Abstract
In the Pistoia Nursery-Ornamental Rural District (Italy), a leader in Europe in ornamental nurseries covering over 5200 hectares with over 2500 different species of plant, plant-parasitic nematodes represent a serious concern. The potential efficacy of a pot cultivation system using commercial substrates to control plant-parasitic nematodes was assessed. On two different plant species, two different pot cultivation managements, potted plants, and potted plants previously cultivated in natural soil were compared to plants only cultivated in natural soil. The entire soil nematode structure with and without plants was evaluated. The relationship between soil properties and soil nematode community was investigated. All the studied substrates were free from plant-parasitic nematodes. Regarding free-living nematodes, Peat–Pumice showed nematode assemblage established by colonizer and extreme colonizer bacterial feeders, whereas Peat–Perlite included both bacterial and fungal feeders, and, finally, coconut fiber also included omnivores and predators. In farming, the substrates rich in organic matter such as coconut fiber could still play an important role in suppressing plant-parasitic nematodes because of the abundance of free-living nematodes. In fact, they are of crucial importance in both the mineralization of organic matter and the antagonistic control of plant-parasitic nematodes. Potting systems equally reduce virus-vector nematodes and improve the prey/predator ratio favoring natural control.
1. Introduction
The Pistoia Nursery-Ornamental Rural District (Italy) is a leader in Europe in ornamental nurseries covering over 5200 hectares, with approximately 1000 hectares of potting plants, 1500 companies, and over 5500 employees. In total, the Gross Saleable Product (GSP) is over 700 million Euros of which 360 is related to export (www.cespevi.it; https://group.intesasanpaolo.com/it/research/monitor-dei-distretti-agroalimentari; accessed on 11 January 2024). Over 2500 plant species are cultivated and plant-parasitic nematodes characterized by high polyphagies represent a serious concern. Even though extensive research has been devoted to the investigation of the effect and management of plant-parasitic nematodes in crops, the effect of these pests on the ornamental plant industry remains a relatively understudied field [1]. There are many categories of plant-parasitic nematodes that affect ornamental plants, with the main genera being Meloidogyne, Aphelenchoides, Paratylenchus, Pratylenchus, Helicotylenchus, Radopholus, Xiphinema, Trichodorus, Paratrichodorus, Rotylenchulus, and Longidorus.
The majority of states in the world have implemented measures to contrast the spread of plant pests by enforcing strict controls on the import/export of plant materials. The new European legislation on protective measures against plant pests (Regulation EU 2016/2031) imposes a “prohibition on the introduction and movement of EU regulated non-quarantine pests on plants for planting”. The nurserymen as “professional operators shall not introduce a Union regulated non-quarantine pest into, or move that pest within, the EU territory on the plants for planting through which it is transmitted” (art. 37 Reg. EU 2016/2031). A list of eighteen nematode species belonging to the genera Ditylenchus, Tylenchulus, Aphelenchoides, Heterodera, Meloidogyne, Pratylenchus, Longidorus, and Xiphinema was established in Annex IV of Regulation (EU) 2019/2072. These plant-parasitic species, already present and widespread in the European Union, represent a phytosanitary risk and potentially determine a severe economic, social, and environmental impact in the EU territory. It is worth remembering that, for these nematodes, the accepted damage tolerance threshold is “zero”. It is worth noting also that in the Pistoia Nursery-Ornamental Rural District (Italy), the application of the EU legislation represents a serious threat to the plant industry because, concurrently, the 91/414 EEC Directive also imposed an important reduction in the number of plant protection products suitable for marketing. For this reason, a progressive elimination of many pesticides is still ongoing and, thus, the control of plant-parasitic nematodes is becoming more difficult.
In this context, it is crucial to understand the role of different commercial substrates or natural soil in reducing the risk of plant-parasitic nematode infestation in the nursery. Using healthy commercial substrates or soil is critical for maintaining low infestation levels of plant-parasitic nematodes. Furthermore, this “exclusion approach” contributes to the prevention of accidental pest introduction.
At the same time, soil and commercial substrates may also host many free-living nematodes playing a significant role in controlling plant-parasitic nematodes [2,3]. Several studies have shown that populations of these nematodes decrease with the increase in saprophytic nematodes (bacterial and fungal feeders) [4,5]. Moreover, soil predator nematodes deserve consideration for their potential activity in pest-regulation services [3].
To date, few studies have been conducted to evaluate the impact of pot cultivation systems of ornamental plants on soil plant-parasitic nematodes. The use of solid substrates such as peat, coir, bark, sawdust, compost, rockwool, perlite, pumice, sand, and vermiculite is considered a valid soil management aimed at controlling plant-parasitic nematodes [6]. Short-term trials on tomatoes demonstrated the absence of plant-parasitic nematodes in pots with coconut and peat substrates [2]. However, other authors asserted that soilless culture systems are not effective in the elimination of these noxious organisms [4,7]. Moreover, plant-parasitic nematodes can be introduced by infested propagation materials, from farm appliances, and by irrigation [8,9].
The aim of this study was to assess the potential efficacy of pot cultivation systems using commercial substrates to avoid plant-parasitic nematode infestations and simultaneously increase free-living nematode populations. Two different pot cultivation managements, potted plants, and potted plants previously cultivated in natural soil, were compared to plants only cultivated in natural soil. In detail, the effect of the pot cultivation system was evaluated on (i) the entire soil nematode structure, with and without plants, in Acer palmatum and x Cupressocyparis leylandii cultivations and (ii) the relationship between soil properties (i.e., physical and chemical) and soil nematode community.
