Repeated Solid Digestate Amendment Increases Denitrifying Enzyme Activity in an Acid Clayey Soil
Abstract
:1. Introduction
2. Materials and Methods
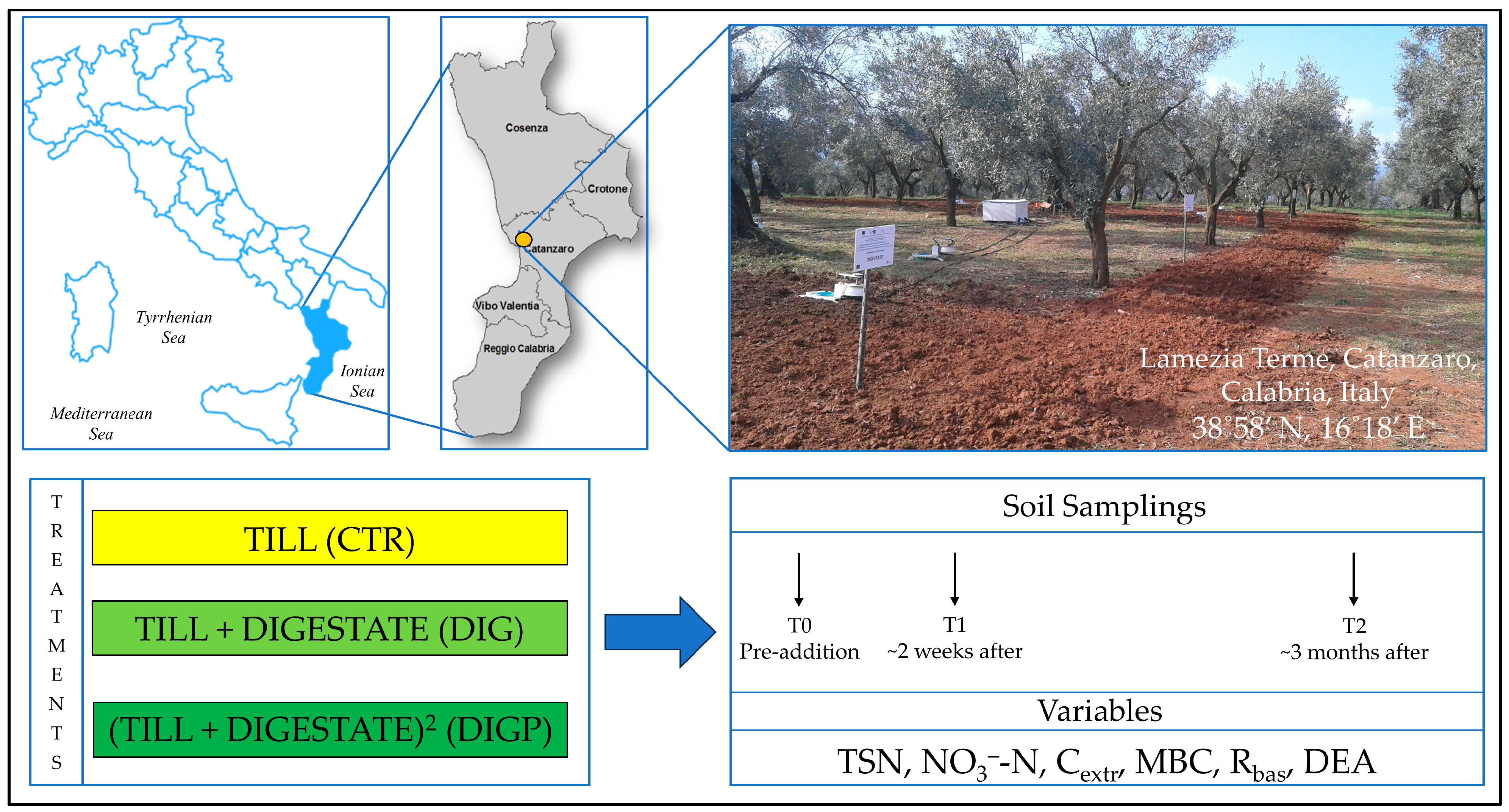
2.1. Experimental Site
2.2. Solid Anaerobic Digestate
2.3. Experimental Design and Soil Management
2.4. Soil Sampling
2.5. Soil Chemical and Biochemical Variables Determination
2.6. Statistics
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wesseler, J. The EU’s farm-to-fork strategy: An assessment from the perspective of agricultural economics. Appl. Econ. Perspect. Policy 2022, 44, 1826–1843. [Google Scholar] [CrossRef]
- Lal, R. Beyond COP 21: Potential and challenges of the “4 per Thousand” initiative. J. Soil Water Conserv. 2016, 71, 20A–25A. [Google Scholar] [CrossRef]
- Lipper, L.; Thornton, P.; Campbell, B.M.; Baedeker, T.; Braimoh, A.; Bwalya, M.; Caron, P.; Cattaneo, A.; Garrity, D.; Henry, K.; et al. Climate-smart agriculture for food security. Nat. Clim. Chang. 2014, 4, 1068–1072. [Google Scholar] [CrossRef]
- Wang, Z.-H.; Li, S.-X. Chapter Three-Nitrate N Loss by Leaching and Surface Runoff in Agricultural Land: A Global Issue (a Review); Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2019; Volume 156, pp. 159–217. ISBN 0065-2113. [Google Scholar]
- Cui, M.; Zeng, L.; Qin, W.; Feng, J. Measures for reducing nitrate leaching in orchards: A review. Environ. Pollut. 2020, 263, 114553. [Google Scholar] [CrossRef] [PubMed]
- Snyder, C.S.; Bruulsema, T.W.; Jensen, T.L.; Fixen, P.E. Review of greenhouse gas emissions from crop production systems and fertilizer management effects. Agric. Ecosyst. Environ. 2009, 133, 247–266. [Google Scholar] [CrossRef]
- Skowrońska, M.; Filipek, T. Life cycle assessment of fertilizers: A review. Int. Agrophysics 2014, 28, 101–110. [Google Scholar] [CrossRef]
- Font-Palma, C. Methods for the Treatment of Cattle Manure—A Review. C 2019, 5, 27. [Google Scholar] [CrossRef]
- Zavattaro, L.; Bechini, L.; Grignani, C.; van Evert, F.K.; Mallast, J.; Spiegel, H.; Sandén, T.; Pecio, A.; Giráldez Cervera, J.V.; Guzmán, G.; et al. Agronomic effects of bovine manure: A review of long-term European field experiments. Eur. J. Agron. 2017, 90, 127–138. [Google Scholar] [CrossRef]
- Diacono, M.; Montemurro, F. Long-term effects of organic amendments on soil fertility. A review. Agron. Sustain. Dev. 2010, 30, 401–422. [Google Scholar] [CrossRef]
- Bernal, M.P.; Alburquerque, J.A.; Moral, R. Composting of animal manures and chemical criteria for compost maturity assessment. A review. Bioresour. Technol. 2009, 100, 5444–5453. [Google Scholar] [CrossRef]
- Le Pera, A.; Sellaro, M.; Bencivenni, E. Composting food waste or digestate? Characteristics, statistical and life cycle assessment study based on an Italian composting plant. J. Clean. Prod. 2022, 350, 131552. [Google Scholar] [CrossRef]
- Chynoweth, D.P.; Owens, J.M.; Legrand, R. Renewable methane from anaerobic digestion of biomass. Renew. Energy 2001, 22, 1–8. [Google Scholar] [CrossRef]
