Shifts in Soil Bacterial Communities under Three-Year Fertilization Management and Multiple Cropping Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Soil Sampling and Measuring
3. Results
3.1. Soil Carbon and Nitrogen Content
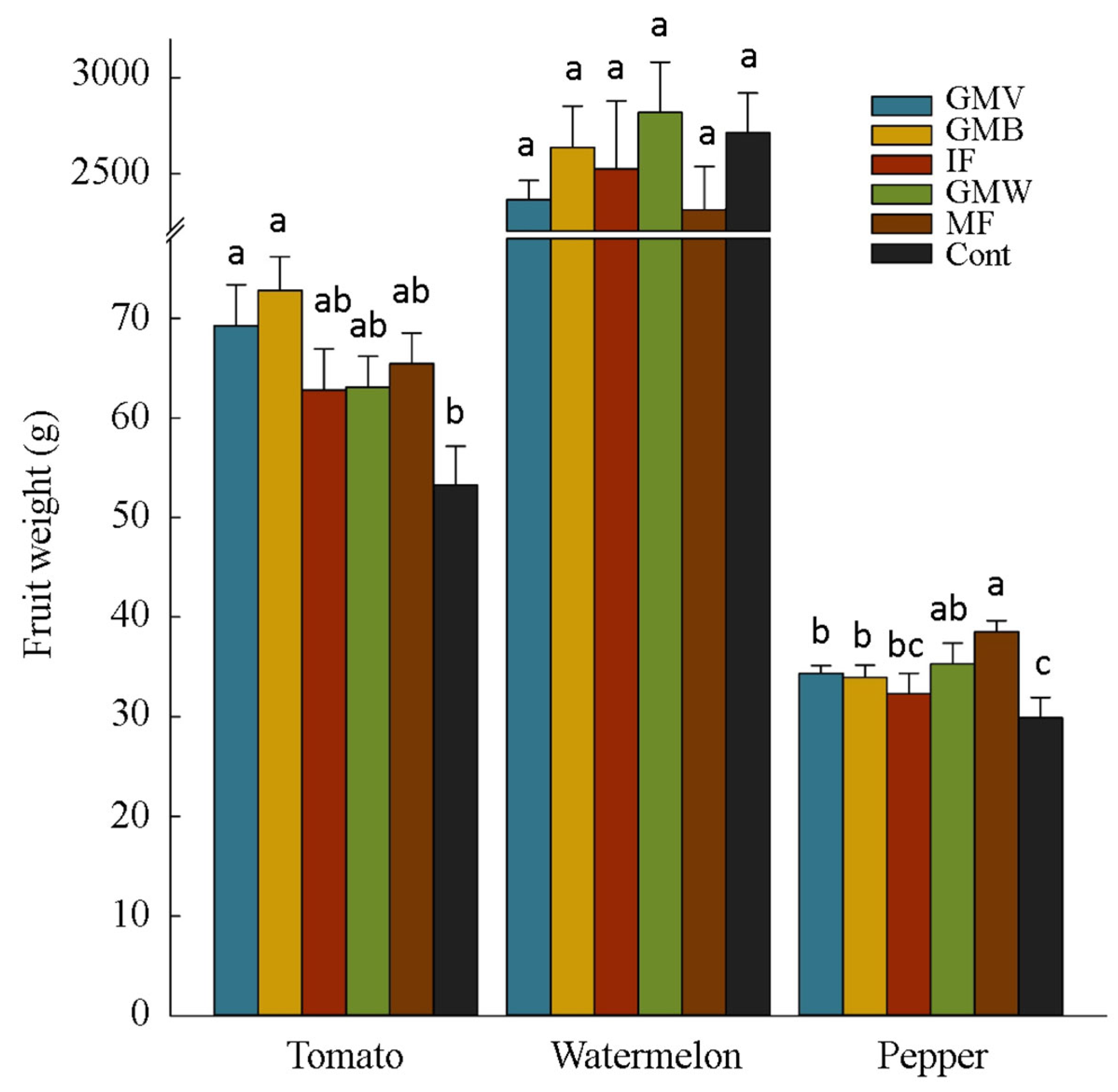
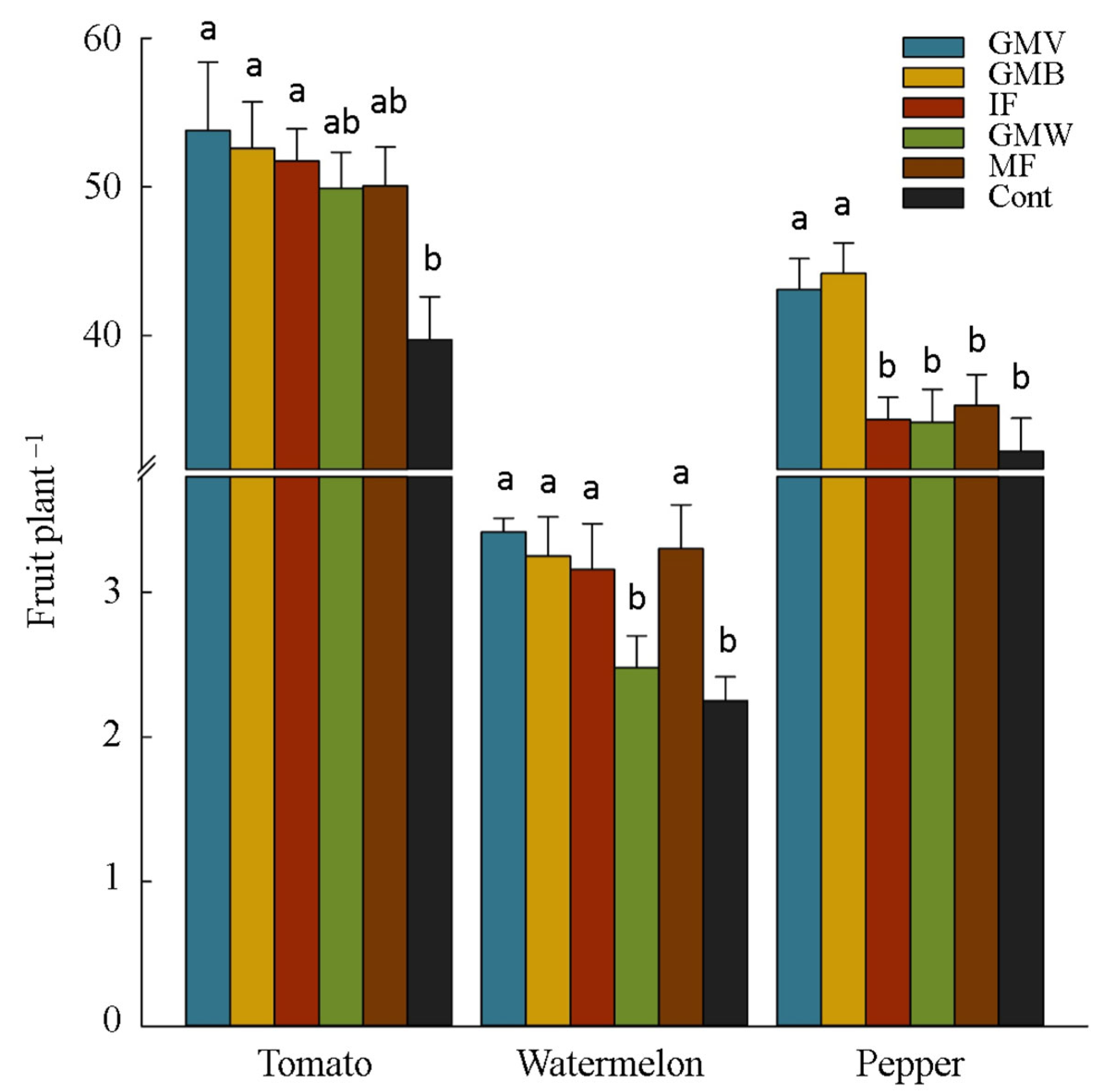
3.2. Plants Yield and Yield Components
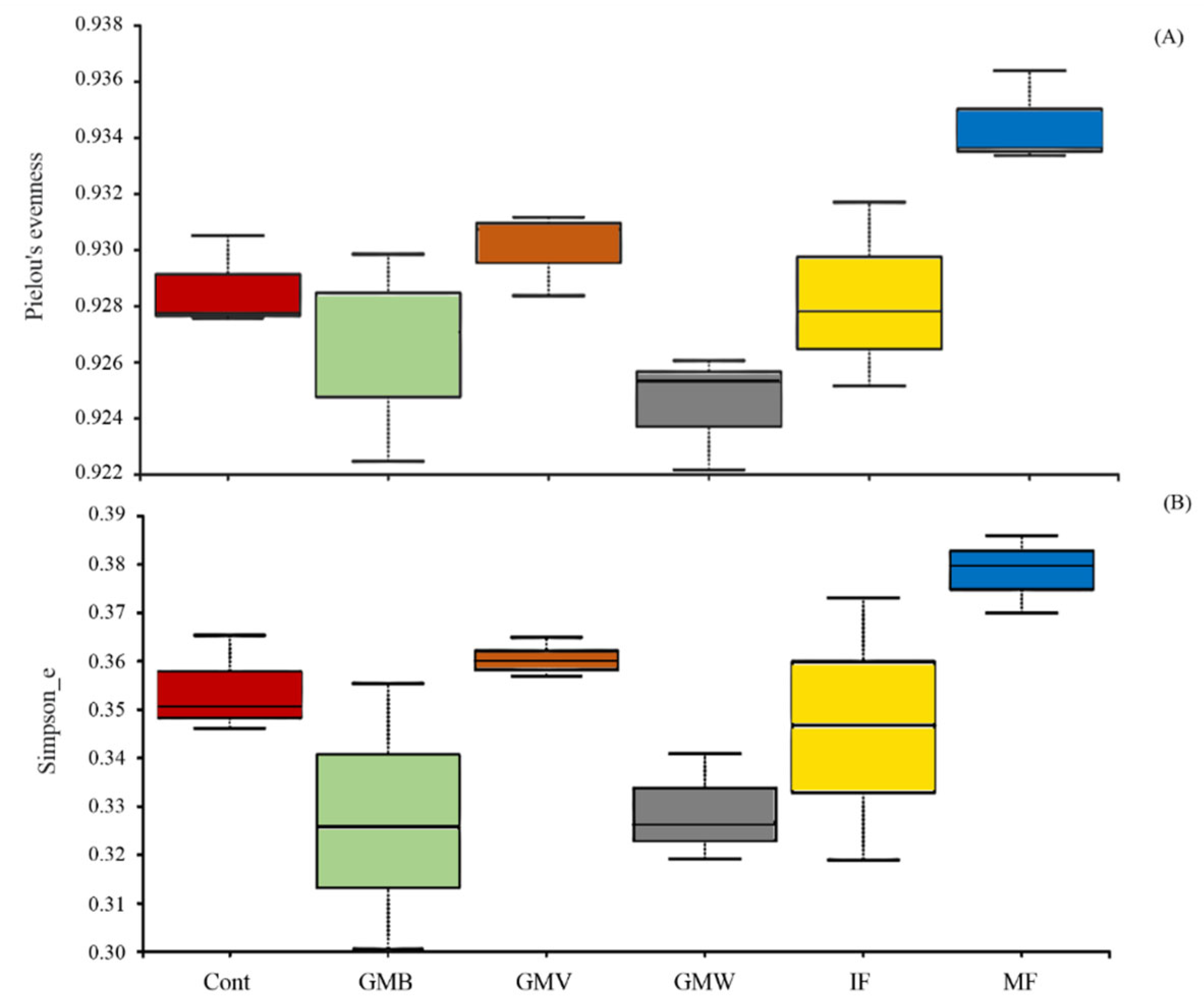
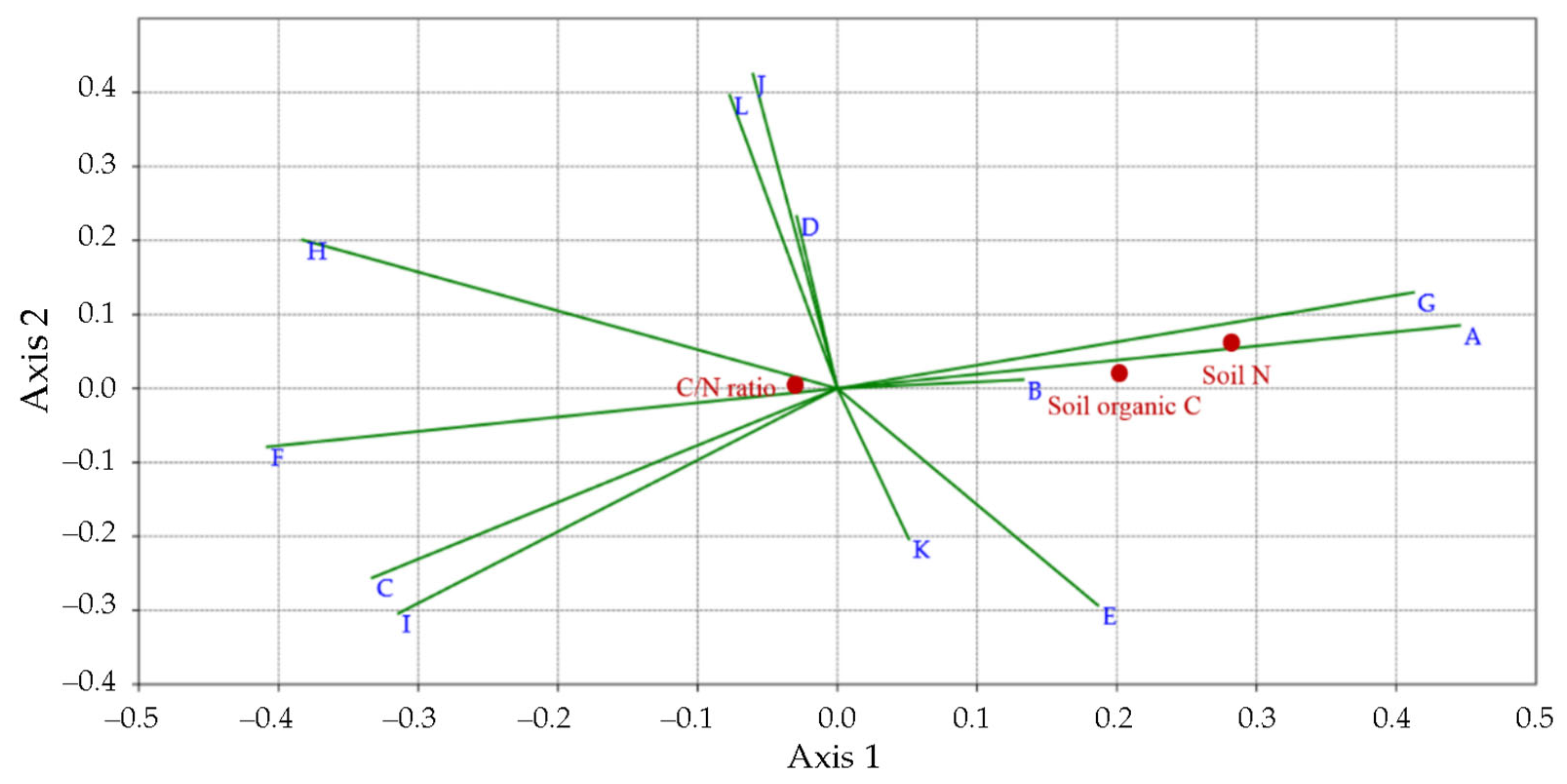
3.3. 16S rDNA Metabarcoding Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kelly, C.; Ferrara, A.; Wilson, G.A.; Ripullone, F.; Nole, A.; Harmer, N.; Salvati, L. Community resilience and land degradation in forest and shrubland socio-ecological systems: Evidence from Gorgoglione, Basilicata, Italy. Land Use Policy 2015, 46, 11–20. [Google Scholar] [CrossRef]
- Yaghoubi Khanghahi, M.; Cucci, G.; Lacolla, G.; Lanzellotti, L.; Crecchio, C. Soil fertility and bacterial community composition in a semiarid Mediterranean agricultural soil under long-term tillage management. Soil Use Manag. 2020, 36, 604–615. [Google Scholar] [CrossRef]
- Van der Bom, F.; Nunes, I.; Raymond, N.S.; Hansen, V.; Bonnichsen, L.; Magid, J.; Nybroe, O.; Jensen, L.S. Long-term fertilisation form, level and duration affect the diversity, structure and functioning of soil microbial communities in the field. Soil Biol. Biochem. 2018, 122, 91–103. [Google Scholar] [CrossRef]
- Rapisarda, S.; Di Biase, G.; Mazzon, M.; Ciavatta, C.; Cavani, L. Nitrogen Availability in Organic Fertilizers from Tannery and Slaughterhouse By-Products. Sustainability 2022, 14, 12921. [Google Scholar] [CrossRef]
- Sądej, W.; Przekwas, K. Fluctuations of nitrogen levels in soil profile under conditions of a long-term fertilization experiment. Plant Soil Environ. 2008, 54, 197–203. [Google Scholar] [CrossRef]
- Luo, G.; Li, L.; Friman, V.-P.; Guo, J.; Guo, S.; Shen, Q.; Ling, N. Organic Amendments Increase Crop Yields by Improving Microbe-Mediated Soil Functioning of Agroecosystems: A Meta-Analysis. Soil Biol. Biochem. 2018, 124, 105–115. [Google Scholar] [CrossRef]