2. Materials and Methods
2.1. Field Site
The experimental site was located at “Vannucci Piante” farm, in the Pistoia district (Central Italy; 43.85096 N, 10.98172 E; 55 m a.s.l.), one of the most important ornamental nursery areas in Europe. The local climate was classified as Cfa (temperate, no dry season, hot summer) by Köppen Climate Types, characterized by a mean air temperature of 15.5 °C and a mean annual precipitation of 1120 mm throughout the two years of the study from 2020 to 2021 (Regione Toscana–Settore Idrologico e Geologico Regionale http://www.sir.toscana.it, accessed on 11 January 2024). The average minimum temperature was 2.5 °C in January 2020, and the average maximum temperatures were 24.2 °C and 26.7 °C in July and August, respectively; the precipitation was concentrated in winter, particularly in December, and it ranged from 261.6 to 326.8 mm in the three years of study.
The plants used in the trial, A. palmatum and x C. leylandii, were cultivated under (i) open-air conditions in natural soil and (ii) shaded structures in pots (18 liters/potted plant) with the standard coconut-fiber substrate.
As regards plant cultivation in the pot, A. palmatum and x C. leylandii seedlings or sprouted vegetative propagation material were initially planted in Peat–Perlite (75:25 v v−1) (for 3–4 months), then transplanted into Peat–Pumice (60:40 v v−1) (for 18–24 months), and finally into coconut fiber (coir dust–coir fiber 70:30 v v−1) for each of the following transplants. All these commercial substrates were “added” with controlled released fertilizers. Concerning commercialization, plants cultivated in natural soil were used to be potted and then left in the nursery for a variable time until marketing. Irrigation and fertigation were the same for all plots; the same phytosanitary treatments were applied, even though no chemical was used to control plant-parasitic nematodes. Specifically, for both plant species, Universol Blue ICL fertilizer (18-11-18 for N, P2O5, and K2O, respectively, plus 2.5 MgO and microelements: B 0.01%, Cu 0.01%, Fe 0.1%, Mn 0.04%, Mo 0.001%, Zn 0.01%) were used (1 g/L twice a week). As regards phytosanitary treatments, one treatment with Sulphoxaflor and another with Acetamiprid were used against aphids on A. palmatum, whereas 2–3 treatments with copper oxychloride were applied against fungal pathogens on C. leylandii.
2.2. Experimental Design and Soil Sampling
First trial: substrates in the absence of plants. In spring 2020, three different substrates, Peat–Perlite (PPE), Peat–Pumice (PPU), and coconut fiber (CF), were compared, before their use, to evaluate soil nematode community structures in the absence of plants. Moreover, as regards coconut fiber (the substrate used for the longest period in the trial), possible changes in the soil nematode community were evaluated at intervals of 0, 10, 30, 60, and 90 days throughout the time in which this material remained in the transplant area of the nursery before usage. To characterize the soil nematode community, eighteen soil samples were collected for each substrate and at each time interval.
Second trial: substrates with a plant. Young plants of A. palmatum and x C. leylandii were selected for the experiments. The absence of plant-parasitic nematodes was confirmed by previous sampling and analysis of the roots of these plants following the EPPO protocol [10]. For each plant species, potted plants (PP) and potted plants previously cultivated in natural soil (PNS) were compared to plants only cultivated in natural soil (NS). A total of fifty plants per plant species and per treatment was used. In 2020 and 2021, three samples of soil were withdrawn in each season, with the purpose of determining soil nematode structure (i.e., 3 samples/treatment, for each season, for 2 years, for a total of 72 samples). Once surface residues were removed, a hand auger was used to carry out each sampling (5 cm diameter inside) from the 30 cm deep top layer of bare soil. For every sample of soil, six cores were randomly withdrawn and subsequently mixed in order to form a single composite sample.
2.3. Soil Physical and Chemical Analysis
The physical and chemical properties of representative soil profiles and the commercial substrates reported in the field site were determined. Regarding soil, considered parameters were texture, according to the International Society of Soil Science (ISSS) standards, pH and EC (1:2.5 and 1:5 water extraction method, respectively), total Kjeldahl nitrogen, N-NO3, N-NH4, P and K content, organic matter percentage, C/N ratio, and active CaCO3. Physical characterization of commercial substrates included the water volume for the determination of available water capacity, the air-filled porosity, the holding water capacity (W −1 kPa), the bulk density (BD), and the total porosity (TP), determined as described by De Boodt et al. [11]. Water tensions at 0, −1, −2, −3, −5, and −10 kPa were used to elaborate the water retention curve. Then, pH was measured according to the EN 13037/1999 method, while EC according to the EN 13038/1999.
2.4. Analysis of Soil Nematode Community
The cotton-wood filter extraction method was used to isolate nematodes from 100 mL of each soil sample. Extraction was performed at room temperature for 48 h at approximately 20 °C. A 25 µm mesh was used to sieve each nematode suspension and nematodes were then counted by a stereomicroscope (50× magnification). Temporary slides were made for all specimens and their identification to genus level was carried out at higher magnification using the keys of Mai and Lyon [12], Bongers [13], and Marinari-Palmisano and Vinciguerra [14]. Taxonomic families were assigned to trophic groups based on Yeates et al. [15] and Okada et al. [16]. Nematode communities were characterized adopting the following criteria: (i) total abundance of individuals; (ii) richness determined by counting the number of taxa; (iii) Maturity (MI) and Plant Parasitic indices (PPI) determined following Bongers [17] and the food web indicators (BI, Basal Index; EI, Enrichment Index; SI, Structure Index; CI, Channel Index) following Ferris et al. [18]; (iv) diversity-weighted abundance (θ) expressed as biomass [19] and categorizing populations of soil nematodes on a functional basis into plant-parasitic nematodes, predators (including omnivores), and detritivores (bacterial and fungal feeders), in accordance with Ferris and Tuomisto [20] for evaluating the eco-system services efficiency; (v) prey-to-predator θ mass ratio aimed at evaluating regulation function for evaluating the ecosystem services efficiency.
