Effects of Plastic versus Straw Mulching Systems on Soil Microbial Community Structure and Enzymes in Strawberry Cultivation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site and Sampling Description
2.2. Physicochemical Characterization
2.3. Abundance and Structure of Soil Microbial Communities
2.4. Soil Enzyme Activity and Functional Gene Abundance
2.5. Data Analysis
3. Results
3.1. Soil Physicochemical Parameters and General Microbial Indices
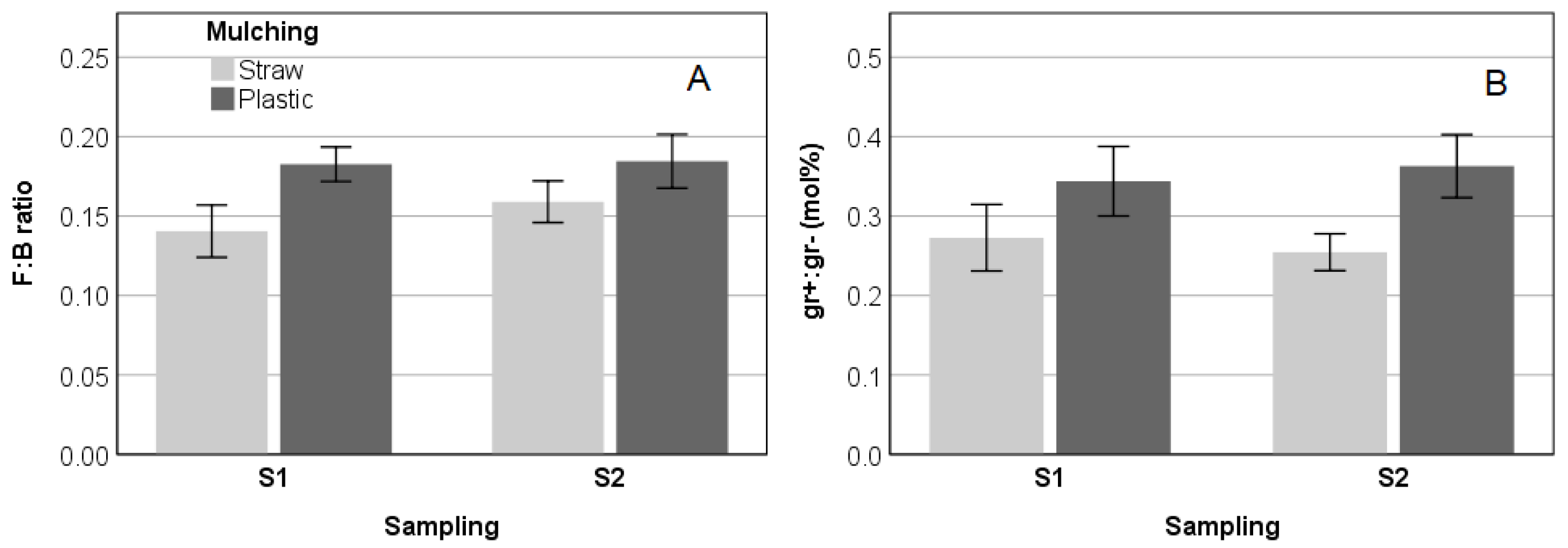
3.2. Abundance and Structure of Soil Microbial Communities
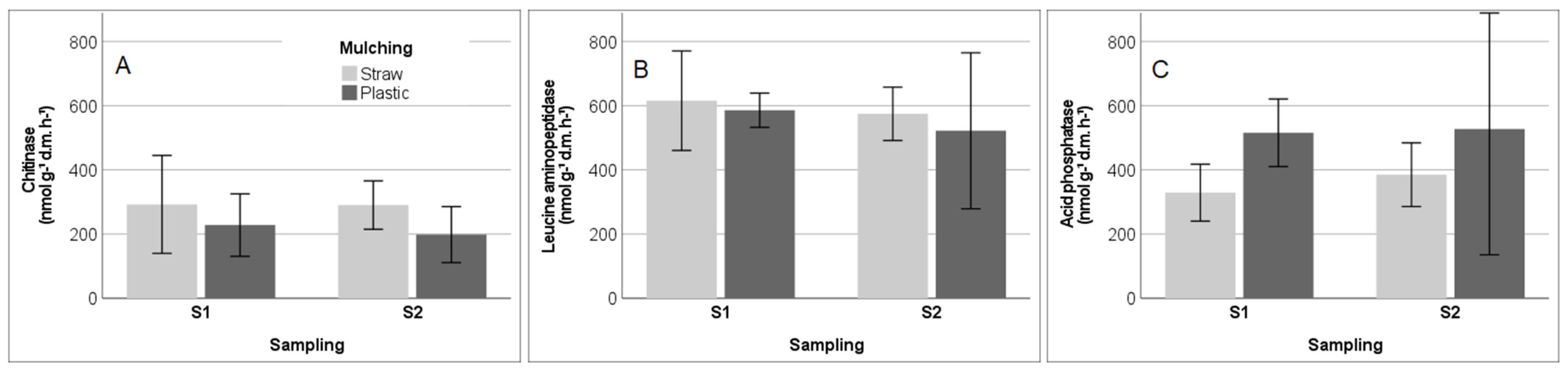
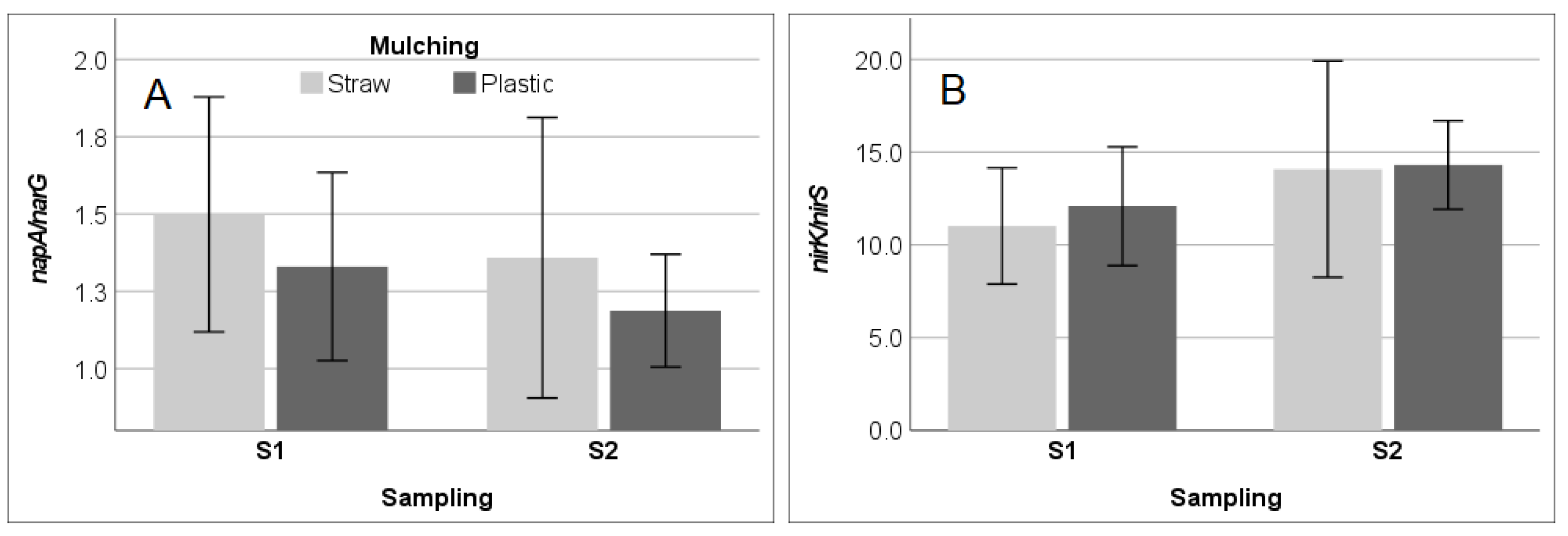
3.3. Soil Enzyme Activities and Functional Gene Abundance in SM and PM Systems
4. Discussion
4.1. Changes in the Abundance and Structure of Microbial Communities as a Consequence of the Physicochemical Soil Parameters under the Mulching Systems
4.2. Soil Enzyme Activity in Response to Soil Parameters under Straw and Plastic Mulching
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mezzetti, B.; Giampieri, F.; Zhang, Y.-T.; Zhong, C.-F. Status of strawberry breeding programs and cultivation systems in Europe and the rest of the world. J. Berry Res. 2018, 8, 205–221. [Google Scholar] [CrossRef]
- Lamont, W.J. Plastics: Modifying the microclimate for the production of vegetable crops. Horttechnology 2005, 15, 477–481. [Google Scholar] [CrossRef]
- Lieten, P.; Hokanson, S.; Jamieson, A. Protected Cultivation of Strawberries in Central Europe. In Strawberry Research to 2001. Proceedings of the 5th North American Strawberry Conference, Niagara Falls, ON, Canada, 14–16 January 2001; ASHS Press: Alexandria, VA, USA, 2002; pp. 102–107. [Google Scholar]
- Weber, R.W.; Hahn, M. Grey mould disease of strawberry in northern Germany: Causal agents, fungicide resistance and management strategies. Appl. Microbiol. Biotechnol. 2019, 103, 1589–1597. [Google Scholar] [CrossRef]
- Paulus, A.O. Fungal diseases of strawberry. HortScience 1990, 25, 885–889. [Google Scholar] [CrossRef] [Green Version]
- Neri, D.; Baruzzi, G.; Massetani, F.; Faedi, W. Strawberry production in forced and protected culture in Europe as a response to climate change. Can. J. Plant Sci. 2012, 92, 1021–1036. [Google Scholar] [CrossRef]
- Stevens, M.D.; Black, B.L.; Lea-Cox, J.D.; Feuz, D. Horticultural and economic considerations in the sustainability of three cold-climate strawberry production systems. HortScience 2011, 46, 445–451. [Google Scholar] [CrossRef] [Green Version]
- Steinmetz, Z.; Wollmann, C.; Schaefer, M.; Buchmann, C.; David, J.; Tröger, J.; Muñoz, K.; Frör, O.; Schaumann, G.E. Plastic mulching in agriculture. Trading short-term agronomic benefits for long-term soil degradation? Sci. Total Environ. 2016, 550, 690–705. [Google Scholar] [CrossRef]