- Neri, A.; Bernardi, B.; Zimbalatti, G.; Benalia, S. An Overview of Anaerobic Digestion of Agricultural By-Products and Food Waste for Biomethane Production. Energies 2023, 16, 6851. [Google Scholar] [CrossRef]
- Slepetiene, A.; Volungevicius, J.; Jurgutis, L.; Liaudanskiene, I.; Amaleviciute-Volunge, K.; Slepetys, J.; Ceseviciene, J. The potential of digestate as a biofertilizer in eroded soils of Lithuania. Waste Manag. 2020, 102, 441–451. [Google Scholar] [CrossRef]
- Jiang, Z.; Zheng, H.; Xing, B. Environmental life cycle assessment of wheat production using chemical fertilizer, manure compost, and biochar-amended manure compost strategies. Sci. Total Environ. 2021, 760, 143342. [Google Scholar] [CrossRef]
- Ren, J.; Liu, X.; Yang, W.; Yang, X.; Li, W.; Xia, Q.; Li, J.; Gao, Z.; Yang, Z. Rhizosphere soil properties, microbial community, and enzyme activities: Short-term responses to partial substitution of chemical fertilizer with organic manure. J. Environ. Manag. 2021, 299, 113650. [Google Scholar] [CrossRef]
- Reuland, G.; Sigurnjak, I.; Dekker, H.; Michels, E.; Meers, E. The Potential of Digestate and the Liquid Fraction of Digestate as Chemical Fertiliser Substitutes under the RENURE Criteria. Agronomy 2021, 11, 1374. [Google Scholar] [CrossRef]
- Abubaker, J.; Risberg, K.; Jönsson, E.; Dahlin, A.S.; Cederlund, H.; Pell, M. Short-term effects of biogas digestates and pig slurry application on soil microbial activity. Appl. Environ. Soil Sci. 2015, 2015, 658542. [Google Scholar] [CrossRef]
- Makádi, M.; Tomócsik, A.; Orosz, V. Digestate: A New Nutrient Source—Review. In Biogas; InTech: London, UK, 2012. [Google Scholar]
- Mondello, G.; Salomone, R.; Ioppolo, G.; Saija, G.; Sparacia, S.; Lucchetti, M.C. Comparative LCA of alternative scenarios for waste treatment: The case of food waste production by the mass-retail sector. Sustainability 2017, 9, 827. [Google Scholar] [CrossRef]
- Badagliacca, G.; Lo Presti, E.; Gelsomino, A.; Monti, M. Effect of Solid Digestate Amendment on The Dynamics of N Soluble Forms in Two Contrasting Soil Profiles under Mediterranean Environment. Agriculture 2023, 13, 1311. [Google Scholar] [CrossRef]
- Riva, C.; Orzi, V.; Carozzi, M.; Acutis, M.; Boccasile, G.; Lonati, S.; Tambone, F.; D’Imporzano, G.; Adani, F. Short-term experiments in using digestate products as substitutes for mineral (N) fertilizer: Agronomic performance, odours, and ammonia emission impacts. Sci. Total Environ. 2016, 547, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Maucieri, C.; Nicoletto, C.; Caruso, C.; Sambo, P.; Borin, M. Effects of digestate solid fraction fertilisation on yield and soil carbon dioxide emission in a horticulture succession. Ital. J. Agron. 2017, 12, 116–123. [Google Scholar] [CrossRef]
- Monti, M.; Badagliacca, G.; Romeo, M.; Gelsomino, A. No-Till and Solid Digestate Amendment Selectively Affect the Potential Denitrification Activity in Two Mediterranean Orchard Soils. Soil Syst. 2021, 5, 31. [Google Scholar] [CrossRef]
