Rapid Recent Recovery from Acidic Deposition in Central Ontario Lakes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Lake and Bulk Precipitation Sampling
2.3. Chemical Analyses
2.4. Statistical Analyses
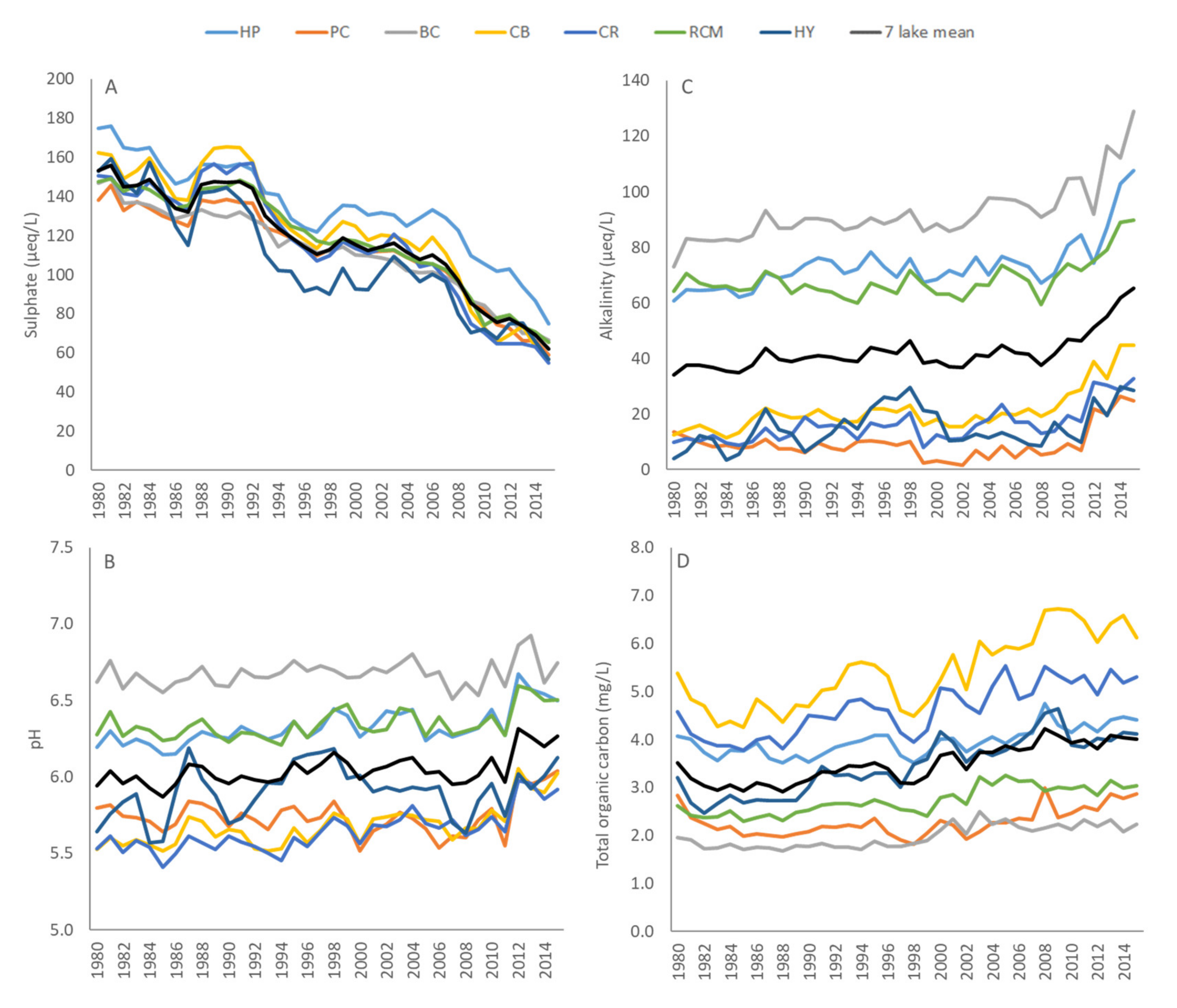
3. Results
3.1. Bulk Deposition
3.2. Lake Chemistry
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Driscoll, C.T.; Driscoll, K.M.; Fakhraei, H.; Civerolo, K. Long-term temporal trends and spatial patterns in the acid-base chemistry of lakes of the Adirondacks region of New York in response to decreases in acid deposition. Atmos. Environ. 2016, 146, 5–14. [Google Scholar] [CrossRef] [Green Version]
- Futter, M.N.; Valinia, S.; Löfgren, S.; Köhler, S.J.; Fölster, J. Long-term trends in water chemistry of acid-sensitive Swedish lakes show slow recovery from historic acidification. Ambio 2015, 43, 77–90. [Google Scholar] [CrossRef] [Green Version]
- Watmough, S.A.; Dillon, P.J. Base cation and nitrogen budgets for seven forested catchments in central Ontario, 1983–1999. For. Ecol. Manag. 2003, 177, 155–177. [Google Scholar] [CrossRef]
- Keller, W.; Heneberry, J.; Edwards, B.A. Recovery of acidified Sudbury, Ontario, Canada, lakes: A multi-decade synthesis and update. Environ. Rev. 2019, 27, 1–16. [Google Scholar] [CrossRef]
- Garmo, O.G.; Skjelkvåle, B.L.; de Wit, H.A.; Colombo, L.; Curtis, C.; Fölster, J.; Hoffmann, A.; Hruška, J.; Høgåsen, T.; Jeffries, D.S.; et al. Trends in surface water chemistry in acidified areas in Europe and North America from 1990 to 2008. Wat. Air Soil Pollut. 2014, 225, 1880. [Google Scholar] [CrossRef] [Green Version]
- Monteith, D.T.; Stoddard, J.L.; Evans, C.D.; de Wit, H.A.; Forsius, M.; Høgåsen, T.; Vesely, J. Dissolved organic carbon trends resulting from changes in atmospheric deposition chemistry. Nature 2007, 450, 537–540. [Google Scholar] [CrossRef] [PubMed]
- Skjelkvåle, B.L.; Stoddard, J.L.; Jeffries, D.S.; Tørseth, K.; Høgåsen, T.; Bowman, J.; Mannio, J.; Monteith, D.T.; Mosello, R.; Rogora, M.; et al. Regional scale evidence for improvements in surface water chemistry 1990–2001. Environ. Pollut. 2005, 137, 165–176. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, M.J.; Lovett, G.; Bailey, S.; Beall, F.; Burns, D.; Buso, D.; Clair, T.A.; Courchesne, F.; Duchesne, L.; Eimers, C.; et al. Comparisons of Watershed Sulfur Budgets in Southeast Canada and Northeast Us: New Approaches and Implications. Biogeochemistry 2011, 103, 181–207. [Google Scholar] [CrossRef]
