Assessing the Role of Melia azedarach Botanical Nematicide in Enhancing the Structure of the Free-Living Nematode Community
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Nematode Extraction and Analysis
2.3. Statistical Analyses
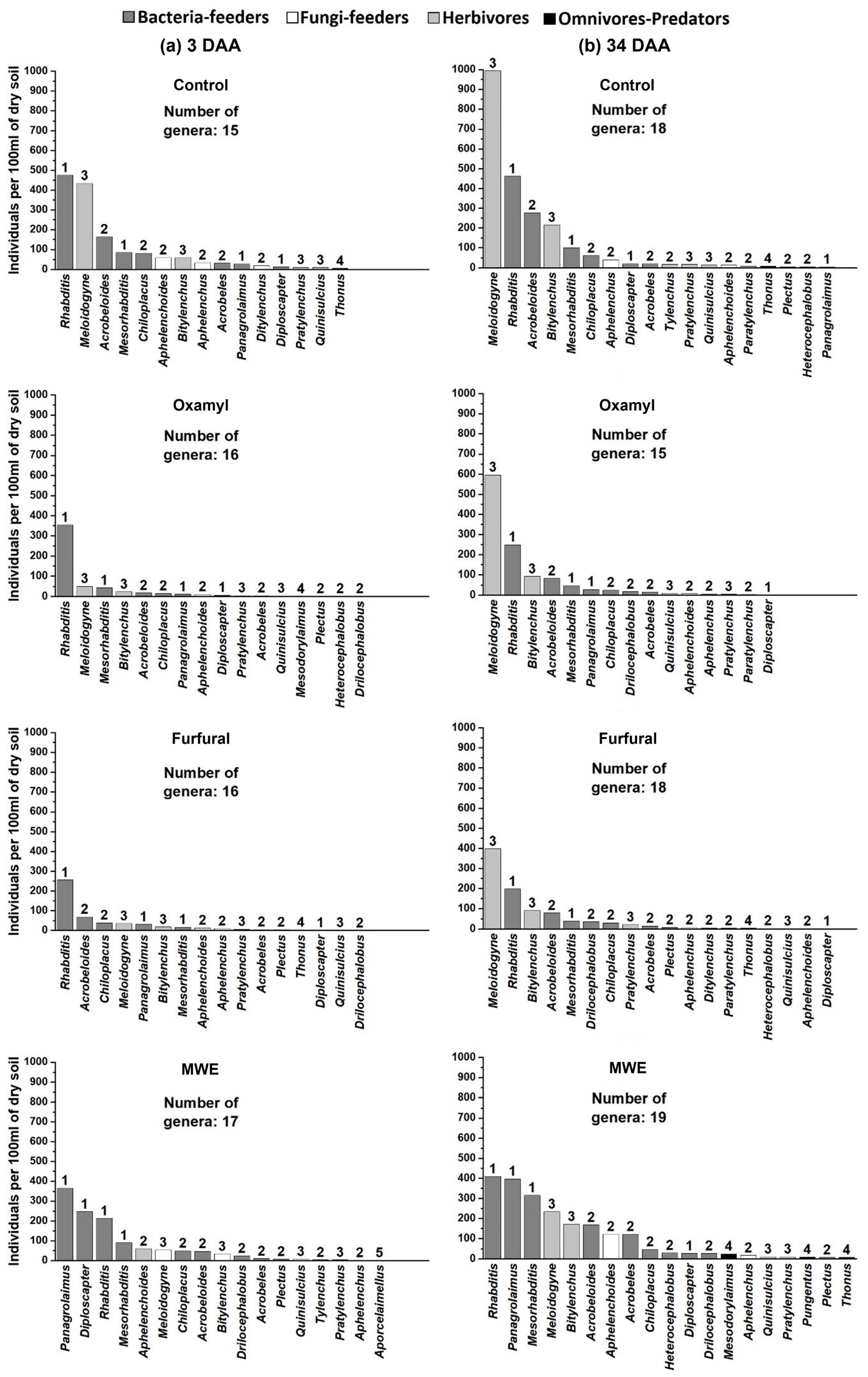
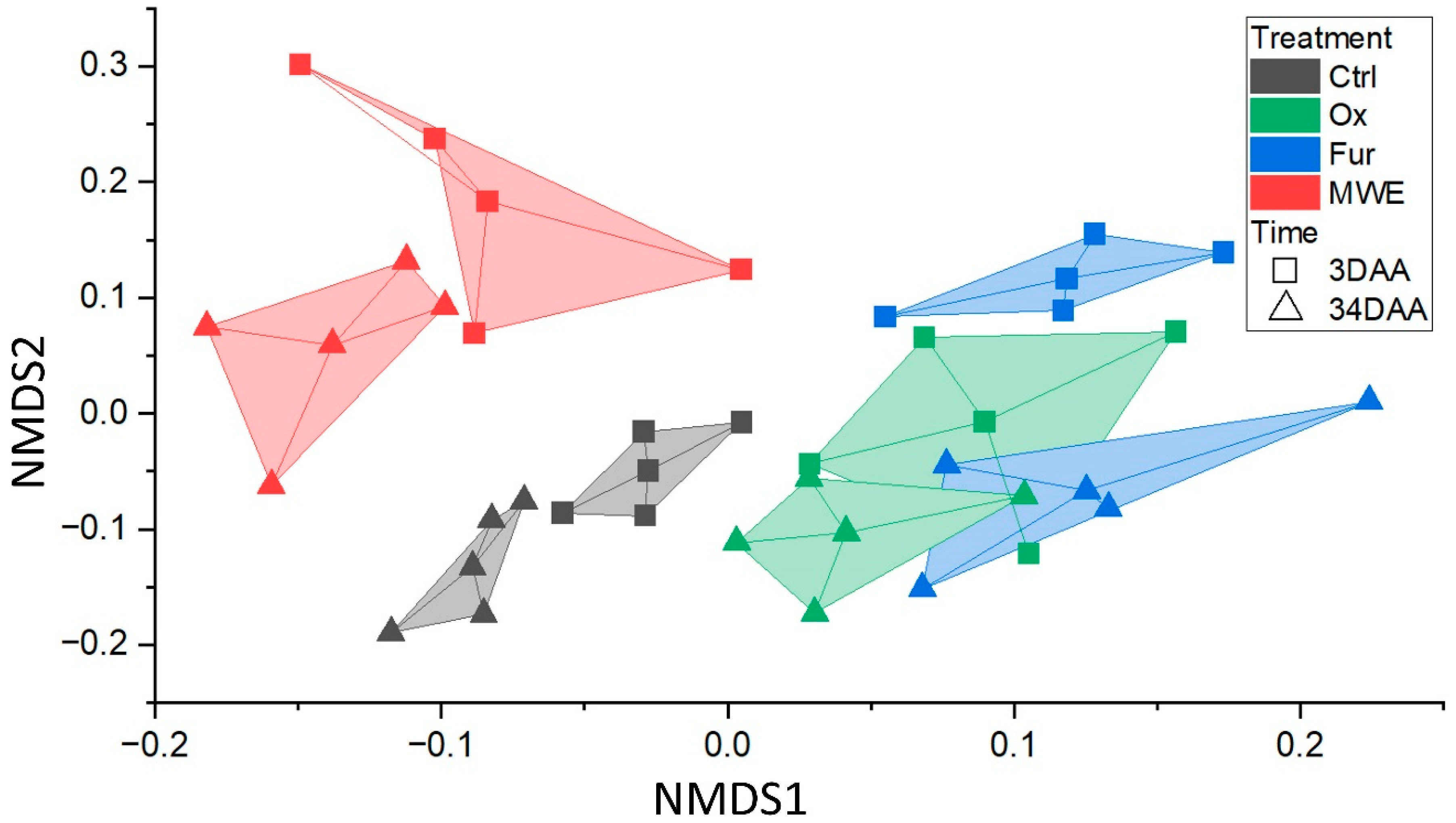
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Topalović, O.; Geisen, S. Nematodes as suppressors and facilitators of plant performance. New Phytol. 2023, 238, 2305–2312. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Khan, M.R.; Walia, R.K. Crop Loss Estimations due to Plant-Parasitic Nematodes in Major Crops in India. Natl. Acad. Sci. Lett. USA 2020, 43, 409–412. [Google Scholar] [CrossRef]
- Jones, J.T.; Haegeman, A.; Danchin, E.G.J.; Gaur, H.S.; Helder, J.; Jones, M.G.K.; Kikuchi, T.; Manzanilla-Lopez, R.; Palomares-Rius, J.E.; Wesemael, W.M.L.; et al. Top 10 Plant-Parasitic Nematodes in Molecular Plant Pathology. Mol. Plant Pathol. 2013, 14, 946–961. [Google Scholar] [CrossRef] [PubMed]
- Sikandar, A.; Zhang, M.; Wang, Y.; Zhu, X.; Liu, X.; Fan, H.; Xuan, Y.; Chen, L.; Duan, Y. Review article: Meloidogyne incognita (Root-knot nematode) a risk to agriculture. Appl. Ecol. Environ. Res. 2020, 18, 1679–1690. [Google Scholar] [CrossRef]
- Curtis, R.H. Plant parasitic nematode proteins and the host–parasite interaction. Brief. Funct. Genom. 2007, 6, 50–58. [Google Scholar] [CrossRef][Green Version]
- Ali, M.A.; Azeem, F.; Li, H.; Bohlmann, H. Smart parasitic nematodes use multifaceted strategies to parasitize plants. Front. Plant Sci. 2017, 8, 1699. [Google Scholar] [CrossRef]
- Ibrahim, H.M.M.; Ahmad, E.M.; Martínez-Medina, A.; Aly, M.A.M. Effective approaches to study the plant-root knot nematode interaction. Plant Physiol. Biochem. 2019, 141, 332–342. [Google Scholar] [CrossRef]
- Khanal, C.; Harshman, D.; Giles, C. On-Farm Evaluations of Nonfumigant Nematicides on Nematode Communities of Peach. Phytopathology 2022, 112, 2218–2223. [Google Scholar] [CrossRef]
- Ntalli, N.G.; Caboni, P. Botanical nematicides: A review. J. Agric. Food Chem. 2012, 60, 9929–9940. [Google Scholar] [CrossRef]
- Kruger, D.; Fourie, J.; Malan, A.P. Cover crops with biofumigation properties for the suppression of plant-parasitic nematodes: A review. S. Afr. J. Enol. Vitic. 2013, 34, 287–295. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Chang, S.X.; Zhang, J.; Jiang, P.; Zhou, G.; Shen, Z. Contrasting effects of bamboo leaf and its biochar on soil CO2 efflux and labile organic carbon in an intensively managed Chinese chestnut plantation. Biol. Fertil. Soils 2014, 50, 1109–1119. [Google Scholar] [CrossRef]
