Pedogenic Processes in a Posidonia oceanica Mat
Abstract
:1. Introduction
2. Material and Methods
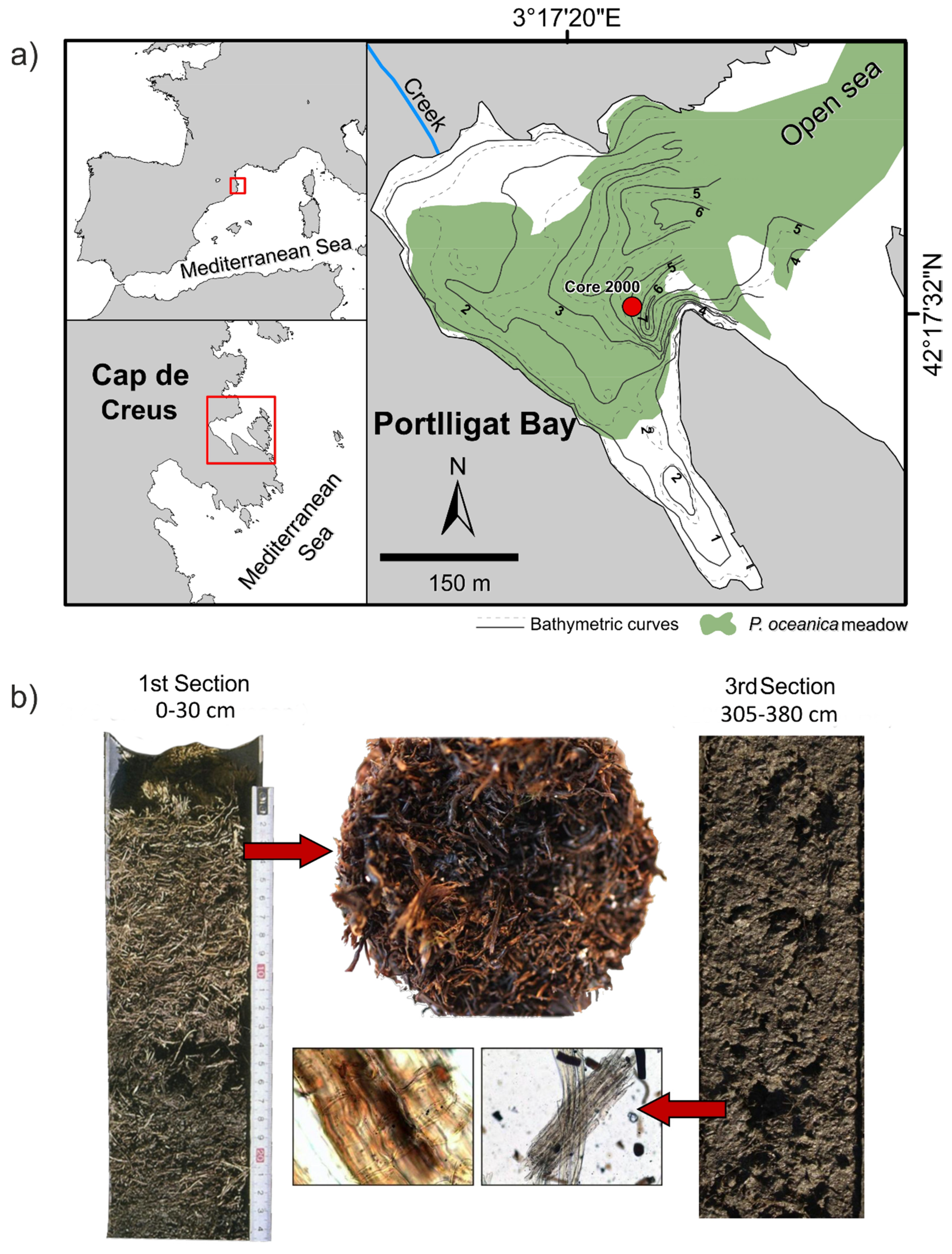
2.1. Study Area and Sampling Methods
2.2. Geochemical Analysis
2.3. Statistical Methods:
3. Results
3.1. Organic Matter, Carbonates and Elemental Composition
3.2. Geochemical Signals
4. Discussion
4.1. Organic VS Inorganic Matter Accumulation
4.2. Accumulation of Carbonates
4.3. Enrichment in Fine Soil Particles
4.4. Fine Organic Matter (FOM)
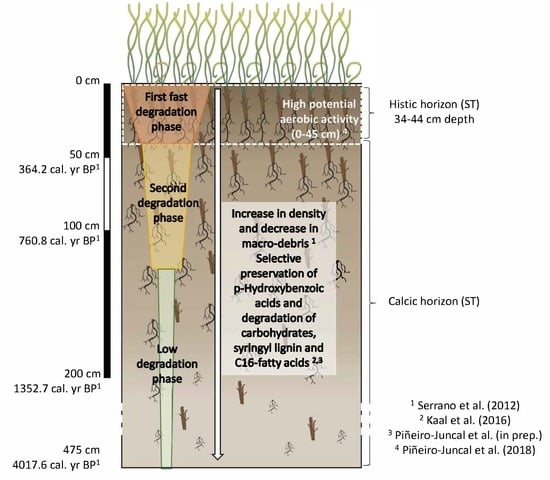
4.5. Soil Classification
4.5.1. Soil Taxonomy (ST)
4.5.2. World Reference Base (WRB)
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Soil Survey Staff. Soil Taxonomy: A Basic System of Soil Classification for Making and Interpreting Soil Surveys; Natural Resourse Conservation Service, USA Department Agriculture: Washington, DC, USA, 1999.
- IUSS Working Group. World reference base for soil resources 2014. In International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports No. 106; Cambridge University Press: Cambridge, UK, 2015; pp. 1–191. [Google Scholar] [CrossRef]
- Nóbrega, G.N.; Romero, D.J.; Otero, X.L.; Ferreira, T.O. Pedological studies of subaqueous soils as a contribution to the protection of seagrass meadows in Brazil. Revista Brasileira Ciência Solo 2018, 42, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Nóbrega, G.N. Subaqueous soils of the Brazilian seagrass meadows: Biogeochemistry, genesis, and classification. Ph.D. Thesis, University of São Paulo, São Paulo, Brasil, 2017. [Google Scholar]
- Serrano, O.; Mateo, M.A.; Renom, P.; Julià, R. Characterization of soils beneath a Posidonia oceanica meadow. Geoderma 2012, 185, 26–36. [Google Scholar] [CrossRef]
- Bradley, M.P.; Stolt, M.H. Subaqueous soil-landscape relationships in a Rhode Island estuary. Soil Sci. Soc. Am. J. 2003, 67, 1487–1495. [Google Scholar] [CrossRef] [Green Version]
- Kristensen, E.; Rabenhorst, M.C. Do marine rooted plants grow in sediment or soil? A critical appraisal on definitions, methodology and communication. Earth Sci. Rev. 2015, 145, 1–8. [Google Scholar] [CrossRef]
- Rueda, J.L.; Salas, C.; Marina, P. Seasonal variation in a deep subtidal Zostera marina L. bed in southern Spain (western Mediterranean Sea). Bot. Mar. 2008, 51, 92–102. [Google Scholar] [CrossRef]
- Van Katwijk, M.M.; Bos, A.R.; Hermus, D.C.R.; Suykerbuyk, W. Sediment modification by seagrass beds: Muddification and sandification induced by plant cover and environmental conditions. Estuar. Coast. Shelf Sci. 2010, 89, 175–181. [Google Scholar] [CrossRef]
