Impact of Short-Term Cultivation on Some Selected Properties of Sandy Soil in an Arid Environment
Abstract
:1. Introduction
2. Materials and Methods
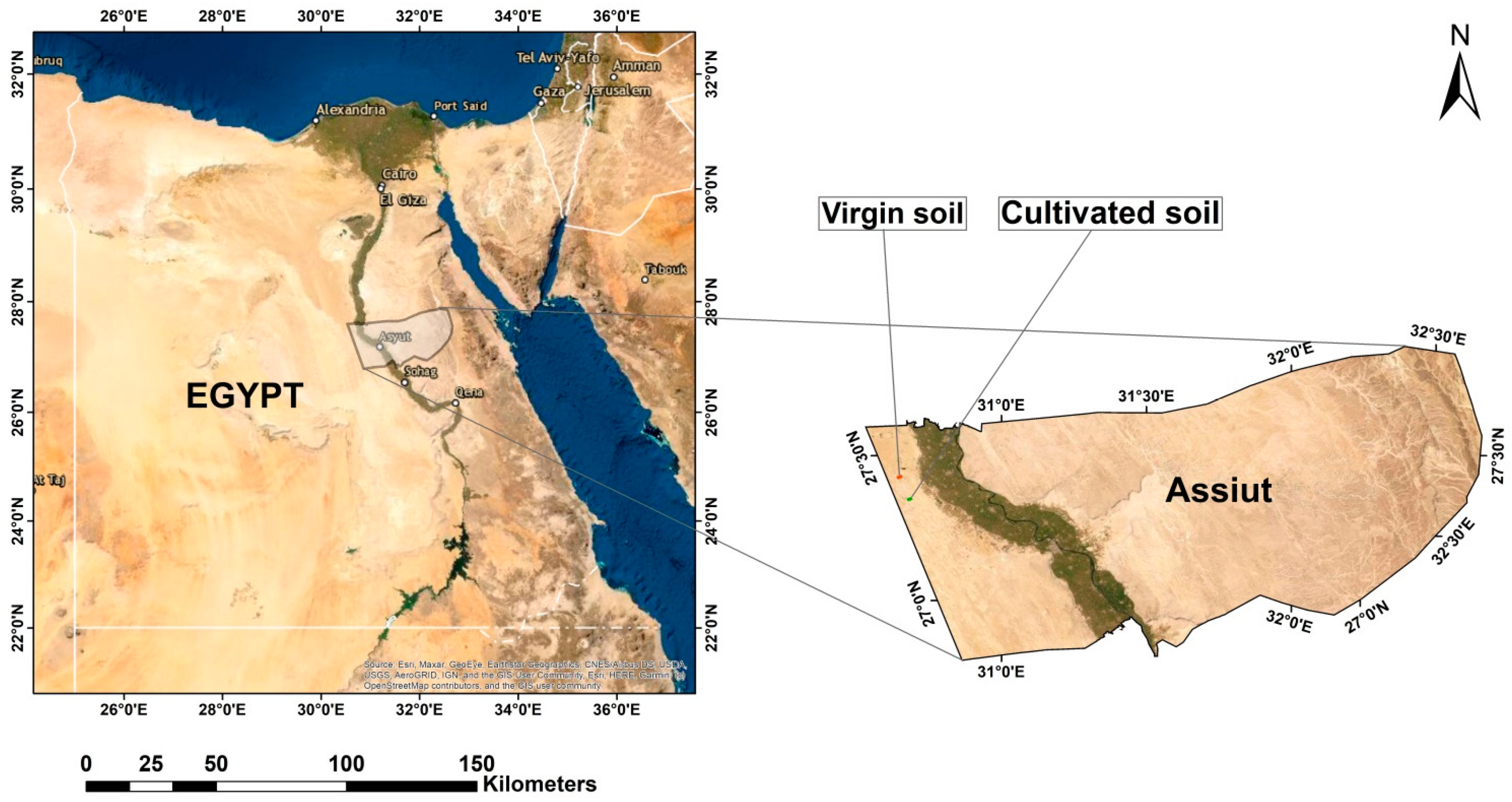
2.1. Study Area
2.2. Soil Sampling
2.3. Laboratory Analysis
2.4. Statistics and Geostatistics Analysis
3. Results
3.1. Soil Property Descriptive Statistics
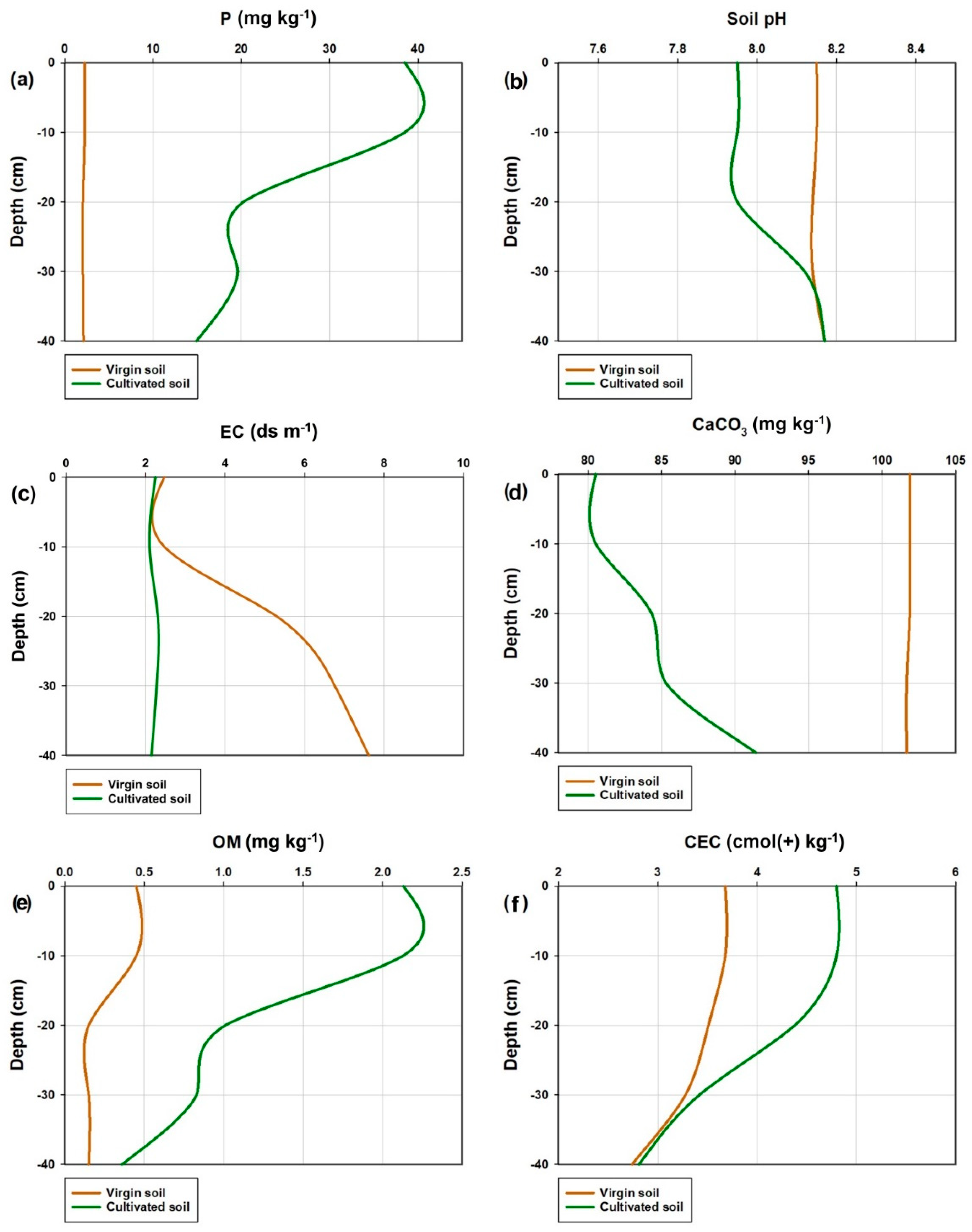
3.1.1. Available Soil Phosphorus
3.1.2. Soil pH
3.1.3. Soil Salinity
3.1.4. Soil Lime Content
3.1.5. Organic Matter Content (OM)
3.1.6. Cation Exchange Capacity (CEC)
3.2. Cultivation-Induced Changes
3.2.1. Soil Phosphorus
3.2.2. Soil pH
3.2.3. Soil Electrical Conductivity (EC)
3.2.4. Soil Lime Content
3.2.5. Soil Organic Matter (OM)
3.2.6. Cation Exchange Capacity (CEC)
3.3. Geostatistical Analysis and Spatial Distribution of Soil Properties
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. The Future of Food and Agriculture, Trends and Challenges; FAO: Rome, Italy, 2017; ISBN 9789251095515. [Google Scholar]
- Lal, R. Soil degradation as a reason for inadequate human nutrition. Food Secur. 2009, 1, 45–57. [Google Scholar] [CrossRef]
- Lal, R. Climate Change and Soil Degradation Mitigation by Sustainable Management of Soils and Other Natural Resources. Agric. Res. 2012, 1, 199–212. [Google Scholar] [CrossRef] [Green Version]
- Verheye, W. Soils of arid and semi-arid areas. In Land Use, Land Cover and Soil Sciences-Volume II: Land Evaluation; Verheye, W., Ed.; Encyclopedia of Life Support Systems (EOLSS): Paris, France, 2009; Volume II, pp. 1–29. [Google Scholar]
- Maleki, S.; Karimi, A.; Zeraatpisheh, M.; Poozeshi, R.; Feizi, H. Long-term cultivation effects on soil properties variations in different landforms in an arid region of eastern Iran. CATENA 2021, 206, 105465. [Google Scholar] [CrossRef]
- Zhang, J.; Song, C.; Yang, W. Effects of cultivation on soil microbiological properties in a freshwater march soil in Northeast China. Soil Tillage Res. 2007, 93, 231–235. [Google Scholar] [CrossRef]
- Ajami, M.; Heidari, A.; Khormali, F.; Gorji, M.; Ayoubi, S. Environmental factors controlling soil organic carbon storage in loess soils of a subhumid region, northern Iran. Geoderma 2016, 281, 1–10. [Google Scholar] [CrossRef]
- Alidoust, E.; Afyuni, M.; Hajabbasi, M.A.; Mosaddeghi, M.R. Soil carbon sequestration potential as affected by soil physical and climatic factors under different land uses in a semiarid region. CATENA 2018, 171, 62–71. [Google Scholar] [CrossRef]
- Song, Q.; Gao, X.; Du, H.; Lei, J.; Li, S.; Li, S. Cultivation impacts on soil texture during oasis expansion in Xinjiang, Northwest China: Wind erosion effects. Aeolian Res. 2021, 50, 100646. [Google Scholar] [CrossRef]
- Zeraatpisheh, M.; Bakhshandeh, E.; Hosseini, M.; Alavi, S. Assessing the effects of deforestation and intensive agriculture on the soil quality through digital soil mapping. Geoderma 2020, 363, 114139. [Google Scholar] [CrossRef]
- Bahrami, S.; Ghahraman, K. Geomorphological controls on soil fertility of semi-arid alluvial fans: A case study of the Joghatay Mountains, Northeast Iran. CATENA 2019, 176, 145–158. [Google Scholar] [CrossRef]
- Delpupo, C.; Schaefer, C.; Roque, M.; Faria, A.; Rosad, K.; Thomazini, A.; de Paula, M. Soil and landform interplay in the dry valley of Edson Hills, Ellsworth Mountains, continental Antarctica. Geomorphology 2017, 295, 134–146. [Google Scholar] [CrossRef]
