Biological Activities in Artificially Heavy-Metal-Contaminated Growing Substrates
Abstract
:1. Introduction
2. Materials and Methods
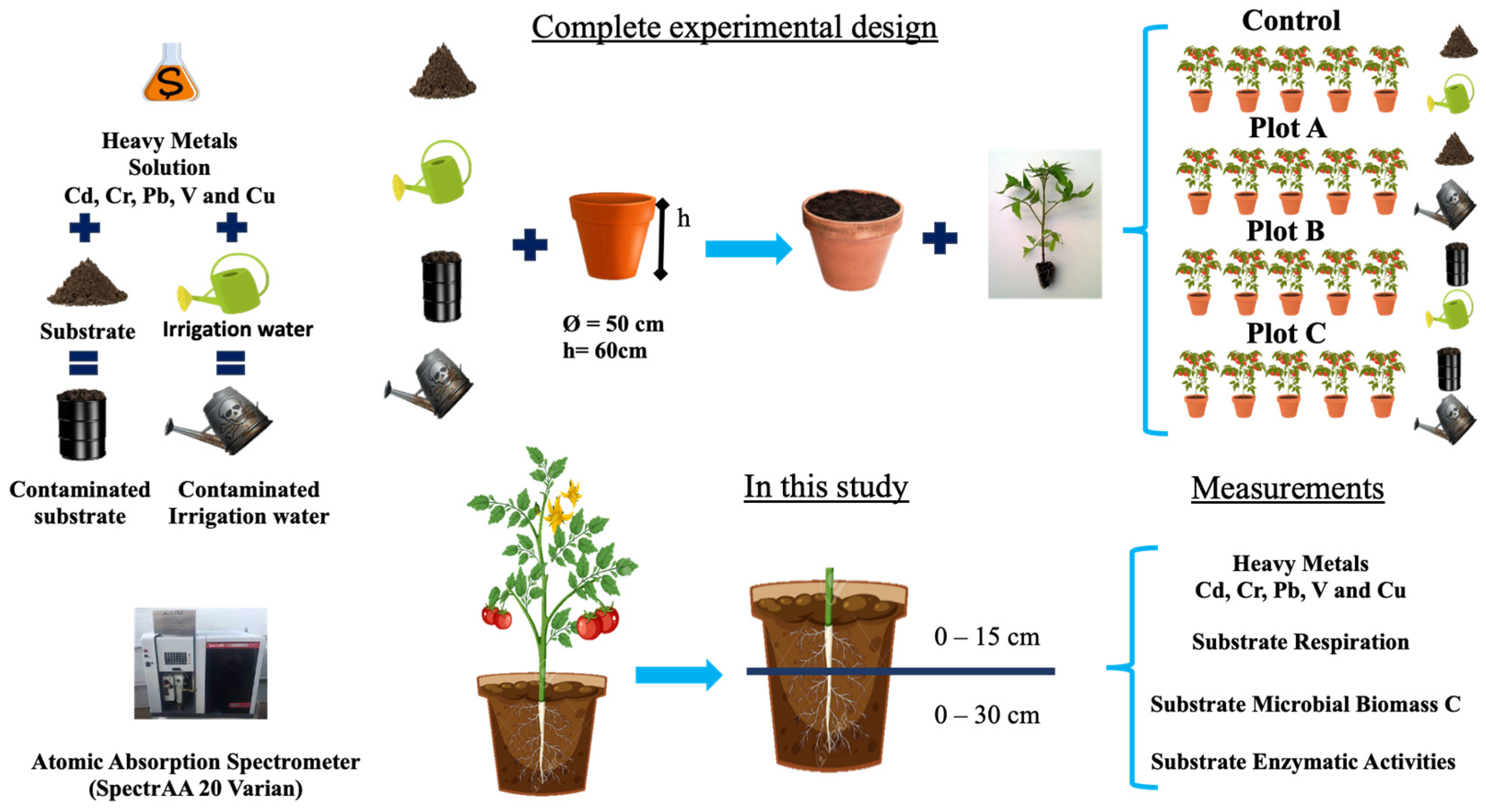
2.1. Experimental Design and Substrate Processing
2.2. Substrate Heavy Metal Assays
2.3. Substrate Biological Properties
2.4. Statistics
3. Results
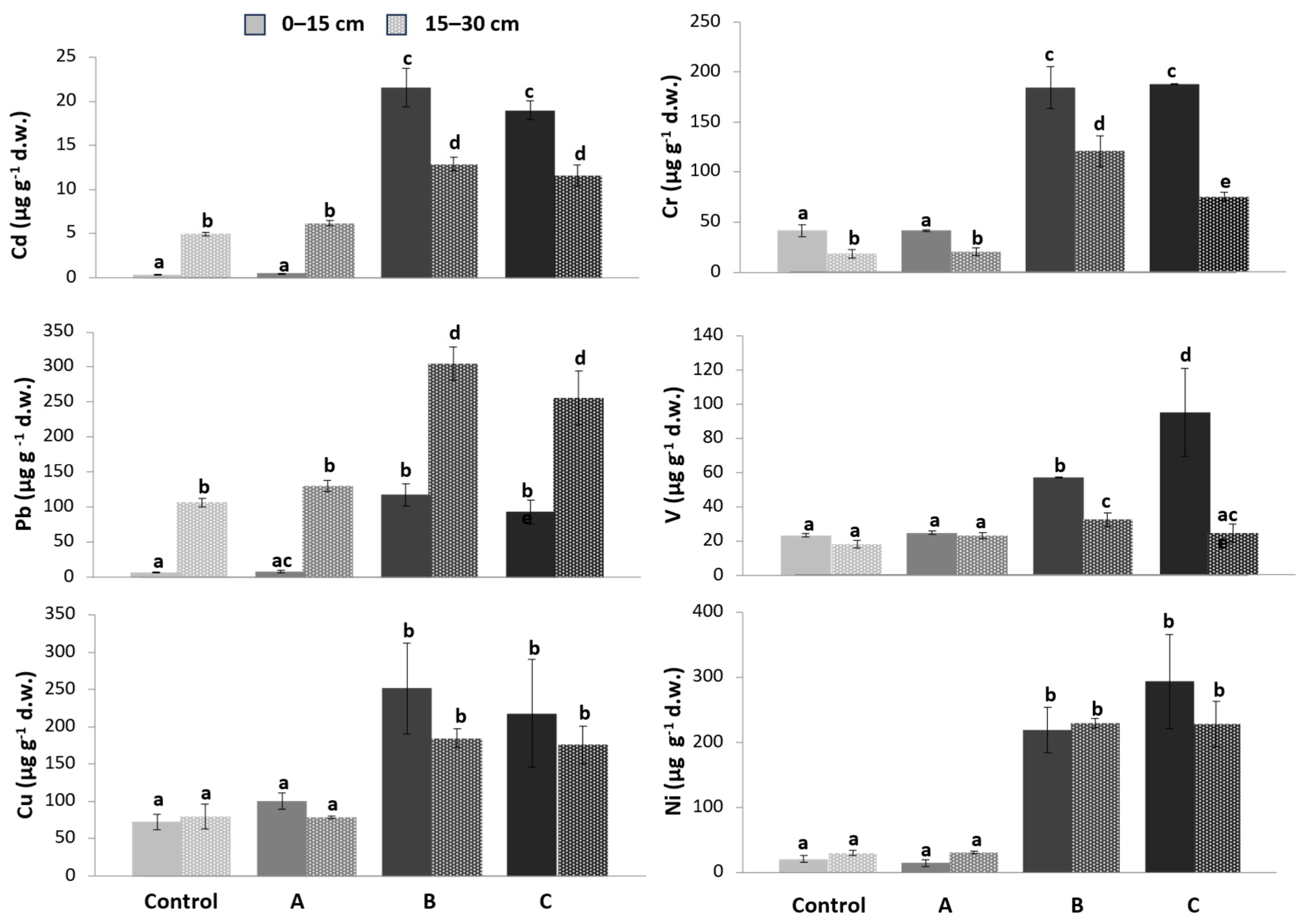
3.1. Heavy Metal Content
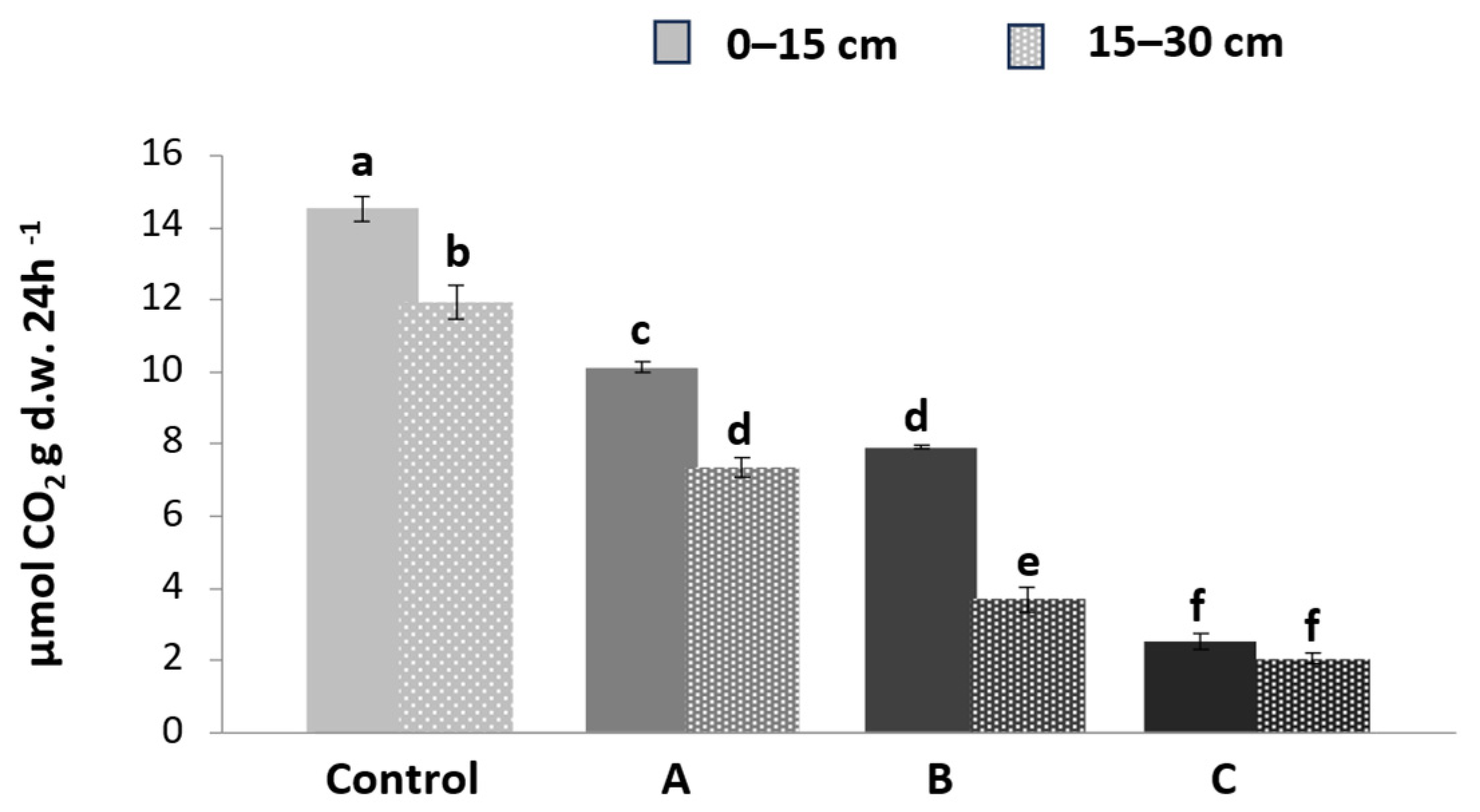
3.2. Soil Respiration
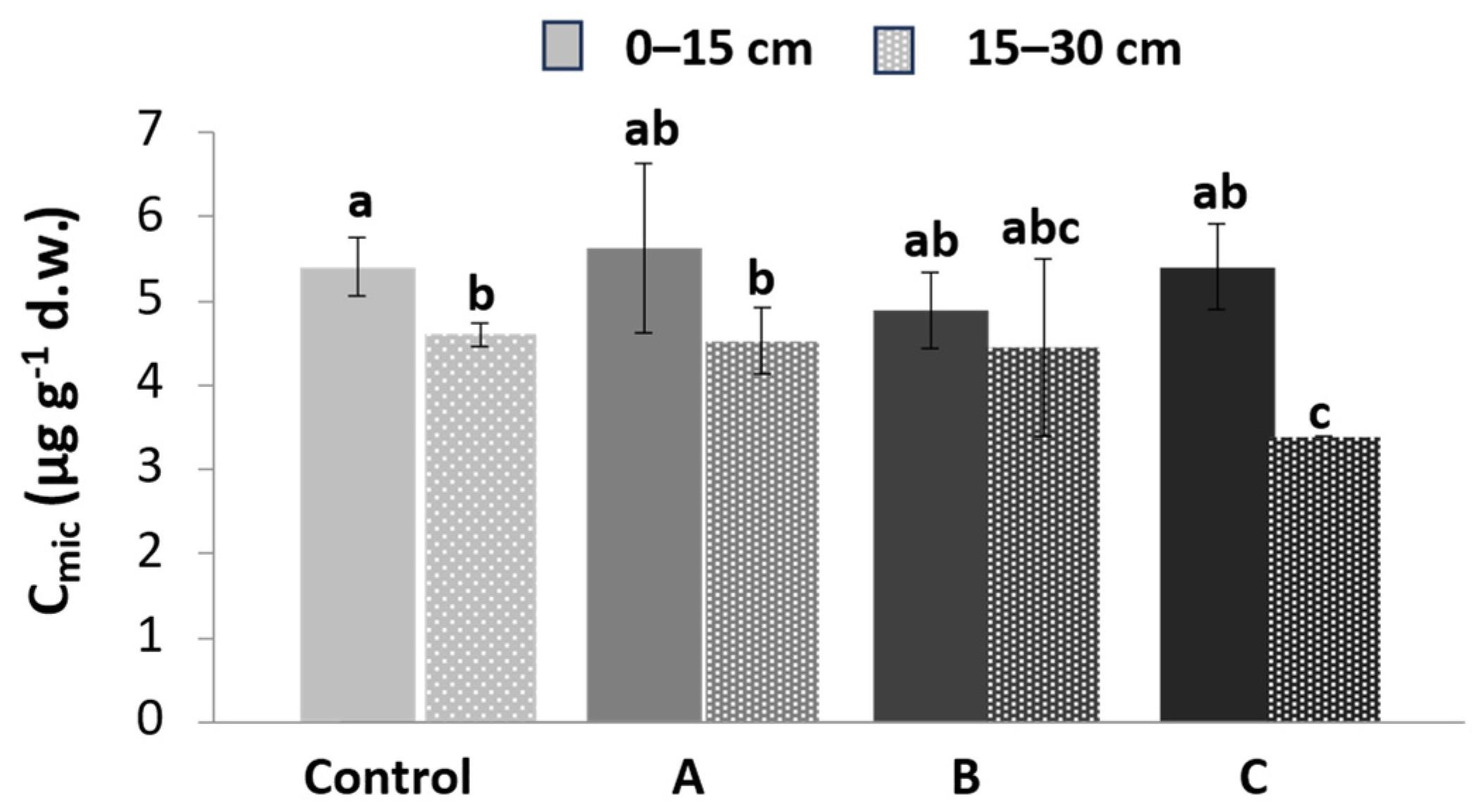
3.3. Microbial Biomass C (Cmic)
3.4. qCO2 Index
3.5. Enzyme Activities
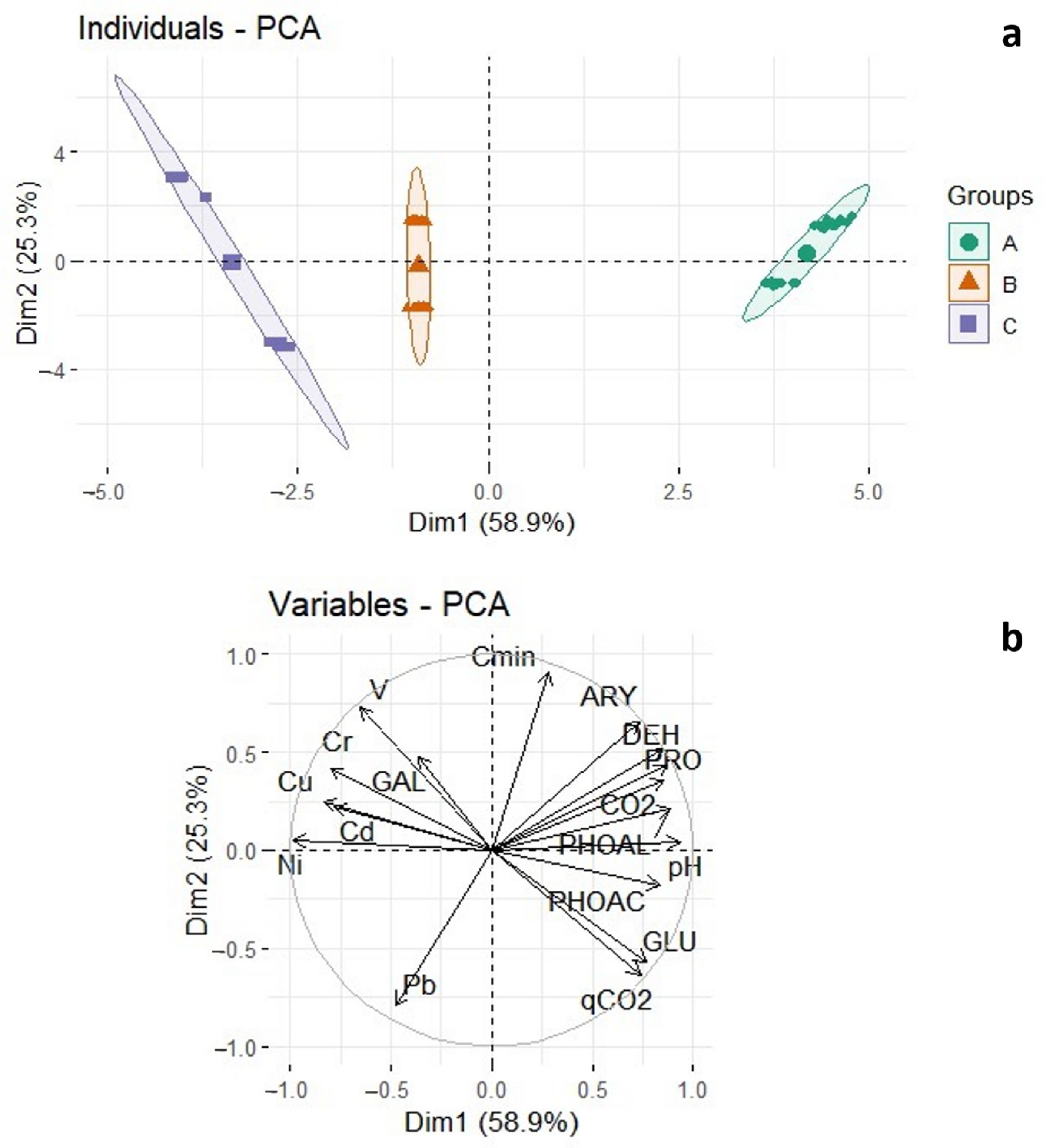
3.6. Correlation between HMs and Biological Parameters, and PCA Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fussy, A.; Papenbrock, J. An Overview of Soil and Soilless Cultivation Techniques-Chances, Challenges and the Neglected Question of Sustainability. Plants 2022, 11, 1153. [Google Scholar] [CrossRef]
- Alnaimy, M.A.; Shahin, S.A.; Vranayova, Z.; Zelenakova, M.; Abdel-Hamed, E.M.W. Long-Term Impact of Wastewater Irrigation on Soil Pollution and Degradation: A Case Study from Egypt. Water 2021, 13, 2245. [Google Scholar] [CrossRef]
- Kumar, S.S.; Kumar, A.; Singh, S.; Malyan, S.K.; Baram, S.; Sharma, J.; Singh, R.; Pugazhendhi, A. Industrial wastes: Fly ash, steel slag, and phosphogypsum-potential candidates to mitigate greenhouse gas emissions from paddy fields. Chemosphere 2020, 241, 124824. [Google Scholar] [CrossRef] [PubMed]