- Fourqurean, J.W.; Duarte, C.M.; Kennedy, H.; Marbà, N.; Holmer, M.; Mateo, M.A.; Apostolaki, E.T.; Kendrick, G.A.; Krause-Jensen, D.; McGlathery, K.J.; et al. Seagrass ecosystems as a globally significant carbon stock. Nat. Geosci. 2012, 5, 505–509. [Google Scholar] [CrossRef]
- Kennedy, H.; Gacia, E.; Kennedy, D.P.; Papadimitriou, S.; Duarte, C.M. Organic carbon sources to SE Asian coastal sediments. Estuar. Coast. Shelf Sci. 2004, 60, 59–68. [Google Scholar] [CrossRef]
- Mazarrasa, I.; Marbà, N.; Lovelock, C.E.; Serrano, O.; Lavery, P.S.; Fourqurean, J.W.; Kennedy, H.; Mateo, M.A.; Krause-Jensen, D.; Steven, A.D.; et al. Seagrass meadows as a globally significant carbonate reservoir. Biogeosciences 2015, 12, 4993–5003. [Google Scholar] [CrossRef] [Green Version]
- Smit, A.J.; Brearley, A.; Hyndes, G.A.; Lavery, P.S.; Walker, D.I. Carbon and nitrogen stable isotope analysis of an Amphibolis griffithii seagrass bed. Estuar. Coast. Shelf Sci. 2005, 65, 545–556. [Google Scholar] [CrossRef]
- Duarte, C.M.; Middelburg, J.J.; Caraco, N. Major role of marine vegetation on the oceanic carbon cycle. Biogeosciences 2005, 2, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Cifuentes, A.; Antón, J.; Benlloch, S.; Donnelly, A.; Herbert, R.A.; Rodríguez-Valera, F. Prokaryotic diversity in Zostera noltii colonized marine sediments. Appl. Environ. Microbiol. 2000, 66, 1715–1719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danovaro, R. Detritus-bacteria-meiofauna interactions in a seagrass bed (Posidonia oceanica) of the NW Mediterranean. Mar. Biol. 1996, 127, 1–13. [Google Scholar] [CrossRef]
- Jones, W.B.; Cifuentes, L.A.; Kaldy, J.E. Stable carbon isotope evidence for coupling between sedimentary bacteria and seagrasses in a sub-tropical lagoon. Mar. Ecol. Prog. Ser. 2003, 255, 15–25. [Google Scholar] [CrossRef] [Green Version]
- Lopez, N.I.; Duarte, C.M.; Vallespinos, F.; Romero, J.; Alcoverro, T. Bacterial activity in NW Mediterranean seagrass (Posodonia oceania) sediments. J. Exp. Mar. Bio. Ecol. 1995, 187, 39–49. [Google Scholar] [CrossRef]
- Borum, J.; Sand-Jensen, K.; Binzer, T.; Pedersen, O.; Greve, T.M. Oxygen movement in seagrasses. In Seagrasses: Biology, Ecology and Conservation; Springer: Dordrecht, The Netherlands, 2006; pp. 255–270. [Google Scholar]
- Hartog, C.; Den Kuo, J. Taxonomy and biogeography of seagrasses. In Seagrasses: Biology, Ecology and Conservation; Springer: Dordrecht, The Netherlands, 2006; pp. 1–23. [Google Scholar]