- Fathizad, H.; Hakimzadeh Ardakani, M.A.; Sodaiezadeh, H.; Kerry, R.; Taghizadeh-Mehrjardi, R. Investigation of the spatial and temporal variation of soil salinity using random forests in the central desert of Iran. Geoderma 2020, 365, 114233. [Google Scholar] [CrossRef]
- Maleki, S.; Khormali, F.; Bagheri Bodaghabadi, M.; Mohammadi, J.; Hoffmeister, D.; Kehl, M. Role of geomorphic surface on the above-ground biomass and soil organic carbon storage in a semi-arid region of Iranian loess plateau. Quat. Int. 2020, 552, 111–121. [Google Scholar] [CrossRef]
- Schlesinger, W.H. An evaluation of abiotic carbon sinks in deserts. Glob. Chang. Biol. 2016, 23, 25–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, A.M.; Gillespie, A.W.; Dhillon, G.S.; Kar, G.; Minielly, C.; Koala, S.; Ouattara, B.; Kimaro, A.A.; Bationo, A.; Schoenau, J.J.; et al. Long-term effects of integrated soil fertility management practices on soil chemical properties in the Sahel. Geoderma 2020, 366, 114207. [Google Scholar] [CrossRef]
- Chen, L.; Qi, X.; Zhang, X.; Li, Q.; Zhang, Y. Effect of agricultural land use changes on soil nutrient use efficiency in an agricultural area, Beijing, China. Chin. Geogr. Sci. 2011, 21, 392–402. [Google Scholar] [CrossRef]
- Dereje, G. Effect of Land use Types on Selected Soil Physical and Chemical Properties at Sire Morose Sub Watershed, Central Highland of Ethiopia. Int. J. Eng. Res. Technol. 2020, 9, 770–779. [Google Scholar] [CrossRef]
- Marzaioli, R.; D’Ascoli, R.; De Pascale, R.A.; Rutigliano, F.A. Soil quality in a Mediterranean area of Southern Italy as related to different land use types. Appl. Soil Ecol. 2010, 44, 205–212. [Google Scholar] [CrossRef]
- Tellen, V.A.; Yerima, B.P.K. Effects of land use change on soil physicochemical properties in selected areas in the North West region of Cameroon. Environ. Syst. Res. 2018, 7, 3. [Google Scholar] [CrossRef] [Green Version]
- Li, X.-G.; Li, F.-M.; Rengel, Z.; Bhupinderpal, S.; Wang, Z.-F. Cultivation effects on temporal changes of organic carbon and aggregate stability in desert soils of Hexi Corridor region in China. Soil Tillage Res. 2006, 91, 22–29. [Google Scholar] [CrossRef]
- Bruun, T.B.; Elberling, B.; de Neergaard, A.; Magid, J. Organic Carbon Dynamics in Different Soil Types After Conversion of Forest to Agriculture. Land Degrad. Dev. 2015, 26, 272–283. [Google Scholar] [CrossRef]
- Yang, T.; Siddique, H.M.; Liu, K. Cropping systems in agriculture and their impact on soil health—A review. Glob. Ecol. Conserv. 2020, 23, e01118. [Google Scholar] [CrossRef]
- Zhang, X.; Davidson, E.A.; Zou, T.; Lassaletta, L.; Quan, Z.; Li, T.; Zhang, W. Quantifying Nutrient Budgets for Sustainable Nutrient Management. Glob. Biogeochem. Cycles 2020, 34, e2018GB00606. [Google Scholar] [CrossRef] [Green Version]
- Yakubu, S. Changes in soil physical properties due to different land uses in part of Nigeria Northern Guinea Savanna. Zaria Geogr. 2010, 18, 47–56. [Google Scholar]
- García-Orenes, F.; Guerrero, C.; Roldan, A.; Mataix-Solera, J.; Cerdà, A.; Campoy, M.; Zornoza, R.; Bárcenas, G.; Caravaca, F. Soil microbial biomass and activity under different agricultural management systems in a semiarid Mediterranean agroecosystem. Soil Tillage Res. 2010, 109, 110–115. [Google Scholar] [CrossRef]
- He, M.; Ji, X.; Bu, D.; Zhi, J. Cultivation effects on soil texture and fertility in an arid desert region of northwestern China. J. Arid Land 2020, 12, 701–715. [Google Scholar] [CrossRef]