- Raven, P.H.; Hassenzahl, D.M.; Hager, M.C.; Gift, N.Y.; Berg, L.R. Environment, 9th ed.; Wiley: Hoboken, NJ, USA, 2019; ISBN 978-1-119-62472-1. [Google Scholar]
- Marshall, M.; Pineda, M.; Yargeau, V. Sensitivity of the LuminoTox tool to monitor contaminants of emerging concern in municipal secondary wastewater effluent. Sci. Total Environ. 2017, 598, 1065–1075. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, M.; Surendran, U.; Raja, P.; Kumar, A.; Senapathi, V. A review of heavy metals accumulation pathways, sources and management in soils. Arab. J. Geosci. 2021, 14, 2156. [Google Scholar] [CrossRef]
- Murtić, S.; Zahirović, Ć.; Čivic, H.; Karić, L.; Furković, J. Uptake of heavy metals by tomato plants (Lycopersicum esculentum Mill.) and their distribution inside the plant. Agric. For. 2018, 64, 251–261. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Rodríguez-Eugenio, N.; McLaughlin, M.; Pennock, D. Soil Pollution: A Hidden Reality; FAO: Rome, Italy, 2018; 142p. [Google Scholar]
- Liu, Z.; Zhuang, J.; Zheng, K.; Luo, C. Differential response of the soil nutrients, soil bacterial community structure and metabolic functions to different risk areas in Lead-Zine tailings. Front. Microbiol. 2023, 14, 1131770. [Google Scholar] [CrossRef]
- Barra Caracciolo, A.; Terenzi, V. Rhizosphere Microbial Communities and Heavy Metals. Microorganisms 2021, 8, 1462. [Google Scholar] [CrossRef]