- Huang, J.; He, P.; Duan, H.; Yang, Z.; Zhang, H.; Lü, F. Leaching risk of antibiotic resistance contamination from organic waste compost in rural areas. Environ. Pollut. 2023, 320, 121108. [Google Scholar] [CrossRef] [PubMed]
- Basso, B.; Ritchie, J.T. Impact of compost, manure and inorganic fertilizer on nitrate leaching and yield for a 6-year maize–alfalfa rotation in Michigan. Agric. Ecosyst. Environ. 2005, 108, 329–341. [Google Scholar] [CrossRef]
- Li, P.; Lang, M.; Li, C.; Thomas, B.W.; Hao, X. Nutrient Leaching from Soil Amended with Manure and Compost from Cattle Fed Diets Containing Wheat Dried Distillers’ Grains with Solubles. Water Air Soil Pollut. 2016, 227, 393. [Google Scholar] [CrossRef]
- Corden, C.; Bougas, K.; Cunningham, E.; Tyrer, D.; Kreißig, J.; Zetti, E.; Gamero, E.; Wildey, R.; Crookes, M. Digestate and Compost as Fertilisers: Risk Assessment and Risk Management Options; European Commission: Brussels, Belgium, 2019. [Google Scholar]
- Samoraj, M.; Mironiuk, M.; Izydorczyk, G.; Witek-Krowiak, A.; Szopa, D.; Moustakas, K.; Chojnacka, K. The challenges and perspectives for anaerobic digestion of animal waste and fertilizer application of the digestate. Chemosphere 2022, 295, 133799. [Google Scholar] [CrossRef]
- Nkoa, R. Agricultural benefits and environmental risks of soil fertilization with anaerobic digestates: A review. Agron. Sustain. Dev. 2014, 34, 473–492. [Google Scholar] [CrossRef]
- Soil Survey, S. Keys to Soil Taxonomy; USDA Natural Resources Conservation Services: Washington, DC, USA, 2014. [Google Scholar]
- WRB, I.W.G. World Reference Base for Soil Resources 2006, 2nd ed.; World Soil Resources Reports No.103; FAO: Roma, Italia, Country, 2006. [Google Scholar]
- Badagliacca, G.; Petrovičovà, B.; Pathan, S.I.; Roccotelli, A.; Romeo, M.; Monti, M.; Gelsomino, A. Use of solid anaerobic digestate and no-tillage practice for restoring the fertility status of two Mediterranean orchard soils with contrasting properties. Agric. Ecosyst. Environ. 2020, 300, 107010. [Google Scholar] [CrossRef]
- Badagliacca, G.; Romeo, M.; Gelsomino, A.; Monti, M. Short-term effects of repeated application of solid digestate on soil C and N dynamics and CO2 emission in a clay soil olive (Olea europaea L.) orchard. Clean. Circ. Bioeconomy 2022, 1, 100004. [Google Scholar] [CrossRef]
- Pathan, S.I.; Roccotelli, A.; Petrovičovà, B.; Romeo, M.; Badagliacca, G.; Monti, M.; Gelsomino, A. Temporal dynamics of total and active prokaryotic communities in two Mediterranean orchard soils treated with solid anaerobic digestate or managed under no-tillage. Biol. Fertil. Soils 2021, 57, 837–861. [Google Scholar] [CrossRef]
- Barra Caracciolo, A.; Bustamante, M.A.; Nogues, I.; Di Lenola, M.; Luprano, M.L.; Grenni, P. Changes in microbial community structure and functioning of a semiarid soil due to the use of anaerobic digestate derived composts and rosemary plants. Geoderma 2015, 245–246, 89–97. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Laudicina, V.; Palazzolo, E.; Badalucco, L. Natural Organic Compounds in Soil Solution: Potential Role as Soil Quality Indicators. Curr. Org. Chem. 2013, 17, 2991–2997. [Google Scholar] [CrossRef]