- Li, Y.Y.; Shao, M.A. Change of soil physical properties under long-term natural vegetation restoration in the Loess Plateau of China. J. Arid Environ. 2006, 64, 77–96. [Google Scholar] [CrossRef]
- Raiesi, F. Carbon and N mineralization as affected by soil cultivation and crop residue in a calcareous wetland ecosystem in Central Iran. Agric. Ecosyst. Environ. 2006, 112, 13–20. [Google Scholar] [CrossRef]
- Islam, K.R.; Weil, R.R. Land use effects on soil quality in a tropical forest ecosystem of Bangladesh. Agric. Ecosyst. Environ. 2000, 79, 9–16. [Google Scholar] [CrossRef]
- Bünemann, E.K.; Bongiorno, G.; Bai, Z.; Creamer, R.E.; De Deyn, G.; de Goede, R.; Fleskens, L.; Geissen, V.; Kuyper, T.W.; Mäder, P.; et al. Soil quality—A critical review. Soil Biol. Biochem. 2018, 120, 105–125. [Google Scholar] [CrossRef]
- Fernández, F.G.; Sorensen, B.A.; Villamil, M.B. A Comparison of Soil Properties after Five Years of No-Till and Strip-Till. Agron. J. 2015, 107, 1339–1346. [Google Scholar] [CrossRef]
- Jia, Z.; Kuzyakov, Y.; Myrold, D.; Tiedje, J. Soil Organic Carbon in a Changing World. Pedosphere 2017, 27, 789–791. [Google Scholar] [CrossRef]
- van Es, H.M.; Karlen, D.L. Reanalysis Validates Soil Health Indicator Sensitivity and Correlation with Long-term Crop Yields. Soil Sci. Soc. Am. J. 2019, 83, 721–732. [Google Scholar] [CrossRef] [Green Version]
- FAO. The State of Food Insecurity in the World: Strengthening the Enabling Environment for Food Security and Nutrition; FAO: Rome, Italy, 2014. [Google Scholar]
- Deng, L.; Wang, K.; Zhu, G.; Liu, Y.; Chen, L.; Shangguan, Z. Changes of soil carbon in five land use stages following 10 years of vegetation succession on the Loess Plateau, China. CATENA 2018, 171, 185–192. [Google Scholar] [CrossRef]
- Liu, Q.; Xu, H.; Yi, H. Impact of Fertilizer on Crop Yield and C:N:P Stoichiometry in Arid and Semi-Arid Soil. Int. J. Environ. Res. Public Health 2021, 18, 4341. [Google Scholar] [CrossRef]
- Rutkowska, A.; Pikuła, D. Effect of crop rotation and nitrogen fertilization on the quality and quantity of soil organic matter. In Soil Processes and Current Trends in Quality Assessment; Soriano, M.C.H., Ed.; Intech Open Book: Rijeka, Croatia, 2013; pp. 249–267. [Google Scholar]
- Šimanský, V.; Jonczak, J.; Horváthová, J.; Igaz, D.; Aydın, E.; Kováčik, P. Does long-term application of mineral fertilizers improve physical properties and nutrient regime of sandy soils? Soil Tillage Res. 2022, 215, 105224. [Google Scholar] [CrossRef]
- Abedi, F.; Amirian-Chakan, A.R.; Faraji, M.; Taghizadeh-Mehrjardi, R.; Kerry, R.; Razmjoue, D.; Scholten, T. Salt dome induced soil salinity in southern Iran: Prediction and mapping with averaging machine learning models. Land Degrad. Dev. 2020, 32, 1540–1554. [Google Scholar] [CrossRef]
- Sarwar, G.; Ibrahim, M.; Tahir, M.A.; Iftikhar, Y.; Haider, M.S.; Sabah, N.; Han, K.H.; Ha, S.G.; Zhang, Y.-S. Effect of Compost and Gypsum Application on the Chemical Properties and Fertility Status of Saline-Sodic Soil. Korean J. Soil Sci. Fertil. 2011, 44, 510–516. [Google Scholar] [CrossRef] [Green Version]
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; USDA-Natural Resources Conservation Service: Washington, DC, USA, 2014.
- Olsen, S.R.; Cole, C.V.; Watanabe, F.S.; Dean, L.A. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; US Department of Agriculture: Washington, DC, USA, 1954; Volume 939, pp. 1–19.