- Rashid, A.; Schutte, B.J.; Ulery, A.; Deyholos, M.K.; Sanogo, S.; Lehnhoff, E.A.; Beck, L. Heavy Metal Contamination in Agricultural Soil: Environmental Pollutants Affecting Crop Health. Agronomy 2023, 13, 1521. [Google Scholar] [CrossRef]
- Jiang, Y.; Chao, S.; Liu, J.; Yang, Y.; Chen, Y.; Zhang, A.; Cao, H. Source apportionment and health risk assessment of heavy metals in soil for a township in Jiangsu Province, China. Chemosphere 2017, 168, 1658–1668. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Meng, D.; Li, J.; Yin, H.; Liu, H.; Liu, X.; Cheng, C.; Xiao, Y.; Liu, Z.; Yan, M. Response of soil microbial communities and microbial interactions to long-term heavy metal contamination. Environ. Pollut. 2017, 231, 908–917. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Liu, C.; Li, B.; Dong, Y. Trifolium repens L. regulated phytoremediation of heavy metal contaminated soil by promoting soil enzyme activities and beneficial rhizosphere associated microorganisms. J. Hazard. Mater. 2021, 402, 123829. [Google Scholar] [CrossRef] [PubMed]
- Yeboah, J.O.; Shi, G.; Shi, W. Effect of heavy metal contamination on soil enzymes activities. J. Geosci. Environ. Prot. 2021, 9, 135–154. [Google Scholar] [CrossRef]
- Changchao, L.; Quan, Q.; Gan, Y.; Dong, J.; Fang, J.; Wang, L.; Liu, J. Effects of heavy metals on microbial communities in sediments and establishment of bioindicators based on microbial taxa and function for environmental monitoring and management. Sci. Total Environ. 2020, 749, 141555. [Google Scholar] [CrossRef]