- Renčo, M.; Kováčik, P. Assessment of the nematicidal potential of vermicompost, vermicompost tea, and urea application on the potato-cyst nematodes Globodera rostochiensis and Globodera pallida. J. Plant Prot. Res. 2015, 55, 187–192. [Google Scholar] [CrossRef]
- Ntalli, N.; Caboni, P. A review of isothiocyanates biofumigation activity on plant parasitic nematodes. Phytochem. Rev. 2017, 16, 827–834. [Google Scholar] [CrossRef]
- Kekelis, P.; Papatheodorou, E.M.; Terpsidou, E.; Dimou, M.; Aschonitis, V.; Monokrousos, N. The Free-Living Nematodes as Indicators of the Soil Quality in Relation to the Clay Content, When Coffee Waste Is Applied. Agronomy 2022, 12, 2702. [Google Scholar] [CrossRef]
- Ferris, H.; Bongers, T.; de Goede, R.G.M. A Framework for Soil Food Web Diagnostics: Extension of the Nematode Faunal Analysis Concept. Appl. Soil Ecol. 2001, 18, 13–29. [Google Scholar] [CrossRef]
- Lu, Q.; Liu, T.; Wang, N.; Dou, Z.; Wang, K.; Zuo, Y. A review of soil nematodes as biological indicators for the assessment of soil health. Front. Agr. Sci. Eng. 2020, 7, 275–281. [Google Scholar] [CrossRef]
- Resasco, J.; Bitters, M.E.; Cunningham, S.A.; Jones, H.I.; McKenzie, V.J.; Davies, K.F. Experimental habitat fragmentation disrupts nematode infections in Australian skinks. Ecology 2019, 100, e02547. [Google Scholar] [CrossRef]
- Brussaard, L.; de Ruiter, P.C.; Brown, G.G. Soil biodiversity for agricultural sustainability. Agr. Ecosyst. Environ. 2007, 121, 233–244. [Google Scholar] [CrossRef]
- Grabau, Z.J.; Mauldin, M.D.; Habteweld, A.; Carter, E.T. Nematicide Efficacy at Managing Meloidogyne arenaria and Non-Target Effects on Free-Living Nematodes in Peanut Production. J. Nematol. 2020, 52, e2020-28. [Google Scholar] [CrossRef]
- Ntalli, N.; Monokrousos, N.; Rumbos, C.; Kontea, D.; Zioga, D.; Argyropoulou, M.D.; Menkissoglu-Spiroudi, U.; Tsiropoulos, N.G. Greenhouse biofumigation with Melia azedarach controls Meloidogyne spp. and enhances soil biological activity. J. Pest Sci. 2018, 91, 29–40. [Google Scholar] [CrossRef]
- Panayiotou, E.; Dimou, M.; Monokrousos, N. The effects of grazing intensity on soil processes in a Mediterranean protected area. Environ. Monit. Assess. 2017, 189, 441. [Google Scholar] [CrossRef] [PubMed]