- Eimers, M.C.; Dillon, P.J. Climate effects on sulphate flux from forested catchments in south-central Ontario. Biogeochemistry 2002, 61, 337–355. [Google Scholar] [CrossRef]
- Stoddard, J.L.; Jeffries, D.S.; Lukewille, A.; Clair, T.A.; Dillon, P.J.; Driscoll, C.T.; Forsius, M.; Johnannessen, M.; Kahl, J.S.; Kellogg, J.H.; et al. Regional trends in aquatic recovery from acidification in North America and Europe. Nature 1999, 401, 575–578. [Google Scholar] [CrossRef]
- Watmough, S.A.; Eimers, M.C.; Baker, S. Impediments to recovery from acid deposition. Atmos. Environ. 2016, 146, 15–27. [Google Scholar] [CrossRef] [Green Version]
- Evans, C.D.; Chapman, P.J.; Clark, J.M.; Monteith, D.T.; Cresser, M.S. Alternative explanations for rising dissolved organic carbon export from organic soils. Glob. Chang. Biol. 2006, 12, 2044–2053. [Google Scholar] [CrossRef]
- Beamish, R.J.; Harvey, H.H. Acidification of the La Cloche Mountain lakes, Ontario and resulting fish mortalities. J. Fish. Res. Board Can. 1972, 29, 1131–1143. [Google Scholar] [CrossRef]
- Dillon, P.J.; Reid, R.A.; de Grosbois, E. The rate of acidification of aquatic ecosystems in Ontario, Canada. Nature 1987, 329, 45–48. [Google Scholar] [CrossRef]
- Vet, R.; Artz, R.S.; Carou, S.; Shaw, M.; Ro, C.; Aas, W.; Baker, A.; Bowersox, V.; Dentener, F.; Galy-Lacaux, C.; et al. A global assessment of precipitation chemistry and deposition of sulfur, nitrogen, sea salt, base cations, organic acids, acidity and pH, and phosphorus. Atmos. Environ. 2014, 93, 3–100. [Google Scholar] [CrossRef]
- Dillon, P.J.; Evans, R.D. Long-term changes in the chemistry of a soft-water lake under changing acid deposition rates and climatic fluctuations. Verh. Internat. Veren. Limnol. 2000, 27, 2615–2619. [Google Scholar]
- Jeffries, D.S.; Clair, T.C.; Couture, S.; Dillon, P.J.; Dupont, J.; Keller, W.; McNicol, D.K.; Turner, M.A.; Vet, R.; Weeber, R. Assessing the recovery of lakes in southeastern Canada from the effects of acid deposition. Ambio 2003, 32, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Palmer, M.E.; Yan, N.D.; Paterson, A.M.; Girard, R.D. Water quality changes in south-central Ontario lakes and the role of local factors in regulating lake response to regional stressors. Can. J. Fish. Aquat. Sci. 2011, 68, 1038–1050. [Google Scholar] [CrossRef]
- Eimers, M.C.; Watmough, S.A.; Buttle, J.M.; Dillon, P.J. Examination of the potential relationship between droughts, sulphate and dissolved organic carbon at a wetland-draining stream. Glob. Chang. Biol. 2008, 14, 938–948. [Google Scholar] [CrossRef]
