Effect of Municipal Solid Waste Compost on Antimony Mobility, Phytotoxicity and Bioavailability in Polluted Soils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil and MSWC Origin, Characteristics and Mesocosms Set Up
2.2. Soil Characterization and Sb Mobility in Treated and Untreated Soils
2.3. Biolog Community-Level Physiological Profiles and Soil Enzyme Activities in Treated and Untreated Sb-Polluted Soils
2.4. Sb Phytotoxicity and Bioavailability in Treated and Untreated Sb-Polluted Soils
2.5. Statistical Analysis
3. Results and Discussion
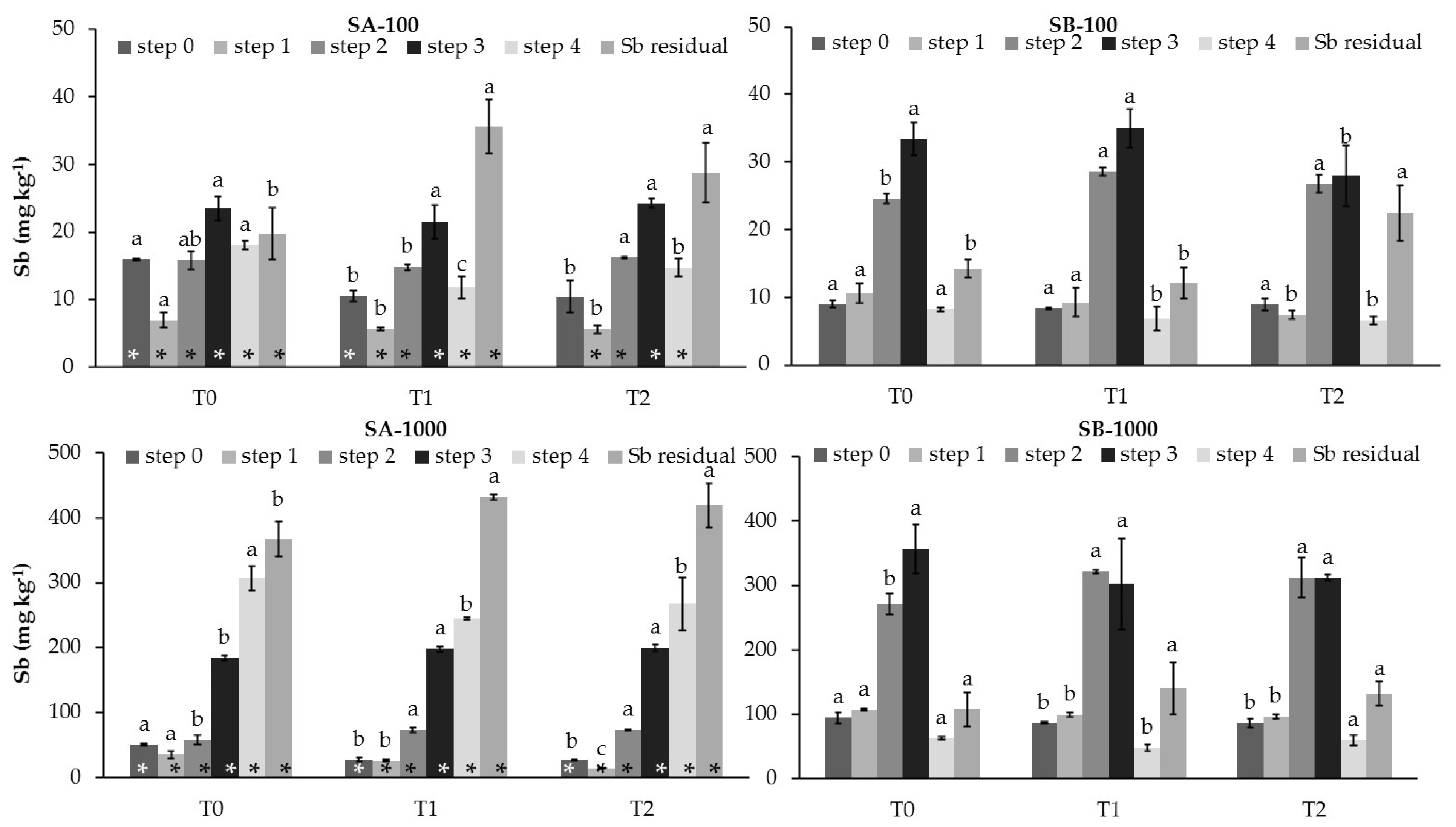
3.1. Influence of MSWC on Selected Chemical Properties and Sb Mobility in Polluted Soils
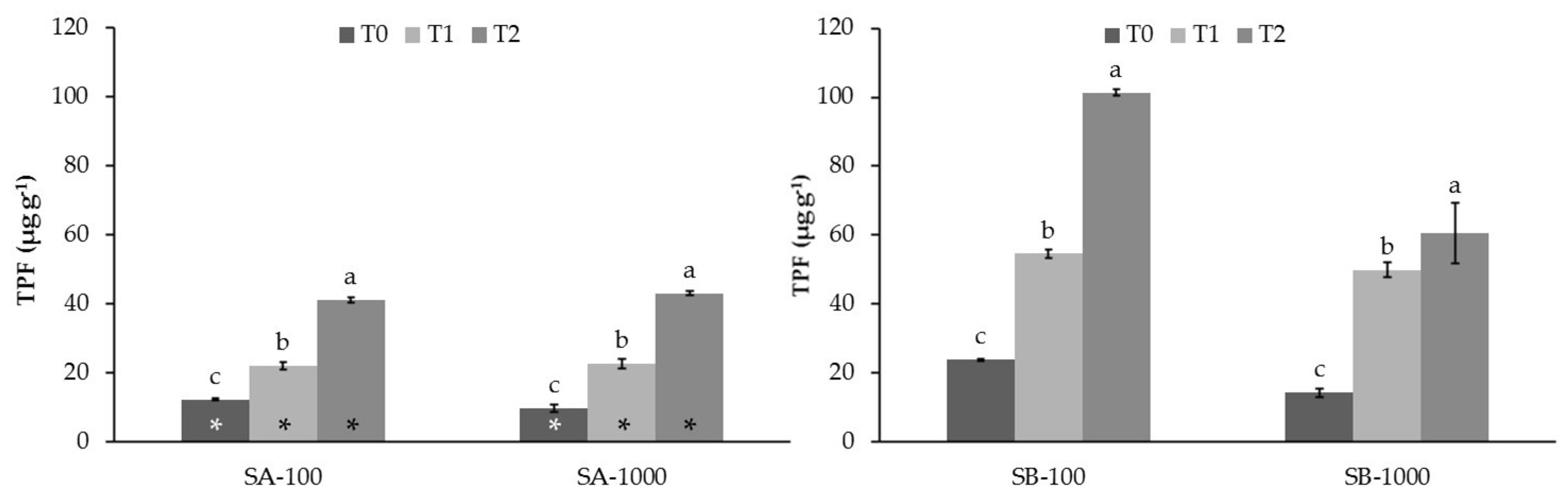
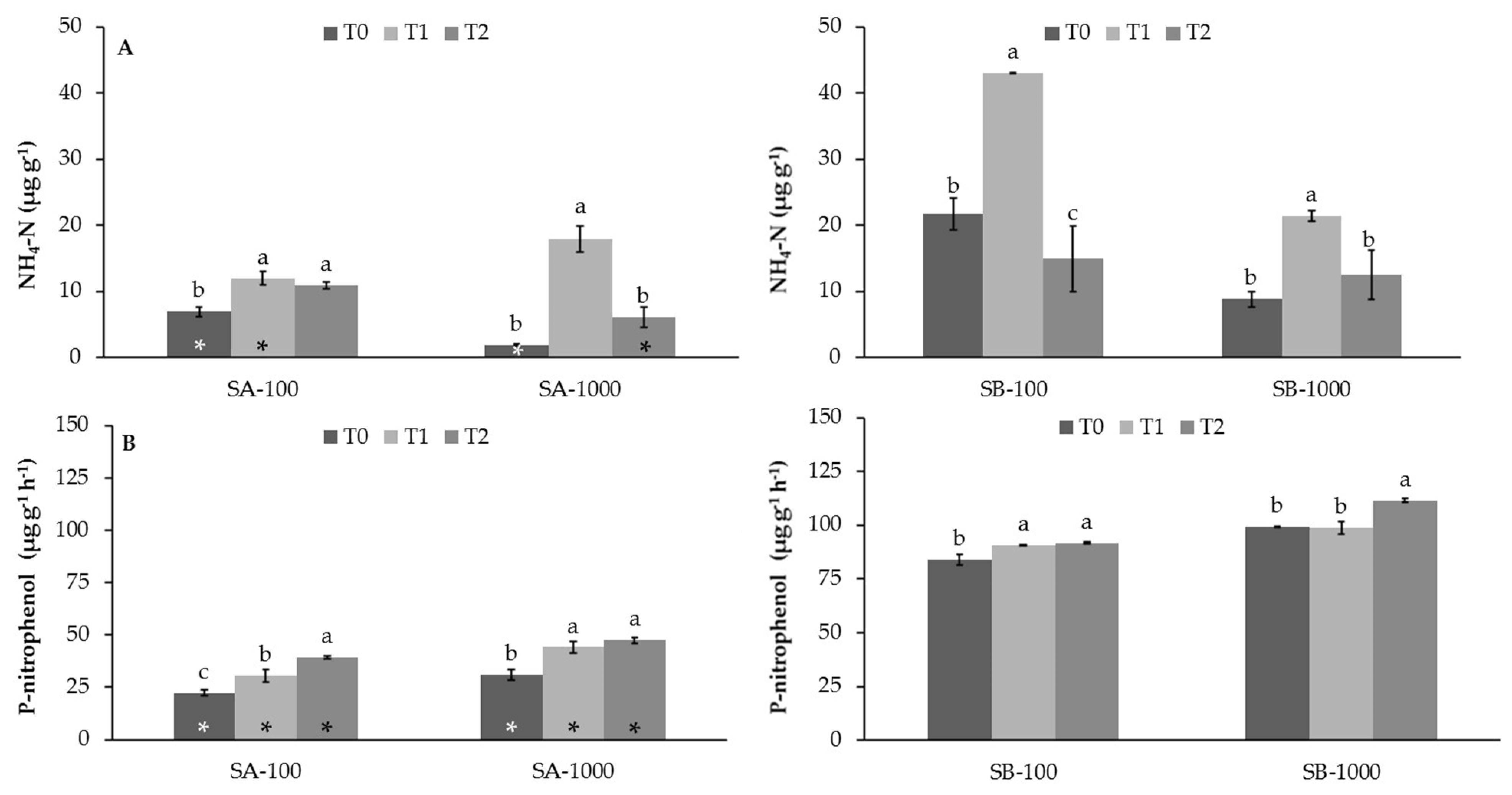
3.2. Influence of MSWC on Soil Enzyme Activities in Sb-Polluted Soils
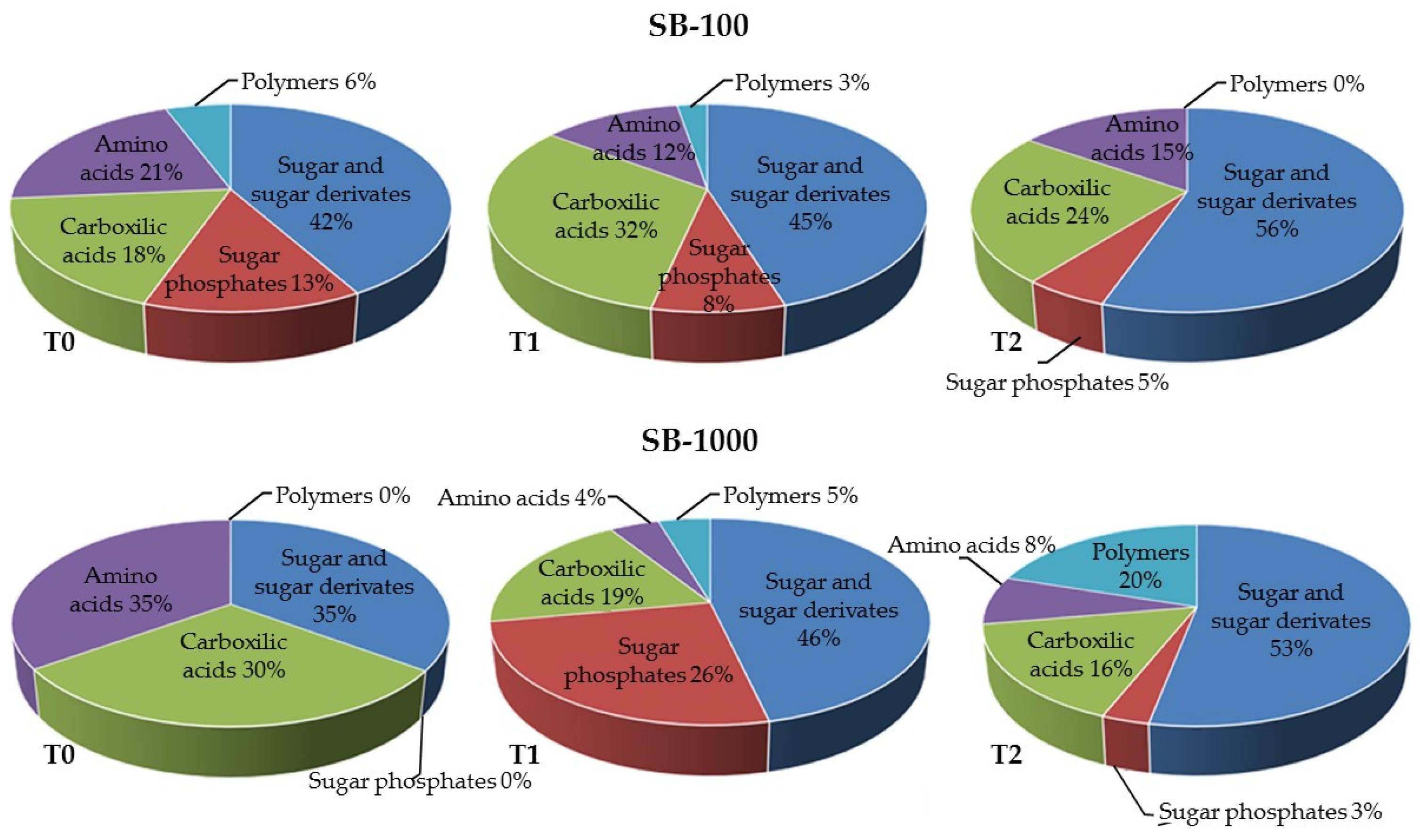
3.3. Influence of MSWC on Soil Microbial Activity and Catabolic Diversity in Sb-Polluted Soils