- Ohlinger, R. Soil respiration by titration. In Methods in Soil Biology; Springer: Berlin/Heidelberg, Germany, 1995; pp. 93–98. [Google Scholar]
- Šimek, M.; Elhottová, D.; Klimeš, F.; Hopkins, D.W. Emissions of N2O and CO2, denitrification measurements and soil properties in red clover and ryegrass stands. Soil Biol. Biochem. 2004, 36, 9–21. [Google Scholar] [CrossRef]
- Allaire, J. RStudio: Integrated development environment for R. Boston MA 2012, 770, 165–171. [Google Scholar]
- Fernández-Bayo, J.D.; Achmon, Y.; Harrold, D.R.; McCurry, D.G.; Hernandez, K.; Dahlquist-Willard, R.M.; Stapleton, J.J.; VanderGheynst, J.S.; Simmons, C.W. Assessment of Two Solid Anaerobic Digestate Soil Amendments for Effects on Soil Quality and Biosolarization Efficacy. J. Agric. Food Chem. 2017, 65, 3434–3442. [Google Scholar] [CrossRef]
- Zuber, S.M.; Villamil, M.B. Meta-analysis approach to assess effect of tillage on microbial biomass and enzyme activities. Soil Biol. Biochem. 2016, 97, 176–187. [Google Scholar] [CrossRef]
- Montanaro, G.; Tuzio, A.C.; Xylogiannis, E.; Kolimenakis, A.; Dichio, B. Carbon budget in a Mediterranean peach orchard under different management practices. Agric. Ecosyst. Environ. 2017, 238, 104–113. [Google Scholar] [CrossRef]
- Eickenscheidt, T.; Freibauer, A.; Heinichen, J.; Augustin, J.; Drösler, M. Short-term effects of biogas digestate and cattle slurry application on greenhouse gas emissions affected by N availability from grasslands on drained fen peatlands and associated organic soils. Biogeosciences 2014, 11, 6187–6207. [Google Scholar] [CrossRef]
- Martin, S.L.; Clarke, M.L.; Othman, M.; Ramsden, S.J.; West, H.M. Biochar-mediated reductions in greenhouse gas emissions from soil amended with anaerobic digestates. Biomass Bioenergy 2015, 79, 39–49. [Google Scholar] [CrossRef]
- Cucina, M.; Tacconi, C.; Ricci, A.; Pezzolla, D.; Sordi, S.; Zadra, C.; Gigliotti, G. Evaluation of benefits and risks associated with the agricultural use of organic wastes of pharmaceutical origin. Sci. Total Environ. 2018, 613–614, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.J.; Jones, D.L.; Chadwick, D.R.; Williams, A.P. Repeated application of anaerobic digestate, undigested cattle slurry and inorganic fertilizer N: Impacts on pasture yield and quality. Grass Forage Sci. 2018, 73, 758–763. [Google Scholar] [CrossRef]
- Cardelli, R.; Giussani, G.; Marchini, F.; Saviozzi, A. Short-term effects on soil of biogas digestate, biochar and their combinations. Soil Res. 2018, 56, 623. [Google Scholar] [CrossRef]
- Alburquerque, J.A.; de la Fuente, C.; Ferrer-Costa, A.; Carrasco, L.; Cegarra, J.; Abad, M.; Bernal, M.P. Assessment of the fertiliser potential of digestates from farm and agroindustrial residues. Biomass Bioenergy 2012, 40, 181–189. [Google Scholar] [CrossRef]
- Blagodatskaya, E.; Blagodatsky, S.; Anderson, T.-H.; Kuzyakov, Y. Microbial Growth and Carbon Use Efficiency in the Rhizosphere and Root-Free Soil. PLoS ONE 2014, 9, e93282. [Google Scholar] [CrossRef]