2.5. Statistical Analysis
One-way ANOVA was performed to assess the influence of substrate on nematode taxa abundance and indicators of nematode community structure (first trial). Two-way ANOVA was carried out to assess management and plant species effects on nematode taxa abundance and nematode indicators in the second trial. When the F-test was significant at p < 0.05, mean treatments were compared using the Student–Newman–Keuls test (SNK) using CoStat Statistical Software 6.4 2021. Moreover, the comparison of nematode communities was performed by the multi-variate methods of the past analysis package [21]. Nematode communities were compared using analysis of similarity (ANOSIM), and SIMPER analysis based on the Bray–Curtis similarity index, nearest neighbor [22]. The nematode abundance data were square root transformed before the analysis. Bonferroni correction was then applied. Canonical Correspondence Analysis (CCA) was performed in order to evaluate the interactions between the communities of nematodes (abundance of nematode taxa and its indicators) and both soil chemical and physical variables (EC, soil pH, and TOC). After the analysis, we selected only the significant environmental axes, which were then graphically represented by vectors. Finally, we assessed the statistical significance of the relationship between environmental and community variables by the permutation test of both the first ordination, axis as well as the combination of both the first and second axes.
3. Results
3.1. Soil Physical and Chemical Properties
Natural soil was classified as loamy sand according to the USDA Soil Taxonomy. The electrical conductivity (EC) values ranged from 448 µS cm−1 (1:2 dry material/water extract) in the x C. leylandii plot to 2680 µS cm−1 in the A. palmatum plot, and the soil pH values were 4.7 and 6.8 for A. palmatum and x C. leylandii areas, respectively. The organic matter content was always less than 2%, which is considered the soil quality critical threshold in temperate regions [23]. Specifically, the natural soils, in which A. palmatum and C. leylandii were cultivated, had an organic matter content of 1.4% and 0.72%, respectively.
Regarding the substrate’s main chemical–physical characterization, data are shown in Table 1, while the retentions curve did not show remarkable trends with respect to the recognized optimal range [24,25].

Table 1.
Chemical–physical characterization of the used substrates for potted plant cultivation.
3.2. First Trial: Substrates in Absence of Plants
Ten families of free-living nematodes were identified in soil samples from three different commercial substrates. No plant-parasitic nematode family was found. Most families were consistently found in CF and, to a lesser extent, in PPE. Only two families were present in PPU. One-way ANOSIM analysis on nematode abundance showed significant differences in nematode taxa abundance (R 0.61 and p = 0.0005). The R values, calculated for substrate CF-PPE and CF-PPU pairwise comparison, were 0.52 (p = 0.05) and 0.57 (p = 0.001), respectively, indicating that the coconut fiber substrate was different from the other ones. No significant difference was found between PPE-PPU.
