Effect of Cathode Material and Its Size on the Abundance of Nitrogen Removal Functional Genes in Microcosms of Integrated Bioelectrochemical-Wetland Systems
Abstract
:1. Introduction
2. Materials and Methods
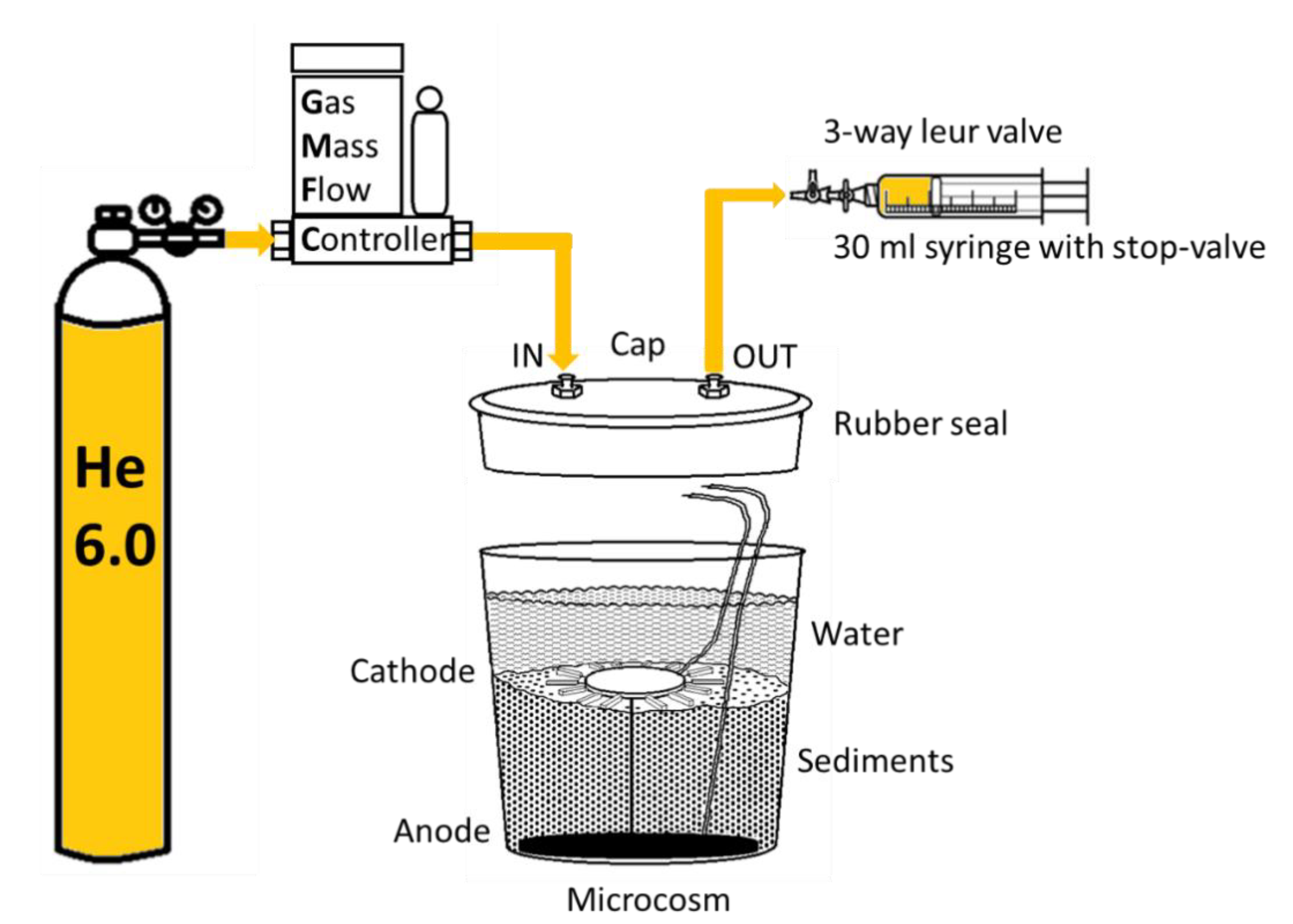
2.1. Experimental Set-Up
2.2. Sediment and Water Sampling
2.3. N2O Sampling and Analysis
2.4. DNA Extraction
2.5. Quantitative Polymerase Chain Reaction (qPCR)
2.6. Electrochemical Study
2.7. Statistical Analysis
3. Results
3.1. Physicochemical Properties, Nitrate Removal and N2O Flux
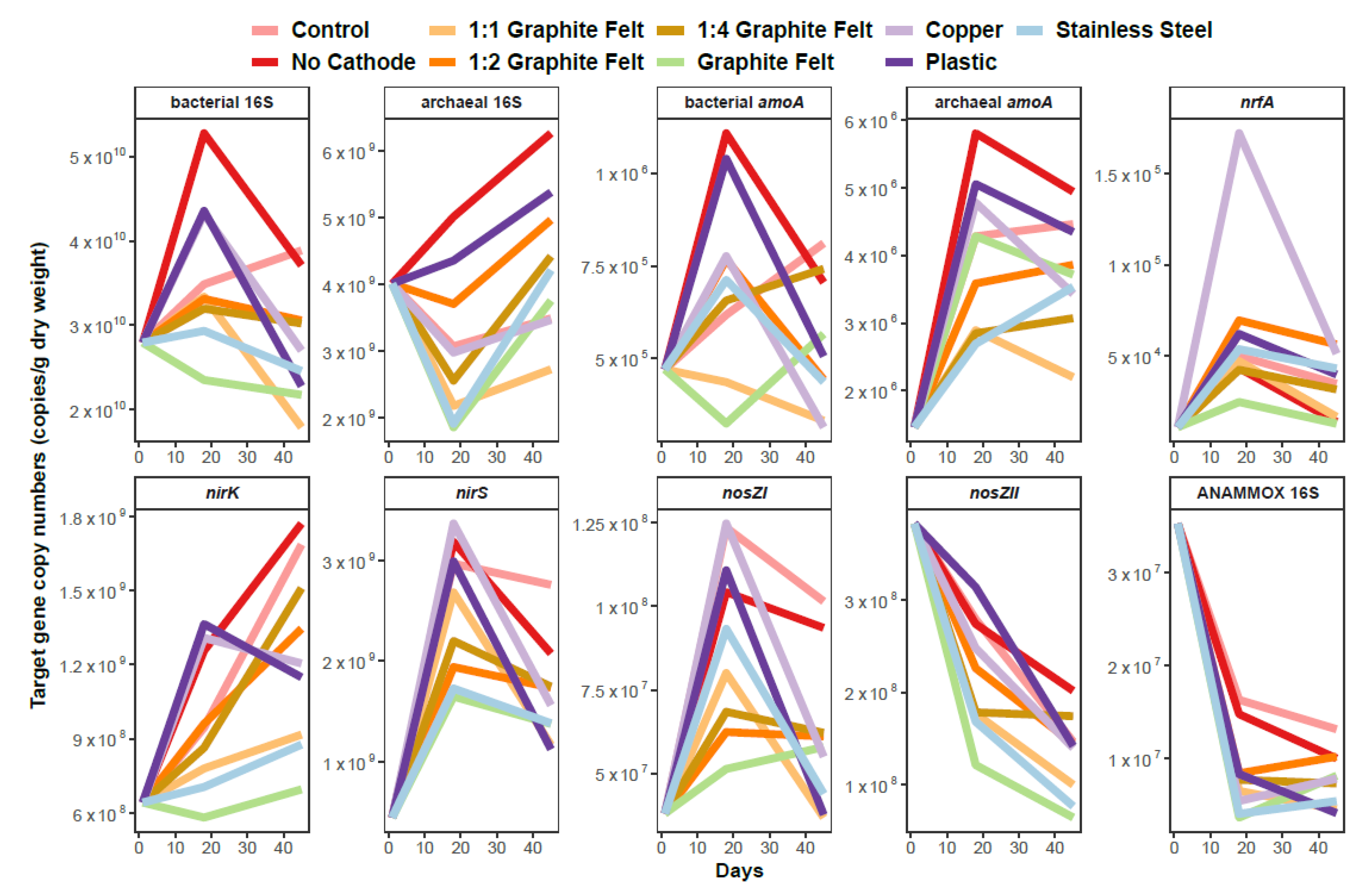
3.2. Sediment Microbial Community Abundance and Proportion in the Prokaryotic Community–Denitrification, Nitrification, ANAMMOX and DNRA Process-Based Genes
3.3. Relationships between Target Genes, Physicochemical Parameters and N2O Flux
3.4. Electrochemical Measurements
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Galloway, J.N.; Leach, A.M.; Bleeker, A.; Erisman, J.W. A chronology of human understanding of the nitrogen cycle. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20130120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rockström, J.; Steffen, W.; Noone, K.; Persson, Å.; Chapin, F.S.; Lambin, E.F.; Lenton, T.M.; Scheffer, M.; Folke, C.; Schellnhuber, H.J.; et al. A safe operating space for humanity. Nature 2009, 461, 472–475. [Google Scholar] [CrossRef] [PubMed]
- Fields, S. Global Nitrogen: Cycling out of Control. Environ. Health Perspect. 2004, 112, A556–A563. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Fletcher, T.D.; Sun, G. Nitrogen removal in constructed wetland systems. Eng. Life Sci. 2009, 9, 11–22. [Google Scholar] [CrossRef]
- Quick, A.M.; Reeder, W.J.; Farrell, T.B.; Tonina, D.; Feris, K.P.; Benner, S.G. Nitrous oxide from streams and rivers: A review of primary biogeochemical pathways and environmental variables. Earth-Sci. Rev. 2019, 191, 224–262. [Google Scholar] [CrossRef]
