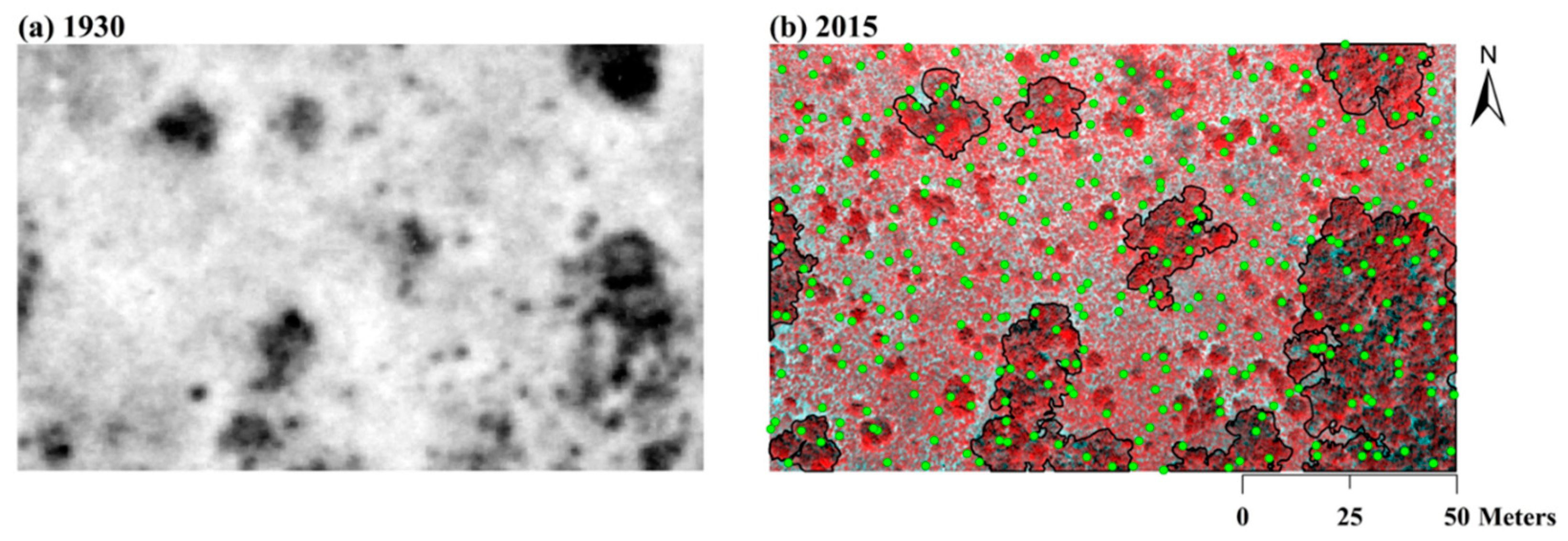



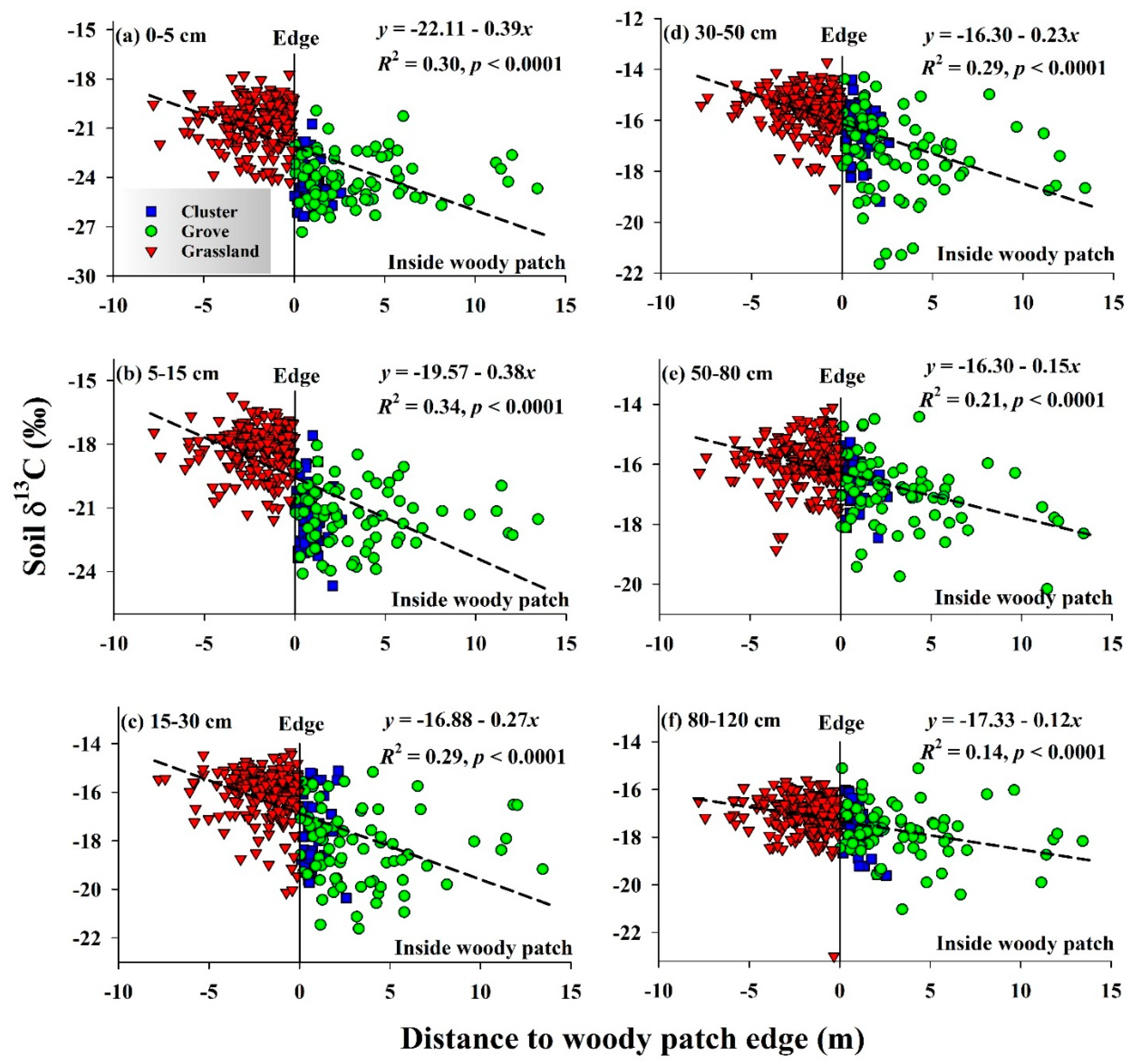
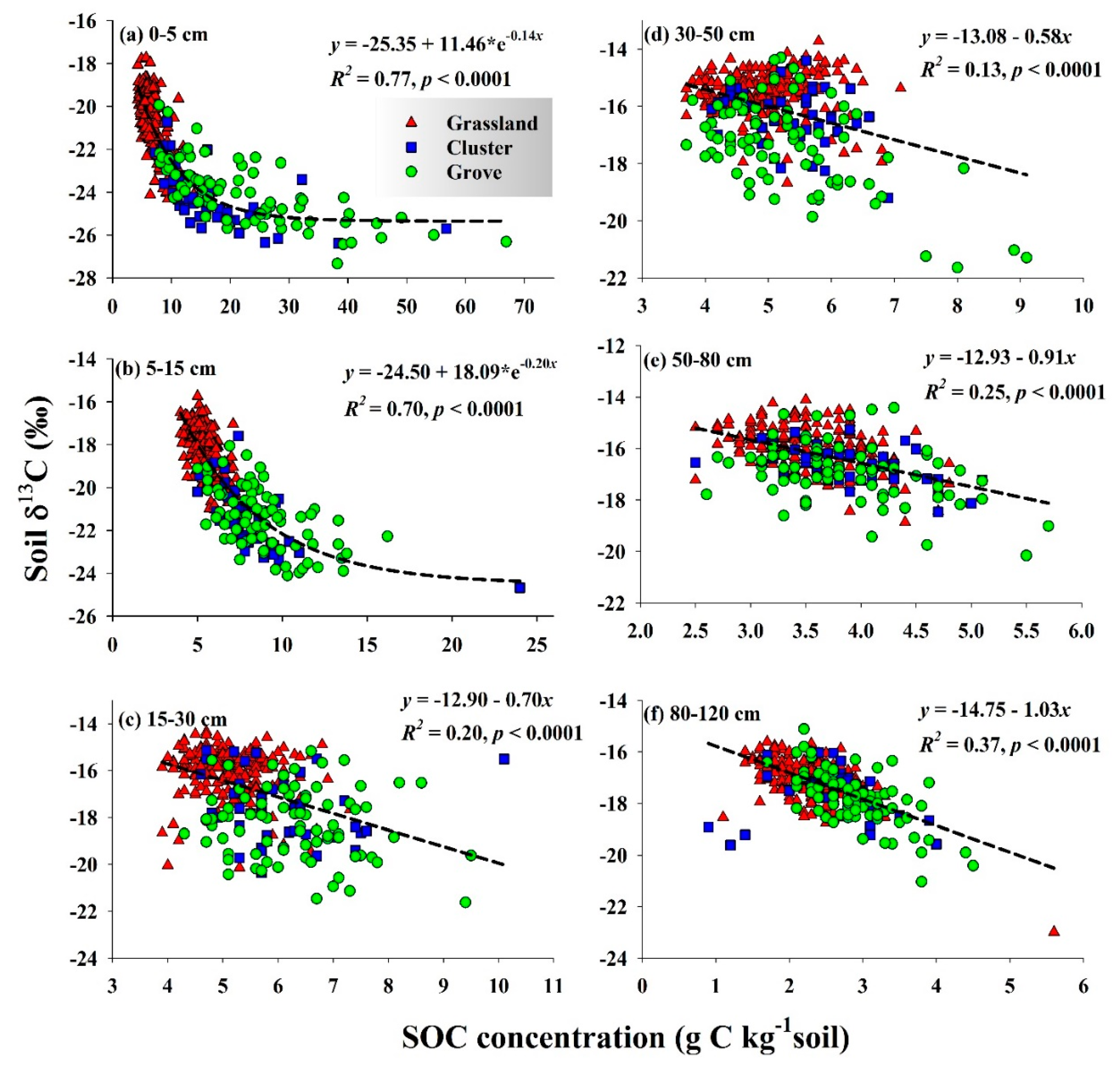
| Depth (cm) | SOC (g C kg−1 soil) * | % SOC Derived from C3 Plants | % of New SOC Derived from Woody Plants ** | |||||
|---|---|---|---|---|---|---|---|---|
| Grassland | Cluster | Grove | Grassland | Cluster | Grove | Cluster | Grove | |
| 0–5 | 6.7 ± 0.1 c | 17.0 ± 1.4 b | 22.1 ± 1.4 a | 51.1 ± 0.6 b | 74.2 ± 1.4 a | 72.7 ± 1.1 a | 60.5 ± 3.6 a | 62.3 ± 3.1 a |
| 5–15 | 5.4 ± 0.1 b | 8.2 ± 0.5 a | 8.8 ± 0.3 a | 35.1 ± 0.5 b | 55.9 ± 1.5 a | 55.6 ± 1.1 a | 37.6 ± 2.8 a | 38.9 ± 2.0 a |
| 15–30 | 5.1 ± 0.0 b | 6.0 ± 0.2 a | 6.3 ± 0.1 a | 20.5 ± 0.5 c | 30.8 ± 1.5 b | 35.0 ± 1.1 a | 15.4 ± 2.2 b | 21.8 ± 1.7 a |
| 30–50 | 5.0 ± 0.1 a | 5.3 ± 0.1 a | 5.3 ± 0.1 a | 16.9 ± 0.4 c | 22.7 ± 1.0 b | 29.0 ± 1.2 a | 8.0 ± 1.4 b | 17.4 ± 1.7 a |