- Aas, W.; Mortier, A.; Bowersox, V.; Cherian, R.; Faluvegi, G.; Fagerli, H.; Hand, J.; Klimont, Z.; Galy-Lacaux, C.; Lehmann, C.M.B.; et al. Global and regional trends of atmospheric sulfur. Sci. Rep. 2019, 9, 953. [Google Scholar] [CrossRef]
- Soil Classification Working Group. The Canadian System of Soil Classification, 3rd ed.; NRC Research Press: Ottawa, ON, Canada, 1998; pp. 53–60. [Google Scholar]
- Ahrens, C.D. Meteorology Today: An Introduction to Weather, Climate, and the Environment; West Publishing Company: St. Paul, MN, USA, 1991; ISBN 0314809058. [Google Scholar]
- Scheider, W.A.; Snyder, W.R.; Clark, B. Deposition of nutrients and major ions by precipitation in south-central Ontario. Wat. Air Soil Pollut. 1979, 12, 171–185. [Google Scholar] [CrossRef]
- Ontario Ministry of Environment. Handbook of Analytical Methods for Environmental Samples; Ontario Ministry of Environment, Lab Services Branch: Toronto, ON, Canada, 1983; p. 682. [Google Scholar]
- Dillon, P.J.; Molot, L.A. Dissolved organic and inorganic carbon mass balances in central Ontario lakes. Biogeochemistry 1997, 36, 29–42. [Google Scholar] [CrossRef]
- Oliver, B.G.; Thurman, E.M.; Malcolm, R.L. The contribution of humic substances to the acidity of colored natural waters. Geochim. Cosmochim. Acta 1983, 47, 2031–2035. [Google Scholar] [CrossRef]
- Hirsch, R.M.; Slack, J.R. A nonparametric trend test for seasonal data with serial dependence. Water Resour. Res. 1984, 20, 727–732. [Google Scholar] [CrossRef] [Green Version]
- Dillon, P.J.; Somers, K.M.; Findeis, J.; Eimers, M.C. Coherent response of lakes in Ontario, Canada to reductions in sulphur deposition: The effects of climate on sulphate concentrations. Hydrol. Earth Syst. Sci. 2003, 7, 583–595. [Google Scholar] [CrossRef]
- Kerr, J.G.; Eimers, M.C.; Creed, I.F.; Adams, M.B.; Beall, F.; Burns, D.; Campbell, J.L.; Christopher, S.F.; Clair, T.A.; Courchesne, F.; et al. The effect of seasonal drying on sulphate dynamics in streams across southeastern Canada and the northeastern USA. Biogeochemistry 2012, 111, 393–409. [Google Scholar] [CrossRef]
- Laudon, H.; Dillon, P.J.; Eimers, M.C.; Semkin, R.G.; Jeffries, D.S. Climate-induced episodic acidification of streams in central Ontario. Environ. Sci. Technol. 2004, 38, 5015–6009. [Google Scholar] [CrossRef]
- Ashforth, D.; Yan, N.D. The interactive effects of Ca concentration and temperature on the survival and reproduction of Daphnia pulex at low and high food concentrations. Limnol. Oceanogr. 2008, 53, 420–432. [Google Scholar] [CrossRef] [Green Version]