- Nelson, R.E. Carbonate and gypsum. In Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; Soil Science Society of America, Inc.: Madison, WI, USA, 1982; pp. 181–196. [Google Scholar]
- Jackson, M.L. Soil Chemical Analysis; Prentice-Hall, Inc.: Englewood Cliffs, NJ, USA, 1973. [Google Scholar]
- Walkley, A.; Black, C.A. An examination of different methods for determining soil organic matter and the proposed modification by the chromic acid titration method. J. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- McLean, E.O. Soil pH and lime requirement. In Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; Soil Science Society of America, Inc.: Madison, WI, USA, 1982; pp. 199–223. [Google Scholar]
- Baruah, T.C.; Barthakur, H.P. A Textbook of Soil Analysis; Vikas Publishing House. Pvt. Ltd.: New Delhi, India, 1997. [Google Scholar]
- Carter, M.R.; Gregorich, E.G. Soil Sampling and Methods of Analysis, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar] [CrossRef]
- Goovaerts, P. Geostatistical tools for characterizing the spatial variability of microbiological and physico-chemical soil properties. Biol. Fertil. Soils 1998, 27, 315–334. [Google Scholar] [CrossRef] [Green Version]
- ESRI. ArcMap Version 10.2.2. User Manual; ESRI: Redlands, CA, USA, 2014. [Google Scholar]
- Selmy, S.; Abd El-Aziz, S.; El-Desoky, A.; El-Sayed, M. Characterizing, predicting, and mapping of soil spatial variability in Gharb El-Mawhoub area of Dakhla Oasis using geostatistics and GIS approaches. J. Saudi Soc. Agric. Sci. 2021, 21, 383–396. [Google Scholar] [CrossRef]
- Kravchenko, A.N. Influence of Spatial Structure on Accuracy of Interpolation Methods. Soil Sci. Soc. Am. J. 2003, 67, 1564–1571. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, T.-B.; Lei, M.; Yang, J.; Guo, Q.-J.; Song, B.; Zhou, X.-Y. Spatial distribution of soil heavy metal pollution estimated by different interpolation methods: Accuracy and uncertainty analysis. Chemosphere 2011, 82, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Mueller, G.T.; Pierce, J.F.; Schabenberger, O.; Warncke, D.D. Map Quality for Site-Specific Fertility Management. Soil Sci. Soc. Am. J. 2001, 65, 1547–1558. [Google Scholar] [CrossRef]
- Al-Busaidi, W.; Janke, R.; Menezes-Blackburn, D.; Khan, M.M. Impact of long-term agricultural farming on soil and water chemical properties: A case study from Al-Batinah regions (Oman). J. Saudi Soc. Agric. Sci. 2021, 21, 397–403. [Google Scholar] [CrossRef]
- Alarima, I.C.; Annan-Afful, E.; Obalum, E.S.; Awotunde, M.J.; Masunaga, T.; Igwe, A.C.; Wakatsuki, T. Comparative assessment of temporal changes in soil degradation under four contrasting land-use options along a tropical toposequence. Land Degrad. Dev. 2020, 31, 439–450. [Google Scholar] [CrossRef]
- Vaz, C.M.P.; de Freitas Iossi, M.; de Mendonça Naime, J.; Macedo, A.; Reichert, J.M.; Reinert, D.J.; Cooper, M. Validation of the Arya and Paris Water Retention Model for Brazilian Soils. Soil Sci. Soc. Am. J. 2005, 69, 577–583. [Google Scholar] [CrossRef] [Green Version]
- Vogelmann, E.S.; Reichert, J.M.; Reinert, D.J.; Mentges, M.I.; Vieira, D.A.; de Barros, C.A.P.; Fasinmirin, J.T. Water repellency in soils of humid subtropical climate of Rio Grande do Sul, Brazil. Soil Tillage Res. 2010, 110, 126–133. [Google Scholar] [CrossRef]
- Fasinmirin, J.T.; Reichert, J.M. Conservation tillage for cassava (Manihot esculenta crantz) production in the tropics. Soil Tillage Res. 2011, 113, 1–10. [Google Scholar] [CrossRef]
- Reichert, J.M.; Morales, C.A.S.; Lima, E.M.; de Bastos, F.; Sampietro, J.A.; Cavalli, J.P.; de Araújo, E.F.; Srinivasan, R. Best tillage practices for early-growth of clonal eucalyptus in soils with distinct granulometry, drainage and profile depth. Soil Tillage Res. 2021, 212, 105038. [Google Scholar] [CrossRef]
- Holthusen, D.; Brandt, A.A.; Reichert, J.M.; Horn, R.; Fleige, H.; Zink, A. Soil functions and in situ stress distribution in subtropical soils as affected by land use, vehicle type, tire inflation pressure and plant residue removal. Soil Tillage Res. 2018, 184, 78–92. [Google Scholar] [CrossRef]
- Goulart, R.Z.; Reichert, J.M.; Rodrigues, M.F.; Chaiben Neto, M.; Ebling, E.D. Comparing tillage methods for growing lowland soybean and corn during wetter-than-normal cropping seasons. Paddy Water Environ. 2021, 19, 401–415. [Google Scholar] [CrossRef]
- França, J.S.; Reichert, J.M.; Holthusen, D.; Rodrigues, M.F.; de Araújo, E.F. Subsoiling and mechanical hole-drilling tillage effects on soil physical properties and initial growth of eucalyptus after eucalyptus on steeplands. Soil Tillage Res. 2021, 207, 104860. [Google Scholar] [CrossRef]
- Bizuhoraho, T.; Kayiranga, A.; Manirakiza, N.; Mourad, K.A. The Effect of Land Use Systems on Soil Properties; A case study from Rwanda. Sustain. Agric. Res. 2018, 7, 30–40. [Google Scholar] [CrossRef] [Green Version]
- Lemenih, M.; Itanna, F. Soil carbon stocks and turnovers in various vegetation types and arable lands along an elevation gradient in southern Ethiopia. Geoderma 2004, 123, 177–188. [Google Scholar] [CrossRef]
- Mandal, A.; Toor, A.S.; Dhaliwal, S.S. Assessment of Sequestered Organic Carbon and Its Pools under Different Agricultural Land-Uses in the Semi-Arid Soils of South-Western Punjab, India. J. Soil Sci. Plant Nutr. 2020, 20, 259–273. [Google Scholar] [CrossRef]