- S’Jacob, J.J.; van Bezooijen, J. A Manual for Practical Work in Nematology; Department of Nematology, Wageningen Agricultural University: Wageningen, The Netherlands, 1984. [Google Scholar]
- Bongers, T. Systematisch gedeelte. In De Nematoden van Nederland: Vormgeving en Technische Realisatie, 2nd ed.; Uitgeverij Pirola: Schoorl, The Netherlands, 1994; pp. 67–383. [Google Scholar]
- Yeates, G.W.; Bongers, T.; De Goede, R.G.; Freckman, D.W.; Georgieva, S. Feeding habits in soil nematode families and genera—An outline for soil ecologists. J. Nematol. 1993, 25, 315. [Google Scholar]
- Bongers, T. The Maturity Index: An Ecological Measure of Environmental Disturbance Based on Nematode Species Composition. Oecologia 1990, 83, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Bongers, T.; Bongers, M. Functional diversity of nematodes. Appl. Soil Ecol. 1998, 10, 239–251. [Google Scholar] [CrossRef]
- Sieriebriennikov, B.; Ferris, H.; de Goede, R.G.M. NINJA: An Automated Calculation System for Nematode-Based Biological Monitoring. Eur. J. Soil Biol. 2014, 61, 90–93. [Google Scholar] [CrossRef]
- Kruskal, J.B. Nonmetric multidimensional scaling: A numerical method. Psychometrika 1964, 29, 115–129. [Google Scholar] [CrossRef]
- Patil, G.P.; Taillie, C. On a variant of the Rao-Rubin theorem. Sankhya: Indian J. Stat. 1979, 41, 129–132. [Google Scholar]
- Rényi, A. On Measures of Entropy and Information. Proc. Fourth Berkeley Symp. Math. Stat. Prob. 1961, 1, 547–561. [Google Scholar]
- Ricotta, C. From theoretical ecology to statistical physics and back: Self-similar landscape metrics as a synthesis of ecological diversity and geometrical complexity. Ecol. Model. 2000, 125, 245–253. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistical Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Butts, C.T. A Relational Event Framework for Social Action. Sociol. Methodol. 2008, 38, 155–200. [Google Scholar] [CrossRef]
- Macarthur, R.; Levins, R. The limiting similarity convergence and divergence of coexisting species. Am. Nat. 1967, 101, 377–385. [Google Scholar] [CrossRef]
- Stamou, G.P.; Argyropoulou, M.D.; Rodriguez-Polo, I.; Boutsis, G.; Kapagianni, P.; Papatheodorou, E.M. A Case Study of Nematode Communities’ Dynamics along Successional Paths in the Reclaimed Landfill. Diversity 2020, 12, 274. [Google Scholar] [CrossRef]
- Borgatti, S.P.; Everett, G.; Freeman, L.C. UCINET 5.0; Version 1.00; Computer Manual; Analytech Technologies: Natick, MA, USA, 1999. [Google Scholar]