- Serrano, O.; Lavery, P.S.; López-Merino, L.; Ballesteros, E.; Mateo, M.A. Location and associated carbon storage of erosional escarpments of seagrass Posidonia mats. Front. Mar. Sci. 2016, 3, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Trevathan-Tackett, S.M.; Seymour, J.R.; Nielsen, D.A.; Macreadie, P.I.; Jeffries, T.C.; Sanderman, J.; Baldock, J.; Howes, J.M.; Steven, A.D.L.; Ralph, P.J. Sediment anoxia limits microbial-driven seagrass carbon remineralization under warming conditions. FEMS Microbiol. Ecol. 2017, 93, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Augier, H.; Boudouresque, C. Premières Observations sur l’herbier de Posidonies et le Détritique Côtier de l’île du Levant (Méditérranée, France), à L’aide du Sous-Marin Griffon de la Marine Nationale; Travaux scientifiques du Parc National de Port-Cros: Hyères, France, 1979; pp. 141–153. [Google Scholar]
- Koch, E.W.; Ackerman, J.D.; Verduin, J.; van Keulen, M. Fluid dynamics in seagrass ecology-from molecules to ecosystems. In Seagrasses: Biology, Ecology and Conservation; Springer: Dordrecht, The Netherlands, 2006; pp. 193–225. [Google Scholar]
- Boudouresque, C.; Meinesz, A. Découverte de l’herbier de posidonie. In Parc National de Port-Cros; Podidonie, G.I.S., Ed.; Parc Naturel Régional de la Corse: Corte, France, 1982. [Google Scholar]
- Kaal, J.; Serrano, O.; Nierop, K.G.J.; Schellekens, J.; Martínez Cortizas, A.; Mateo, M.-Á. Molecular composition of plant parts and sediment organic matter in a Mediterranean seagrass (Posidonia oceanica) mat. Aquat. Bot. 2016, 133, 50–61. [Google Scholar] [CrossRef] [Green Version]
- Canfield, D.E. Factors influencing organic-carbon preservation in marine-sediments. Chem. Geol. 1994, 114, 315–329. [Google Scholar] [CrossRef]
- Duarte, C.M.; Losada, I.J.; Hendriks, I.E.; Mazarrasa, I.; Marbà, N. The role of coastal plant communities for climate change mitigation and adaptation. Nat. Clim. Chang. 2013, 3, 961–968. [Google Scholar] [CrossRef] [Green Version]
- Serrano, O.; Serrano, E.; Inostroza, K.; Lavery, P.S.; Mateo, M.A.; Ballesteros, E. Seagrass meadows provide 3D Habitat for Reef Fish. Front. Mar. Sci. 2017, 4, 3–5. [Google Scholar] [CrossRef] [Green Version]
- Ondiviela, B.; Losada, I.J.; Lara, J.L.; Maza, M.; Galván, C.; Bouma, T.J.; van Belzen, J. The role of seagrasses in coastal protection in a changing climate. Coast. Eng. 2014, 87, 158–168. [Google Scholar] [CrossRef]
- Serrano, O.; Mateo, M.; Dueñas-Bohórquez, A.; Renom, P.; López-Sáez, J.A.; Martínez Cortizas, A. The Posidonia oceanica marine sedimentary record: A Holocene archive of heavy metal pollution. Sci. Total Environ. 2011, 409, 4831–4840. [Google Scholar] [CrossRef] [PubMed]
- Krause-Jensen, D.; Serrano, O.; Apostolaki, E.T.; Gregory, D.J.; Duarte, C.M. Seagrass sedimentary deposits as security vaults and time capsules of the human past. Ambio 2019, 48, 325–335. [Google Scholar] [CrossRef] [Green Version]