- Jarosławiecka, A.K.; Piotrowska-Seget, Z. The Effect of Heavy Metals on Microbial Communities in Industrial Soil in the Area of Piekary Śląskie and Bukowno (Poland). Microbiol. Res. 2022, 13, 626–642. [Google Scholar] [CrossRef]
- Zhang, J.; Jin, K.; Luo, Y.; Du, L.; Tian, R.; Wang, S.; Shen, Y.; Zhang, J.; Li, N.; Shao, W.; et al. Responses of Soil Enzyme Activity to Long-Term Nitrogen Enrichment and Water Addition in a Typical Steppe. Agronomy 2023, 13, 1920. [Google Scholar] [CrossRef]
- Serrani, D.; Ajmone-Marsan, F.; Corti, G.; Cocco, S.; Cardelli, V.; Adamo, P. Heavy metal load and effects on biochemical properties in urban soils of a medium-sized city, Ancona, Italy. Environ. Geochem. Health 2022, 44, 3425–3449. [Google Scholar] [CrossRef]
- Goutam Mukherjee, A.; Ramesh Wanjari, U.; Eladl, M.A.; El-Sherbiny, M.; Elsherbini, D.M.A.; Sukumar, A.; Kannampuzha, S.; Ravichandran, M.; Renu, K.; Vellingiri, B.; et al. Mixed contaminants: Occurrence, interactions, toxicity, detection, and remediation. Molecules 2022, 27, 2577. [Google Scholar] [CrossRef]
- Çakmakçı, R.; Salık, M.A.; Çakmakçı, S. Assessment and Principles of Environmentally Sustainable Food and Agriculture Systems. Agriculture 2023, 13, 1073. [Google Scholar] [CrossRef]
- Abreu, C.A.D.; van Raij, B.; Abreu, M.F.D.; González, A.P. Routine soil testing to monitor heavy metals and boron. Sci. Agric. 2005, 62, 564–571. [Google Scholar] [CrossRef]
- Papa, S.; Bartoli, G.; Alvarez Romero, M.; Mottola, S.; Fioretto, A. Trace metals accumulation in Fragaria ananassa and its possible use as a bioaccumulator. Fresenius Environ. Bull. 2017, 26, 475–482. [Google Scholar]