- Jeziorski, A.; Tanentzap, A.J.; Yan, N.D.; Paterson, A.M.; Palmer, M.E.; Korosi, J.B.; Rusak, J.A.; Arts, M.T.; Keller, W.B.; Ingram, R.; et al. The jellification of north temperate lakes. Proc. R. Soc. B Biol. Sci. 2014, 282, 20142449. [Google Scholar] [CrossRef]
- Tan, Q.; Wang, W. Interspecies differences in calcium content and requirement in four freshwater cladocerans explained by biokinetic parameters. Limnol. Oceanogr. 2010, 55, 1426–1434. [Google Scholar] [CrossRef]
- Strock, K.E.; Nelson, S.J.; Kahl, J.S.; Saros, J.E.; McDowell, W.H. Decadal trends reveal recent acceleration in the rate of recovery from acidification in the northeastern US. Environ. Sci. Tech. 2015, 48, 4681–4689. [Google Scholar] [CrossRef]
- Clair, T.A.; Dennis, I.F.; Vet, R. Water chemistry and dissolved organic carbon trends in lakes from Canada’s Atlantic Provinces: No recovery from acidification measured after 25 years of lake monitoring. Can. J. Fish. Aquat. Sci. 2011, 68, 663–674. [Google Scholar] [CrossRef]
- Lawrence, G.B.; Hazlett, P.W.; Fernandez, I.J.; Ouimet, R.; Bailey, S.W.; Shortle, W.C.; Smith, K.T.; Antidormi, M.R. Declining acidic deposition begins reversal of forest-soil acidification in the northeastern US and eastern Canada. Environ. Sci. Technol. 2015, 49, 13103–13111. [Google Scholar] [CrossRef] [PubMed]
- Kirchner, J.W.; Dillon, P.J.; LaZerte, B.D. Predicted response of stream chemistry to acid loading tested in Canadian catchments. Nature 1992, 358, 478–482. [Google Scholar] [CrossRef]
- Bottomley, D.J.; Craig, D.; Johnston, L.M. Neutralization of acid runoff by groundwater discharge to streams in Canadian Precambrian Shield watersheds. J. Hydrol. 1984, 75, 1–26. [Google Scholar] [CrossRef]
- Kirschbaum, M.U.F. The temperature-dependence of soil organic matter decomposition, and the effect of global warming on soil organic C storage. Soil Biol. Biochem. 1995, 27, 753–760. [Google Scholar] [CrossRef]
- Knorr, W.; Prentice, I.C.; House, J.I.; Holland, E.A. Long-term sensitivity of soil carbon turnover to warming. Nature 2005, 433, 298–301. [Google Scholar] [CrossRef]
- Klaminder, J.; Grip, H.; Morth, C.M.; Laudon, H. Carbon mineralization and pyrite oxidation in groundwater: Importance for silicate weathering in boreal forest soils and stream base-flow chemistry. Appl. Geochem. 2011, 26, 319–324. [Google Scholar] [CrossRef]
- Hedin, L.O.; Granat, L.; Likens, G.E.; Buishand, T.A.; Galloway, J.N.; Butler, T.J.; Rodhe, H. Steep declines in atmospheric base cations in regions of Europe and North America. Nature 1994, 367, 351–354. [Google Scholar] [CrossRef]