The SIMPER analysis showed 70.36% of the overall dissimilarity and family breakdown of similarity indicating that eight families accounted for 95% of this similarity (Table 2). Differences were mainly due to a higher proportion of Neotylenchidae, Aphelenchoidae, and Dorylaimidae. Following the one-way ANOVA analysis, the abundance of individuals belonging to the Dorylaimidae was significantly higher in CF than in other substrates. In general, the fungal feeder nematodes were more abundant in PPE and, albeit to a lesser extent, in CF: specifically, Neotylenchidae and Anguinidae (mainly Ditylenchus myceliophagus) were more numerous in PPE than in CF and PPU; and the abundance of Aphelenchoidae (mainly Aphelenchoides composticola, Bursaphelenchus fungivorus, and Ektaphelenchoides sp.) was higher in PPE and CF than PPU. In PPU, only bacterial feeder nematodes were found.

Table 2.
Percentage contribution to the Bray–Curtis dissimilarity in family nematode abundance among the different substrates (SIMPER analysis). Mean values and standard errors are reported. Different letters for the same parameter indicate significantly different values (SNK test, p < 0.05).
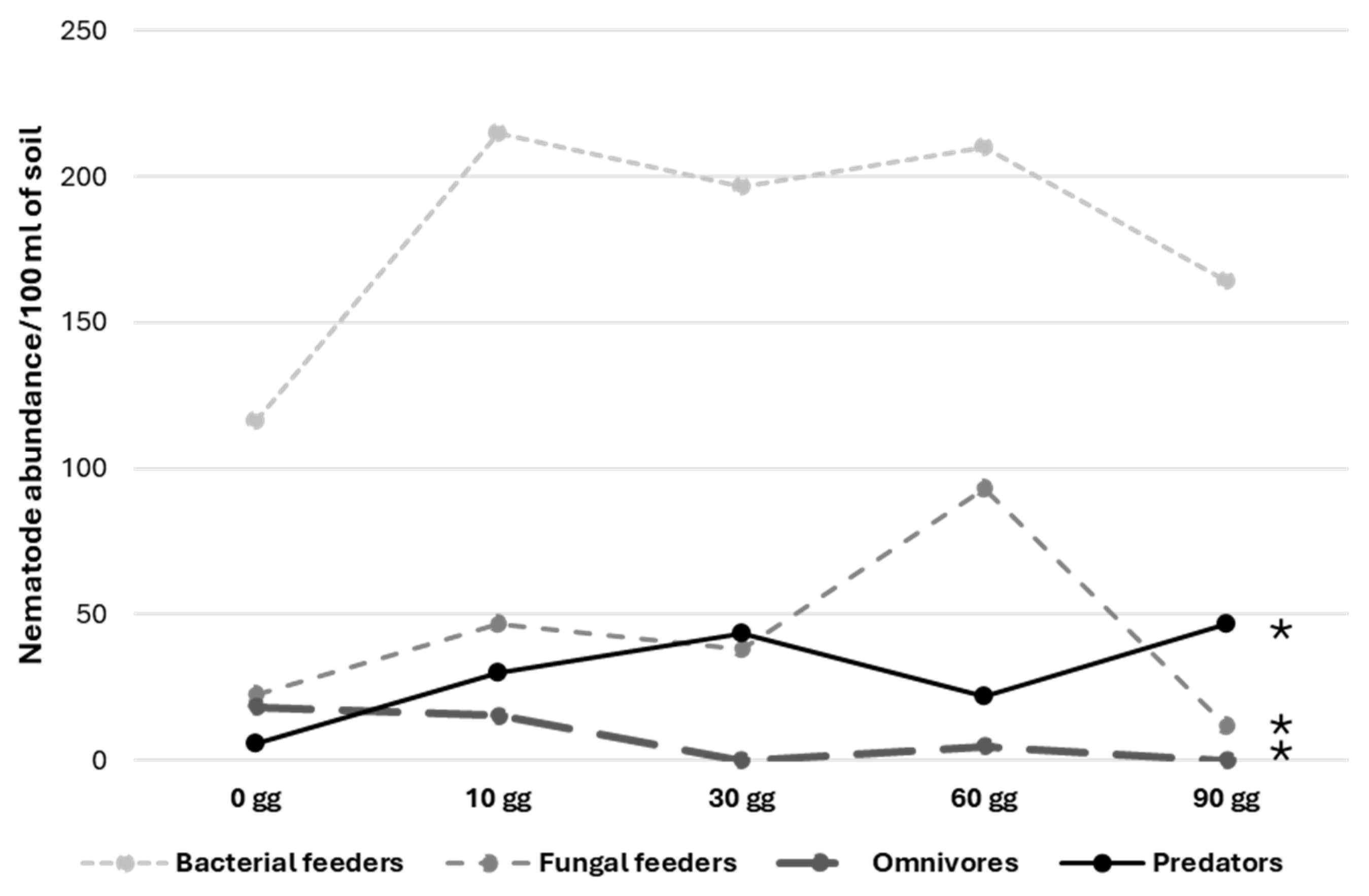
The evaluation of coconut fiber throughout the time in which this material remained in the transplant area (at 10, 30, and 60 days before usage) showed a significant increase in omnivores, predators, and fungal feeder nematodes (Figure 1). Bacterial feeder nematodes remained the dominant trophic group, even though no significant evidence emerged.
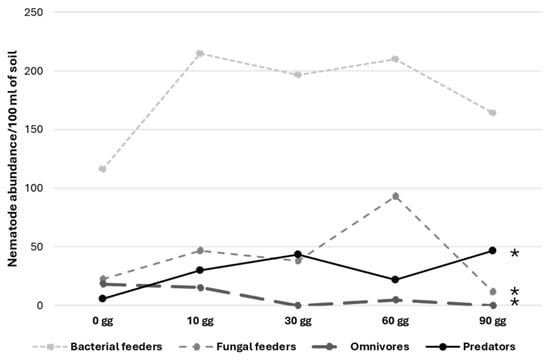
Figure 1.
Time trends of different nematode trophic groups in coconut fiber placed in the transplant area before usage. ANOVA analysis * = significant 0.01 < p < 0.05.
In Table 3, MI values ranged from 1.5 to 1.9, indicating the conspicuous presence of generalist and opportunistic species; the significantly highest values were found in CF and PPE. EI was higher in PPU and CF, while SI was significantly higher in CF than in other substrates. Finally, CI showed values lower than 50 in all types of substrates, and the highest values were found in PPE.

Table 3.
Soil nematode indices refer to different commercial substrates. Different letters for the same parameter indicate significantly different values (SNK test, p < 0.05).
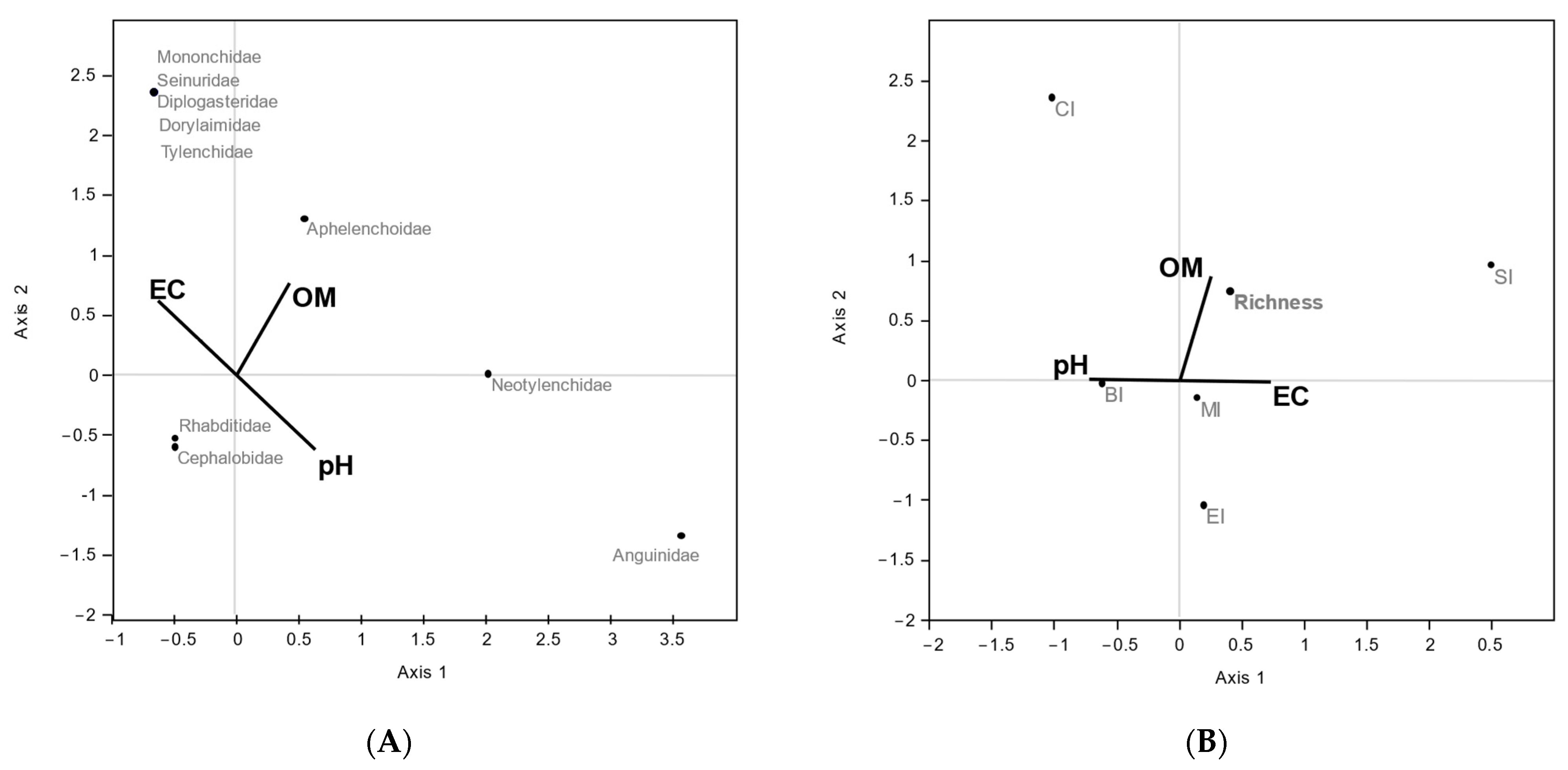
The CCA conducted between nematode taxa abundance and commercial substrate variables (organic matter content (OM), EC, and pH), evidenced that axis 1 was dominated by EC (−0.63) and pH (0.63), while axis 2 was dominated by OM (0.77). Tylenchidae, Diplogasteridae, Seinuridae, Dorylaimidae, and Mononchidae families were positively associated with organic matter content while EC Neotylenchidae and Anguinidae were positively associated with pH. The bacterial feeder families of Rhabditidae and Cephalobidae were poorly affected by soil parameters (Figure 2A).
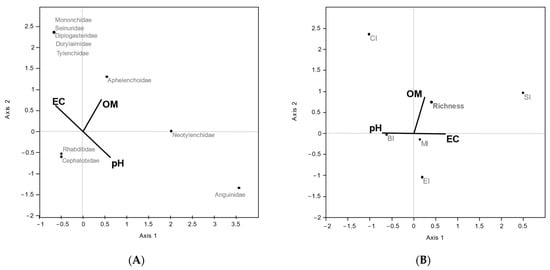
Figure 2.
Scatter plot of CCA ordination showing relationships between soil properties and nematode taxa abundance (A) and soil nematode indicators (B). (A) Percentages of variance were 69.27% (p < 0.002) for axis 1 and 30.73% (p < 0.001) for axis 2; (B) percentages of variance were 62.65% (p < 0.01) for axis 1 and 37.35% (p < 0.002) for axis 2.