- Whalen, J.K.; Thomas, B.W.; Sharifi, M. Novel Practices and Smart Technologies to Maximize the Nitrogen Fertilizer Value of Manure for Crop Production in Cold Humid Temperate Regions. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2019; Volume 153, pp. 1–85. ISBN 978-0-12-817404-3. [Google Scholar]
- Luján Soto, R.; Martínez-Mena, M.; Cuéllar Padilla, M.; de Vente, J. Restoring soil quality of woody agroecosystems in Mediterranean drylands through regenerative agriculture. Agric. Ecosyst. Environ. 2021, 306, 107191. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Walthall, C.L. Soil biological fertility: Foundation for the next revolution in agriculture? Commun. Soil Sci. Plant Anal. 2015, 46, 753–762. [Google Scholar] [CrossRef]
- Bünemann, E.K.; Schwenke, G.D.; Van Zwieten, L. Impact of agricultural inputs on soil organisms: A review. Soil Res. 2006, 44, 379–406. [Google Scholar] [CrossRef]
- Ma, T.; He, X.; Chen, S.; Li, Y.; Huang, Q.; Xue, C.; Shen, Q. Long-term organic–inorganic fertilization regimes alter bacterial and fungal communities and rice yields in paddy soil. Front. Microbiol. 2022, 13, 890712. [Google Scholar] [CrossRef]
- Francioli, D.; Schulz, E.; Lentendu, G.; Wubet, T.; Buscot, F.; Reitz, T. Mineral vs. organic amendments: Microbial community structure, activity and abundance of agriculturally relevant microbes are driven by long-term fertilization strategies. Front. Microbiol. 2016, 7, 1446. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Khafipour, E.; Krause, D.O.; Entz, M.H.; De Kievit, T.R.; Fernando, W.G.D. Pyrosequencing reveals the influence of organic and conventional farming systems on bacterial communities. PLoS ONE 2012, 7, e51897. [Google Scholar] [CrossRef]