3.4. Influence of MSWC on Sb Phytotoxicity and Bioavailability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Herath, I.; Vithanage, M.; Bundschuh, J. Antimony as a global dilemma: Geochemistry, mobility, fate and transport. Environ. Pollut. 2017, 223, 545–559. [Google Scholar] [CrossRef]
- Pierart, A.; Shahid, M.; Sejalon-Delmas, N.; Dumat, C. Antimony bioavailability: Knowledge and research perspectives for sustainable agricultures. J. Hazard. Mater. 2015, 289, 219–234. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.Q.; He, M.C.; Xi, J.H.; Lu, X.F. Antimony distribution and mobility in rivers around the world’s largest antimony mine of Xikuangshan, Hunan Province, China. Microchem. J. 2011, 97, 4–11. [Google Scholar] [CrossRef]
- Li, X.D.; Thornton, I. Arsenic, Antimony and Bismuth in Soil and Pasture Herbage in Some Old Metalliferous Mining Areas in England. Environ. Geochem. Health 1993, 15, 135–144. [Google Scholar] [CrossRef]
- Dietl, C.; Reifenhauser, W.; Peichl, L. Association of antimony with traffic-occurrence in airborne dust, deposition and accumulation in standardized grass cultures. Sci. Total Environ. 1997, 205, 235–244. [Google Scholar] [CrossRef]
- Foldi, C.; Sauermann, S.; Dohrmann, R.; Mansfeldt, T. Traffic-related distribution of antimony in roadside soils. Environ. Pollut. 2018, 237, 704–712. [Google Scholar] [CrossRef]
- Manta, D.S.; Angelone, M.; Bellanca, A.; Neri, R.; Sprovieri, M. Heavy metals in urban soils: A case study from the city of Palermo (Sicily), Italy. Sci. Total Environ. 2002, 300, 229–243. [Google Scholar] [CrossRef]
- Biver, M.; Turner, A.; Filella, M. Antimony release from polyester textiles by artificial sweat solutions: A call for a standardized procedure. Regul. Toxicol. Pharm. 2021, 119, 104824. [Google Scholar] [CrossRef]
- G.U. GU del 14 aprile 2006, Decreto Legislativo 3 aprile 2006, n. 152, Norme in Materia Ambientale. 2006. Available online: https://www.gazzettaufficiale.it/ (accessed on 30 September 2021).
- United States Environmental Protection Agency (USEPA). National Primary Drinking Water Regulations; United States Environmental Protection Agency: Washington, DC, USA, 2009.