The biplot of CCA including soil nematode indicators and the OM, EC, and pH environmental variables showed axis 1 dominated by EC (0.73) and pH (−0.73) and axis 2 by OM (0.87). SI was positively influenced by EC and organic matter content, and, instead, CI was positively correlated with organic matter and to a lesser extent with pH (Figure 2B).
3.3. Second Trial: Substrates with Plants
Seventeen plant-parasitic and free-living nematode families were identified in soil samples collected. In detail, 16 and 15 families were found on A. palmatum and x C. leylandii, respectively. Regarding management, consistent differences in the number of taxa were found: the lowest number of families was in PP (7 families), especially for plant-parasitic nematodes (only one family), compared to PNS (16 families) and NS (15 families). In general, ANOSIM analysis on nematode abundance confirmed the low difference between the two plant species (R 0.23, p = 0.0001) and higher differences per soil management (R 0.36, p = 0.0001). Pot conditions were different from cultivation in natural soil: R values in pairwise comparison were for the management PP-PNS 0.44 (p = 0.002); for PP-NS, 0.53 (p = 0.0003); and for PNS-NS 0.31 (p = 0.001). The analysis of the contribution of family abundance to the average Bray–Curtis dissimilarity, among managements using SIMPER, was 57.27% of the overall dissimilarity; the family breakdown of similarity showed that 15 families accounted for 95% of this similarity. Differences were mainly due to a higher proportion of Rhabditidae, Cephalobidae, and Dorylaimidae in PNS than in the PP and NS.
Two-way ANOVA for plant factor further evidenced that Diphterophoridae, Telotylenchidae (mainly Trophurus spp.), Heteroderidae, and Hoplolaimidae (mainly Rotylenchus spp. and to a lesser extent Helicotylenchus spp. and Scutellonema spp.) were significantly higher in A. palmatum than x C. leylandii, while Aphelenchoidae, Tylenchidae, Psilenchidae, Pratylenchidae, and Trichodoridae were significantly more abundant in x C. leylandii than in A. palmatum. As regards management, Seinuridae, Tylenchidae, Psilenchidae, and Pratylenchidae (mainly Pratylenchus vulnus) were significantly higher in PNS than others (Table 4). The virus-vector plant-parasitic nematodes Trichodoridae and Longidoridae showed the highest abundance in NS. Moreover, significant interactions between plant and management factors were found.

Table 4.
Effects of different managements in A. palmatum and x C. leylandii on the abundance of nematode taxa (mean number ± SE of nematodes/100 mL soil). Samples were collected from PP, potted plants; PNS, potted plants previously cultivated in natural soil; NS, plants only cultivated in natural soil. Different letters for the same parameter indicate significantly different values (SNK test, p < 0.05).
The soil nematode indicators showed few differences between the two plant species: BI was significantly higher in A. palmatum than x C. leylandii, while an opposite result was recorded for SI Table 5. On the contrary, many differences were found per management. Soil nematode communities in natural soil showed the highest MI, PPI, and CI values, while BI and EI values were significantly higher in PNS. As expected, the plants only cultivated in pots using coconut fiber substrate exhibited the lowest MI, PPI, and BI values.