- Zhou, Z.; Zeng, X.; Chen, K.; Li, Z.; Guo, S.; Shangguan, Y.; Yu, H.; Tu, S.; Qin, Y. Long-term straw mulch effects on crop yields and soil organic carbon fractions at different depths under a no-till system on the Chengdu Plain, China. J. Soils Sediments 2019, 19, 2143–2152. [Google Scholar] [CrossRef]
- Almeida, D.d.O.; Klauberg Filho, O.; Almeida, H.C.; Gebler, L.; Felipe, A.F. Soil microbial biomass under mulch types in an integrated apple orchard from Southern Brazil. Sci. Agric. 2011, 68, 217–222. [Google Scholar] [CrossRef]
- Fu, X.; Wang, J.; Sainju, U.M.; Zhao, F.; Liu, W. Soil microbial community and carbon and nitrogen fractions responses to mulching under winter wheat. Appl. Soil Ecol. 2019, 139, 64–68. [Google Scholar] [CrossRef]
- Huang, F.; Liu, Z.; Mou, H.; Li, J.; Zhang, P.; Jia, Z. Impact of farmland mulching practices on the soil bacterial community structure in the semiarid area of the loess plateau in China. Eur. J. Soil Biol. 2019, 92, 8–15. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, Y.; Duan, C.; Wang, X.; Zhang, X.; Ju, W.; Chen, H.; Yue, S.; Wang, Y.; Li, S.; et al. Ecoenzymatic stoichiometry reveals microbial phosphorus limitation decreases the nitrogen cycling potential of soils in semi-arid agricultural ecosystems. Soil Tillage Res. 2020, 197, 104463. [Google Scholar] [CrossRef]
- Akhtar, K.; Wang, W.; Ren, G.; Khan, A.; Feng, Y.; Yang, G. Changes in soil enzymes, soil properties, and maize crop productivity under wheat straw mulching in Guanzhong, China. Soil Tillage Res. 2018, 182, 94–102. [Google Scholar] [CrossRef]
- Masciandaro, G.; Ceccanti, B.; Benedicto, S.; Lee, H.; Cook, H. Enzyme activity and C and N pools in soil following application of mulches. Can. J. Soil Sci. 2004, 84, 19–30. [Google Scholar] [CrossRef]
- Muñoz, K.; Buchmann, C.; Meyer, M.; Schmidt-Heydt, M.; Steinmetz, Z.; Diehl, D.; Thiele-Bruhn, S.; Schaumann, G. Physicochemical and microbial soil quality indicators as affected by the agricultural management system in strawberry cultivation using straw or black polyethylene mulching. Appl. Soil Ecol. 2017, 113, 36–44. [Google Scholar] [CrossRef]
- Guo, B.; Meng, J.; Wang, X.; Yin, C.; Hao, W.; Ma, B.; Tao, Z. Quantification of pesticide residues on plastic mulching films in typical farmlands of the North China. Front. Environ. Sci. Eng. 2020, 14, 2. [Google Scholar] [CrossRef]
- Meyer, M.; Diehl, D.; Schaumann, G.E.; Muñoz, K. Agricultural mulching and fungicides—impacts on fungal biomass, mycotoxin occurrence, and soil organic matter decomposition. Environ. Sci. Pollut. Res. 2021, 28, 36535–36550. [Google Scholar] [CrossRef]