- Papa, S.; Curcio, E.; Lombardi, A.; D’Oriano, P.; Fioretto, A. Soil microbial activity in three evergreen oak (Quercus ilex) woods in a Mediterranean area. In Developments in Soil Science; Violante, A., Huang, P.M., Bollag, J.M., Gianfreda, L., Eds.; Elsevier Science B.V.: Amsterdam, The Netherland; Boston, MA, USA, 2002; Volume 28B, pp. 229–237. ISSN 0166-2481. [Google Scholar]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Anderson, T.H.; Domsch, K.H. The metabolic quotient for CO2 (qCO2) a specific activity parameter to assess the effects of environmental conditions, such as pH, on the microbial biomass of forest soils. Soil Biol. Biochem. 1993, 25, 393–395. [Google Scholar] [CrossRef]
- Eivazi, F.; Tabatabai, M.A. Glucosidases and galactosidases in soils. Soil Biol. Biochem. 1988, 20, 601–606. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Ladd, J.N.; Bulter, J.H.A. Short-term assays of soil proteolytic enzyme activities using proteins and dipeptide derivatives as substrates. Soil Biol. Biochem. 1972, 4, 19–30. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Arylsulfatase activity of soils. Soil Sci. Soc. Am. J. 1970, 34, 225–229. [Google Scholar] [CrossRef]
- Von Mersi, W.; Schinner, F. An improved and accurate method for determining the dehydrogenase activity of soils with iodonitrotetrazolium chloride. Biol. Fertil. Soils 1991, 11, 216–220. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- RStudio Team. R Studio: Integrated Development for R; PBC: Boston, MA, USA, 2020; Available online: http://www.rstudio.com/ (accessed on 6 March 2023).