- Geyer, K.M.; Kyker-Snowman, E.; Grandy, A.S.; Frey, S.D. Microbial carbon use efficiency: Accounting for population, community, and ecosystem-scale controls over the fate of metabolized organic matter. Biogeochemistry 2016, 127, 173–188. [Google Scholar] [CrossRef]
- Wrage, N.; Velthof, G.L.; van Beusichem, M.L.; Oenema, O. Role of nitrifier denitrification in the production of nitrous oxide. Soil Biol. Biochem. 2001, 33, 1723–1732. [Google Scholar] [CrossRef]
- Chen, J.; Strous, M. Denitrification and aerobic respiration, hybrid electron transport chains and co-evolution. Biochim. Biophys. Acta-Bioenerg. 2013, 1827, 136–144. [Google Scholar] [CrossRef]
- Jones, C.M.; Stres, B.; Rosenquist, M.; Hallin, S. Phylogenetic Analysis of Nitrite, Nitric Oxide, and Nitrous Oxide Respiratory Enzymes Reveal a Complex Evolutionary History for Denitrification. Mol. Biol. Evol. 2008, 25, 1955–1966. [Google Scholar] [CrossRef]
- Tiedje, J.M.; Simkins, S.; Groffman, P.M. Perspectives on measurement of denitrification in the field including recommended protocols for acetylene based methods. Plant Soil 1989, 115, 261–284. [Google Scholar] [CrossRef]
- Gardner, L.M.; White, J.R. Denitrification Enzyme Activity as an Indicator of Nitrate Movement through a Diversion Wetland. Soil Sci. Soc. Am. J. 2010, 74, 1037–1047. [Google Scholar] [CrossRef]
- Mania, D.; Woliy, K.; Degefu, T.; Frostegård, Å. A common mechanism for efficient N2O reduction in diverse isolates of nodule-forming bradyrhizobia. Environ. Microbiol. 2020, 22, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Jonassen, K.R.; Hagen, L.H.; Vick, S.H.W.; Arntzen, M.Ø.; Eijsink, V.G.H.; Frostegård, Å.; Lycus, P.; Molstad, L.; Pope, P.B.; Bakken, L.R. Nitrous oxide respiring bacteria in biogas digestates for reduced agricultural emissions. ISME J. 2022, 16, 580–590. [Google Scholar] [CrossRef]
- Badagliacca, G.; Benítez, E.; Amato, G.; Badalucco, L.; Giambalvo, D.; Laudicina, V.A.; Ruisi, P. Long-term effects of contrasting tillage on soil organic carbon, nitrous oxide and ammonia emissions in a Mediterranean Vertisol under different crop sequences. Sci. Total Environ. 2018, 619–620, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Köster, J.R.; Cárdenas, L.M.; Bol, R.; Lewicka-Szczebak, D.; Senbayram, M.; Well, R.; Giesemann, A.; Dittert, K. Anaerobic digestates lower N2O emissions compared to cattle slurry by affecting rate and product stoichiometry of denitrification—An N2O isotopomer case study. Soil Biol. Biochem. 2015, 84, 65–74. [Google Scholar] [CrossRef]
- Baral, K.R.; Labouriau, R.; Olesen, J.E.; Petersen, S.O. Nitrous oxide emissions and nitrogen use efficiency of manure and digestates applied to spring barley. Agric. Ecosyst. Environ. 2017, 239, 188–198. [Google Scholar] [CrossRef]
- Philippot, L.; Hallin, S.; Schloter, M. Ecology of Denitrifying Prokaryotes in Agricultural Soil. Adv. Agron. 2007, 96, 249–305. [Google Scholar]