- Filella, M.; Belzile, N.; Chen, Y.W. Antimony in the environment: A review focused on natural waters II. Relevant solution chemistry. Earth Sci. Rev. 2002, 59, 265–285. [Google Scholar] [CrossRef]
- Filella, M.; Williams, P.A.; Belzile, N. Antimony in the environment: Knowns and unknowns. Environ. Chem. 2009, 6, 95–105. [Google Scholar] [CrossRef] [Green Version]
- Filella, M.; Belzile, N.; Chen, Y.W. Antimony in the environment: A review focused on natural waters I. Occurrence. Earth-Sci. Rev. 2002, 57, 125–176. [Google Scholar] [CrossRef]
- He, M.C.; Wang, N.N.; Long, X.J.; Zhang, C.J.; Ma, C.L.; Zhong, Q.Y.; Wang, A.H.; Wang, Y.; Pervaiz, A.; Shan, J. Antimony speciation in the environment: Recent advances in understanding the biogeochemical processes and ecological effects. J. Environ. Sci. 2019, 75, 14–39. [Google Scholar] [CrossRef]
- Johnston, S.G.; Bennett, W.W.; Doriean, N.; Hockmann, K.; Karimian, N.; Burton, E.D. Antimony and arsenic speciation, redox-cycling and contrasting mobility in a mining-impacted river system. Sci. Total Environ. 2020, 710, 136354. [Google Scholar] [CrossRef]
- Castaldi, P.; Silvetti, M.; Manzano, R.; Brundu, G.; Roggero, P.P.; Garau, G. Mutual effect of Phragmites australis, Arundo donax and immobilization agents on arsenic and trace metals phytostabilization in polluted soils. Geoderma 2018, 314, 63–72. [Google Scholar] [CrossRef]
- Diquattro, S.; Garau, G.; Lauro, G.; Silvetti, M.; Deiana, S.; Castaldi, P. Municipal solid waste compost as a novel sorbent for antimony(V): Adsorption and release trials at acidic pH. Environ. Sci. Pollut. Res. 2018, 25, 5603–5615. [Google Scholar] [CrossRef] [PubMed]
- Filella, M.; Williams, P.A. Antimony interactions with heterogeneous complexants in waters, sediments and soils: A review of binding data for homologous compounds. Chem. Erde-Geochem. 2012, 72, 49–65. [Google Scholar] [CrossRef]
- Nakamaru, Y.M.; Altansuvd, J. Speciation and bioavailability of selenium and antimony in non-flooded and wetland soils: A review. Chemosphere 2014, 111, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Tighe, M.; Lockwood, P.; Wilson, S. Adsorption of antimony(v) by floodplain soils, amorphous iron(III) hydroxide and humic acid. J. Environ. Monit. 2005, 7, 1177–1185. [Google Scholar] [CrossRef] [Green Version]
- Diquattro, S.; Garau, G.; Mangia, N.P.; Drigo, B.; Lombi, E.; Vasileiadis, S.; Castaldi, P. Mobility and potential bioavailability of antimony in contaminated soils: Short-term impact on microbial community and soil biochemical functioning. Ecotoxicol. Environ. Saf. 2020, 196, 110576. [Google Scholar] [CrossRef]
- Garau, G.; Silvetti, M.; Vasileiadis, S.; Donner, E.; Diquattro, S.; Deiana, S.; Lombi, E.; Castaldi, P. Use of municipal solid wastes for chemical and microbiological recovery of soils contaminated with metal(loid)s. Soil Biol. Biochem. 2017, 111, 25–35. [Google Scholar] [CrossRef]
- Yu, H.; Zheng, X.F.; Weng, W.L.; Yan, X.Z.; Chen, P.B.; Liu, X.Y.; Peng, T.; Zhong, Q.P.; Xu, K.; Wang, C.; et al. Synergistic effects of antimony and arsenic contaminations on bacterial, archaeal and fungal communities in the rhizosphere of Miscanthus sinensis: Insights for nitrification and carbon mineralization. J. Hazard. Mater. 2021, 411, 125094. [Google Scholar] [CrossRef] [PubMed]
- Busby, R.R.; Barbato, R.A.; Jung, C.M.; Bednar, A.J.; Douglas, T.A.; Ringelberg, D.B.; Indest, K.J. Alaskan plants and their assembled rhizosphere communities vary in their responses to soil antimony. Appl. Soil Ecol. 2021, 167, 104031. [Google Scholar] [CrossRef]
- Yadav, V.; Arif, N.; Kovac, J.; Singh, V.P.; Tripathi, D.K.; Chauhan, D.K.; Vaculik, M. Structural modifications of plant organs and tissues by metals and metalloids in the environment: A review. Plant. Physiol. Biochem. 2021, 159, 100–112. [Google Scholar] [CrossRef]
- Zhu, Y.M.; Yang, J.G.; Wang, L.Z.; Lin, Z.T.; Dai, J.X.; Wang, R.J.; Yu, Y.S.; Liu, H.; Rensing, C.; Feng, R.W. Factors influencing the uptake and speciation transformation of antimony in the soil-plant system, and the redistribution and toxicity of antimony in plants. Sci. Total Environ. 2020, 738, 140232. [Google Scholar] [CrossRef]
- Wang, X.Q.; Li, F.B.; Yuan, C.L.; Li, B.; Liu, T.X.; Liu, C.S.; Du, Y.H.; Liu, C.P. The translocation of antimony in soil-rice system with comparisons to arsenic: Alleviation of their accumulation in rice by simultaneous use of Fe(II) and NO3-. Sci. Total Environ. 2019, 650, 633–641. [Google Scholar] [CrossRef]
- Teng, F.Y.; Zhang, Y.X.; Wang, D.Q.; Shen, M.C.; Hu, D.F. Iron-modified rice husk hydrochar and its immobilization effect for Pb and Sb in contaminated soil. J. Hazard. Mater. 2020, 398, 122977. [Google Scholar] [CrossRef]
- Almas, A.R.; Pironin, E.; Okkenhaug, G. The partitioning of Sb in contaminated soils after being immobilization by Fe-based amendments is more dynamic compared to Pb. Appl. Geochem. 2019, 108, 104378. [Google Scholar] [CrossRef]
- Nakamaru, Y.M.; Peinado, F.J.M. Effect of soil organic matter on antimony bioavailability after the remediation process. Environ. Pollut. 2017, 228, 425–432. [Google Scholar] [CrossRef]
- Verbeeck, M.; Thiry, Y.; Smolders, E. Soil organic matter affects arsenic and antimony sorption in anaerobic soils. Environ. Pollut. 2020, 257, 113566. [Google Scholar] [CrossRef] [PubMed]
- Lewinska, K.; Karczewska, A. A release of toxic elements from military shooting range soils as affected by pH and treatment with compost. Geoderma 2019, 346, 1–10. [Google Scholar] [CrossRef]
- Clemente, R.; Hartley, W.; Riby, P.; Dickinson, N.M.; Lepp, N.W. Trace element mobility in a contaminated soil two years after field-amendment with a greenwaste compost mulch. Environ. Pollut. 2010, 158, 1644–1651. [Google Scholar] [CrossRef]
- Abou Jaoude, L.; Castaldi, P.; Nassif, N.; Pinna, M.V.; Garau, G. Biochar and compost as gentle remediation options for the recovery of trace elements-contaminated soils. Sci. Total Environ. 2020, 711, 134511. [Google Scholar] [CrossRef]
- Garau, M.; Garau, G.; Diquattro, S.; Roggero, P.P.; Castaldi, P. Mobility, bioaccessibility and toxicity of potentially toxic elements in a contaminated soil treated with municipal solid waste compost. Ecotoxicol. Environ. Saf. 2019, 186, 109766. [Google Scholar] [CrossRef]
- G.U. GU del 21 ottobre 1999, Decreto Ministeriale 13 settembre 1999, Approvazione dei Metodi Ufficiali di Analisi Chimica del Suolo. 1999. Available online: https://www.gazzettaufficiale.it/ (accessed on 30 September 2021).