- Fox, J.; Carvalho, M.S. The RcmdrPlugin. Survival Package: Extending the R Commander Interface to Survival Analysis. J. Stat. Softw. 2012, 49, 1–32. [Google Scholar] [CrossRef]
- Ashraf, M.N.; Waqas, M.A.; Rahman, S. Microbial metabolic quotient is a dynamic indicator of soil health: Trends, implications, and perspectives. Eurasian Soil Sci. 2022, 55, 1794–1803. [Google Scholar] [CrossRef]
- Ananyeva, N.D.; Ivashchenko, K.V.; Sushko, S.V. Microbial indicators of urban soils and their role in the assessment of ecosystem services: A review. Eurasian Soil Sci. 2021, 54, 1517–1531. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; Li, Y.; Li, L.; Tang, M.; Hu, W.; Chen, L.; Ai, S. Speciation of heavy metals in soils and their immobilization at micro-scale interfaces among diverse soil components. Sci. Total Environ. 2022, 825, 153862. [Google Scholar] [CrossRef]
- Sintorini, M.M.; Widyatmoko, H.; Sinaga, E.; Aliyah, N. Effect of pH on metal mobility in the soil. IOP Conf. Ser. Earth Environ. Sci. 2021, 737, 012071. [Google Scholar] [CrossRef]
- García, I.; Dorronsoro, C. Contaminación por Metales Pesados. En Tecnología de Suelos. Universidad de Granada. Departamento de Edafología y Química Agrícola. 2005. Available online: http://edafologia.ugr.es (accessed on 14 June 2023).