- O’Flynn, C.J.; Fenton, O.; Wall, D.; Brennan, R.B.; McLaughlin, M.J.; Healy, M.G. Influence of soil phosphorus status, texture, pH and metal content on the efficacy of amendments to pig slurry in reducing phosphorus losses. Soil Use Manag. 2018, 34, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Solomon, D.; Lehmann, J.; Mamo, T.; Fritzsche, F.; Zech, W. Phosphorus forms and dynamics as influenced by land use changes in the sub-humid Ethiopian highlands. Geoderma 2002, 105, 21–48. [Google Scholar] [CrossRef]
- Tesfaye, W. Status of Selected Physicochemical Properties of Soils under Long Term Sugarcane Cultivation Fields at Wonji-Shoa Sugar Estate. Am. J. Agric. For. 2021, 9, 397–408. [Google Scholar] [CrossRef]
- Vandereijk, D.; Janssen, B.H.; Oenema, O. Initial and residual effects of fertilizer phosphorus on soil phosphorus and maize yields on phosphorus fixing soils: A case study in south-west Kenya. Agric. Ecosyst. Environ. 2006, 116, 104–120. [Google Scholar] [CrossRef]
- Zhang, W.; An, L.Y.; Chen, P.X. Phosphorus fractionation related to environmental risks resulting from intensive vegetable cropping and fertilization in a subtropical region. Environ. Pollut. 2021, 269, 116098. [Google Scholar] [CrossRef]
- Bewket, W.; Stroosnijder, L. Effects of agroecological land use succession on soil properties in Chemoga watershed, Blue Nile basin, Ethiopia. Geoderma 2003, 111, 85–98. [Google Scholar] [CrossRef]
- Whitbread, A.M.; Lefroy, R.D.B.; Blair, G.J. A survey of the impact of cropping on soil physical and chemical properties in north-western New South Wales. Soil Res. 1998, 36, 669–682. [Google Scholar] [CrossRef]
- Kiflu, A.; Beyene, S. Effects of different land use systems on selected soil properties in South Ethiopia. J. Soil Sci. Environ. Manag. 2013, 4, 100–107. [Google Scholar] [CrossRef]
- Cooper, J.; Reed, E.Y.; Hörtenhuber, S.; Lindenthal, T.; Løes, A.-K.; Mäder, P.; Magid, J.; Oberson, A.; Kolbe, H.; Möller, K. Phosphorus availability on many organically managed farms in Europe. Nutr. Cycl. Agroecosys. 2018, 110, 227–239. [Google Scholar] [CrossRef] [Green Version]
- Menezes-Blackburn, D.; Giles, C.; Darch, T.; George, T.S.; Blackwell, M.; Stutter, M.; Shand, C.; Lumsdon, D.; Cooper, P.; Wendler, R.; et al. Opportunities for mobilizing recalcitrant phosphorus from agricultural soils: A review. Plant Soil 2018, 427, 5–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solomon, D.; Fritzsche, F.; Tekalign, M.; Lehmann, J.; Zech, W. Soil Organic Matter Composition in the Subhumid Ethiopian Highlands as Influenced by Deforestation and Agricultural Management. Soil Sci. Soc. Am. J. 2002, 66, 68–82. [Google Scholar] [CrossRef] [Green Version]
- Zinn, Y.; Lal, R.; Resck, D. Changes in soil organic carbon stocks under agriculture in Brazil. Soil Tillage Res. 2005, 48, 28–40. [Google Scholar] [CrossRef]
- Dang, M. Quantitative and qualitative soil quality assessments of tea enterprises in Northern Vietnam. Afr. J. Agric. Res. 2007, 2, 455–462. [Google Scholar] [CrossRef]
- Samadi, A.; Gilkes, R.J. Forms of phosphorus in virgin and fertilized calcareous soils of Western Australia. Soil Res. 1998, 36, 585–601. [Google Scholar] [CrossRef]
- Carrow, R.N.; Stowell, L.; Gelernter, W.; Davis, S.; Duncan, R.R.; Skorulski, J. Clarifying soil testing: III. SLAN sufficiency ranges and recommendations. Golf. Course Manag. 2004, 72, 194–198. [Google Scholar]
- Nega, E.; Heluf, G. Effect of land use changes and soil depth on soil organic matter, total nitrogen and available phosphorus contents of soils in Senbat watershed, western Ethiopia. J. Agric. Biol. Sci. 2013, 8, 206–212. [Google Scholar]
- Foth, H.; Ellis, B. Soil Fertility, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1997. [Google Scholar] [CrossRef]
- Agoume’, V.; Birang, A.M. Impact of land-use systems on some physical and chemical soil properties of an Oxisol in the humid forest Zone of Southern Cameroon. Tropicultura 2009, 27, 15–20. [Google Scholar]
- Fu, B.; Chen, L.; Ma, K.; Zhou, H.; Wang, J. The relationships between land use and soil conditions in the hilly area of the loess plateau in northern Shaanxi, China. CATENA 2000, 39, 69–78. [Google Scholar] [CrossRef]
- Gol, C. The effects of land use change on soil properties and organic carbon at Dagdami river catchment in Turkey. J. Environ. Biol. 2009, 30, 825–830. [Google Scholar] [PubMed]
- Wasihun, M.; Muktar, M.; Teshome, Y. Evaluation of the effect of land use types on selected soil physico-chemical properties in Itang-kir Area of Gambella Regional, Ethiopia. J. Biol. Agric. Healthc. 2015, 5, 92–102. [Google Scholar]
- Zhao, Q.; Zeng, H.D.; Fan, P.Z.; Lee, K.D. Effect of Land Cover Change on Soil Phosphorus Fractions in Southeastern Horqin Sandy Land, Northern China. Pedosphere 2008, 18, 741–748. [Google Scholar] [CrossRef]
- Deng, Y.; Xia, D.; Cai, C.; Ding, S. Effects of land uses on soil physic-chemical properties and erodibility in collapsing-gully alluvial fan of Anxi County, China. J. Integr. Agric. 2016, 15, 1863–1873. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, R.P.; Pand, K.K.; Thakur, S. Soil Properties Affected by Land Use Systems in Western Chitwan, Nepal. Int. J. Appl. Sci. Biotechnol. 2014, 2, 398–402. [Google Scholar] [CrossRef] [Green Version]
- Mengistu, T.; Dereje, T. Impact of land use types and soil depths on selected soil physicochemical properties in Fasha District, Konso Zone, Southern Ethiopia. J. Soil Sci. Environ. Manag. 2021, 12, 10–16. [Google Scholar] [CrossRef]