- Brandstetter, A.; Sletten, R.S.; Mentler, A.; Wenzel, W.W. Estimating dissolved organic carbon in natural waters by UV absorbance (254 nm). Z Pflanz Bodenkunde 1996, 159, 605–607. [Google Scholar] [CrossRef]
- Wenzel, W.W.; Kirchbaumer, N.; Prohaska, T.; Stingeder, G.; Lombi, E.; Adriano, D.C. Arsenic fractionation in soils using an improved sequential extraction procedure. Anal. Chim. Acta 2001, 436, 309–323. [Google Scholar] [CrossRef]
- Insam, H. A New Set of Substrates Proposed for Community Characterization in Environmental Samples. In Microbial Communities; Insam, H., Rangger, A., Eds.; Springer: Berlin/Heidelberg, Germany, 1997. [Google Scholar]
- Niklinska, M.; Chodak, M.; Laskowski, R. Pollution-induced community tolerance of microorganisms from forest soil organic layers polluted with Zn or Cu. Appl. Soil Ecol. 2006, 32, 265–272. [Google Scholar] [CrossRef]
- Garland, J.L. Analytical approaches to the characterization of samples of microbial communities using patterns of potential C source utilization. Soil Biol. Biochem. 1996, 28, 213–221. [Google Scholar] [CrossRef]
- Garau, G.; Castaldi, P.; Santona, L.; Deiana, P.; Melis, P. Influence of red mud, zeolite and lime on heavy metal immobilization, culturable heterotrophic microbial populations and enzyme activities in a contaminated soil. Geoderma 2007, 142, 47–57. [Google Scholar] [CrossRef]
- Zak, J.C.; Willig, M.R.; Moorhead, D.L.; Wildman, H.G. Functional Diversity of Microbial Communities: A Quantitative Approach. Soil Biol. Biochem. 1994, 26, 1101–1108. [Google Scholar] [CrossRef]
- Alef, K.; Nannipieri, P. Methods in Applied Soil Microbiology and Biochemistry; Academic Press: San Diego, CA, USA, 1995. [Google Scholar]
- Garau, G.; Diquattro, S.; Lauro, G.P.; Deiana, S.; Castaldi, P. Influence of Pb(II) in the sorption of As(V) by a Ca-polygalacturonate network, a root mucilage model. Soil Sci. Plant. Nutr. 2019, 65, 305–315. [Google Scholar] [CrossRef]
- Garau, G.; Mele, E.; Castaldi, P.; Lauro, G.P.; Deiana, S. Role of polygalacturonic acid and the cooperative effect of caffeic and malic acids on the toxicity of Cu(II) towards triticale plants (x Triticosecale Wittm). Biol. Fertil. Soils 2015, 51, 535–544. [Google Scholar] [CrossRef]
- Garau, G.; Palma, A.; Lauro, G.P.; Mele, E.; Senette, C.; Manunza, B.; Deiana, S. Detoxification Processes from Vanadate at the Root Apoplasm Activated by Caffeic and Polygalacturonic Acids. PLoS ONE 2015, 10, e0141041. [Google Scholar] [CrossRef] [Green Version]
- Silvetti, M.; Garau, G.; Demurtas, D.; Marceddu, S.; Deiana, S.; Castaldi, P. Influence of lead in the sorption of arsenate by municipal solid waste composts: Metal(loid) retention, desorption and phytotoxicity. Bioresour. Technol. 2017, 225, 90–98. [Google Scholar] [CrossRef]
- Giannakis, G.V.; Kourgialas, N.N.; Paranychianakis, N.V.; Nikolaidis, N.P.; Kalogerakis, N. Effects of Municipal Solid Waste Compost on Soil Properties and Vegetables Growth. Compost Sci. Util. 2014, 22, 116–131. [Google Scholar] [CrossRef]
- Manzano, R.; Silvetti, M.; Garau, G.; Deiana, S.; Castaldi, P. Influence of iron-rich water treatment residues and compost on the mobility of metal(loid)s in mine soils. Geoderma 2016, 283, 1–9. [Google Scholar] [CrossRef]
- Reddy, N.; Crohn, D.M. Compost Induced Soil Salinity: A New Prediction Method and Its Effect on Plant Growth. Compost Sci. Util. 2012, 20, 133–140. [Google Scholar] [CrossRef]
- Zhang, M.; Heaney, D.; Henriquez, B.; Solberg, E.; Bittner, E. A four-year study on influence of biosolids/MSW cocompost application in less productive soils in Alberta: Nutrient dynamics. Compost Sci. Util. 2006, 14, 68–80. [Google Scholar] [CrossRef]