- Wang, Q.; Wang, J.; Cheng, J.; Zhu, Y.; Geng, J.; Wang, X.; Feng, X.; Hou, H. A New Method for Ecological Risk Assessment of Combined Contaminated Soil. Toxics 2023, 11, 411. [Google Scholar] [CrossRef] [PubMed]
- Minnikova, T.; Kolesnikov, S.; Khoroshaev, D.; Tsepina, N.; Evstegneeva, N.; Timoshenko, A. Assessment of the Health of Soils Contaminated with Ag, Bi, Tl, and Te by the Intensity of Microbiological Activity. Life 2023, 13, 1592. [Google Scholar] [CrossRef]
- Aponte, H.; Meli, P.; Butler, B.; Paolini, J.; Matus, F.; Merino, C.; Cornejo, P.; Kuzyakov, Y. Meta-analysis of heavy metal effects on soil enzyme activities. Sci. Total Environ. 2020, 737, 139744. [Google Scholar] [CrossRef]
- Nannipieri, P.; Greco, S.; Ceccanti, B. Ecological significance of the biological activity in soil. Soil Biochem. 2017, 6, 293–356. [Google Scholar]
- Gao, Y.; Zhou, P.; Mao, L.; Zhi, Y.E.; Shi, W.J. Assessment of effects of heavy metals combined pollution on soil enzyme activities and microbial community structure: Modified ecological dose–response model and PCR-RAPD. Environ. Earth Sci. 2010, 60, 603–612. [Google Scholar] [CrossRef]
- Ilakiya, T.; Swarnapriya, R.; Pugalendhi, L.; Geethalakshmi, V.; Lakshmanan, A.; Kumar, M.; Lorenzo, J.M. Carbon Accumulation, Soil Microbial and Enzyme Activities in Elephant Foot Yam-Based Intercropping System. Agriculture 2023, 13, 187. [Google Scholar] [CrossRef]
- Malik, K.M.; Khan, K.S.; Billah, M.; Akhtar, M.S.; Rukh, S.; Alam, S.; Munir, A.; Mahmood Aulakh, A.; Rahim, M.; Qaisrani, M.M.; et al. Organic Amendments and Elemental Sulfur Stimulate Microbial Biomass and Sulfur Oxidation in Alkaline Subtropical Soils. Agronomy 2021, 11, 2514. [Google Scholar] [CrossRef]
- Lucheta, A.R.; Lambais, M.R. Sulfur in agriculture. Rev. Bras. De Ciência Do Solo 2012, 36, 1369–1379. [Google Scholar] [CrossRef]
- Xu, Y.; Seshadri, B.; Bolan, N.; Sarkar, B.; Sik Ok, Y.; Zhang, W.; Rumpel, C.; Sparks, D.; Farrell, M.; Hall, T.; et al. Microbial functional diversity and carbon use feedback in soils as affected by heavy metals. Environ. Int. 2019, 125, 478–488. [Google Scholar] [CrossRef]
- Zhao, H.; Yu, L.; Yu, M.; Afzal, M.; Dai, Z.; Brookes, P.; Xu, J. Nitrogen combined with biochar changed the feedback mechanism between soil nitrification and Cd availability in an acidic soil. J. Hazard. Mater. 2020, 390, 121631. [Google Scholar] [CrossRef]
- Francisca, F.M.; Glatstein, D.A. Environmental application of basic oxygen furnace slag for the removal of heavy metals from leachates. J. Hazard. Mater. 2020, 384, 121294. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Zhang, L.; Zhang, J.; Ren, L.; Zhou, Y.; Zheng, Y.; Luo, L.; Yang, Y.; Huang, H.; Chen, A. Physicochemical features, metal availability and enzyme activity in heavy metal-polluted soil remediated by biochar and compost. Sci. Total Environ. 2020, 701, 134751. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.K.; Kim, J.W.; Lee, S.P.; Yang, J.E.; Kim, S.C. Heavy metal remediation in soil with chemical amendments and its impact on activity of antioxidant enzymes in Lettuce (Lactuca sativa) and soil enzymes. Appl. Biol. Chem. 2020, 63, 42. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, T.; Zhou, L.; Lou, W.; Zeng, W.; Liu, T.; Yin, H.; Liu, H.; Liu, X.; Mathivanan, K.; et al. Soil microbial community assembly model in response to heavy metal pollution. Environ. Res. 2022, 213, 113576. [Google Scholar] [CrossRef]
- Naylo, A.; Almeida Pereira, S.I.; Benidire, L.; El Khalil, H.; Castro, P.M.; Ouvrard, S.; Schwartz, C.; Boularbah, A. Trace and major element contents, microbial communities, and enzymatic activities of urban soils of Marrakech city along an anthropization gradient. J. Soils Sediments 2019, 19, 2153–2165. [Google Scholar] [CrossRef]