- Reganold, J.P.; Andrews, P.K.; Reeve, J.R.; Carpenter-Boggs, L.; Schadt, C.W.; Alldredge, J.R.; Ross, C.F.; Davies, N.M.; Zhou, J. Fruit and soil quality of organic and conventional strawberry agroecosystems. PLoS ONE 2010, 5, e12346. [Google Scholar] [CrossRef]
- Wang, F.; Li, X.; Zhu, L.; Du, Z.; Zhang, C.; Wang, J.; Wang, J.; Lv, D. Responses of Soil Microorganisms and Enzymatic Activities to Azoxystrobin in Cambisol. Pol. J. Environ. Stud. 2018, 27, 2775–2783. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, T.; Zhu, L.; Du, Z.; Li, B.; Wang, J.; Wang, J.; Sun, Y. Using enzyme activities and soil microbial diversity to understand the effects of fluoxastrobin on microorganisms in fluvo-aquic soil. Sci. Total Environ. 2019, 666, 89–93. [Google Scholar] [CrossRef]
- Meyer, M.; Hausbeck, M. Using soil-applied fungicides to manage Phytophthora crown and root rot on summer squash. Plant Dis. 2013, 97, 107–112. [Google Scholar] [CrossRef] [Green Version]
- Nannipieri, P.; Trasar-Cepeda, C.; Dick, R.P. Soil enzyme activity: A brief history and biochemistry as a basis for appropriate interpretations and meta-analysis. Biol. Fertil. Soils 2018, 54, 11–19. [Google Scholar] [CrossRef]
- Tan, X.; Nie, Y.; Ma, X.; Guo, Z.; Liu, Y.; Tian, H.; Megharaj, M.; Shen, W.; He, W. Soil chemical properties rather than the abundance of active and potentially active microorganisms control soil enzyme kinetics. Sci. Total Environ. 2021, 770, 144500. [Google Scholar] [CrossRef]
- Meyer, M.; Diehl, D.; Schaumann, G.; Muñoz, K. Analysis of biogeochemical processes in plastic-covered soil during establishment period in strawberry cultivation. SN Appl. Sci. 2020, 2, 1749. [Google Scholar] [CrossRef]
- Lieten, P. Strawberry Production in Central Europe. Int. J. Fruit Sci. 2005, 5, 91–105. [Google Scholar] [CrossRef]
- Wedge, D.E.; Smith, B.J.; Quebedeaux, J.P.; Constantin, R.J. Fungicide management strategies for control of strawberry fruit rot diseases in Louisiana and Mississippi. Crop Prot. 2007, 26, 1449–1458. [Google Scholar] [CrossRef]
- Wang, H.; Guo, Q.; Li, X.; Li, X.; Yu, Z.; Li, X.; Yang, T.; Su, Z.; Zhang, H.; Zhang, C. Effects of long-term no-tillage with different straw mulching frequencies on soil microbial community and the abundances of two soil-borne pathogens. Appl. Soil Ecol. 2020, 148, 103488. [Google Scholar] [CrossRef]
- Petrasch, S.; Knapp, S.J.; van Kan, J.A.L.; Blanco-Ulate, B. Grey mould of strawberry, a devastating disease caused by the ubiquitous necrotrophic fungal pathogen Botrytis cinerea. Mol. Plant Pathol. 2019, 20, 877–892. [Google Scholar] [CrossRef] [Green Version]
- Lewis, K.; Green, A.; Tzilivakis, J.; Warner, D. The Pesticide Properties Database (PPDB) Developed by the Agriculture & Environment Research Unit (AERU). 2015. Available online: http://sitem.herts.ac.uk/aeru/ppdb/ (accessed on 14 December 2021).