- Garau, G.; Roggero, P.P.; Diquattro, S.; Garau, M.; Pinna, M.V.; Castaldi, P. Innovative amendments derived from industrial and municipal wastes enhance plant growth and soil functions in PTE-polluted environments. Ital. J. Agron. 2021, 4, 159–170. [Google Scholar] [CrossRef]
- Penalver-Alcala, A.; Alvarez-Rogel, J.; Conesa, H.M.; Gonzalez-Alcaraz, M.N. Biochar and urban solid refuse ameliorate the inhospitality of acidic mine tailings and foster effective spontaneous plant colonization under semiarid climate. J. Environ. Manag. 2021, 292, 112824. [Google Scholar] [CrossRef]
- Slukovskaya, M.V.; Vasenev, V.I.; Ivashchenko, K.V.; Dolgikh, A.V.; Novikov, A.I.; Kremenetskaya, I.P.; Ivanova, L.A.; Gubin, S.V. Organic matter accumulation by alkaline-constructed soils in heavily metal-polluted area of Subarctic zone. J. Soils Sediment 2021, 21, 2071–2088. [Google Scholar] [CrossRef]
- Diquattro, S.; Castaldi, P.; Ritch, S.; Juhasz, A.L.; Brunetti, G.; Scheckel, K.G.; Garau, G.; Lombi, E. Insights into the fate of antimony (Sb) in contaminated soils: Ageing influence on Sb mobility, bioavailability, bioaccessibility and speciation. Sci. Total Environ. 2021, 770, 145354. [Google Scholar] [CrossRef]
- Dousova, B.; Lhotka, M.; Filip, J.; Kolousek, D. Removal of arsenate and antimonate by acid-treated Fe-rich clays. J. Hazard. Mater. 2018, 357, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Vandenbohede, A.; Wallis, I.; Alleman, T. Trace metal behavior during in-situ iron removal tests in Leuven, Belgium. Sci. Total Environ. 2019, 648, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Garau, G.; Porceddu, A.; Sanna, M.; Silvetti, M.; Castaldi, P. Municipal solid wastes as a resource for environmental recovery: Impact of water treatment residuals and compost on the microbial and biochemical features of As and trace metal-polluted soils. Ecotoxicol. Environ. Saf. 2019, 174, 445–454. [Google Scholar] [CrossRef]
- Garau, G.; Morillas, L.; Roales, J.; Castaldi, P.; Mangia, N.P.; Spano, D.; Mereu, S. Effect of monospecific and mixed Mediterranean tree plantations on soil microbial community and biochemical functioning. Appl. Soil Ecol. 2019, 140, 78–88. [Google Scholar] [CrossRef]
- Garcia, C.; Hernandez, T.; Costa, F. Potential use of dehydrogenase activity as an index of microbial activity in degraded soils. Commun. Soil Sci. Plant Anal. 1997, 28, 123–134. [Google Scholar] [CrossRef]
- Nannipieri, P.; Ascher, J.; Ceccherini, M.T.; Landi, L.; Pietramellara, G.; Renella, G. Microbial diversity and soil functions. Eur. J. Soil Sci. 2003, 54, 655–670. [Google Scholar] [CrossRef]
- Abad-Valle, P.; Iglesias-Jimenez, E.; Alvarez-Ayuso, E. A comparative study on the influence of different organic amendments on trace element mobility and microbial functionality of a polluted mine soil. J. Environ. Manag. 2017, 188, 287–296. [Google Scholar] [CrossRef]
- Garau, G.; Silvetti, M.; Deiana, S.; Deiana, P.; Castaldi, P. Long-term influence of red mud on As mobility and soil physico-chemical and microbial parameters in a polluted sub-acidic soil. J. Hazard. Mater. 2011, 185, 1241–1248. [Google Scholar] [CrossRef]
- Alvarenga, P.; Goncalves, A.P.; Fernandes, R.M.; de Varennes, A.; Vallini, G.; Duarte, E.; Cunha-Queda, A.C. Evaluation of composts and liming materials in the phytostabilization of a mine soil using perennial ryegrass. Sci. Total Environ. 2008, 406, 43–56. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharyya, K.G.; Sen Gupta, S. Adsorption of a few heavy metals on natural and modified kaolinite and montmorillonite: A review. Adv. Colloid Interface Sci. 2008, 140, 114–131. [Google Scholar] [CrossRef]
- Raiesi, F.; Dayani, L. Compost application increases the ecological dose values in a non-calcareous agricultural soil contaminated with cadmium. Ecotoxicology 2021, 30, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, M.; Li, Z.W.; Zhang, C.; Wan, C.L.; Zhang, Y.; Lee, D.J. Inhibition of urease activity by humic acid extracted from sludge fermentation liquid. Bioresour. Technol. 2019, 290, 121767. [Google Scholar] [CrossRef] [PubMed]