- Chirinda, N.; Carter, M.S.; Albert, K.R.; Ambus, P.; Olesen, J.E.; Porter, J.R.; Petersen, S.O. Emissions of nitrous oxide from arable organic and conventional cropping systems on two soil types. Agric. Ecosyst. Environ. 2010, 136, 199–208. [Google Scholar] [CrossRef]
- Jha, N.; Saggar, S.; Giltrap, D.; Tillman, R.; Deslippe, J. Soil properties impacting denitrifier community size, structure, and activity in New Zealand dairy-grazed pasture. Biogeosci. Discuss. 2017, 14, 4243–4253. [Google Scholar] [CrossRef]
- Shrewsbury, L.H.; Smith, J.L.; Huggins, D.R.; Carpenter-Boggs, L.; Reardon, C.L. Denitrifier abundance has a greater influence on denitrification rates at larger landscape scales but is a lesser driver than environmental variables. Soil Biol. Biochem. 2016, 103, 221–231. [Google Scholar] [CrossRef]
- Askri, A.; Laville, P.; Trémier, A.; Houot, S. Influence of Origin and Post-treatment on Greenhouse Gas Emissions After Anaerobic Digestate Application to Soil. Waste Biomass Valorization 2016, 7, 293–306. [Google Scholar] [CrossRef]
- Sun, S.; Liu, J.; Li, Y.; Jiang, P.; Chang, S.X. Similar quality and quantity of Dissolved Organic Carbon under different land use systems in two Canadian and Chinese soils. J. Soils Sediments 2012, 13, 34–42. [Google Scholar] [CrossRef]
- Alburquerque, J.A.; de la Fuente, C.; Bernal, M.P. Chemical properties of anaerobic digestates affecting C and N dynamics in amended soils. Agric. Ecosyst. Environ. 2012, 160, 15–22. [Google Scholar] [CrossRef]
- Pezzolla, D.; Bol, R.; Gigliotti, G.; Sawamoto, T.; López, A.L.; Cardenas, L.; Chadwick, D. Greenhouse gas (GHG) emissions from soils amended with digestate derived from anaerobic treatment of food waste. Rapid Commun. Mass Spectrom. 2012, 26, 2422–2430. [Google Scholar] [CrossRef] [PubMed]
- Butterbach-bahl, K.; Baggs, E.M.; Dannenmann, M.; Kiese, R.; Zechmeister-boltenstern, S. Nitrous oxide emissions from soils: How well do we understand the processes and their controls? Philos. Trans. R Soc. Lond. B Biol. Sci. 2013, 368, 20130122. [Google Scholar] [CrossRef] [PubMed]
- Badagliacca, G.; Rees, R.M.; Giambalvo, D.; Saia, S. Vertisols and Cambisols had contrasting short term greenhouse gas responses to crop residue management. Plant Soil Environ. 2020, 66, 222–233. [Google Scholar] [CrossRef]
- Fiedler, S.R.; Augustin, J.; Wrage-Mönnig, N.; Jurasinski, G.; Gusovius, B.; Glatzel, S. Potential short-term losses of N2O and N2 from high concentrations of biogas digestate in arable soils. Soil 2017, 3, 161–176. [Google Scholar] [CrossRef]
- Tatti, E.; Goyer, C.; Zebarth, B.J.; Burton, D.L.; Giovannetti, L.; Viti, C. Short-Term Effects of Mineral and Organic Fertilizer on Denitrifiers, Nitrous Oxide Emissions and Denitrification in Long-Term Amended Vineyard Soils. Soil Sci. Soc. Am. J. 2013, 77, 113–122. [Google Scholar] [CrossRef]
- Vallejo, A.; Skiba, U.; García-Torres, L.; Arce, A.; López-Fernández, S.; Sánchez-Martín, L. Nitrogen oxides emission from soils bearing a potato crop as influenced by fertilization with treated pig slurries and composts. Soil Biol. Biochem. 2006, 38, 2782–2793. [Google Scholar] [CrossRef]