- Yang, H.; Wang, F.; Yu, J.; Huang, K.; Zhang, H.; Fu, Z. An improved weighted index for the assessment of heavy metal pollution in soils in Zhejiang, China. Environ. Res. 2021, 192, 110246. [Google Scholar] [CrossRef] [PubMed]


| Parameter | ||
|---|---|---|
| Nutrient content | ||
| Nitrogen | 1% | |
| Phosphorus | 0.4% | |
| Potassium | 0.75% | |
| pH | 7.30 | |
| Electrical conductivity | 75 mS/m | |
| Laboratory compacted bulk density | 0.357 kg/L | |
| Dry matter | 38% | |
| Organic matter on dry matter % | 67% |
| Trace Metal | A mg/kg | B µg/L |
|---|---|---|
| Cd | 15 | 5 |
| Cr | 150 | 50 |
| Ni | 120 | 20 |
| Pb | 100 | 10 |
| Cu | 120 | 1000 |
| V | 90 | 50 |
| S0 | S1 | IW0 | IW1 | |
|---|---|---|---|---|
| V | 22.3 ± 1.33 | 123 ± 7.38 | 10 ± 0.6 | 60 ± 3.6 |
| Ni | 21.3 ± 1.27 | 382 ± 22.9 | 5.7 ± 0.34 | 25 ± 1.5 |
| Cd | 0.49 ± 0.03 | 21.2 ± 1.27 | 0.35 ± 0.021 | 6 ± 0.36 |
| Pb | 8.31 ± 0.49 | 151 ± 9.06 | 0.49 ± 0.029 | 12 ± 0.72 |
| Cu | 76 ± 4.56 | 384 ± 23. | 10 ± 0.6 | 1100 ± 66 |
| Cr | 41 ± 2.46 | 253 ± 15.2 | 22 ± 1.32 | 60 ± 3.6 |
| Cd | Pb | Cu | Ni | V | Cr | CO2 | Cmin | qCO2 | PHOal | PHOac | ARY | GLU | GAL | PRO | DEH | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cd | 1 | |||||||||||||||
| Pb | 0.488 * | 1 | ||||||||||||||
| Cu | 0.948 *** | 0.431 * | 1 | |||||||||||||
| Ni | 0.911 *** | 0.581 * | 0.927 *** | 1 | ||||||||||||
| V | 0.744 *** | −0.049 | 0.732 *** | 0.738 *** | 1 | |||||||||||
| Cr | 0.912 *** | 0.309 | 0.961 *** | 0.909 *** | 0.846 *** | 1 | ||||||||||
| CO2 | −0.705 *** | −0.731 *** | −0.684 *** | −0.836 *** | −0.492 * | −0.607 ** | 1 | |||||||||
| Cmin | −0.237 | −0.815 *** | −0.127 | −0.268 | 0.347 | 0.079 | 0.498 * | 1 | ||||||||
| qCO2 | −0.746 *** | −0.085 | −0.796 *** | −0.799 *** | −0.938 *** | −0.852 *** | 0.667 ** | −0.240 | 1 | |||||||
| PHOal | −0.676 *** | −0.603 ** | −0.714 *** | −0.807 *** | −0.536 * | −0.655 ** | 0.959 *** | 0.318 | 0.741 *** | 1 | ||||||
| PHOac | −0.708 *** | −0.339 | −0.825 *** | −0.816 *** | −0.629 ** | −0.753 *** | 0.795 *** | 0.143 | 0.816 *** | 0.885 *** | 1 | |||||
| ARY | −0.655 *** | −0.737 *** | −0.665 ** | −0.747 *** | −0.337 | −0.534 * | 0.957 *** | 0.561 * | 0.562 * | 0.955 *** | 0.823 *** | 1 | ||||
| GLU | −0.738 *** | −0.134 | −0.799 *** | −0.803 *** | −0.847 *** | −0.814 *** | 0.716 *** | −0.097 | 0.935 *** | 0.815 *** | 0.926 *** | 0.675 *** | 1 | |||
| GAL | −0.637 ** | −0.236 | −0.745 *** | −0.687 *** | −0.604 ** | −0.699 *** | 0.731 *** | 0.017 | 0.798 *** | 0.875 *** | 0.955 *** | 0.796 *** | 0.914 *** | 1 | ||
| PRO | −0.803 *** | −0.741 *** | −0.793 *** | −0.862 *** | −0.459 * | −0.668 *** | 0.956 *** | 0.566 * | 0.633 ** | 0.924 *** | 0.844 *** | 0.969 *** | 0.723 *** | 0.773 *** | 1 | |
| DEH | −0.704 *** | −0.698 *** | −0.756 *** | −0.816 *** | −0.416 | −0.652 ** | 0.934 *** | 0.459 * | 0.629 ** | 0.967 *** | 0.891 *** | 0.976 *** | 0.753 *** | 0.855 *** | 0.961 *** | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papa, S.; Alvarez-Romero, M. Biological Activities in Artificially Heavy-Metal-Contaminated Growing Substrates. Soil Syst. 2023, 7, 111. https://doi.org/10.3390/soilsystems7040111
Papa S, Alvarez-Romero M. Biological Activities in Artificially Heavy-Metal-Contaminated Growing Substrates. Soil Systems. 2023; 7(4):111. https://doi.org/10.3390/soilsystems7040111
Chicago/Turabian StylePapa, Stefania, and Marta Alvarez-Romero. 2023. "Biological Activities in Artificially Heavy-Metal-Contaminated Growing Substrates" Soil Systems 7, no. 4: 111. https://doi.org/10.3390/soilsystems7040111