- Huisman, M.; Van Duijn, M.A.J. Software for social network analysis. In Models and Methods in Social Network Analysis; Carrington, P.J., Scott, J., Wasserman, S., Eds.; Cambridge University Press: New York, NY, USA, 2005; pp. 270–316. [Google Scholar]
- O’Malley, A.J.; Marsden, P.V. The Analysis of Social Networks. Health Serv. Outcomes Res. Methodol. 2008, 8, 222–269. [Google Scholar] [CrossRef]
- Grabau, Z.J.; Liu, C.; Sandoval-Ruiz, R. Meloidogyne incognita management by nematicides in tomato production. J. Nematol. 2021, 53, e2021–e2055. [Google Scholar] [CrossRef]
- Abdelnabby, H.; Wang, Y.H.; Xiao, X.Q.; Wang, G.F.; Yang, F.; Xiao, Y.N. Impact of direct and indirect application of rising furfural concentrations on viability, infectivity and reproduction of the root knot nematode, Meloidogyne incognita in Pisum sativum. Microb. Pathog. 2016, 96, 26–34. [Google Scholar] [CrossRef]
- Abdelnabby, H.; Hu, Z.; Wang, H.; Zhang, X. Furfural–biochar-based formulations show synergistic and potentiating effects against Meloidogyne incognita in tomato. Pestic. Sci. 2018, 91, 203–218. [Google Scholar] [CrossRef]
- Pandiarajan, P.; Baskaran, P.G.; Kathiresan, M.; Kanth, S. Physico chemical characterization of fiber from Melia azedarach barks as an effective reinforcement in polymer matrices. J. Nat. Fibers. 2022, 19, 2093–2105. [Google Scholar] [CrossRef]
- Gebremikael, M.T.; Steel, H.; Buchan, D.; Bert, W.; De Neve, S. Nematodes enhance plant growth and nutrient uptake under C and N-rich conditions. Sci. Rep. 2016, 6, 32862. [Google Scholar] [CrossRef]
- Boutsis, G.; Stamou, G.; Argyropoulou, M. Short term effects of soil disinfection with metham sodium and organic alternatives on nematode communities. Community Ecol. 2011, 12, 161–170. [Google Scholar] [CrossRef]
- Ntalli, N.; Zioga, D.; Argyropoulou, M.D.; Papatheodorou, E.M.; Menkissoglu-Spiroudi, U.; Monokrousos, N. Anise, parsley and rocket as nematicidal soil amendments and their impact on non-target soil organisms. Appl. Soil Ecol. 2019, 143, 17–25. [Google Scholar] [CrossRef]
- Waldo, B.D.; Grabau, Z.J.; Mengistu, T.M.; Crow, W.T. Nematicide effects on non-target nematodes in bermudagrass. J. Nematol. 2019, 51, e2019-09. [Google Scholar] [CrossRef] [PubMed]
- Ferris, H.; Tuomisto, H. Unearthing the role of biological diversity in soil health. Soil Biol. Biochem. 2015, 85, 101–109. [Google Scholar] [CrossRef]