- Tao, R.; Wakelin, S.A.; Liang, Y.; Hu, B.; Chu, G. Nitrous oxide emission and denitrifier communities in drip-irrigated calcareous soil as affected by chemical and organic fertilizers. Sci. Total Environ. 2018, 612, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Fan, F.; Song, A.; Li, Z.; Yu, W.; Liang, Y. Different denitrification potential of aquic brown soil in Northeast China under inorganic and organic fertilization accompanied by distinct changes of nirS- and nirK-denitrifying bacterial community. Eur. J. Soil Biol. 2014, 65, 47–56. [Google Scholar] [CrossRef]
- Vallejo, A.; Díez, J.A.; López-Valdivia, L.M.; Cartagena, M.C.; Tarquis, A.; Hernáiz, P. Denitrification from an irrigated soil fertilized with pig slurry under Mediterranean conditions. Biol. Fertil. Soils 2004, 40, 93–100. [Google Scholar] [CrossRef]
- Wang, J.; Chadwick, D.R.; Cheng, Y.; Yan, X. Global analysis of agricultural soil denitrification in response to fertilizer nitrogen. Sci. Total Environ. 2018, 616–617, 908–917. [Google Scholar] [CrossRef]
- Gee, G.W.; Bauder, J.W. Particle-size Analysis. In Methods of Soil Analysis: Part 1 Physical and Mineralogical Methods; Klute, A., Ed.; American Society of Agronomy, Inc.: Madison, WI, USA; Soil Science of America, Inc.: Madison, WI, USA, 1986; Volume 4, pp. 383–411. [Google Scholar]
- Blake, G.R.; Hartge, K.H. Core method. In Methods of Soil Analysis: Part 1 Physical and Mineralogical Methods; Klute, A., Ed.; American Society of Agronomy, Inc.: Madison, WI, USA; Soil Science of America, Inc.: Madison, WI, USA, 1986; Volume 4, pp. 364–367. [Google Scholar]
- Thomas, G.W. Soil pH and Soil Acidity. In Methods of Soil Analysis: Part 3 Chemical Methods; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Summer, M.E., Eds.; American Society of Agronomy, Inc.: Madison, WI, USA; Soil Science of America, Inc.: Madison, WI, USA, 1996; Volume 4, pp. 475–490. [Google Scholar]
- Rhoades, J.D. Salinity: Electrical Conductivity and Total Dissolved Solids. In Methods of Soil Analysis: Part 3 Chemical Methods; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Summer, M.E., Eds.; American Society of Agronomy, Inc.: Madison, WI, USA; Soil Science of America, Inc.: Madison, WI, USA, 1996; Volume 4, pp. 417–436. [Google Scholar]
- Loeppert, R.H.; Suarez, D.L. Carbonate and Gypsum. In Methods of Soil Analysis: Part 3 Chemical Methods; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Summer, M.E., Eds.; American Society of Agronomy, Inc.: Madison, WI, USA; Soil Science of America, Inc.: Madison, WI, USA, 1996; Volume 4, pp. 437–474. [Google Scholar]