- ISO 10390:2005-12; Soil Quality-Determination of pH. ISO: Geneva, Switzerland, 2005.
- DIN1484, German Institute for Standarization; Water Analysis—Guidelines for the Determination of Total Organic Carbon (TOC) and Dissolved Organic Carbon (DOC). 1997. Available online: https://www.din.de/en/getting-involved/standards-committees/textilnorm/publications/wdc-beuth:din21:301999219 (accessed on 14 December 2021).
- Joergensen, R.G.; Emmerling, C. Methods for evaluating human impact on soil microorganisms based on their activity, biomass, and diversity in agricultural soils. J. Plant Nutr. Soil Sci. 2006, 169, 295–309. [Google Scholar] [CrossRef]
- Anderson, T.-H. Microbial eco-physiological indicators to asses soil quality. Agric. Ecosyst. Environ. 2003, 98, 285–293. [Google Scholar] [CrossRef]
- ISO14240-2; Soil Quality—Determination of Soil Microbial Biomass—Part 2: Fumigation-Extraction Method. ISO: Geneva, Switzerland, 1997.
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. Microbial biomass measurements in forest soils: The use of the chloroform fumigation-incubation method in strongly acid soils. Soil Biol. Biochem. 1987, 19, 697–702. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar]
- White, D.; Davis, W.; Nickels, J.; King, J.; Bobbie, R. Determination of the sedimentary microbial biomass by extractible lipid phosphate. Oecologia 1979, 40, 51–62. [Google Scholar] [CrossRef]
- Steinmetz, Z.; Kurtz, M.P.; Zubrod, J.P.; Meyer, A.H.; Elsner, M.; Schaumann, G.E. Biodegradation and photooxidation of phenolic compounds in soil—A compound-specific stable isotope approach. Chemosphere 2019, 230, 210–218. [Google Scholar] [CrossRef]
- Kenngott, K.G.; Riess, K.; Muñoz, K.; Schaumann, G.E.; Buhk, C.; Diehl, D. Flood Pulse Irrigation of Meadows Shapes Soil Chemical and Microbial Parameters More Than Mineral Fertilization. Soil Syst. 2021, 5, 24. [Google Scholar] [CrossRef]
- Gómez-Brandón, M.; Lores, M.; Domínguez, J. A new combination of extraction and derivatization methods that reduces the complexity and preparation time in determining phospholipid fatty acids in solid environmental samples. Bioresour. Technol. 2010, 101, 1348–1354. [Google Scholar] [CrossRef]
- Frostegård, Å.; Tunlid, A.; Bååth, E. Use and misuse of PLFA measurements in soils. Soil Biol. Biochem. 2011, 43, 1621–1625. [Google Scholar] [CrossRef]
- Kaiser, C.; Frank, A.; Wild, B.; Koranda, M.; Richter, A. Negligible contribution from roots to soil-borne phospholipid fatty acid fungal biomarkers 18: 2ω6, 9 and 18: 1ω9. Soil Biol. Biochem. 2010, 42, 1650–1652. [Google Scholar] [CrossRef] [Green Version]
- Van Aarle, I.M.; Olsson, P.A. Fungal lipid accumulation and development of mycelial structures by two arbuscular mycorrhizal fungi. Appl. Environ. Microbiol. 2003, 69, 6762–6767. [Google Scholar] [CrossRef] [Green Version]
- Olsson, P.A.; Bååth, E.; Jakobsen, I.; Söderström, B. The use of phospholipid and neutral lipid fatty acids to estimate biomass of arbuscular mycorrhizal fungi in soil. Mycol. Res. 1995, 99, 623–629. [Google Scholar] [CrossRef]
- Frostegård, Å.; Bååth, E. The use of phospholipid fatty acid analysis to estimate bacterial and fungal biomass in soil. Biol. Fertil. Soils 1996, 22, 59–65. [Google Scholar] [CrossRef]
- Klamer, M.; Bååth, E. Estimation of conversion factors for fungal biomass determination in compost using ergosterol and PLFA 18: 2ω6, 9. Soil Biol. Biochem. 2004, 36, 57–65. [Google Scholar] [CrossRef]
- Buyer, J.S.; Teasdale, J.R.; Roberts, D.P.; Zasada, I.A.; Maul, J.E. Factors affecting soil microbial community structure in tomato cropping systems. Soil Biol. Biochem. 2010, 42, 831–841. [Google Scholar] [CrossRef]
- Marx, M.-C.; Wood, M.; Jarvis, S. A microplate fluorimetric assay for the study of enzyme diversity in soils. Soil Biol. Biochem. 2001, 33, 1633–1640. [Google Scholar] [CrossRef]
- Keil, D.; Meyer, A.; Berner, D.; Poll, C.; Schützenmeister, A.; Piepho, H.-P.; Vlasenko, A.; Philippot, L.; Schloter, M.; Kandeler, E.; et al. Influence of land-use intensity on the spatial distribution of N-cycling microorganisms in grassland soils. FEMS Microbiol. Ecol. 2011, 77, 95–106. [Google Scholar] [CrossRef]