- Espenberg, M.; Truu, M.; Mander, Ü.; Kasak, K.; Nõlvak, H.; Ligi, T.; Oopkaup, K.; Maddison, M.; Truu, J. Differences in microbial community structure and nitrogen cycling in natural and drained tropical peatland soils. Sci. Rep. Nat. Publ. Group 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, Y.; Tong, S.; Zheng, M.; Zhao, Y.; Tian, C.; Liu, H.; Feng, C. Enhancement of bacterial denitrification for nitrate removal in groundwater with electrical stimulation from microbial fuel cells. J. Power Sources 2014, 268, 423–429. [Google Scholar] [CrossRef]
- Jain, A.; He, Z. Cathode-enhanced wastewater treatment in bioelectrochemical systems. Npj Clean Water 2018, 1, 1–5. [Google Scholar] [CrossRef]
- Lust, R.; Nerut, J.; Kasak, K.; Mander, Ü. Enhancing Nitrate Removal from Waters with Low Organic Carbon Concentration Using a Bioelectrochemical System—A Pilot-Scale Study. Water 2020, 12, 516. [Google Scholar] [CrossRef] [Green Version]
- Hoareau, M.; Erable, B.; Bergel, A. Microbial electrochemical snorkels (MESs): A budding technology for multiple applications. A mini review. Electrochem. Commun. 2019, 104, 106473. [Google Scholar] [CrossRef]
- Logan, B.E.; Rossi, R.; Ragab, A.; Saikaly, P.E. Electroactive microorganisms in bioelectrochemical systems. Nat. Rev. Microbiol. 2019, 17, 307–319. [Google Scholar] [CrossRef]
- Guadarrama-Pérez, O.; Gutiérrez-Macías, T.; García-Sánchez, L.; Guadarrama-Pérez, V.H.; Estrada-Arriaga, E.B. Recent advances in constructed wetland-microbial fuel cells for simultaneous bioelectricity production and wastewater treatment: A review. Int. J. Energy Res. 2019, 43, 5106–5127. [Google Scholar] [CrossRef]
- Ferapontova, E.E. Effect of cation adsorption on the kinetics of anion electroreduction: Part II. Effect of the adsorption of organic cations in small concentrations on the kinetics of anion electroreduction. J. Electroanal. Chem. 1999, 476, 37–45. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, X.; Ning, X.; Yang, Q. Research progress of microbial fuel cell and constructed wetland coupling system. IOP Conf. Ser. Earth Environ. Sci. 2018, 199, 052014. [Google Scholar] [CrossRef]
- Santoro, C.; Arbizzani, C.; Erable, B.; Ieropoulos, I. Microbial fuel cells: From fundamentals to applications. A review. J. Power Sources 2017, 356, 225–244. [Google Scholar] [CrossRef]