- Van, M.K.; Jucker, T.; Svenning, J.C. Unifying the concepts of stability and resilience in ecology. J. Ecol. 2021, 109, 3114–3132. [Google Scholar]
- Liu, X.D.; Zhang, D.X.; Li, H.X.; Qi, X.X.; Gao, Y.; Zhang, Y.B.; Han, Y.L.; Jiang, Y.; Li, H. Soil nematode community and crop productivity in response to 5-year biochar and manure addition to yellow cinnamon soil. BMC Ecol. 2020, 20, 39. [Google Scholar] [CrossRef]
- Du Preez, G.; Daneel, M.; De Goede, R.; Du Toit, M.J.; Ferris, H.; Fourie, H.; Geisen, S.; Kakouli-Duarte, T.; Korthals, G.; Sánchez-Moreno, S.; et al. Nematode-based indices in soil ecology: Application, utility, and future directions. Soil Biol. Biochem. 2022, 169, 108640. [Google Scholar] [CrossRef]
- Ferris, H. Contribution of nematodes to the structure and function of the soil food web. J. Nematol. 2010, 42, 63–67. [Google Scholar]
- Dimou, M.D.; Monokrousos, N.; Katapodis, P.; Diamantopoulou, P.A.; Argyropoulou, M.D.; Papatheodorou, E.M. Use of Microbially Treated Olive Mill Wastewaters as Soil Organic Amendments; Their Short-Term Effects on the Soil Nematode Community. Diversity 2023, 15, 497. [Google Scholar] [CrossRef]
- Allesina, S.; Bodini, A.; Pascual, M. Functional links and robustness in food webs. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 1701–1709. [Google Scholar] [CrossRef]
- Yang, F.; Chen, Q.; Zhang, Q.; Long, C.; Jia, W.; Cheng, X. Keystone species affect the relationship between soil microbial diversity and ecosystem function under land use change in subtropical China. Funct. Ecol. 2021, 35, 1159–1170. [Google Scholar] [CrossRef]
- Tomar, V.; Ahmad, W. Food web diagnostics and functional diversity of soil inhabiting nematodes in a natural woodland. Helminthologia 2009, 46, 183–189. [Google Scholar] [CrossRef]
- Gupta, D.; Bhandari, S.; Bhusal, D.R. Variation of Nematode Indices under Contrasting Pest Management Practices in a Tomato Growing Agro-Ecosystem. Heliyon 2019, 5, e02621. [Google Scholar] [CrossRef] [PubMed]





| Treatment | Number of Ties | Connectedness | Fragmentation | Average Distance |
|---|---|---|---|---|
| Control | 22 | 0.071 | 0.928 | 1.222 |
| Ox | 8 | 0.038 | 0.961 | 0.533 |
| Fur | 28 | 0.091 | 0.908 | 1.555 |
| MWE | 54 | 0.263 | 0.736 | 2.842 |
| Treatment | MI | CI | EI | SI | |
| 3 DAA | Control | 1.36 ± 0.09 ab | 5.11 ± 1.2 a | 84.89 ± 4.02 b | 3.23 ± 1.23 |
| Ox | 1.15 ± 0.03 c | 0.76 ± 0.37 c | 89.76 ± 1.21 ab | 11.11 ± 5.11 | |