Table 5.
Effects of different managements in A. palmatum and C. leylandii on soil nematode indices (±SE). PP, potted plants; PNS, potted plants previously cultivated in natural soil; NS, plants only cultivated in natural soil. Different letters for the same parameter indicate significantly different values (SNK test, p < 0.05).
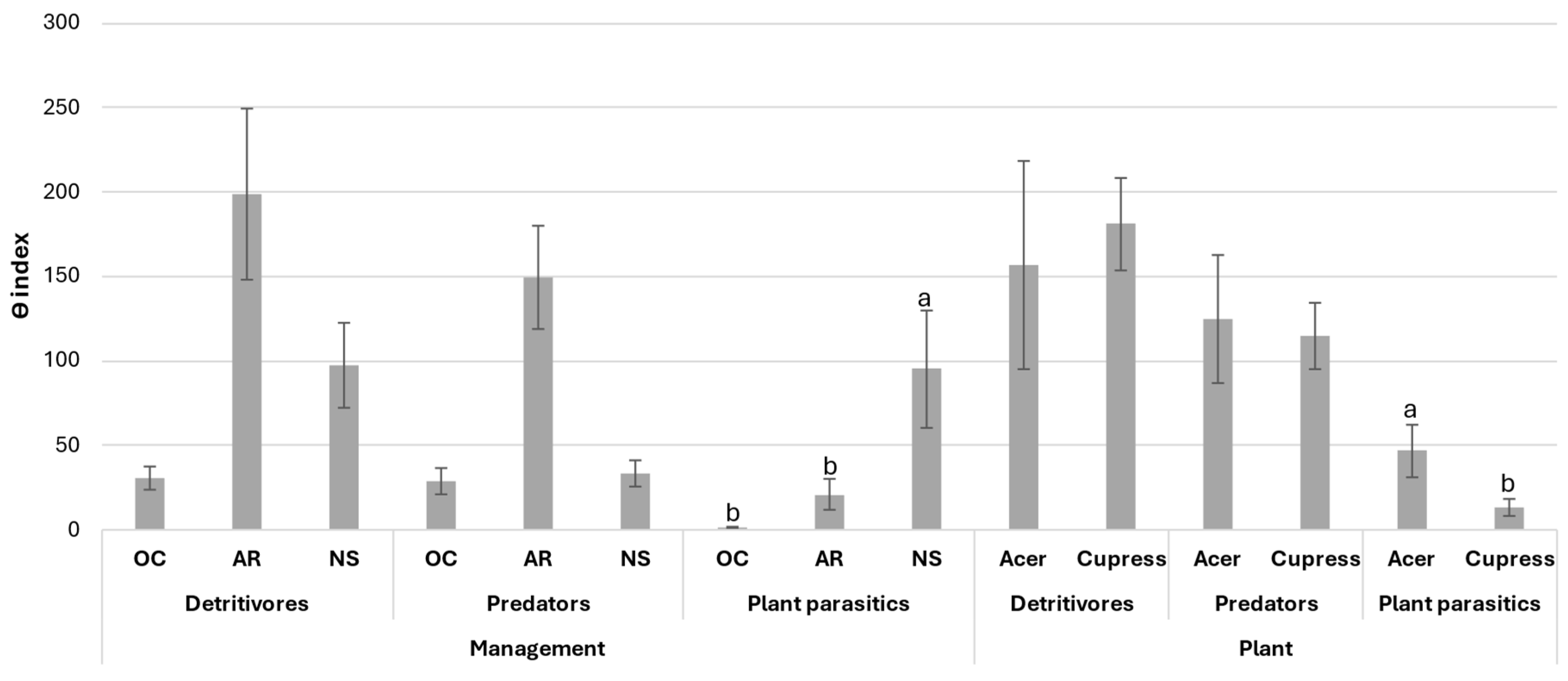
The average values of the diversity-weighted abundance (θ) index are reported in Figure 3. Significant differences were found only in the plant-parasitic channel: the highest value was reported in NS. The regulation functions of opportunistic and plant-parasitic nematodes by predation were greater in PP and to a lesser extent in PNS; they ranged from 1 to 1.1 and from 1 to 1.47 for PP and PNS, respectively. By contrast in NS, the high θ value of the plant-parasitic channel together with the low θ value in the predator channel, caused a predator/prey ratio of 1:5.74 indicating an insufficient regulation. No differences were found between the two different plant species: the predator/prey ratio ranged from 1:1.63 to 1:1.69 for A. palmatum and x C. leylandii, respectively.
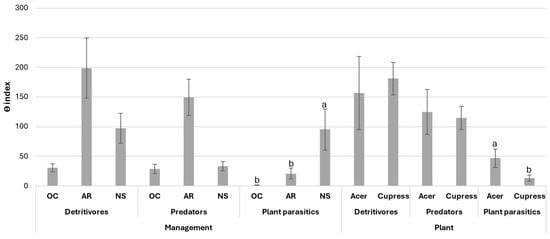
Figure 3.
Diversity-weighted abundance (θ) index for functional classes of soil nematode assemblage. PP, potted plants; PNS, potted plants previously cultivated in natural soil; NS, plants only cultivated in natural soil. Standard errors are reported. Different letters are significant differences at p < 0.05.