- Hallin, S.; Jones, C.M.; Schloter, M.; Philippot, L. Relationship between N-cycling communities and ecosystem functioning in a 50-year-old fertilization experiment. ISME J. 2009, 3, 597–605. [Google Scholar] [CrossRef]
- Heuer, H.; Smalla, K. Manure and sulfadiazine synergistically increased bacterial antibiotic resistance in soil over at least two months. Environ. Microbiol. 2007, 9, 657–666. [Google Scholar] [CrossRef]
- Marhan, S.; Philippot, L.; Bru, D.; Rudolph, S.; Franzaring, J.; Högy, P.; Fangmeier, A.; Kandeler, E. Abundance and activity of nitrate reducers in an arable soil are more affected by temporal variation and soil depth than by elevated atmospheric [CO2]. FEMS Microbiol. Ecol. 2011, 76, 209–219. [Google Scholar] [CrossRef] [Green Version]
- Bárta, J.; Melichová, T.; Vaněk, D.; Picek, T.; Šantrůčková, H. Effect of pH and dissolved organic matter on the abundance of nirK and nirS denitrifiers in spruce forest soil. Biogeochemistry 2010, 101, 123–132. [Google Scholar] [CrossRef]
- Tu, C.; Ristaino, J.B.; Hu, S. Soil microbial biomass and activity in organic tomato farming systems: Effects of organic inputs and straw mulching. Soil Biol. Biochem. 2006, 38, 247–255. [Google Scholar] [CrossRef]
- Bajoriene, K.; Jodaugiene, D.; Pupaliene, R.; Sinkeviciene, A. Effect of organic mulches on the content of organic carbon in the soil. Est. J. Ecol. 2013, 62, 100. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, W.; Wei, W.; He, Z.; Kuzyakov, Y.; Bol, R.; Hu, R. Soil organic matter priming and carbon balance after straw addition is regulated by long-term fertilization. Soil Biol. Biochem. 2019, 135, 383–391. [Google Scholar] [CrossRef]
- Rousk, J.; Brookes, P.C.; Bååth, E. Contrasting Soil pH Effects on Fungal and Bacterial Growth Suggest Functional Redundancy in Carbon Mineralization. Appl. Environ. Microbiol. 2009, 75, 1589–1596. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Wen, X.; Sun, Y.; Zhang, J.; Wu, W.; Liao, Y. Mulching practices altered soil bacterial community structure and improved orchard productivity and apple quality after five growing seasons. Sci. Hortic. 2014, 172, 248–257. [Google Scholar] [CrossRef]
- Bååth, E.; Anderson, T.H. Comparison of soil fungal/bacterial ratios in a pH gradient using physiological and PLFA-based techniques. Soil Biol. Biochem. 2003, 35, 955–963. [Google Scholar] [CrossRef]
- Wang, C.; Li, W.; Yang, Z.; Chen, Y.; Shao, W.; Ji, J. An invisible soil acidification: Critical role of soil carbonate and its impact on heavy metal bioavailability. Sci. Rep. 2015, 5, 12735. [Google Scholar] [CrossRef] [Green Version]
- Amelung, W.; Blume, H.-P.; Fleige, H.; Horn, R.; Kandeler, E.; Kögel-Knabner, I.; Kretzschmar, R.; Stahr, K.; Wilke, B.-M. Chemische Eigenschaften und Prozesse. In Scheffer/Schachtschabel Lehrbuch der Bodenkunde; Springer: Berlin/Heidelberg, Germany, 2018; pp. 151–211. [Google Scholar]
- Pietri, J.A.; Brookes, P. Relationships between soil pH and microbial properties in a UK arable soil. Soil Biol. Biochem. 2008, 40, 1856–1861. [Google Scholar] [CrossRef]
- Torres-Olivar, V.; Ibarra-Jiménez, L.; Cárdenas-Flores, A.; Lira-Saldivar, R.H.; Valenzuela-Soto, J.H.; Castillo-Campohermoso, M.A. Changes induced by plastic film mulches on soil temperature and their relevance in growth and fruit yield of pickling cucumber. Acta Agric. Scand. Sect. B-Soil Plant Sci. 2018, 68, 97–103. [Google Scholar] [CrossRef]
- Allison, S.D.; Wallenstein, M.D.; Bradford, M.A. Soil-carbon response to warming dependent on microbial physiology. Nat. Geosci. 2010, 3, 336–340. [Google Scholar] [CrossRef]
- An, T.; Schaeffer, S.; Li, S.; Fu, S.; Pei, J.; Li, H.; Zhuang, J.; Radosevich, M.; Wang, J. Carbon fluxes from plants to soil and dynamics of microbial immobilization under plastic film mulching and fertilizer application using 13C pulse-labeling. Soil Biol. Biochem. 2015, 80, 53–61. [Google Scholar] [CrossRef]
- Jayalath, N.; Mosley, L.; Fitzpatrick, R.; Marschner, P. Addition of organic matter influences pH changes in reduced and oxidised acid sulfate soils. Geoderma 2016, 262, 125–132. [Google Scholar] [CrossRef]