- Yao, H.; McConnell, C.; Somers, K.M.; Yan, N.D.; Watmough, S.; Scheider, W. Nearshore human interventions reverse patterns of decline in lake calcium budgets in central Ontario as demonstrated by mass-balance analyses. Water Resour. Res. 2011, 47, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Reid, C.; Watmough, S.A. Spatial patterns, trends and the potential long-term impacts of tree harvesting on lake calcium levels in the Muskoka River Watershed, Ontario, Canada. Can. J. Fish. Aquat. Sci. 2016, 73, 382–393. [Google Scholar] [CrossRef]



| 1982–2015 (n = 34) | 1982–2005 (n = 24) | 2005–2015 (n = 11) | |
|---|---|---|---|
| SO4 (meq m−2 yr−1) | − 1.32 *** | − 1.35 *** | − 2.08 ** |
| NO3 (meq m−2 yr−1) | − 0.64 *** | − 0.42 ** | − 0.58 (not sig) |
| pH | + 0.03 *** | + 0.01 *** | + 0.04 *** |
| Sum of base cations (meq m−2 yr−1) | No trend | − 0.22 * | + 2.02 ** |
| Sen’s Slopes | 1982–2015 (n = 34) | 1982–2005 (n = 24) | 2005–2015 (n = 11) |
|---|---|---|---|
| SO4 (µeq L−1 yr−1) | −2.4 (−2.0 to −2.9) 7/7 | −1.7 (−1.3 to −2.6) 7/7 | −5.0 (−3.8 to −6.5) 7/7 |
| Gran Alk (µeq L−1 yr−1) | 0.38 (0.01 to 0.59) 5/7 | 0.26 (−0.17 to 0.43) 3/7 | 2.1 (1.5 to 2.9) 5/7 |
| NO3-N (µeq L−1 yr−1) | −0.03 (−0.04 to 0.07) 3/7 | 0.002 (−0.1 to 0.05) 0/7 | 0.09 (−0.01 to 0.16) 1/7 |
| ANC (µeq L−1 yr−1) | 0.70 (0.23 to 1.0) 6/7 | 0.46 (−0.39 to 0.94) 4/7 | 2.3 (1.1 to 3.4) 5/7 |
| pH (pH units yr−1) | 0.005 (0 to 0.01) 5/7 | 0.007 (0 to 0.01) 4/7 | 0.028 (0.02 to 0.05) 4/7 |
| DOC (mg L−1 yr−1) | 0.025 (0.02 to 0.07) 7/7 | 0.027 (0 to 0.06) 6/7 | 0.020 (−0.02 to 0.06) 2/7 |
| Org acid adj ANC (µeq L−1 yr−1) | 0.55 (0.17 to 0.97) 6/7 | 0.25 (−0.36 to 0.88) 4/7 | 2.3 (1.2 to 3.1) 5/7 |
| Ca (µeq L−1 yr−1) | −1.4 (−1.6 to −0.60) 7/7 | −1.0 (−1.3 to −0.38) 6/7 | −2.0 (−2.5 to −1.1) 5/7 |
| SBC (µeq L−1 yr−1) | −1.9 (−2.2 to −1.0) 7/7 | −1.5 (−1.8 to −0.6) 7/7 | −2.7 (−3.6 to −1.8) 6/7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watmough, S.A.; Eimers, M.C. Rapid Recent Recovery from Acidic Deposition in Central Ontario Lakes. Soil Syst. 2020, 4, 10. https://doi.org/10.3390/soilsystems4010010
Watmough SA, Eimers MC. Rapid Recent Recovery from Acidic Deposition in Central Ontario Lakes. Soil Systems. 2020; 4(1):10. https://doi.org/10.3390/soilsystems4010010
Chicago/Turabian StyleWatmough, Shaun A., and M. Catherine Eimers. 2020. "Rapid Recent Recovery from Acidic Deposition in Central Ontario Lakes" Soil Systems 4, no. 1: 10. https://doi.org/10.3390/soilsystems4010010