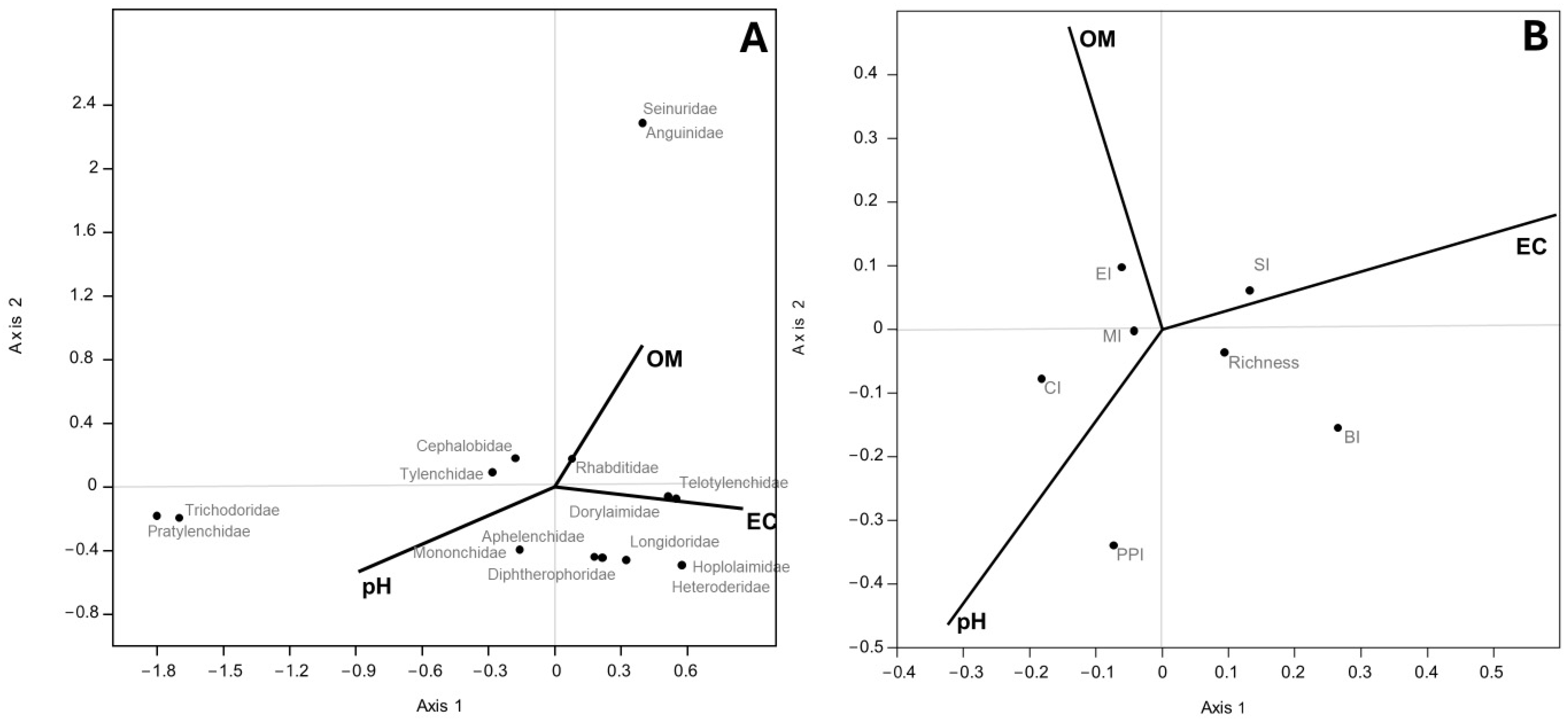
The CCA, conducted between nematode taxa abundance from PP and NS and soil variables (organic content, soil pH, and EC) showed that axis 1 was dominated by EC (0.85) and pH (−0.89), while axis 2 was by OM (0.89). The families of Seinuridae and Anguinidae were positively associated with OM; instead, the families of Trichodoridae and Pratylenchidae were related to pH. The biplot of CCA conducted between soil nematode indicators and the same soil variable showed that axis 2 was dominated by OM (0.48 and pH −0.46); the PPI, which plotted furthest from the origin and so varied the most within this environmental gradient, was positively associated with the pH and inversely with OM (Figure 4A).
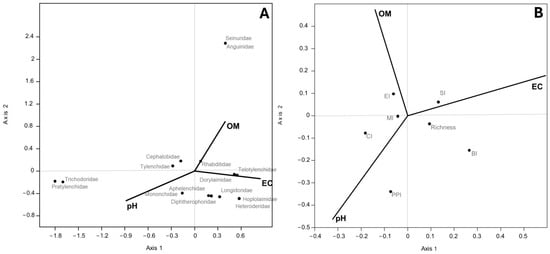
Figure 4.
Scatter plot of CCA ordination showing relationships between soil properties and nematode taxa abundance (A) and soil nematode indicators (B). (A) Percentages of variance were 54.96% (p < 0.001) for axis 1 and 45.04% (p < 0.001) for axis 2; (B) percentages of variance were 30.08% (p < 0.016) for axis 2 and no significant axis 1.
4. Discussion
This study investigated the response of the soil nematode community (i.e., both plant-parasitic and free-living nematodes) to artificial substrates both before and during agricultural practices. It is worth noting that it is relatively difficult to obtain a comprehensive picture of the general problem, as ornamental nurseries represent a complex network formed by a large variety of plants, with their own cultivation, nutritional, and phytosanitary requirements. In this study, the context was simplified by choosing only two different plant species. Specifically, X Cupressocyparis leylandii and A. palmatum were selected as they are representative of conifers and broad-leaved trees and because they are economically relevant cultivated species in the Pistoia district.