- Khaitov, B.; Yun, H.J.; Lee, Y.; Ruziev, F.; Le, T.H.; Umurzokov, M.; Bo, A.; Cho, K.M.; Park, K.W. Impact of organic manure on growth, nutrient content and yield of chilli pepper under various temperature environments. Int. J. Environ. Res. Public Health 2019, 16, 3031. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zheng, H.; Wu, L. Responses of soil organic and inorganic carbon to organic and phosphorus fertilization in a saline–alkaline paddy field. Geosci. Lett. 2023, 10, 15. [Google Scholar] [CrossRef]
- Liu, R.; Zhou, G.-P.; Chang, D.-N.; Gao, S.-J.; Han, M.; Zhang, J.-D.; Sun, X.-F.; Cao, W.-D. Transfer characteristics of nitrogen fixed by leguminous green manure crops when intercropped with maize in northwestern China. J. Integr. Agric. 2022, 21, 1177–1187. [Google Scholar] [CrossRef]
- Ge, S.; Zhu, Z.; Jiang, Y. Long-term impact of fertilization on soil pH and fertility in an apple production system. J. Soil Sci. Plant Nutr. 2018, 18, 282–293. [Google Scholar] [CrossRef]
- Li, C.; Aluko, O.O.; Yuan, G.; Li, J.; Liu, H. The responses of soil organic carbon and total nitrogen to chemical nitrogen fertilizers reduction base on a meta-analysis. Sci. Rep. 2022, 29, 16326. [Google Scholar] [CrossRef]
- Liu, Y.; Li, C.; Cai, G.; Sauheitl, L.; Xiao, M.; Shibistova, O.; Ge, T.; Guggenberger, G. Meta-analysis on the effects of types and levels of N, P, and K fertilization on organic carbon in cropland soils. Geoderma 2023, 437, 116580. [Google Scholar] [CrossRef]
- Zhao, Z.; Gunina, T.G.A.; Li, Y.; Zhu, Z.; Peng, P.; Wu, J.; Kuzyakov, Y. Carbon and nitrogen availability in paddy soil affects rice photosynthate allocation, microbial community composition, and priming: Combining continuous 13C labeling with PLFA analysis. Plant Soil 2019, 445, 137–152. [Google Scholar] [CrossRef]
- Del Amor, F.M. Yield and fruit quality response of sweet pepper to organic and mineral fertilization. Renew. Agric. Food Syst. 2007, 22, 233–238. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Yun, G.-S.; Jeon, Y.-M.; Kim, E.-J.; Noh, S.-J.; Park, S.-W.; Kim, T.-I.; Hong, S.T. Effects of winter cultivation of green manure crop and application of organic resources on watermelon yield and soil physico-chemical properties in greenhouse. Korean J. Soil Sci. Fertil. 2019, 52, 530–540. [Google Scholar] [CrossRef]