- Drenovsky, R.E.; Vo, D.; Graham, K.J.; Scow, K.M. Soil Water Content and Organic Carbon Availability Are Major Determinants of Soil Microbial Community Composition. Microb. Ecol. 2004, 48, 424–430. [Google Scholar] [CrossRef]
- Holland, E.; Coleman, D.C. Litter placement effects on microbial and organic matter dynamics in an agroecosystem. Ecology 1987, 68, 425–433. [Google Scholar] [CrossRef]
- Fanin, N.; Kardol, P.; Farrell, M.; Nilsson, M.-C.; Gundale, M.J.; Wardle, D.A. The ratio of Gram-positive to Gram-negative bacterial PLFA markers as an indicator of carbon availability in organic soils. Soil Biol. Biochem. 2019, 128, 111–114. [Google Scholar] [CrossRef]
- Rousk, J.; Bååth, E.; Brookes, P.C.; Lauber, C.L.; Lozupone, C.; Caporaso, J.G.; Knight, R.; Fierer, N. Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J. 2010, 4, 1340–1351. [Google Scholar] [CrossRef]
- Liu, J.; Li, S.; Yue, S.; Tian, J.; Chen, H.; Jiang, H.; Siddique, K.H.; Zhan, A.; Fang, Q.; Yu, Q. Soil microbial community and network changes after long-term use of plastic mulch and nitrogen fertilization on semiarid farmland. Geoderma 2021, 396, 115086. [Google Scholar] [CrossRef]
- Kramer, C.; Gleixner, G. Soil organic matter in soil depth profiles: Distinct carbon preferences of microbial groups during carbon transformation. Soil Biol. Biochem. 2008, 40, 425–433. [Google Scholar] [CrossRef]
- Luo, S.; Wang, S.; Yao, P.; Guo, D.; Li, X.; Li, S.; Tian, C. Soil microbial communities under film mulching and N fertilization in semiarid farmland. Nutr. Cycl. Agroecosystems 2019, 114, 157–170. [Google Scholar] [CrossRef]
- Wan, X.; Huang, Z.; He, Z.; Yu, Z.; Wang, M.; Davis, M.R.; Yang, Y. Soil C:N ratio is the major determinant of soil microbial community structure in subtropical coniferous and broadleaf forest plantations. Plant Soil 2015, 387, 103–116. [Google Scholar] [CrossRef]
- Barreiro, A.; Bååth, E.; Díaz-Raviña, M. Bacterial and fungal growth in burnt acid soils amended with different high C/N mulch materials. Soil Biol. Biochem. 2016, 97, 102–111. [Google Scholar] [CrossRef]
- Dick, R.; Rasmussen, P.; Kerle, E. Influence of long-term residue management on soil enzyme activities in relation to soil chemical properties of a wheat-fallow system. Biol. Fertil. Soils 1988, 6, 159–164. [Google Scholar] [CrossRef]
- Hendriksen, N.B.; Creamer, R.E.; Stone, D.; Winding, A. Soil exo-enzyme activities across Europe—The influence of climate, land-use and soil properties. Appl. Soil Ecol. 2016, 97, 44–48. [Google Scholar] [CrossRef]
- Rodriguez-Kabana, R.; Godoy, G.; Morgan-Jones, G.; Shelby, R.A. The determination of soil chitinase activity: Conditions for assay and ecological studies. Plant Soil 1983, 75, 95–106. [Google Scholar] [CrossRef]
- Turner, B.L. Variation in pH Optima of Hydrolytic Enzyme Activities in Tropical Rain Forest Soils. Appl. Environ. Microbiol. 2010, 76, 6485–6493. [Google Scholar] [CrossRef] [Green Version]
- Niemi, R.M.; Vepsäläinen, M. Stability of the fluorogenic enzyme substrates and pH optima of enzyme activities in different Finnish soils. J. Microbiol. Methods 2005, 60, 195–205. [Google Scholar] [CrossRef]
- Della Mónica, I.F.; Godoy, M.S.; Godeas, A.M.; Scervino, J.M. Fungal extracellular phosphatases: Their role in P cycling under different pH and P sources availability. J. Appl. Microbiol. 2018, 124, 155–165. [Google Scholar] [CrossRef] [Green Version]
- Meyer, M.; Diehl, D.; Schaumann, G.E.; Muñoz, K. Multiannual soil mulching in agriculture: Analysis of biogeochemical soil processes under plastic and straw mulches in a 3-year field study in strawberry cultivation. J. Soils Sediments 2021, 21, 3733–3752. [Google Scholar] [CrossRef]
- Luo, G.; Sun, B.; Li, L.; Li, M.; Liu, M.; Zhu, Y.; Guo, S.; Ling, N.; Shen, Q. Understanding how long-term organic amendments increase soil phosphatase activities: Insight into phoD-and phoC-harboring functional microbial populations. Soil Biol. Biochem. 2019, 139, 107632. [Google Scholar] [CrossRef]