- Bhunia, G.S.; Shit, P.K.; Maiti, R. Spatial variability of soil organic carbon under different land use using radial basis function (RBF). Model. Earth Syst. Environ. 2016, 2, 17. [Google Scholar] [CrossRef]
- Muche, M.; Kokeb, A.; Molla, E. Assessing the Physicochemical Properties of Soil under Different Land Use Types. J. Environ. Anal. Toxicol. 2015, 5, 309. [Google Scholar] [CrossRef]
- Yu, P.; Han, D.; Liu, S.; Wen, X.; Huang, Y.; Jia, H. Soil quality assessment under different land uses in an alpine grassland. CATENA 2018, 171, 280–287. [Google Scholar] [CrossRef]
- Dong, S.; Wan, S.; Kang, Y.; Li, X. Prospects of using drip irrigation for ecological conservation and reclaiming highly saline soils at the edge of Yinchuan Plain. Agric. Water Manag. 2020, 239, 106255. [Google Scholar] [CrossRef]
- Gorji, T.; Yıldırım, A.; Hamzehpour, N.; Tanik, A.; Sertel, E. Soil salinity analysis of Urmia Lake Basin using Landsat-8 OLI and Sentinel-2A based spectral indices and electrical conductivity measurements. Ecol. Indic. 2020, 112, 106173. [Google Scholar] [CrossRef]
- Huang, Y.J.; Li, Z.; Zhuo, Z.Q.; Xing, A.; Huang, Y.F. Soil water and salt dynamics under different irrigation and drainage management scenarios based on SahysMod model. Trans. Chin. Soc. Agric. Eng. 2020, 36, 129–140. [Google Scholar]
- Li, P.; Qian, H.; Wu, J. Conjunctive use of groundwater and surface water to reduce soil salinization in the Yinchuan Plain, North-West China. Int. J. Water Resour. Dev. 2018, 34, 337–353. [Google Scholar] [CrossRef]
- Mandal, A.; Toor, A.S.; Dhaliwal, S.S.; Singh, P.; Singh, V.K.; Sharma, V.; Gupta, R.K.; Naresh, R.K.; Kumar, Y.; Pramanick, B.; et al. Long-Term Field and Horticultural Crops Intensification in Semiarid Regions Influence the Soil Physiobiochemical Properties and Nutrients Status. Agronomy 2022, 12, 1010. [Google Scholar] [CrossRef]
- Alnaimy, M.; Zelenakova, M.; Vranayova, Z.; Abu-Hashim, M. Effects of Temporal Variation in Long-Term Cultivation on Organic Carbon Sequestration in Calcareous Soils: Nile Delta, Egypt. Sustainability 2020, 12, 4514. [Google Scholar] [CrossRef]
- Bughio, M.A.; Wang, P.; Meng, F.; Qing, C.; Kuzyakov, Y.; Wang, X.; Junejo, S.A. Neoformation of pedogenic carbonates by irrigation and fertilization and their contribution to carbon sequestration in soil. Geoderma 2016, 262, 12–19. [Google Scholar] [CrossRef]
- Chmiel, S.; Hałas, S.; Głowacki, S.; Sposób, J.; Maciejewska, E.; Trembaczowski, A. Concentration of soil CO2 as an indicator of the decalcification rate after liming treatment. Int. Agrophysics 2016, 30, 143–150. [Google Scholar] [CrossRef] [Green Version]
- Mikhailova, E.A.; Post, C.J. Effects of Land Use on Soil Inorganic Carbon Stocks in the Russian Chernozem. J. Environ. Qual. 2006, 35, 1384–1388. [Google Scholar] [CrossRef] [PubMed]
- Sanderman, J. Can management induced changes in the carbonate system drive soil carbon sequestration? A review with particular focus on Australia. Agric. Ecosyst. Environ. 2012, 155, 70–77. [Google Scholar] [CrossRef]
- Doran, J.W.; Sarrantonio, M.; Liebig, M.A. Soil Health and Sustainability. Adv. Agron. 1996, 56, 1–54. [Google Scholar] [CrossRef]
- Lal, R.; Delgado, J.A.; Groffman, P.M.; Millar, N.; Dell, C.; Rotz, A. Management to mitigate and adapt to climate change. J. Soil Water Conserv. 2011, 66, 276–285. [Google Scholar] [CrossRef]
- Berns, A.E.; Knicker, H. Soil Organic Matter. eMagRes 2014, 3, 43–54. [Google Scholar] [CrossRef]
- Bormann, H.; Klaassen, K. Seasonal and land use dependent variability of soil hydraulic and soil hydrological properties of two Northern German soils. Geoderma 2008, 145, 295–302. [Google Scholar] [CrossRef]
- Botta, G.F.; Becerra, A.T.; Tourn, F.B. Effect of the number of tractor passes on soil rut depth and compaction in two tillage regimes. Soil Tillage Res. 2009, 103, 381–386. [Google Scholar] [CrossRef]
- Brady, N.C.; Weil, R.R. The Nature and Properties of Soils, 13th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2002; p. 960. [Google Scholar]
- Celik, I. Land-use effects on organic matter and physical properties of soil in a southern Mediterranean highland of Turkey. Soil Tillage Res. 2005, 83, 270–277. [Google Scholar] [CrossRef]
- Feller, C.; Beare, M. Physical control of soil organic matter dynamics in the tropics. Geoderma 1997, 79, 69–116. [Google Scholar] [CrossRef]
- Gangwar, K.S.; Singh, K.K.; Sharma, S.K.; Tomar, O.K. Alternative tillage and crop residue management in wheat after rice in sandy loam soils of Indo-Gangetic plains. Soil Tillage Res. 2006, 88, 242–252. [Google Scholar] [CrossRef]
- Ghorbani-Dashtaki, S.; Homaee, M.; Loiskandl, W. Towards using pedotransfer functions for estimating infiltration parameters. Hydrol. Sci. J. 2016, 61, 1477–1488. [Google Scholar] [CrossRef] [Green Version]
- Haghighi, F.; Gorji, M.; Shorafa, M. A study of the effects of land use changes on soil physical properties and organic matter. Land Degrad. Dev. 2010, 21, 496–502. [Google Scholar] [CrossRef]
- Karami, A.; Homaee, M.; Afzalinia, S.; Ruhipour, H.; Basirat, S. Organic resource management: Impacts on soil aggregate stability and other soil physico-chemical properties. Agric. Ecosyst. Environ. 2012, 148, 22–28. [Google Scholar] [CrossRef]
- Kimetu, J.M.; Lehmann, J.; Ngoze, S.O.; Mugendi, D.N.; Kinyangi, J.M.; Riha, S.; Verchot, L.; Recha, J.; Pell, A.N. Reversibility of Soil Productivity Decline with Organic Matter of Differing Quality Along a Degradation Gradient. Ecosystems 2008, 11, 726–739. [Google Scholar] [CrossRef]