- Fracchiolla, M.; Lasorella, C.; Santamaria, P.; Renna, M.; Signore, A.; Cazzato, E. Response of organically grown mini watermelon (Citrullus lanatus (Thunb.) Matsum. & Nakai) to different green manure crops and nitrogen fertilization. Acta Hortic. 2020, 1294, 85–90. [Google Scholar] [CrossRef]
- Gao, F.; Li, H.; Mu, X.; Gao, H.; Zhang, Y.; Li, R.; Cao, K.; Ye, L. Effects of organic fertilizer application on tomato yield and quality: A meta-analysis. Appl. Sci. 2023, 13, 2184. [Google Scholar] [CrossRef]
- Özbolat, O.; Sánchez-Navarro, V.; Zornoza, R.; Egea-Cortines, M.; Cuartero, J.; Ros, M.; Pascual, J.A.; Boix-Fayos, C.; Almagro, M.; de Vente, J.; et al. Long-term adoption of reduced tillage and green manure improves soil physicochemical properties and increases the abundance of beneficial bacteria in a Mediterranean rainfed almond orchard. Geoderma 2023, 429, 116218. [Google Scholar] [CrossRef]
- Lin, S.; Pi, Y.; Long, D.; Duan, J.; Zhu, X.; Wang, X.; He, J.; Zhu, Y. Impact of Organic and Chemical Nitrogen Fertilizers on the Crop Yield and Fertilizer Use Efficiency of Soybean–Maize Intercropping Systems. Agriculture 2022, 12, 1428. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, C.; Stahr, K.; Kuzyakov, Y.; Wei, X. The effect of microorganisms on soil carbonate recrystallization and abiotic CO2 uptake of soil. Catena 2020, 192, 104592. [Google Scholar] [CrossRef]
- Tang, L.; Ren, F.; Li, Y.; Duan, Y.; Sun, N.; Zhao, P.; Li, Y.; Xu, M. The inorganic carbon fixation improved by long-term manure fertilization in kastanozems under rotation system of north China. Agronomy 2023, 13, 641. [Google Scholar] [CrossRef]
- Shanmugam, S.; Buehring, N.W.; Prevost, J.D.; Kingery, W.L. Soil bacterial community diversity and composition as affected by tillage intensity treatments in corn-soybean production systems. Microbiol. Res. 2021, 12, 157–172. [Google Scholar] [CrossRef]