- López-Sáez, J.A.; López-Merino, L.; Mateo, M.Á.; Serrano, Ó.; Pérez-Díaz, S.; Serrano, L. Palaeoecological potential of the marine organic deposits of Posidonia oceanica: A case study in the NE Iberian Peninsula. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2009, 271, 215–224. [Google Scholar] [CrossRef]
- López-Merino, L.; Colás-Ruiz, N.R.; Adame, M.F.; Serrano, O.; Martínez Cortizas, A.; Mateo, M.A. A six thousand-year record of climate and land-use change from Mediterranean seagrass mats. J. Ecol. 2017, 1–12. [Google Scholar] [CrossRef]
- De los Santos, C.B.; Krause-Jensen, D.; Alcoverro, T.; Marbà, N.; Duarte, C.M.; van Katwijk, M.M.; Pérez, M.; Romero, J.; Sánchez-Lizaso, L.J.; Roca, G.; et al. Recent trend reversal for declining European seagrass meadows. Nat. Commun. 2019, 10, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Zieman, J.C.; Fourqurean, J.W.; Frankovich, T.A. Seagrass die-off in Florida Bay: Long-term trends in abundance and growth of turtle grass. Thalassia testudinum. Estuaries 1999, 22, 460–470. [Google Scholar] [CrossRef]
- Lavery, P.S.; Mateo, M.Á.; Serrano, O.; Rozaimi, M. Variability in the carbon storage of seagrass habitats and its implications for global estimates of Blue Carbon ecosystem service. PLoS ONE 2013, 8, e73748. [Google Scholar] [CrossRef] [Green Version]
- Telesca, L.; Belluscio, A.; Criscoli, A.; Ardizzone, G.; Apostolaki, E.T.; Fraschetti, S.; Gristina, M.; Knittweis, L.; Martin, C.S. Seagrass meadows (Posidonia oceanica) distribution and trajectories of change. Sci. Rep. 2015, 5, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Marbà, N.; Holmer, M.; Gacia, E.; Barrón, C. Seagrass beds and coastal biogeochemistry. In Seagrasses: Biology, Ecology and Conservation; Springer: Dordrecht, The Netherlands, 2006; pp. 133–155. [Google Scholar]
- Piñeiro-Juncal, N.; Mateo, M.Á.; Holmer, M.; Martínez-Cortizas, A. Potential microbial functional activity along a Posidonia oceanica soil profile. Aquat. Microb. Ecol. 2018, 81, 189–200. [Google Scholar] [CrossRef]
- Lo Iacono, C.; Mateo, M.A.; Grácia, E.; Guasch, L.; Carbonell, R.; Serrano, L.; Serrano, O.; Dañobeitia, J. Very high-resolution seismo-acoustic imaging of seagrass meadows (Mediterranean Sea): Implications for carbon sink estimates. Geophys. Res. Lett. 2008, 35, 1–5. [Google Scholar] [CrossRef]
- Institut Geològico de Catalunya. Geologic map Alt Emporda (1:50000). 2006. Available online: https://www.icgc.cat/en/Public-Administration-and-Enterprises/Downloads/Geological-and-geothematic-cartography/Geological-cartography/Geological-map-1-50-000/Regional-geological-map-of-Catalonia-1-50-000 (accessed on 16 January 2020).