- Alonso, R.M.; San-Martín, M.I.; Mateos, R.; Morán, A.; Escapa, A. Scale-Up of Bioelectrochemical Systems for Energy Valorization of Waste Streams. In Microbial Electrochemical Technologies; Tiquia-Arashiro, S.M., Pant, D., Eds.; CRC Press: Boca Raton, FL, USA, 2000; pp. 447–459. [Google Scholar] [CrossRef]
- Sánchez, C.; Dessì, P.; Duffy, M.; Lens, P.N.L. Microbial electrochemical technologies: Electronic circuitry and characterization tools. Biosens. Bioelectron. 2020, 150, 111884. [Google Scholar] [CrossRef]
- Erable, B.; Etcheverry, L.; Bergel, A. From microbial fuel cell (MFC) to microbial electrochemical snorkel (MES): Maximizing chemical oxygen demand (COD) removal from wastewater. Biofouling 2011, 27, 319–326. [Google Scholar] [CrossRef] [Green Version]
- Ramírez-Vargas, C.A.; Arias, C.A.; Carvalho, P.; Zhang, L.; Esteve-Núñez, A.; Brix, H. Electroactive biofilm-based constructed wetland (EABB-CW): A mesocosm-scale test of an innovative setup for wastewater treatment. Sci. Total Environ. 2019, 659, 796–806. [Google Scholar] [CrossRef]
- Xiao, Y.; Zheng, Y.; Wu, S.; Yang, Z.-H.; Zhao, F. Bacterial community structure of autotrophic denitrification biocathode by 454 pyrosequencing of the 16S rRNA gene. Microb. Ecol. 2015, 69, 492–499. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, J.; Li, X.; Wang, S.; Hu, B.; Ding, X. The Denitrification Characteristics and Microbial Community in the Cathode of an MFC with Aerobic Denitrification at High Temperatures. Front. Microbiol. 2017, 8, 9. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Guo, Y.; Cai, J.; Wen, H.; Mao, Z.; Zhang, H.; Wang, X.; Ma, L.; Zhu, M. Electricity production and the analysis of the anode microbial community in a constructed wetland-microbial fuel cell. RSC Adv. 2019, 9, 21460–21472. [Google Scholar] [CrossRef] [Green Version]
- Van Doan, T.; Lee, T.K.; Shukla, S.K.; Tiedje, J.M.; Park, J. Increased nitrous oxide accumulation by bioelectrochemical denitrification under autotrophic conditions: Kinetics and expression of denitrification pathway genes. Water Res. 2013, 47, 7087–7097. [Google Scholar] [CrossRef] [PubMed]