- Zheng, W.; Gong, Q.; Zhao, Z.; Liu, J.; Zhai, B.; Wang, Z.; Li, Z. Changes in the soil bacterial community structure and enzyme activities after intercrop mulch with cover crop for eight years in an orchard. Eur. J. Soil Biol. 2018, 86, 34–41. [Google Scholar] [CrossRef]
- Hermans, S.M.; Buckley, H.L.; Case, B.S.; Curran-Cournane, F.; Taylor, M.; Lear, G. Using soil bacterial communities to predict physico-chemical variables and soil quality. Microbiome 2020, 8, 79. [Google Scholar] [CrossRef]
- Yaghoubi Khanghahi, M.; Murgese, P.; Strafella, S.; Crecchio, C. Soil biological fertility and bacterial community response to land use intensity: A case study in the mediterranean area. Diversity 2019, 11, 211. [Google Scholar] [CrossRef]
- Fierer, N.; Bradford, M.A.; Jackson, R.B. Toward an ecological classification of soil bacteria. Ecology 2007, 88, 1354–1364. [Google Scholar] [CrossRef] [PubMed]
- Lupwayi, N.Z.; Lafond, G.P.; Ziadi, N.; Grant, C.A. Soil microbial response to nitrogen fertilizer and tillage in barley and corn. Soil Tillage Res. 2012, 118, 139–146. [Google Scholar] [CrossRef]
- Fontaine, S.; Mariotti, A.; Abbadie, L. The priming effect of organic matter: A question of microbial competition? Soil Biol. Biochem. 2003, 35, 837–843. [Google Scholar] [CrossRef]
- Xun, W.; Zhao, J.; Xue, C.; Zhang, G.; Ran, W.; Wang, B. Significant alteration of soil bacterial communities and organic carbon decomposition by different long-term fertilization management conditions of extremely low-productivity arable soil in South China. Environ. Microbiol. 2016, 18, 1907–1917. [Google Scholar] [CrossRef]
- Ho, A.; Di Lonardo, D.P.; Bodelier, P.L.E. Revisiting life strategy concepts in environmental microbial ecology. FEMS Microbiol. Ecol. 2017, 93, fix006. [Google Scholar] [CrossRef]
- Torsvik, V.; Øvreås, L. Microbial diversity and function in soil: From genes to ecosystems. Curr. Opin. Microbiol. 2002, 5, 240–245. [Google Scholar] [CrossRef]
- Romero, F.; Hilfiker, S.; Edlinger, A.; Held, A.; Hartman, K.; Labouyrie, M.; van der Heijden, M.G.A. Soil microbial biodiversity promotes crop productivity and agro-ecosystem functioning in experimental microcosms. Sci. Total Environ. 2023, 885, 163683. [Google Scholar] [CrossRef]