- Leiva-Dueñas, C.; López-Merino, L.; Serrano, O.; Martínez Cortizas, A.; Mateo, M.A. Millennial-scale trends and controls in Posidonia oceanica (L. Delile) ecosystem productivity. Glob. Planet Chang. 2018, 169, 92–104. [Google Scholar] [CrossRef]
- Cheburkin, A.K.; Shotyk, W. An Energy-dispersive miniprobe multielement analyzer (EMMA) for direct analysis of Pb and other trace elements in peats. Fresenius J. Anal. Chem. 1996, 354, 688–691. [Google Scholar] [CrossRef]
- Weiss, D.; Shotyk, W.; Cheburkin, A.K.; Gloor, M. Determination of Pb in the ash fraction of plants and peats using the energy-dispersive miniprobe multielement analyser (EMMA). Analyst 1998, 123, 2097–2102. [Google Scholar] [CrossRef]
- Revelle, W. Psych: Procedures for Personality and Psychological Research; Northwestern University: Evanston, IL, USA, 2017. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2013. [Google Scholar]
- Aitchison, J. The statistical analysis of compositional data. J. R. Stat. Soc. Ser. B 1982, 44, 139–177. [Google Scholar] [CrossRef]
- Gallagher, K.; Bodin, T.; Sambridge, M.; Weiss, D.; Kylander, M.; Large, D. Inference of abrupt changes in noisy geochemical records using transdimensional changepoint models. Earth Planet Sci. Lett. 2011, 311, 182–194. [Google Scholar] [CrossRef] [Green Version]
- Serrano, O.; Martínez-Cortizas, A.; Mateo, M.A.; Biester, H.; Bindler, R. Millennial scale impact on the marine biogeochemical cycle of mercury from early mining on the Iberian Peninsula. Global Biogeochem. Cycles 2013, 27, 21–30. [Google Scholar] [CrossRef] [Green Version]
- Piñeiro-Juncal, N.; Kaal, J.; Fornazier Moreira, J.C.; Martínez-Cortizas, A.; Rodrigues Lambais, M.; Otero, X.L.; Mateo, M.A. Cover loss of Posidonia oceanica meadows causes organic matter remineralization and shifts in the prokaryotic communities in the underlying soil. in preparation.
- Rothwell, R.G.; Croudace, I.W. Twenty years of XRF core scanning marine sediments: What do geochemical proxies tell us? In Micro-XRF Studies of Sediment Cores: Applications of a Non-Destructive Tool for the Environmental Sciences; Springer: Berlin/Heidelberg, Germany, 2015; pp. 25–102. [Google Scholar]
- Dembitsky, V.M. Bromo and iodo containing alkaloids from marine microorganisms and sponges. Russ. J. Bioorganic. Chem. 2002, 28, 170–182. [Google Scholar] [CrossRef]
- Gribble, G.W. The natural production of organobromine compounds. Environ. Sci. Pollut. Res. 2000, 7, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, M.; Jilbert, T.; De Lange, G.J.; Lourens, L.J.; Reichart, G.J. Bromine counts from XRF scanning as an estimate of the marine organic carbon content of sediment cores. Geochem. Geophys. Geosyst. 2008, 9, 1–6. [Google Scholar] [CrossRef]
- Leri, A.C.; Mayer, L.M.; Thornton, K.R.; Ravel, B. Bromination of marine particulate organic matter through oxidative mechanisms. Geochim. Cosmochim. Acta. 2014, 142, 53–63. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Cortizas, A.; Ferro-Vázquez, C.; Kaal, J.; Biester, H.; Casais, M.C.; Rodríguez, T.T.; Rodríguez Lado, L. Bromine accumulation in acidic black colluvial soils. Geochimica Cosmochimica Acta 2016, 174, 143–155. [Google Scholar] [CrossRef]