- Vilar-Sanz, A.; Puig, S.; García-Lledó, A.; Trias, R.; Balaguer, M.D.; Colprim, J.; Bañeras, L. Denitrifying Bacterial Communities Affect Current Production and Nitrous Oxide Accumulation in a Microbial Fuel Cell. PLoS ONE 2013, 8, e63460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srinivasan, V.N.; Butler, C.S. Ecological and Transcriptional Responses of Anode-Respiring Communities to Nitrate in a Microbial Fuel Cell. Environ. Sci. Technol. 2017, 51, 5334–5342. [Google Scholar] [CrossRef] [PubMed]
- Cecconet, D.; Bolognesi, S.; Callegari, A.; Capodaglio, A.G. Controlled sequential biocathodic denitrification for contaminated groundwater bioremediation. Sci. Total Environ. 2019, 651, 3107–3116. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tian, Y.; Liu, H.; Zhao, X.; Peng, S. The influence of incorporating microbial fuel cells on greenhouse gas emissions from constructed wetlands. Sci. Total Environ. 2019, 656, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Kasak, K.; Kill, K.; Pärn, J.; Mander, Ü. Efficiency of a newly established in-stream constructed wetland treating diffuse agricultural pollution. Ecol. Eng. 2018, 119, 1–7. [Google Scholar] [CrossRef]
- Loftfield, N.; Flessa, H.; Augustin, J.; Beese, F. Automated Gas Chromatographic System for Rapid Analysis of the Atmospheric Trace Gases Methane, Carbon Dioxide, and Nitrous Oxide. J. Environ. Qual. 1997, 26, 560–564. [Google Scholar] [CrossRef]
- Kandeler, E.; Deiglmayr, K.; Tscherko, D.; Bru, D.; Philippot, L. Abundance of narG, nirS, nirK, and nosZ Genes of Denitrifying Bacteria during Primary Successions of a Glacier Foreland. Appl. Environ. Microbiol. 2006, 72, 5957–5962. [Google Scholar] [CrossRef] [Green Version]
- Ruijter, J.M.; Ramakers, C.; Hoogaars, W.M.H.; Karlen, Y.; Bakker, O.; Van den Hoff, M.J.B.; Moorman, A.F.M. Amplification efficiency: Linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Res. 2009, 37, e45. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Naumann, U.; Wright, S.T.; Warton, D.I. Mvabund–an R package for model-based analysis of multivariate abundance data. Methods Ecol. 2012, 3, 471–474. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Aulenta, F.; Puig, S.; Esteve-Núñez, A.; He, Y.; Mu, Y.; Rabaey, K. Microbial electrochemistry for bioremediation. Environ. Sci. Ecotechnol. 2020, 1, 100013. [Google Scholar] [CrossRef]