- Bender, S.F.; van der Heijden, M.G.A. Soil biota enhance agricultural sustainability by improving crop yield, nutrient uptake and reducing nitrogen leaching losses. J. Appl. Ecol. 2015, 52, 228–239. [Google Scholar] [CrossRef]
- Wagg, C.; Bender, S.F.; Widmer, F.; van der Heijden, M.G.A. Soil biodiversity and soil community composition determine ecosystem multifunctionality. Proc. Natl. Acad. Sci. USA 2014, 111, 5266–5270. [Google Scholar] [CrossRef]
- Xiong, J.; Peng, S.; Liu, Y.; Yin, H.; Zhou, L.; Zhou, Z.; Tan, G.; Gu, Y.; Zhang, H.; Huang, J.; et al. Soil properties, rhizosphere bacterial community, and plant performance respond differently to fumigation and bioagent treatment in continuous cropping fields. Front. Microbiol. 2022, 13, 923405. [Google Scholar] [CrossRef] [PubMed]
- Allison, S.D.; Martiny, J.B.H. Resistance, resilience, and redundancy in microbial communities. Proc. Natl. Acad. Sci. USA 2008, 105, 11512–11519. [Google Scholar] [CrossRef] [PubMed]
- Geisseler, D.; Scow, K.M. Long-term effects of mineral fertilizers on soil microorganisms—A review. Soil Biol. Biochem. 2014, 75, 54–63. [Google Scholar] [CrossRef]




| Soil Texture | Clay Loam |
|---|---|
| pH (H2O) | 7.5 |
| Electrical conductivity (EC) | 0.9 dS m−1 |
| Organic carbon | 1.3% |
| Organic matter | 2.2% |
| Nitrogen (N) | 1.4 g kg−1 |
| C/N ratio | 9.3 |
| Calcium carbonate (CaCO3) | 223 g kg−1 |
| Available phosphorus | 18.5 mg kg−1 |
| Treatment | First Year | Second Year | Third Year |
|---|---|---|---|
| Tomato (Solanum lycopersicum L. cv. Dobler) | Watermelon (Citrullus lanatus cv. Modellino) | Pepper (Capsicum annuum L. cv. Senise) | |
| GMV | Green manure vetch + 75 kg N ha−1 of organic N fertilizer | Green manure vetch + 75 kg N ha−1 of organic N fertilizer | Green manure vetch |
| GMB | Green manure field bean + 75 kg N ha−1 of organic N fertilizer | Green manure field bean + 75 kg N ha−1 of organic N fertilizer | Green manure field bean |