- Myneni, S.C.B. Formation of stable chlorinated hydrocarbons in weathering plant material. Science 2002, 295, 1039–1041. [Google Scholar] [CrossRef] [Green Version]
- Trevathan-Tackett, S.M.; Macreadie, P.I.; Sanderman, J.; Baldock, J.; Howes, J.M.; Ralph, P.J. A global assessment of the chemical recalcitrance of seagrass tissues: Implications for long-term carbon sequestration. Front. Plant Sci. 2017, 8, 1–18. [Google Scholar] [CrossRef]
- Kaal, J.; Serrano, O.; del Río, J.C.; Rencoret, J. Radically different lignin composition in Posidonia species may link to differences in organic carbon sequestration capacity. Org. Geochem. 2018. [Google Scholar] [CrossRef]
- Holmer, M.; Duarte, C.M.; Marbà, N. Sulfur cycling and seagrass (Posidonia oceanica) status in carbonate sediments. Biogeochemistry 2003, 66, 223–239. [Google Scholar] [CrossRef]
- Benito, G.; Macklin, M.G.; Zielhofer, C.; Jones, A.F.; Machado, M.J. Holocene flooding and climate change in the Mediterranean. Catena 2015, 130, 13–33. [Google Scholar] [CrossRef] [Green Version]
- Canals, M.; Ballesteros, E. Production of carbonate sediments by phytobenthic communities in the Mallorca-Minorca Shelf, northwestern Mediterranean Sea. Deep Res. 1997, 44, 611–629. [Google Scholar]
- Mateu-Vicens, G.; Brandano, M.; Gaglianone, G.; Baldassarre, A. Seagrass-meadow sedimentary facies in a mixed siliciclastic-carbonate temperate system in the Tyrrhenian Sea (Pontinian Islands, Western Mediterranean). J. Sediment. Res. 2012, 82, 451–463. [Google Scholar] [CrossRef]
- De Falco, G.; Baroli, M.; Cucco, A.; Simeone, S. Intrabasinal conditions promoting the development of a biogenic carbonate sedimentary facies associated with the seagrass. Posidonia oceanica. Cont. Shelf Res. 2008, 28, 797–812. [Google Scholar] [CrossRef]
- Saderne, V.; Geraldi, N.R.; Macreadie, P.I.; Maher, D.T.; Middelburg, J.J.; Serrano, O.; Almahasheer, H.; Arias-Ortiz, A.; Cusack, M.; Eyre, B.D.; et al. Role of carbonate burial in Blue Carbon budgets. Nat. Commun. 2019, 10, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyngby, J.E.; Brix, H. Heavy metals in eelgrass (Zostera marina L.) during growth and decomposition. Hydrobiologia 1989, 176–177, 189–196. [Google Scholar] [CrossRef]
- Ragsdale, H.L.; Thorhaug, A. Trace metal cycling in the USA coastal zone: A synthesis. Am. J. Bot. 1980, 67, 1102–1112. [Google Scholar] [CrossRef]
- Fenchel, T.; King, G.M.; Blackburn, H. Bacterial Biogeochemistry: The Ecophysiology of Mineral Cycling, 3rd ed.; Elsevier Academic Press: San Diego, CA, USA, 1998. [Google Scholar]
- Schuetz, L. Atmospheric mineral dust properties and source markers. In Paleoclimatology and Paleometeorology: Modern and Past Patterns of Global Atmospheric Transport; Leinen, M., Sarnthein, M., Eds.; Springer: Dordrecht, The Netherlands, 1989; pp. 359–383. [Google Scholar]