- Pous, N.; Koch, C.; Colprim, J.; Puig, S.; Harnisch, F. Extracellular electron transfer of biocathodes: Revealing the potentials for nitrate and nitrite reduction of denitrifying microbiomes dominated by Thiobacillus sp. Electrochem. Commun. 2014, 49, 93–97. [Google Scholar] [CrossRef] [Green Version]
- Kato, S.; Hashimoto, K.; Watanabe, K. Microbial interspecies electron transfer via electric currents through conductive minerals. Proc. Natl. Acad. Sci. USA 2012, 109, 10042–10046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Summers, Z.M.; Fogarty, H.E.; Leang, C.; Franks, A.E.; Malvankar, N.S.; Lovley, D.R. Direct exchange of electrons within aggregates of an evolved syntrophic coculture of anaerobic bacteria. Science 2010, 330, 1413–1415. [Google Scholar] [CrossRef] [Green Version]
- Qu, Y.; Feng, Y.; Wang, X.; Logan, B.E. Use of a Coculture to Enable Current Production by Geobacter sulfurreducens. Appl. Environ. Microbiol. 2012, 78, 3484–3487. [Google Scholar] [CrossRef] [Green Version]
- Bourdakos, N.; Marsili, E.; Mahadevan, R. A defined co-culture of Geobacter sulfurreducens and Escherichia coli in a membrane-less microbial fuel cell. Biotechnol. Bioeng. 2014, 111, 709–718. [Google Scholar] [CrossRef]
- Morris, B.E.L.; Henneberger, R.; Huber, H.; Moissl-Eichinger, C. Microbial syntrophy: Interaction for the common good. FEMS Microbiol. Rev. 2013, 37, 384–406. [Google Scholar] [CrossRef]
- Moissl-Eichinger, C.; Pausan, M.; Taffner, J.; Berg, G.; Bang, C.; Schmitz, R.A. Archaea Are Interactive Components of Complex Microbiomes. Trends Microbiol. 2018, 26, 70–85. [Google Scholar] [CrossRef]
- Stein, L.Y. Insights into the physiology of ammonia-oxidizing microorganisms. Curr. Opin. Chem. Biol. 2019, 49, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Hallin, S.; Philippot, L.; Löffler, F.E.; Sanford, R.A.; Jones, C.M. Genomics and Ecology of Novel N2O-Reducing Microorganisms. Trends Microbiol. 2018, 26, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Lisa, J.A.; Tobias, C.R. Linking DNRA community structure and activity in a shallow lagoonal estuarine system. Front. Microbiol. 2014, 5, 460. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Treatment | Nitrate Removal | Nitrite Removal |