- Margalef, O.; Sardans, J.; Fernández-Martínez, M.; Molowny-Horas, R.; Janssens, I.A.; Ciais, P.; Goll, D.; Richter, A.; Obersteiner, M.; Asensio, D.; et al. Global patterns of phosphatase activity in natural soils. Sci. Rep. 2017, 7, 1337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ratliff, T.J.; Fisk, M.C. Phosphatase activity is related to N availability but not P availability across hardwood forests in the northeastern United States. Soil Biol. Biochem. 2016, 94, 61–69. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, T.; Wang, S.; Wang, Z. Soil pH and C/N ratio determines spatial variations in soil microbial communities and enzymatic activities of the agricultural ecosystems in Northeast China: Jilin Province case. Appl. Soil Ecol. 2020, 155, 103629. [Google Scholar] [CrossRef]
- Monkiedje, A.; Ilori, M.O.; Spiteller, M. Soil quality changes resulting from the application of the fungicides mefenoxam and metalaxyl to a sandy loam soil. Soil Biol. Biochem. 2002, 34, 1939–1948. [Google Scholar] [CrossRef]
- Wightwick, A.M.; Salzman, S.A.; Reichman, S.M.; Allinson, G.; Menzies, N.W. Effects of copper fungicide residues on the microbial function of vineyard soils. Environ. Sci. Pollut. Res. 2013, 20, 1574–1585. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.L.; Shan, M.; Fang, H.; Wang, X.; Chu, X.Q. Responses of soil microorganisms and enzymes to repeated applications of chlorothalonil. J. Agric. Food Chem. 2006, 54, 10070–10075. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, J.; Wu, M.; Xie, X.; Wu, J.; Wei, W. Differentiated Response of Denitrifying Communities to Fertilization Regime in Paddy Soil. Microb. Ecol. 2012, 63, 446–459. [Google Scholar] [CrossRef]
- Chen, Z.; Luo, X.; Hu, R.; Wu, M.; Wu, J.; Wei, W. Impact of Long-Term Fertilization on the Composition of Denitrifier Communities Based on Nitrite Reductase Analyses in a Paddy Soil. Microb. Ecol. 2010, 60, 850–861. [Google Scholar] [CrossRef]
- Enwall, K.; Philippot, L.; Hallin, S. Activity and Composition of the Denitrifying Bacterial Community Respond Differently to Long-Term Fertilization. Appl. Environ. Microbiol. 2005, 71, 8335–8343. [Google Scholar] [CrossRef] [Green Version]
- Čuhel, J.; Šimek, M.; Laughlin, R.J.; Bru, D.; Chèneby, D.; Watson, C.J.; Philippot, L. Insights into the Effect of Soil pH on N2O and N2 Emissions and Denitrifier Community Size and Activity. Appl. Environ. Microbiol. 2010, 76, 1870–1878. [Google Scholar] [CrossRef] [Green Version]
- Levy-Booth, D.J.; Prescott, C.E.; Grayston, S.J. Microbial functional genes involved in nitrogen fixation, nitrification and denitrification in forest ecosystems. Soil Biol. Biochem. 2014, 75, 11–25. [Google Scholar] [CrossRef]
- Ligi, T.; Truu, M.; Truu, J.; Nõlvak, H.; Kaasik, A.; Mitsch, W.J.; Mander, Ü. Effects of soil chemical characteristics and water regime on denitrification genes (nirS, nirK, and nosZ) abundances in a created riverine wetland complex. Ecol. Eng. 2014, 72, 47–55. [Google Scholar] [CrossRef]
- Yang, Y.-D.; Hu, Y.-G.; Wang, Z.-M.; Zeng, Z.-H. Variations of the nirS-, nirK-, and nosZ-denitrifying bacterial communities in a northern Chinese soil as affected by different long-term irrigation regimes. Environ. Sci. Pollut. Res. 2018, 25, 14057–14067. [Google Scholar] [CrossRef] [PubMed]
- Bach, H.J.; Tomanova, J.; Schloter, M.; Munch, J.C. Enumeration of total bacteria and bacteria with genes for proteolytic activity in pure cultures and in environmental samples by quantitative PCR mediated amplification. J. Microbiol. Methods 2002, 49, 235–245. [Google Scholar] [CrossRef]
- Braker, G.; Fesefeldt, A.; Witzel, K.P. Development of PCR primer systems for amplification of nitrite reductase genes (nirK and nirS) to detect denitrifying bacteria in environmental samples. Appl. Environ. Microbiol. 1998, 64, 3769–3775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Throbäck, I.N.; Enwall, K.; Jarvis, A.; Hallin, S. Reassessing PCR primers targeting nirS, nirK and nosZ genes for community surveys of denitrifying bacteria with DGGE. FEMS Microbiol. Ecol. 2004, 49, 401–417. [Google Scholar] [CrossRef]
- Henry, S.; Baudoin, E.; López-Gutiérrez, J.C.; Martin-Laurent, F.; Brauman, A.; Philippot, L. Quantification of denitrifying bacteria in soils by nirK gene targeted real-time PCR. J. Microbiol. Methods 2004, 59, 327–335. [Google Scholar] [CrossRef]