- Taboada, T.; Cortizas, A.M.; García, C.; García-Rodeja, E. Particle-size fractionation of titanium and zirconium during weathering and pedogenesis of granitic rocks in NW Spain. Geoderma 2006, 131, 218–236. [Google Scholar] [CrossRef]
- Riley, J.P.; Chester, R. Introduction to Marine Chemistry; Academic Press: London, UK, 1971. [Google Scholar]
- Martínez-Cortizas, A.; Pontevedra-Pombal, X.; Nóvoa Muñoz, J.C.; García-Rodeja, E. Four thousand years of atmospheric Pb, Cd and Zn deposition recorded by the ombrotrophic peat bog of Penido Vello (Northwestern Spain). Water Air Soil Pollut. 1997, 387–403. [Google Scholar] [CrossRef]
- Pedersen, O.; Borum, J.; Duarte, C.M.; Fortes, M.D. Oxygen dynamics in the rhizosphere of Cymodocea rotundata. Mar. Ecol. Prog. Ser. 1998, 169, 283–288. [Google Scholar] [CrossRef]
- Connell, E.L.; Colmer, T.D.; Walker, D.I. Radial oxygen loss from intact roots of Halophila ovalis as a function of distance behind the root tip and shoot illumination. Aquat. Bot. 1999, 63, 219–228. [Google Scholar] [CrossRef]
- Greve, T.M.; Borum, J.; Pedersen, O. Meristematic oxygen variability in eelgrass (Zostera marina). Limnol. Oceanogr. 2003, 48, 210–216. [Google Scholar] [CrossRef]



| PC1 | PC2 | PC3 | PC4 | Com | |
|---|---|---|---|---|---|
| Si | 0.94 | 0.15 | 0.08 | −0.11 | 0.93 |
| K | 0.88 | −0.01 | 0.04 | −0.18 | 0.81 |
| Al | 0.88 | 0.24 | 0.20 | −0.01 | 0.87 |
| S | −0.85 | −0.15 | 0.03 | −0.21 | 0.79 |
| Rb | 0.83 | −0.09 | 0.22 | −0.01 | 0.75 |
| P | −0.83 | 0.14 | 0.14 | −0.01 | 0.72 |
| Br | −0.81 | −0.42 | −0.07 | 0.14 | 0.85 |
| Cl | −0.67 | −0.37 | −0.25 | 0.16 | 0.66 |
| Sr | 0.18 | 0.91 | 0.11 | −0.28 | 0.95 |
| Ca | −0.01 | 0.88 | −0.02 | −0.36 | 0.90 |
| COM | −0.15 | −0.87 | 0.06 | −0.00 | 0.78 |
| Mg | −0.02 | 0.84 | −0.08 | −0.02 | 0.71 |
| Cr | 0.24 | −0.82 | −0.27 | −0.09 | 0.82 |
| As | −0.42 | −0.82 | −0.03 | −0.04 | 0.85 |
| CaCO3 | 0.19 | 0.76 | −0.13 | −0.53 | 0.91 |
| Zr | 0.46 | 0.70 | 0.26 | −0.16 | 0.79 |
| Fe | 0.13 | −0.01 | 0.92 | 0.03 | 0.87 |
| Ti | 0.61 | −0.01 | 0.70 | 0.13 | 0.88 |
| Pb | 0.14 | −0.40 | −0.50 | 0.62 | 0.81 |
| FOM | −0.13 | −0.12 | 0.14 | 0.92 | 0.89 |
| Var. % | 32.9 | 30.5 | 9.9 | 9.5 | |
| Ac. Var. % | 32.9 | 63.4 | 73.3 | 82. 8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piñeiro-Juncal, N.; Leiva-Dueñas, C.; Serrano, O.; Mateo, M.Á.; Martínez-Cortízas, A. Pedogenic Processes in a Posidonia oceanica Mat. Soil Syst. 2020, 4, 18. https://doi.org/10.3390/soilsystems4020018
Piñeiro-Juncal N, Leiva-Dueñas C, Serrano O, Mateo MÁ, Martínez-Cortízas A. Pedogenic Processes in a Posidonia oceanica Mat. Soil Systems. 2020; 4(2):18. https://doi.org/10.3390/soilsystems4020018
Chicago/Turabian StylePiñeiro-Juncal, Nerea, Carmen Leiva-Dueñas, Oscar Serrano, Miguel Ángel Mateo, and Antonio Martínez-Cortízas. 2020. "Pedogenic Processes in a Posidonia oceanica Mat" Soil Systems 4, no. 2: 18. https://doi.org/10.3390/soilsystems4020018
APA StylePiñeiro-Juncal, N., Leiva-Dueñas, C., Serrano, O., Mateo, M. Á., & Martínez-Cortízas, A. (2020). Pedogenic Processes in a Posidonia oceanica Mat. Soil Systems, 4(2), 18. https://doi.org/10.3390/soilsystems4020018