|---|---|---|
| Control | 66.51 ± 1.54 | 98.66 ± 0.42 |
| No Cathode | 53.88 ± 23.52 | 99.37 ± 0.08 |
| 1:1 Graphite Felt | 48.39 ± 29.05 | 98.07 ± 2.31 |
| 1:2 Graphite Felt | 41.35 ± 15.27 | 99.40 ± 0.06 |
| 1:4 Graphite Felt | 41.52 ± 6.99 | 99.33 ± 0.11 |
| Graphite Felt | 41.18 ± 13.67 | 99.00 ± 0.20 |
| Copper | 40.55 ± 26.70 | 99.39 ± 0.27 |
| Plastic | 74.61 ± 7.22 | 99.39 ± 0.27 |
| Stainless steel | 40.34 ± 9.88 | 99.25 ± 0.22 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gadegaonkar, S.S.; Philippon, T.; Rogińska, J.M.; Mander, Ü.; Maddison, M.; Etienne, M.; Barrière, F.; Kasak, K.; Lust, R.; Espenberg, M. Effect of Cathode Material and Its Size on the Abundance of Nitrogen Removal Functional Genes in Microcosms of Integrated Bioelectrochemical-Wetland Systems. Soil Syst. 2020, 4, 47. https://doi.org/10.3390/soilsystems4030047
Gadegaonkar SS, Philippon T, Rogińska JM, Mander Ü, Maddison M, Etienne M, Barrière F, Kasak K, Lust R, Espenberg M. Effect of Cathode Material and Its Size on the Abundance of Nitrogen Removal Functional Genes in Microcosms of Integrated Bioelectrochemical-Wetland Systems. Soil Systems. 2020; 4(3):47. https://doi.org/10.3390/soilsystems4030047
Chicago/Turabian StyleGadegaonkar, Sharvari S., Timothé Philippon, Joanna M. Rogińska, Ülo Mander, Martin Maddison, Mathieu Etienne, Frédéric Barrière, Kuno Kasak, Rauno Lust, and Mikk Espenberg. 2020. "Effect of Cathode Material and Its Size on the Abundance of Nitrogen Removal Functional Genes in Microcosms of Integrated Bioelectrochemical-Wetland Systems" Soil Systems 4, no. 3: 47. https://doi.org/10.3390/soilsystems4030047
APA StyleGadegaonkar, S. S., Philippon, T., Rogińska, J. M., Mander, Ü., Maddison, M., Etienne, M., Barrière, F., Kasak, K., Lust, R., & Espenberg, M. (2020). Effect of Cathode Material and Its Size on the Abundance of Nitrogen Removal Functional Genes in Microcosms of Integrated Bioelectrochemical-Wetland Systems. Soil Systems, 4(3), 47. https://doi.org/10.3390/soilsystems4030047