| IF | Calcium nitrate (150 kg N ha−1) | Calcium nitrate (100 kg N ha−1) | Calcium nitrate (150 kg N ha−1) |
| GMW | Green manure wheat + 75 kg N ha−1 of organic N fertilizer | Green manure wheat + 75 kg N ha−1 of organic N fertilizer | Green manure wheat |
| MF | Livestock manure fertilization (40 t ha−1) + 75 kg N ha−1 of organic N fertilizer | Livestock manure fertilization (40 t ha−1) + 75 kg N ha−1 of organic N fertilizer | Livestock manure fertilization (40 t ha−1) |
| Cont | Control treatment (without nitrogen fertilization) | ||
| DB (t ha −1) | N Content (% of DB) | Supplied N (kg ha−1) | DB (t ha −1) | N Content (% of DB) | Supplied N (kg ha−1) | DB (t ha −1) | N Content (% of DB) | Supplied N (kg ha−1) | |
|---|---|---|---|---|---|---|---|---|---|
| First Year | Second Year | Third Year | |||||||
| GMV | 8.7 | 2.64 | 230 | 10.8 | 2.36 | 255 | 7.1 | 2.52 | 179 |
| GMB | 9.3 | 2.59 | 240 | 9.7 | 2.49 | 241 | 12.5 | 2.34 | 292 |
| GMW | 3.1 | 1.54 | 50 | 9.1 | 1.34 | 122 | 7.1 | 1.46 | 104 |
| MF | 8.0 | 1.50 | 120 | 8.0 | 1.50 | 120 | 8.0 | 1.50 | 120 |
| A | GMV | GMB | IF | GMW | MF | Cont |
|---|---|---|---|---|---|---|
| Phylum | ||||||
| Proteobacteria | 23.94 ± 0.60 a | 24.23 ± 0.47 a | 22.51 ± 1.12 ab | 23.18 ± 0.97 a | 23.12 ± 0.55 a | 20.76 ± 0.26 b |
| Actinobacteriota | 21.39 ± 0.55 a | 21.40 ± 2.08 a | 21.71 ± 0.99 a | 19.76 ± 3.22 a | 20.95 ± 3.48 a | 19.75 ± 0.70 a |
| Acidobacteriota | 9.72 ± 0.29 c | 9.71 ± 0.26 c | 11.28 ± 0.47 b | 10.95 ± 0.93 bc | 12.14 ± 0.54 ab | 13.11 ± 0.44 a |
| Bacteroidota | 10.56 ± 0.40 a | 11.51 ± 1.24 a | 10.45 ± 0.96 a | 12.90 ± 1.60 a | 8.59 ± 4.76 a | 11.10 ± 1.19 a |
| Chloroflexi | 8.16 ± 0.38 ab | 7.84 ± 0.25 ab | 7.49 ± 0.92 b | 7.49 ± 0.44 b | 10.32 ± 1.98 a | 7.91 ± 0.10 ab |
| Firmicutes | 4.29 ± 0.33 b | 4.23 ± 0.38 b | 7.38 ± 1.26 a | 5.53 ± 1.09 ab | 4.58 ± 0.18 b | 6.38 ± 0.55 ab |
| Planctomycetota | 7.24 ± 1.40 a | 6.35 ± 0.13 ab | 4.38 ± 1.12 b | 5.56 ± 1.32 ab | 6.59 ± 0.69 ab | 4.92 ± 0.33 b |