- Sumner, M.E.; Miller, W.P. Cation Exchange Capacity and Exchange Coefficients. In Methods of Soil Analysis: Part 3 Chemical Methods; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Summer, M.E., Eds.; American Society of Agronomy, Inc.: Madison, WI, USA; Soil Science of America, Inc.: Madison, WI, USA, 1996; Volume 4, pp. 1201–1230. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total Carbon, Organic Carbon, and Organic Matter. In Methods of Soil Analysis: Part 3 Chemical Methods; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Summer, M.E., Eds.; American Society of Agronomy, Inc.: Madison, WI, USA; Soil Science of America, Inc.: Madison, WI, USA, 1996; Volume 4, pp. 961–1010. [Google Scholar]
- Bremner, J.M. Nitrogen – Total. In Methods of Soil Analysis: Part 3 Chemical Methods; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Summer, M.E., Eds.; American Society of Agronomy, Inc.: Madison, WI, USA; Soil Science of America, Inc.: Madison, WI, USA, 1996; Volume 4, pp. 1085–1122. [Google Scholar]
- Olsen, S.R.; Cole, C.V.; Watandbe, F.; Dean, L. Estimation of Available Phosphorus in Soil by Extraction with sodium Bicarbonate. J. Chem. Inf. Model. 1954, 53, 1689–1699. [Google Scholar]

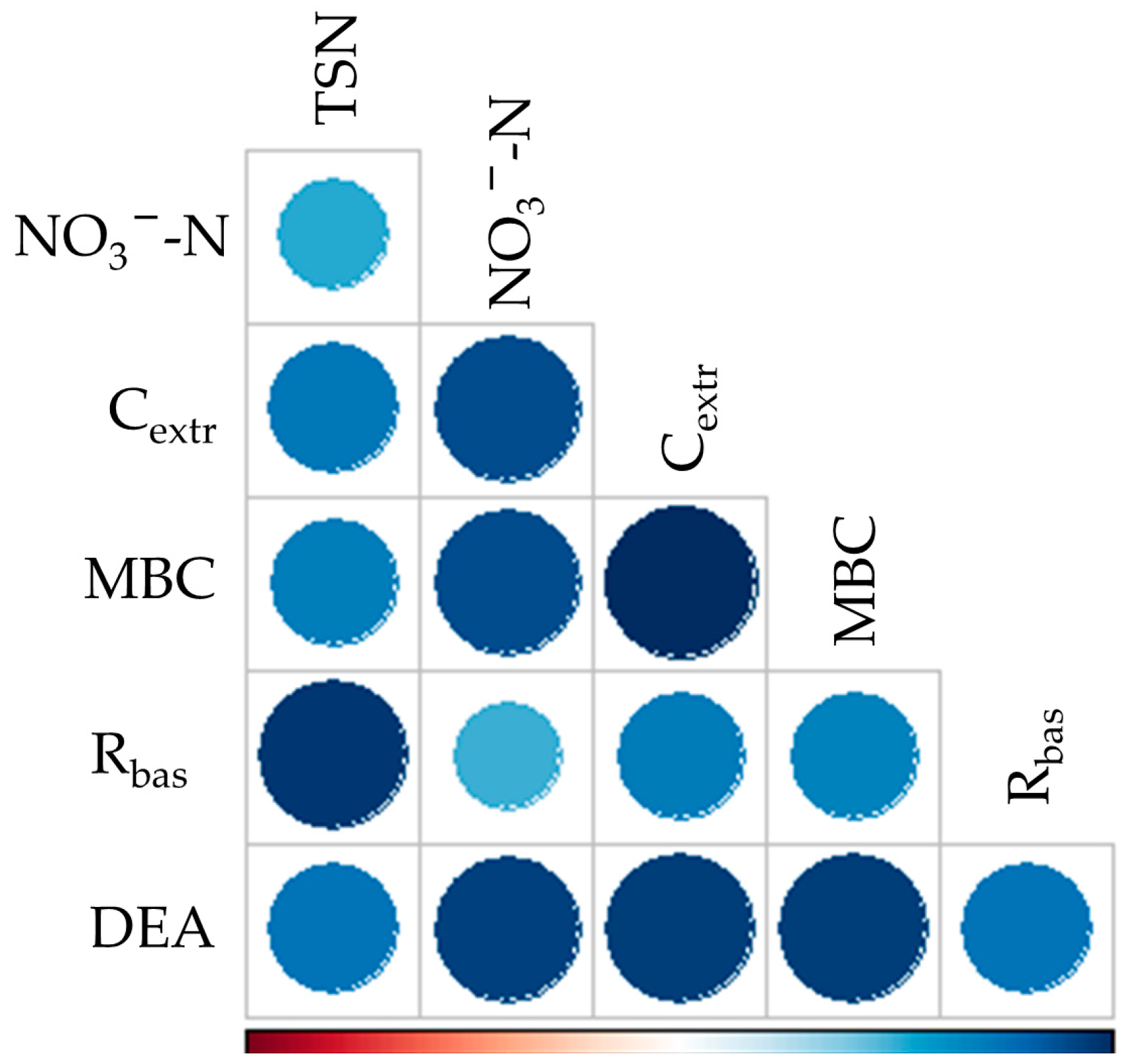
| TSN [mg N kg−1] | NO3−-N [mg N kg−1] | |||||
|---|---|---|---|---|---|---|
| CTR | DIG | DIGP | CTR | DIG | DIGP | |
| T0 | 31.0 b | 26.8 b | 43.8 a | 2.84 b | 2.70 b | 5.05 a |
| T1 | 39.2 c | 73.5 b | 105.3 a | 2.86 c | 4.62 b | 11.69 a |
| T2 | 28.6 c | 63.7 b | 83.9 a | 4.11 c | 18.56 b | 26.9 a |
| p-values | ||||||
| Trt | <0.0001 | <0.0001 | ||||
| Time | <0.0001 | <0.0001 | ||||
| Trt × Time | <0.0001 | <0.0001 | ||||
| Cextr [mg C kg−1] | MBC [mg C kg−1] | Rbas [mg CO2-C kg−1 (28d)−1] | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CTR | DIG | DIGP | CTR | DIG | DIGP | CTR | DIG | DIGP | |
| T0 | 104.1 b | 81.6 b | 147.9 a | 236.7 b | 185.5 b | 329.0 a | 157.2 b | 157.7 b | 296.0 a |
| T1 | 130.3 c | 237.1 b | 329.7 a | 296.1 c | 455.8 b | 659.5 a | 222.0 c | 837.6 b | 1313.9 a |
| T2 | 168.2 c | 309.6 b | 464.7 a | 403.1 c | 594.2 b | 893.4 a | 139.0 c | 563.8 b | 1007.4 a |
| p-values | |||||||||
| Trt | <0.0001 | <0.0001 | <0.0001 | ||||||
| Time | <0.0001 | <0.0001 | <0.0001 | ||||||
| Trt × Time | <0.0001 | <0.0001 | <0.0001 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badagliacca, G.; Lo Presti, E.; Gelsomino, A.; Monti, M. Repeated Solid Digestate Amendment Increases Denitrifying Enzyme Activity in an Acid Clayey Soil. Soil Syst. 2024, 8, 14. https://doi.org/10.3390/soilsystems8010014
Badagliacca G, Lo Presti E, Gelsomino A, Monti M. Repeated Solid Digestate Amendment Increases Denitrifying Enzyme Activity in an Acid Clayey Soil. Soil Systems. 2024; 8(1):14. https://doi.org/10.3390/soilsystems8010014
Chicago/Turabian StyleBadagliacca, Giuseppe, Emilio Lo Presti, Antonio Gelsomino, and Michele Monti. 2024. "Repeated Solid Digestate Amendment Increases Denitrifying Enzyme Activity in an Acid Clayey Soil" Soil Systems 8, no. 1: 14. https://doi.org/10.3390/soilsystems8010014
APA StyleBadagliacca, G., Lo Presti, E., Gelsomino, A., & Monti, M. (2024). Repeated Solid Digestate Amendment Increases Denitrifying Enzyme Activity in an Acid Clayey Soil. Soil Systems, 8(1), 14. https://doi.org/10.3390/soilsystems8010014