- Castaldi, P.; Garau, G.; Melis, P. Maturity assessment of compost from municipal solid waste through the study of enzyme activities and water-soluble fractions. Waste Manag. 2008, 28, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Feigl, V.; Ujaczki, E.; Vaszita, E.; Molnar, M. Influence of red mud on soil microbial communities: Application and comprehensive evaluation of the Biolog EcoPlate approach as a tool in soil microbiological studies. Sci. Total Environ. 2017, 595, 903–911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lebrun, M.; Miard, F.; Van Poucke, R.; Tack, F.M.G.; Scippa, G.S.; Bourgerie, S.; Morabito, D. Effect of fertilization, carbon-based material, and redmud amendments on the bacterial activity and diversity of a metal(loid) contaminated mining soil. Land Degrad. Dev. 2021, 32, 11. [Google Scholar] [CrossRef]
- Lombi, E.; Zhao, F.J.; Wieshammer, G.; Zhang, G.Y.; McGrath, S.P. In situ fixation of metals in soils using bauxite residue: Biological effects. Environ. Pollut. 2002, 118, 445–452. [Google Scholar] [CrossRef]
- Tu, C.; Guan, F.; Sun, Y.H.; Guo, P.P.; Liu, Y.; Li, L.Z.; Scheckel, K.G.; Luo, Y.M. Stabilizing effects on a Cd polluted coastal wetland soil using calcium polysulphide. Geoderma 2018, 332, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Garau, M.; Castaldi, P.; Patteri, G.; Roggero, P.P.; Garau, G. Evaluation of Cynara cardunculus L. and municipal solid waste compost for aided phytoremediation of multi potentially toxic element-contaminated soils. Environ. Sci. Pollut. Res. 2021, 28, 3253–3265. [Google Scholar] [CrossRef] [PubMed]
- Kuzyakov, Y.; Friedel, J.K.; Stahr, K. Review of mechanisms and quantification of priming effects. Soil Biol. Biochem. 2000, 32, 1485–1498. [Google Scholar] [CrossRef]
- Song, Y.Y.; Liu, C.; Song, C.C.; Wang, X.W.; Ma, X.Y.; Gao, J.L.; Gao, S.Q.; Wang, L.L. Linking soil organic carbon mineralization with soil microbial and substrate properties under warming in permafrost peatlands of Northeastern China. Catena 2021, 203, 105348. [Google Scholar] [CrossRef]
- Zhu, P.F.; Zhu, J.R.; Pang, J.Y.; Xu, W.W.; Shu, L.Z.; Hu, H.Q.; Wu, Y.; Tang, C.P. Biochar Improves the Growth Performance of Maize Seedling in Response to Antimony Stress. Water Air Soil Pollut. 2020, 231, 154. [Google Scholar] [CrossRef]
- Hiradate, S.; Ma, J.F.; Matsumoto, H. Strategies of plants to adapt to mineral stresses in problem soils. Adv. Agron. 2007, 96, 65–132. [Google Scholar] [CrossRef]
- Kraemer, S.M.; Crowley, D.E.; Kretzschmar, R. Geochemical aspects of phytosiderophore-promoted iron acquisition by plants. Adv. Agron. 2006, 91, 1–46. [Google Scholar] [CrossRef]
- Mitra, R.; Singh, S.B.; Singh, B. Radiochemical evidence validates the involvement of root released organic acid and phytosiderphore in regulating the uptake of phosphorus and certain metal micronutrients in wheat under phosphorus and iron deficiency. J. Adioanalytical Nucl. Chem. 2020, 326, 893–910. [Google Scholar] [CrossRef]
- Nakib, D.; Slatni, T.; Di Foggia, M.; Rombola, A.D.; Abdelly, C. Changes in organic compounds secreted by roots in two Poaceae species (Hordeum vulgare and Polypogon monspenliensis) subjected to iron deficiency. J. Plant Res. 2021, 134, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Nedjimi, B. Phytoremediation: A sustainable environmental technology for heavy metals decontamination. SN Appl. Sci. 2021, 3, 286. [Google Scholar] [CrossRef]

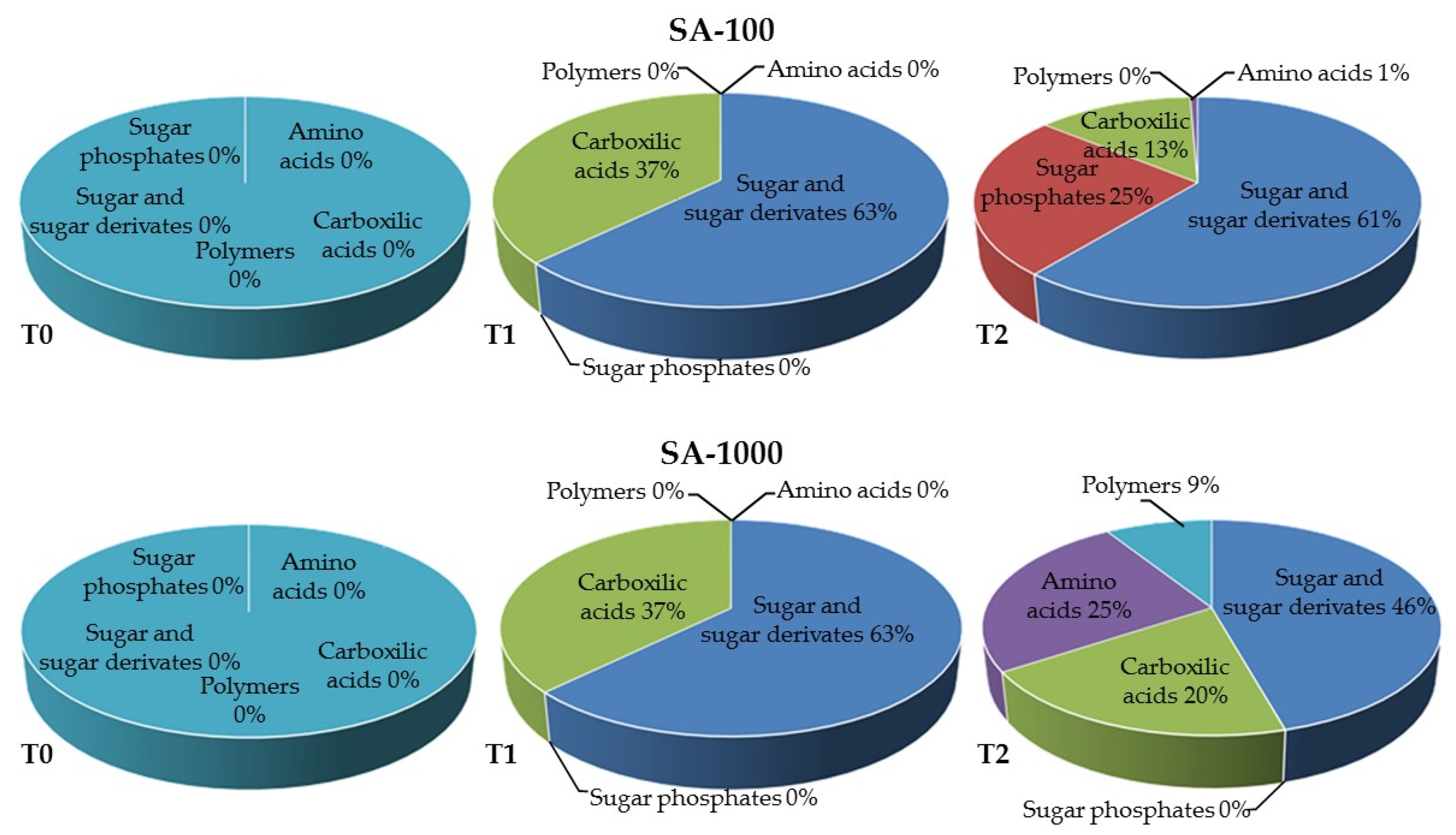

| pH | EC (mS m−1) | DOC (mg g−1) | CEC (cmol(+)·kg−1) | P Olsen (mg kg−1) | TOC (g kg−1) | TN (g kg−1) | |