| 50–80 | 3.5 ± 0.0 b | 3.9 ± 0.1 a | 3.9 ± 0.1 a | 19.5 ± 0.4 b | 24.5 ± 0.8 a | 26.5 ± 0.8 a | 7.2 ± 1.1 a | 10.2 ± 1.2 a |
| 80–120 | 2.3 ± 0.0 b | 2.6 ± 0.1 a | 2.9 ± 0.1 a | 27.0 ± 0.4 b | 30.8 ± 0.9 a | 32.0 ± 0.8 a | 6.5 ± 1.5 a | 8.4 ± 1.3 a |
| Time | Grove Cover | Cluster Cover | Total Woody Cover | |||
|---|---|---|---|---|---|---|
| m2 | % | m2 | % | m2 | % | |
| 1930 | 2659 | 16.6 | 693 | 4.3 | 3352 | 20.9 |
| 2015 | 4375 | 27.3 | 1647 | 10.3 | 6024 | 37.6 |
| Net change (1930–2015) | +1716 | +10.7 | +956 | +6.0 | +2672 | +16.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Boutton, T.W.; Wu, X.B. A Three-Dimensional Assessment of Soil δ13C in a Subtropical Savanna: Implications for Vegetation Change and Soil Carbon Dynamics. Soil Syst. 2019, 3, 73. https://doi.org/10.3390/soilsystems3040073
Zhou Y, Boutton TW, Wu XB. A Three-Dimensional Assessment of Soil δ13C in a Subtropical Savanna: Implications for Vegetation Change and Soil Carbon Dynamics. Soil Systems. 2019; 3(4):73. https://doi.org/10.3390/soilsystems3040073
Chicago/Turabian StyleZhou, Yong, Thomas W. Boutton, and X. Ben Wu. 2019. "A Three-Dimensional Assessment of Soil δ13C in a Subtropical Savanna: Implications for Vegetation Change and Soil Carbon Dynamics" Soil Systems 3, no. 4: 73. https://doi.org/10.3390/soilsystems3040073
APA StyleZhou, Y., Boutton, T. W., & Wu, X. B. (2019). A Three-Dimensional Assessment of Soil δ13C in a Subtropical Savanna: Implications for Vegetation Change and Soil Carbon Dynamics. Soil Systems, 3(4), 73. https://doi.org/10.3390/soilsystems3040073