| Myxococcota | 3.42 ± 0.08 ab | 3.52 ± 0.22 a | 4.11 ± 0.17 a | 3.25 ± 0.42 ab | 2.62 ± 0.56 b | 3.91 ± 0.10 a |
| Gemmatimonadota | 2.47 ± 0.42 a | 2.68 ± 0.20 a | 2.75 ± 0.19 a | 2.43 ± 0.30 a | 2.79 ± 0.25 a | 2.83 ± 0.19 a |
| Verrucomicrobiota | 3.70 ± 0.13 a | 3.29 ± 0.20 a | 3.07 ± 0.17 a | 3.76 ± 1.02 a | 2.38 ± 1.19 a | 3.57 ± 0.26 a |
| Entotheonellaeota | 1.36 ± 0.12 a | 1.34 ± 0.04 a | 1.18 ± 0.27 a | 1.33 ± 0.04 a | 1.64 ± 0.31 a | 1.51 ± 0.12 a |
| Nitrospirota | 1.37 ± 0.03 a | 1.41 ± 0.29 a | 1.25 ± 0.12 a | 1.24 ± 0.10 a | 0.95 ± 0.15 a | 1.30 ± 0.14 a |
| B | ||||||
| Ratio of Proteobacteria to Acidobacteria | 2.46 ± 0.36 a | 2.50 ± 0.42 a | 2.00 ± 0.38 b | 2.12 ± 0.26 b | 1.90 ± 0.23 b | 1.58 ± 0.19 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yaghoubi Khanghahi, M.; Curci, M.; Cazzato, E.; Lasorella, C.; Traversa, A.; Crecchio, C.; Spagnuolo, M. Shifts in Soil Bacterial Communities under Three-Year Fertilization Management and Multiple Cropping Systems. Soil Syst. 2024, 8, 5. https://doi.org/10.3390/soilsystems8010005
Yaghoubi Khanghahi M, Curci M, Cazzato E, Lasorella C, Traversa A, Crecchio C, Spagnuolo M. Shifts in Soil Bacterial Communities under Three-Year Fertilization Management and Multiple Cropping Systems. Soil Systems. 2024; 8(1):5. https://doi.org/10.3390/soilsystems8010005
Chicago/Turabian StyleYaghoubi Khanghahi, Mohammad, Maddalena Curci, Eugenio Cazzato, Cesare Lasorella, Andreina Traversa, Carmine Crecchio, and Matteo Spagnuolo. 2024. "Shifts in Soil Bacterial Communities under Three-Year Fertilization Management and Multiple Cropping Systems" Soil Systems 8, no. 1: 5. https://doi.org/10.3390/soilsystems8010005
APA StyleYaghoubi Khanghahi, M., Curci, M., Cazzato, E., Lasorella, C., Traversa, A., Crecchio, C., & Spagnuolo, M. (2024). Shifts in Soil Bacterial Communities under Three-Year Fertilization Management and Multiple Cropping Systems. Soil Systems, 8(1), 5. https://doi.org/10.3390/soilsystems8010005