- Li, X.-G.; Li, F.-M.; Zed, R.; Zhan, Z.-Y.; Bhupinderpal, S. Soil physical properties and their relations to organic carbon pools as affected by land use in an alpine pastureland. Geoderma 2007, 139, 98–105. [Google Scholar] [CrossRef]
- Murphy, B.W. Impact of soil organic matter on soil properties—A review with emphasis on Australian soils. Soil Res. 2015, 53, 605–635. [Google Scholar] [CrossRef]
- Tesfaye, W.; Kibebew, K.; Bobe, B.; Melesse, T.; Teklu, E. Effects of long term sugarcane production on soils physicochemical properties at Finchaa sugar Estate. J. Soil Sci. Environ. Manag. 2020, 11, 30–40. [Google Scholar] [CrossRef] [Green Version]
- Toohey, R.C.; Boll, J.; Brooks, E.S.; Jones, J.R. Effects of land use on soil properties and hydrological processes at the point, plot, and catchment scale in volcanic soils near Turrialba, Costa Rica. Geoderma 2018, 315, 138–148. [Google Scholar] [CrossRef]
- Tolera, E.; Tesfaye, W. The Effect of Application of Vermicompost and NPS Fertilizer on Selected Soil Properties and Yield of Maize (Zea may L.) at Toke Kutaye, Ethiopia. Int. J. Appl. Agric. Sci. 2021, 7, 247–257. [Google Scholar]
- Angelova, V.R.; Akova, I.V.; Artinova, S.N.; Ivanov, K. The effect of organic amendments on soil chemical characteristics. Bulg. J. Agric. Sci. 2013, 19, 958–971. [Google Scholar]
- Davari, M.; Gholami, L.; Nabiollahi, K.; Homaee, M.; Jafari, J.H. Deforestation and cultivation of sparse forest impacts on soil quality (case study: West Iran, Baneh). Soil Tillage Res. 2020, 198, 104504. [Google Scholar] [CrossRef]
- Plante, A.; Conant, R.T. Soil Organic Matter Dynamics, Climate Change Effects. In Global Environmental Change; Freedman, B., Ed.; Springer: Dordrecht, The Netherlands, 2014; Volume 1, pp. 317–323. [Google Scholar] [CrossRef]
- Gao, G.; Change, C. Changes in Cation exchange capacity and particle size distribution of soil as associated with long-term annual application of cattle feed lot manure. Soil Sci. 1996, 161, 115–120. [Google Scholar] [CrossRef]
- Urioste, A.; Hevia, G.; Hepper, E.; Anton, L.; Bono, A.; Buschiazzo, D. Cultivation effects on the distribution of organic carbon, total nitrogen and phosphorus in soils of the semiarid region of Argentinian Pampas. Geoderma 2006, 136, 621–630. [Google Scholar] [CrossRef]
- Fasinmirin, J.T.; Olorunfemi, I.E. Comparison of hydraulic conductivity of soils of the forest vegetative zone of Nigeria. Appl. Trop. Agric. 2012, 17, 64–77. [Google Scholar]
- Nega, E.; Heluf, G. Influence of land use changes and soil depth on cation exchange capacity and contents of exchangeable bases in the soils of Senbat Watershed, western Ethiopia. Ethiop. J. Nat. Resour. 2009, 11, 195–206. [Google Scholar]
- Rashidi, M.; Seilsepour, M. Modeling of soil cation exchange capacity based on soil organic carbon. ARPN J. Agric. Biol. Sci. 2008, 3, 41–45. [Google Scholar]
- Johnston, K.; Ver Hoef, J.M.; Krivoruchko, K.; Lucas, N. Using ArcGIS Geostatistical Analyst; Esri: Redlands, CA, USA, 2001; Volume 380. [Google Scholar]
- Reza, S.K.; Dutta, D.; Bandyopadhyay, S.; Singh, S.K. Spatial Variability Analysis of Soil Properties of Tinsukia District, Assam, India. Agric. Res. 2019, 8, 231–238. [Google Scholar] [CrossRef]
- Selmy, S.; Abd El-Aziz, S.; Gameh, M.; Abdelsalam, A. Characterization and mapping spatial variability of Entisols derived from shale in Dakhla Oasis, Egypt. Arab. J. Geosci. 2020, 13, 592. [Google Scholar] [CrossRef]
- Guan, F.; Tang, X.; Fan, S.; Zhao, J.; Peng, C. Changes in soil carbon and nitrogen stocks followed the conversion from secondary forest to Chinese fir and Moso bamboo plantations. CATENA 2015, 133, 455–460. [Google Scholar] [CrossRef]
- Denton, O.A.; Aduramigba-Modupe, V.O.; Ojo, A.O.; Adeoyolanu, O.D.; Are, K.S.; Adelana, A.O.; Oyedele, A.O.; Adetayo, A.O.; Oke, A.O. Assessment of spatial variability and mapping of soil properties for sustainable agricultural production using geographic information system techniques (GIS). Cogent Food Agric. 2017, 3, 1279366. [Google Scholar] [CrossRef]
- Zhang, B.; Niu, L.; Jia, T.; Yu, X.; She, D. Spatial variability of soil organic matter and total nitrogen and the influencing factors in Huzhu County of Qinghai Province, China. Acta Agric. Scand. B Soil Plant Sci. 2022, 72, 576–588. [Google Scholar] [CrossRef]
- Gajda, A.; Czyż, E.; Furtak, K.; Jończyk, K. Effects of crop production practices on soil characteristics and metabolic diversity of microbial communities under winter wheat. Soil Res. 2019, 57, 124–131. [Google Scholar] [CrossRef]
- Ukalska-Jaruga, A.; Klimkowicz-Pawlas, A.; Smreczak, B. Characterization of organic matter fractions in the top layer of soils under different land uses in Central-Eastern Europe. Soil Use Manag. 2019, 35, 595–606. [Google Scholar] [CrossRef]




| Property | Depth (cm) | Mean | Std. D | CV% |
|---|---|---|---|---|
| P (mg kg−1) | 0–10 | 2.25 | 0.26 | 11.2 |
| 10–20 | 2.04 | 0.24 | 11.8 | |
| 20–30 | 2.04 | 0.28 | 14.2 | |
| 30–40 | 2.11 | 0.30 | 13.8 | |
| pH | 0–10 | 8.15 | 0.03 | 0.4 |
| 10–20 | 8.14 | 0.05 | 0.6 | |
| 20–30 | 8.14 | 0.05 | 0.6 | |
| 30–40 | 8.17 | 0.06 | 0.8 | |
| EC (dS m−1) | 0–10 | 2.47 | 0.68 | 27.8 |
| 10–20 | 5.30 | 0.90 | 16.9 | |
| 20–30 | 6.76 | 0.91 | 13.5 | |
| 30–40 | 7.62 | 0.99 | 13.0 | |
| Ca2CO3 (mg kg−1) | 0–10 | 101.9 | 7.92 | 7.8 |
| 10–20 | 101.9 | 2.61 | 2.6 | |
| 20–30 | 101.7 | 6.51 | 6.4 | |
| 30–40 | 101.7 | 3.59 | 3.5 | |