- Bru, D.; Sarr, A.; Philippot, L. Relative abundances of proteobacterial membrane-bound and periplasmic nitrate reductases in selected environments. Appl. Environ. Microbiol. 2007, 73, 5971–5974. [Google Scholar] [CrossRef] [Green Version]

| Depth (cm) | SWC (%) | pH | IC (%) | SOC (%) | DOC (mg kg−1) | Ntot (%) | C:N | MBC (mg kg−1) | MBC:SOC | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | SM | 0–5 | 19.6 ± 0.7 | 6.4 ± 0.3 | 0.11 ± 0.01 | 1.1 ± 0.1 | 24.8 ± 3.4 | 0.135 ± 0.006 | 7.9 ± 0.2 | 172.5 ± 16.6 | 1.6 ± 0.1 |
| 5–10 | 18.3 ± 1.3 | 6.3 ± 0.3 | 0.13 ± 0.01 | 1.0 ± 0.1 | 24.7 ± 5.0 | 0.128 ± 0.010 | 7.7 ± 0.2 | 155.2 ± 15.8 | 1.6 ± 0.2 | ||
| Mean | 19.0 ± 1.2 | 6.4 ± 0.3 | 0.12 ± 0.01 | 1.0 ± 0.1 | 24.7 ± 3.9 | 0.131 ± 0.008 | 7.8 ± 0.2 | 163.9 ± 17.6 | 1.6 ± 0.2 | ||
| PM | 0–5 | 18.6 ± 0.4 | 7.4 ± 0.3 | 0.15 ± 0.01 | 1.3 ± 0.1 | 31.9 ± 4.4 | 0.155 ± 0.010 | 8.7 ± 0.9 | 155.8 ± 17.5 | 1.2 ± 0.1 | |
| 5–10 | 19.1 ± 0.7 | 7.5 ± 0.3 | 0.15 ± 0.01 | 1.3 ± 0.1 | 23.4 ± 4.8 | 0.168 ± 0.010 | 7.5 ± 0.5 | 156.2 ± 16.8 | 1.2 ± 0.1 | ||
| Mean | 18.9 ± 0.6 | 7.4 ± 0.2 | 0.15 ± 0.01 | 1.3 ± 0.1 | 27.6 ± 6.2 | 0.161 ± 0.011 | 8.1 ± 0.9 | 156.0 ± 15.9 | 1.2 ± 0.1 | ||
| S2 | SM | 0–5 | 12.5 ± 1.7 | 6.6 ± 0.3 | 0.13 ± 0.01 | 1.1 ± 0.1 | 32.4 ± 1.9 | 0.131 ± 0.001 | 8.4 ± 0.4 | 162.6 ± 16.0 | 1.5 ± 0.1 |
| 5–10 | 14.1 ± 1.4 | 6.6 ± 0.2 | 0.12 ± 0.01 | 1.0 ± 0.1 | 26.4 ± 3.4 | 0.127 ± 0.003 | 8.1 ± 0.3 | 155.6 ± 11.1 | 1.5 ± 0.1 | ||
| Mean | 13.3 ± 1.5 | 6.6 ± 0.2 | 0.12 ± 0.01 | 1.1 ± 0.1 | 29.4 ± 4.1 | 0.129 ± 0.003 | 8.3 ± 0.3 | 159.1 ± 13.3 | 1.5 ± 0.1 | ||
| PM | 0–5 | 13.8 ± 0.6 | 7.6 ± 0.5 | 0.45 ± 0.02 | 1.2 ± 0.1 | 48.5 ± 7.9 | 0.150 ± 0.005 | 8.3 ± 0.4 | 137.4 ± 31.0 | 1.1 ± 0.3 | |
| 5–10 | 15.4 ± 0.3 | 7.9 ± 0.2 | 0.40 ± 0.02 | 1.0 ± 0.1 | 35.8 ± 5.6 | 0.144 ± 0.005 | 7.0 ± 0.4 | 143.6 ± 13.5 | 1.4 ± 0.2 | ||
| Mean | 14.6 ± 0.9 | 7.8 ± 0.4 | 0.43 ± 0.01 | 1.1 ± 0.2 | 42.1 ± 9.3 | 0.147 ± 0.006 | 7.6 ± 0.8 | 140.5 ± 22.4 | 1.3 ± 0.3 |
| Chitinase | Leucine Aminopeptidase | Acid Phosphatase | napA/narG | nirK/nirS | ||||||
| r | p | r | p | r | p | r | p | r | p | |
| SWC (%) | 0.024 | 0.897 | 0.268 | 0.138 | −0.094 | 0.609 | 0.307 | 0.088 | −0.519 | 0.002 |
| pH | −0.601 | <0.001 | −0.414 | 0.018 | 0.580 | 0.001 | −0.542 | 0.001 | 0.348 | 0.051 |
| DOC (mg kg−¹) | −0.286 | 0.112 | −0.222 | 0.223 | 0.304 | 0.091 | −0.451 | 0.010 | 0.543 | 0.001 |
| C:N | 0.265 | 0.143 | 0.381 | 0.031 | 0.196 | 0.283 | −0.066 | 0.722 | 0.090 | 0.626 |
| MBC:SOC | 0.402 | 0.022 | −0.083 | 0.651 | −0.641 | <0.001 | 0.443 | 0.011 | −0.096 | 0.602 |
| Total PLFAs (nmol g−¹) | 0.705 | <0.001 | 0.335 | 0.061 | −0.383 | 0.030 | 0.474 | 0.006 | −0.041 | 0.824 |
| F:B | −0.623 | <0.001 | −0.168 | 0.357 | 0.609 | <0.001 | −0.352 | 0.048 | 0.177 | 0.331 |
| gr+/gr– | −0.589 | <0.001 | −0.325 | 0.070 | 0.332 | 0.072 | −0.448 | 0.010 | 0.154 | 0.400 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz, K.; Thiele-Bruhn, S.; Kenngott, K.G.J.; Meyer, M.; Diehl, D.; Steinmetz, Z.; Schaumann, G.E. Effects of Plastic versus Straw Mulching Systems on Soil Microbial Community Structure and Enzymes in Strawberry Cultivation. Soil Syst. 2022, 6, 21. https://doi.org/10.3390/soilsystems6010021
Muñoz K, Thiele-Bruhn S, Kenngott KGJ, Meyer M, Diehl D, Steinmetz Z, Schaumann GE. Effects of Plastic versus Straw Mulching Systems on Soil Microbial Community Structure and Enzymes in Strawberry Cultivation. Soil Systems. 2022; 6(1):21. https://doi.org/10.3390/soilsystems6010021
Chicago/Turabian StyleMuñoz, Katherine, Sören Thiele-Bruhn, Kilian G. J. Kenngott, Maximilian Meyer, Dörte Diehl, Zacharias Steinmetz, and Gabriele E. Schaumann. 2022. "Effects of Plastic versus Straw Mulching Systems on Soil Microbial Community Structure and Enzymes in Strawberry Cultivation" Soil Systems 6, no. 1: 21. https://doi.org/10.3390/soilsystems6010021
APA StyleMuñoz, K., Thiele-Bruhn, S., Kenngott, K. G. J., Meyer, M., Diehl, D., Steinmetz, Z., & Schaumann, G. E. (2022). Effects of Plastic versus Straw Mulching Systems on Soil Microbial Community Structure and Enzymes in Strawberry Cultivation. Soil Systems, 6(1), 21. https://doi.org/10.3390/soilsystems6010021