|---|---|---|---|---|---|---|---|
| SA-100 control | 5.14 ± 0.59 b | 77.7 ± 0.78 c | 0.81 ± 0.01 c | 10.6 ± 0.35 b | 30.5 ± 0.33 b | 13.8 ± 0.31 c | 0.96 ± 0.12 b |
| SA-100 + 1% MSWC | 6.16 ± 0.09 a | 102 ± 0.78 b | 1.09 ± 0.03 b | 15.2 ± 0.63 a | 36.2 ± 0.58 a | 17.6 ± 0.03 b | 1.36 ± 0.00 a |
| SA-100 + 2% MSWC | 6.65 ± 0.26 a | 116 ± 2.26 a | 1.17 ± 0.02 a | 16.0 ± 0.06 a | 35.9 ± 0.56 a | 18.8 ± 0.56 a | 1.57 ± 0.14 a |
| SA-1000 control | 5.53 ± 0.71 b | 71.8 ± 5.30 b | 0.82 ± 0.02 c | 11.8 ± 0.01 b | 30.3 ± 0.39 b | 13.5 ± 0.09 c | 1.12 ± 0.04 b |
| SA-1000 + 1% MSWC | 6.46 ± 0.06 a | 102 ± 1.91 a | 1.00 ± 0.03 b | 13.3 ± 0.52 a | 34.4 ± 0.62 a | 16.8 ± 0.34 b | 1.24 ± 0.01 a,b |
| SA-1000 + 2% MSWC | 6.98 ± 0.16 a | 108 ± 2.83 a | 1.09 ± 0.01 a | 14.2 ± 0.75 a | 33.4 ± 0.22 a | 18.5 ± 0.52 a | 1.30 ± 0.10 a |
| SB-100 control | 7.84 ± 0.05 a | 57.8 ± 1.48 b | 0.29 ± 0.00 c | 22.4 ± 0.86 b | 10.1 ± 0.43 c | 12.7 ± 0.20 c | 1.06 ± 0.02 c |
| SB-100 + 1% MSWC | 7.93 ± 0.01 a | 67.7 ± 3.82 b | 0.40 ± 0.01 b | 24.0 ± 0.15 a,b | 15.9 ± 0.46 b | 15.0 ± 0.20 b | 1.13 ± 0.01 b |
| SB-100 + 2% MSWC | 7.93 ± 0.00 a | 88.9 ± 2.97 a | 0.69 ± 0.02 a | 26.2 ± 1.05 a | 19.4 ± 0.68 a | 16.6 ± 0.05 a | 1.19 ± 0.01 a |
| SB-1000 control | 7.92 ± 0.04 a | 63.0 ± 0.07 c | 0.30 ± 0.00 b | 23.2 ± 1.83 b | 9.73 ± 0.43 c | 13.0 ± 0.16 c | 1.02 ± 0.00 b |
| SB-1000 + 1% MSWC | 7.94 ± 0.08 a | 70.7 ± 2.40 b | 0.50 ± 0.01 a | 25.2 ± 0.58 a,b | 15.6 ± 0.59 b | 15.3 ± 0.19 b | 1.21 ± 0.01 a |
| SB-1000 + 2% MSWC | 7.97 ± 0.13 a | 91.3 ± 0.85 a | 0.51 ± 0.01 a | 27.2 ± 1.10 a | 20.3 ± 0.11 a | 16.9 ± 0.22 a | 1.22 ± 0.03 a |
| Root Dry Weight | Root Length | Sb Uptakeroot | DHG | URE | GLU | AWCD | H’ | Richness | |
|---|---|---|---|---|---|---|---|---|---|
| S-100 | −0.62 ** | −0.72 *** | 0.94 *** | −0.55 * | −0.61 ** | −0.76 *** | −0.85 *** | −0.92 *** | −0.80 *** |
| S1-1000 | −0.85 ** | −0.79 * | 0.72 * | −0.85 ** | −0.6 NS | −0.43 NS | −0.67 * | −0.88 ** | −0.78 * |
| S2-1000 | −0.74 * | −0.67 * | −0.92 *** | −0.80 ** | −0.69 * | −0.98 *** | −0.73 * | −0.91 *** | −0.90 *** |
| SA-100 | SA-1000 | SB-100 | SB-1000 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sb Uptake | T0 | T1 | T2 | T0 | T1 | T2 | T0 | T1 | T2 | T0 | T1 | T2 |
| Shoot | 1.61 b | 1.42 b | 2.54 a | 3.36 b | 5.61 a | 6.31 a | 0.53 b | 0.92 a | 0.91 a | 12.38 a | 10.30 a | 9.66 a |
| Root | 1.35 a | 0.26 b | 0.24 b | 1.52 b | 1.85 a | 1.82 a | 0.28 a | 0.01 b | 0.01 b | 1.15 a | 0.79 b | 0.43 c |
| TF | 1.2 c | 5.5 b | 11 a | 2.2 b | 3.0 a,b | 3.5 a | 1.9 b | 92 a | 91 a | 11 b | 13 b | 22 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diquattro, S.; Garau, G.; Garau, M.; Lauro, G.P.; Pinna, M.V.; Castaldi, P. Effect of Municipal Solid Waste Compost on Antimony Mobility, Phytotoxicity and Bioavailability in Polluted Soils. Soil Syst. 2021, 5, 60. https://doi.org/10.3390/soilsystems5040060
Diquattro S, Garau G, Garau M, Lauro GP, Pinna MV, Castaldi P. Effect of Municipal Solid Waste Compost on Antimony Mobility, Phytotoxicity and Bioavailability in Polluted Soils. Soil Systems. 2021; 5(4):60. https://doi.org/10.3390/soilsystems5040060
Chicago/Turabian StyleDiquattro, Stefania, Giovanni Garau, Matteo Garau, Gian Paolo Lauro, Maria Vittoria Pinna, and Paola Castaldi. 2021. "Effect of Municipal Solid Waste Compost on Antimony Mobility, Phytotoxicity and Bioavailability in Polluted Soils" Soil Systems 5, no. 4: 60. https://doi.org/10.3390/soilsystems5040060
APA StyleDiquattro, S., Garau, G., Garau, M., Lauro, G. P., Pinna, M. V., & Castaldi, P. (2021). Effect of Municipal Solid Waste Compost on Antimony Mobility, Phytotoxicity and Bioavailability in Polluted Soils. Soil Systems, 5(4), 60. https://doi.org/10.3390/soilsystems5040060