| Fur | 1.42 ± 0.04 a | 1.75 ± 0.48 bc | 88.77 ± 1.95 b | 10.27 ± 5.55 | |
| MWE | 1.21 ± 0.05 bc | 1.74 ± 0.5 bc | 94.33 ± 1.24 a | 5.17 ± 2.17 | |
| 34 DAA | Control | 1.43 ± 0.10 ab | 2.2 ± 0.34 bc | 83.71 ± 4.88 b | 4.55 ± 2.55 |
| Ox | 1.37 ± 0.10 c | 1.47 ± 0.54 bc | 85.66 ± 5.62 ab | 0 ± 0 | |
| Fur | 1.50 ± 0.10 a | 1.66 ± 0.66 bc | 78.43 ± 6.60 b | 5.24 ± 3.22 | |
| MWE | 1.39 ± 0.05 bc | 2.68 ± 0.98 bc | 90.39 ± 1.15 a | 20.33 ± 10.37 | |
| S | ** | ns | ** | ns | |
| T | * | ns | * | ns | |
| SxT | ns | * | ns | ns |
| Treatment | Fungivore Footprint | Bacterivore Footprint | |
| 3 DAA | Control | 18.21 ± 6.91 a | 1080.61 ± 265.15 a |
| Ox | 0.77 ± 0.43 b | 771.78 ± 331.76 b | |
| Fur | 3.58 ± 1.25 b | 568.86 ± 199.41 b | |
| MWE | 4.71 ± 1.73 ab | 871.43 ± 123.13 ab | |
| 34 DAA | Control | 11.12 ± 2.53 a | 1067.18 ± 147.72 a |
| Ox | 2.15 ± 0.76 b | 568.83 ± 204.72 b | |
| Fur | 2.87 ± 1.42 b | 461.03 ± 169.61 b | |
| MWE | 13.29 ± 7.26 ab | 1188.17 ± 199.47 ab | |
| S | ns | ns | |
| T | * | * | |
| SxT | ns | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Theofilidou, A.; Argyropoulou, M.D.; Ntalli, N.; Kekelis, P.; Mourouzidou, S.; Zafeiriou, I.; Tsiropoulos, N.G.; Monokrousos, N. Assessing the Role of Melia azedarach Botanical Nematicide in Enhancing the Structure of the Free-Living Nematode Community. Soil Syst. 2023, 7, 80. https://doi.org/10.3390/soilsystems7040080
Theofilidou A, Argyropoulou MD, Ntalli N, Kekelis P, Mourouzidou S, Zafeiriou I, Tsiropoulos NG, Monokrousos N. Assessing the Role of Melia azedarach Botanical Nematicide in Enhancing the Structure of the Free-Living Nematode Community. Soil Systems. 2023; 7(4):80. https://doi.org/10.3390/soilsystems7040080
Chicago/Turabian StyleTheofilidou, Aphrodite, Maria D. Argyropoulou, Nikoletta Ntalli, Panagiotis Kekelis, Snezhana Mourouzidou, Ioannis Zafeiriou, Nikolaos G. Tsiropoulos, and Nikolaos Monokrousos. 2023. "Assessing the Role of Melia azedarach Botanical Nematicide in Enhancing the Structure of the Free-Living Nematode Community" Soil Systems 7, no. 4: 80. https://doi.org/10.3390/soilsystems7040080
APA StyleTheofilidou, A., Argyropoulou, M. D., Ntalli, N., Kekelis, P., Mourouzidou, S., Zafeiriou, I., Tsiropoulos, N. G., & Monokrousos, N. (2023). Assessing the Role of Melia azedarach Botanical Nematicide in Enhancing the Structure of the Free-Living Nematode Community. Soil Systems, 7(4), 80. https://doi.org/10.3390/soilsystems7040080