| OM (mg kg−1) | 0–10 | 0.45 | 0.15 | 32.2 |
| 10–20 | 0.15 | 0.03 | 21.2 | |
| 20–30 | 0.15 | 0.03 | 18.7 | |
| 30–40 | 0.15 | 0.03 | 18.7 | |
| CEC (cmol(+)kg−1) | 0–10 | 3.68 | 0.43 | 11.7 |
| 10–20 | 3.51 | 0.48 | 13.7 | |
| 20–30 | 3.28 | 0.27 | 8.1 | |
| 30–40 | 2.74 | 0.50 | 18.1 |
| Property | Depth (cm) | Mean | Std. D | CV% |
|---|---|---|---|---|
| P (mg kg−1) | 0–10 | 38.52 | 5.72 | 14.9 |
| 10–20 | 20.21 | 4.16 | 20.6 | |
| 20–30 | 19.60 | 4.39 | 22.4 | |
| 30–40 | 14.90 | 1.80 | 12.1 | |
| pH | 0–10 | 7.95 | 0.14 | 1.8 |
| 10–20 | 7.95 | 0.13 | 1.7 | |
| 20–30 | 8.13 | 0.18 | 2.3 | |
| 30–40 | 8.17 | 0.21 | 2.6 | |
| EC (dS m−1) | 0–10 | 2.25 | 0.49 | 22.0 |
| 10–20 | 2.31 | 0.60 | 26.0 | |
| 20–30 | 2.28 | 0.60 | 26.4 | |
| 30–40 | 2.15 | 0.56 | 25.9 | |
| Ca2CO3 (mg kg−1) | 0–10 | 80.5 | 3.43 | 4.3 |
| 10–20 | 84.3 | 2.69 | 3.2 | |
| 20–30 | 85.3 | 2.30 | 2.7 | |
| 30–40 | 91.4 | 1.83 | 2.0 | |
| OM (mg kg−1) | 0–10 | 2.13 | 0.43 | 20.0 |
| 10–20 | 1.01 | 0.07 | 6.7 | |
| 20–30 | 0.83 | 0.05 | 5.7 | |
| 30–40 | 0.36 | 0.04 | 11.5 | |
| CEC (cmol(+) kg−1) | 0–10 | 4.80 | 0.05 | 1.0 |
| 10–20 | 4.38 | 0.02 | 0.5 | |
| 20–30 | 3.42 | 0.09 | 2.5 | |
| 30–40 | 2.81 | 0.52 | 18.6 |
| Property | Depth (cm) | Soil | Best-Fitted Model | Prediction Errors | ||||
|---|---|---|---|---|---|---|---|---|
| ME | RMSE | ASE | MSE | RMSSE | ||||
| P | 0–10 | V | Gaussian | 0.00 | 0.13 | 0.11 | 0.07 | 1.09 |
| C | Rational Quadratic * | 0.03 | 2.33 | 2.33 | 0.04 | 1.06 | ||
| 10–20 | V | Gaussian | 0.01 | 0.10 | 0.10 | 0.09 | 1.01 | |
| C | Gaussian | 0.01 | 3.14 | 3.36 | 0.01 | 1.00 | ||
| 20–30 | V | Gaussian * | 0.01 | 0.18 | 0.19 | 0.00 | 1.02 | |
| C | J-Bessel * | 0.07 | 4.23 | 4.22 | 0.02 | 1.00 | ||
| 30–40 | V | K-Bessel | 0.00 | 0.16 | 0.16 | 0.02 | 1.06 | |
| C | J-Bessel | 0.10 | 1.35 | 1.30 | 0.05 | 1.01 | ||
| pH | 0–10 | V | Gaussian * | 0.00 | 0.03 | 0.03 | 0.00 | 1.00 |
| C | Spherical | 0.00 | 0.10 | 0.09 | 0.00 | 1.06 | ||
| 10–20 | V | Gaussian | 0.00 | 0.03 | 0.03 | −0.07 | 0.93 | |
| C | Gaussian | 0.00 | 0.09 | 0.09 | 0.00 | 1.01 | ||
| 20–30 | V | Rational Quadratic | 0.00 | 0.06 | 0.05 | 0.00 | 1.10 | |
| C | Gaussian * | 0.00 | 0.18 | 0.19 | 0.02 | 0.97 | ||
| 30–40 | V | Gaussian | 0.00 | 0.07 | 0.06 | 0.00 | 1.08 | |
| C | Rational Quadratic * | 0.00 | 0.16 | 0.14 | 0.01 | 1.13 | ||
| EC | 0–10 | V | Gaussian | 0.04 | 0.24 | 0.60 | 0.10 | 0.47 |
| C | Hole Effect | 0.02 | 0.28 | 0.30 | 0.07 | 0.86 | ||
| 10–20 | V | Rational Quadratic | 0.01 | 0.62 | 0.82 | 0.01 | 0.72 | |
| C | Rational Quadratic | 0.02 | 0.45 | 0.44 | 0.02 | 1.00 | ||
| 20–30 | V | Rational Quadratic | 0.00 | 0.59 | 0.69 | 0.00 | 0.81 | |
| C | Hole Effect | 0.04 | 0.37 | 0.42 | 0.06 | 0.85 | ||
| 30–40 | V | Rational Quadratic | −0.01 | 0.77 | 0.73 | −0.01 | 1.00 | |
| C | Rational Quadratic | 0.02 | 0.43 | 0.43 | 0.03 | 0.97 | ||
| CaCO3 | 0–10 | V | Hole Effect | −0.13 | 5.06 | 5.87 | −0.02 | 0.84 |
| C | Rational Quadratic | −0.12 | 1.14 | 2.20 | −0.04 | 0.55 | ||
| 10–20 | V | Hole Effect | −0.04 | 2.16 | 2.00 | −0.01 | 1.00 | |
| C | Gaussian | −0.04 | 1.48 | 1.59 | −0.02 | 1.04 | ||
| 20–30 | V | Rational Quadratic | −0.04 | 2.60 | 4.14 | −0.01 | 0.63 | |
| C | Rational Quadratic | 0.00 | 0.58 | 0.62 | −0.01 | 1.04 | ||
| 30–40 | V | Gaussian | −0.06 | 3.11 | 2.65 | −0.02 | 1.09 | |
| C | Pentaspherical | 0.05 | 1.65 | 1.65 | 0.03 | 1.02 | ||
| OM | 0–10 | V | Hole Effect | −0.01 | 0.14 | 0.14 | −0.04 | 1.00 |
| C | Hole Effect | 0.01 | 0.40 | 0.39 | 0.03 | 1.02 | ||
| 10–20 | V | Hole Effect | 0.00 | 0.04 | 0.04 | 0.05 | 1.01 | |
| C | J-Bessel | 0.00 | 0.07 | 0.07 | 0.00 | 1.07 | ||
| 20–30 | V | Rational Quadratic | −0.01 | 0.10 | 0.10 | −0.04 | 1.01 | |
| C | Gaussian | 0.00 | 0.05 | 0.05 | 0.02 | 0.97 | ||
| 30–40 | V | Stable | 0.00 | 0.01 | 0.01 | 0.01 | 1.00 | |
| C | Hole Effect | 0.00 | 0.08 | 0.07 | 0.03 | 1.03 | ||
| CEC | 0–10 | V | Rational Quadratic | −0.01 | 0.27 | 0.27 | −0.01 | 1.00 |
| C | Stable | 0.00 | 0.02 | 0.02 | −0.02 | 1.02 | ||
| 10–20 | V | Hole Effect | −0.01 | 0.29 | 0.29 | −0.01 | 1.02 | |
| C | J-Bessel | 0.00 | 0.01 | 0.01 | 0.01 | 1.04 | ||
| 20–30 | V | J-Bessel | 0.01 | 0.09 | 0.10 | 0.04 | 1.01 | |
| C | Gaussian | 0.00 | 0.07 | 0.08 | −0.01 | 0.92 | ||
| 30–40 | V | Gaussian | 0.00 | 0.18 | 0.17 | 0.00 | 1.02 | |
| C | Gaussian | 0.00 | 0.20 | 0.23 | −0.01 | 1.01 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selmy, S.A.H.; Abd Al-Aziz, S.H.; Ibrahim, A.G.; Jiménez-Ballesta, R. Impact of Short-Term Cultivation on Some Selected Properties of Sandy Soil in an Arid Environment. Soil Syst. 2022, 6, 82. https://doi.org/10.3390/soilsystems6040082
Selmy SAH, Abd Al-Aziz SH, Ibrahim AG, Jiménez-Ballesta R. Impact of Short-Term Cultivation on Some Selected Properties of Sandy Soil in an Arid Environment. Soil Systems. 2022; 6(4):82. https://doi.org/10.3390/soilsystems6040082
Chicago/Turabian StyleSelmy, Salman A. H., Salah H. Abd Al-Aziz, Ahmed G. Ibrahim, and Raimundo Jiménez-Ballesta. 2022. "Impact of Short-Term Cultivation on Some Selected Properties of Sandy Soil in an Arid Environment" Soil Systems 6, no. 4: 82. https://doi.org/10.3390/soilsystems6040082
APA StyleSelmy, S. A. H., Abd Al-Aziz, S. H., Ibrahim, A. G., & Jiménez-Ballesta, R. (2022). Impact of Short-Term Cultivation on Some Selected Properties of Sandy Soil in an Arid Environment. Soil Systems, 6(4), 82. https://doi.org/10.3390/soilsystems6040082