4.1. Effect of Pot Cultivation on Artificial Substrate Soil Nematode Community Structure
As previously reported by several authors, the use of artificial substrates, such as perlite, pumice, and coconut fiber, represents a valid practice aimed at reducing the introduction of plant-parasitic nematodes in nurseries [2,6]. This study provided a reasonable explanation of this trend. These substrates created an unsuitable environment for plant-parasitic nematodes due to the absence of plants and the abundance of free-living nematodes showing a composition typical of organic materials (i.e., compost) [26]. It is well known that as free-living nematodes increase, plant-parasitic nematodes decrease [4,27]. The dominant trophic groups were bacterial and fungal feeders, both involved in the organic matter decomposition. As reported by Ferris & Matute [26] and Georgieva et al. [28], after the application of compost, it is possible to identify a food web succession driving the mineralization process: colonizer and extreme colonizer bacterial feeders start the activity of decomposition, while fungal feeders may subsequently develop. The three analyzed substrates were presumably different phases of this ecological succession: PPU was characterized by the presence of only colonizer and extreme colonizer bacterial feeders, PPE included both bacterial and fungal feeders, and, finally, CF also included omnivores and predators. These differences might be attributed to different content in organic matter, higher in CF than others, and the different characteristics of inert materials such as pumice and perlite. Moreover, fungal feeders, omnivores, and predators increased throughout the period in which CF remained in the transplant area exposed to the air. Substrate aeration probably stimulated microorganism and nematode growth and consequently allowed predator development. The soil nematode indicators confirmed this trend: CF showed the highest values of MI and SI, indicating both the presence of colonizers and a better soil nematode community structure than PPE and PPU. On the contrary, CF showed the lowest EI value. CI indicated that the composition channel was driven by bacteria, even if in PPE the fungal channel was also relevant.
Pot cultivation changed the composition of the nematode community, as shown by similarity analysis. In general, the soilless practice conducted only in pot conditions produced a decrease in the total number of taxa and abundance of nematodes when compared with natural soil conditions. The potted plants, previously cultivated in natural soil, showed an opposite trend. In general, free-living nematodes were moderately affected by pot cultivation, and, in all cases, the dominant trophic group was represented by bacterial feeders followed by fungal feeders, omnivores, and predators. The high presence of free-living nematodes involved in the detritus food web and the graze on bacteria and fungi in the soil may regulate decomposition and nitrogen mineralization in soil ecosystems, as well as in pot cultivations [2,29]. On the contrary, plant-parasitic nematodes were the trophic group mainly affected by variations due to plant species and management. Plant-parasitic nematode communities were peculiar to each examined plant species. Palomares-Rius et al. [30] found that nematode community populations in the rhizosphere of cultivated olives differed according to plant genotype. Although the families were the same, Telotylenchidae, Hoplolaimidae, and Longidoridae were dominant in A. palmatum, while Pratylenchidae, Psilenchidae, and Trichodoridae were more numerous in x C. leylandii. A key role could be played by host–parasite interaction, a factor scarcely investigated yet.
Soilless farming in pots reduced the number of plant-parasitic nematodes in A. palmatum and x C. leylandii plants more than in cultivation in natural soil. In this regard, only a few individuals belonging to the Telotylenchidae family were found. Because coconut fiber substrates were nematode-free, it can be assumed that these plant-parasitic nematodes were accidentally introduced [8,9]. As reported by Hug and Malan [31], the risk of contamination with plant-parasitic nematodes was low only when capped boreholes were the source of irrigation water. According to the same authors, the plant-parasitic nematodes of economic importance found in irrigation water belonged mainly to the genera Meloidogyne, Xiphinema, Tylenchulus, Trichodorus, Criconemoides, and Pratylenchus. On the contrary, in potted plants previously cultivated in natural soil, plant-parasitic nematodes found environments more suitable for growth. The abundance of the families Telotylenchidae, Pratylenchidae, and Hoplolaimidae increased, while virus-vector nematodes belonging to Longidoridae and Trichodoridae decreased. The more homogeneous fertilization and irrigation in pot probably favored root development and consequently enhanced plant-parasitic nematodes, leading to their exponential growth. On the contrary, the constrained space negatively affected the development of virus-vector nematodes, as evidenced by Ali et al. [32] and Landi et al. [33] reporting that the intensification of agricultural practices disfavoured these nematodes.
The nematode indicators confirmed that differences were mainly due to management rather than to plant species. Pot cultivation reduced PPI because of the decrease in k-strategist plant-parasitic nematodes such as virus vectors from natural soil. Moreover, soilless conditions also reduced MI due to the increment of colonizer species, especially in PP [3]. BI and EI were higher after repotting, suggesting that the fungal feeders benefited from these practices, probably due to the high organic matter content supplied by the coconut fiber substrate. CI evidenced that the channel decomposition was driven by bacteria. In terms of ecosystem services, the application of diversity-weighted abundance, expressed as biomass, evidenced that free-living nematodes implicated in nutrient mineralization and plant-parasitic nematodes appeared more regulated in pot farming than in natural soil. However, PP and PNL showed optimal (prey/predator ratio 1:1) and suboptimal regulation values, respectively.
4.2. Soil Factor Influencing Soil Nematode Structure
In general, the commercial substrates were rich in organic matter, especially CF. As reported by Landi et al. [3,5], many families were positively affected by organic carbon, especially the predators Mononchidae and the omnivores Dorylaimidae, while the Anguinidae family was favored by soil pH. On the whole, the soil nematode indicators confirmed that organic matter improved the soil nematode community structure. SI and CI were mainly positively correlated with organic matter. In accordance with several authors, during farming, the presence of roots in soil confirmed that organic matter was the relevant factor for the development of free-living nematodes and the decrease in plant-parasitic nematodes [3,4]. The families Seinuridae (predators) and Anguinidae (fungal feeder nematodes) were always favored by organic matter, whereas the families Pratylenchidae and Trichodoridae (plant-parasitic nematodes) were mostly influenced by soil pH. Regarding soil nematode indicators, the studied indices were only moderately influenced by the explored environmental variables. Only PPI, and to an even lesser extent BI, were weakly influenced by soil pH and CE, respectively.
5. Conclusions
This work shows that substrates rich in organic matter such as coconut fiber, even though unable to prevent accidental introduction during cultivation, could still play an important role in suppressing plant-parasitic nematodes. This is due to the abundance of free-living nematodes, which are of crucial importance in both the mineralization of organic matter and antagonistic control of plant-parasitic nematodes. Potting systems, also, reduce virus-vector nematodes and improve the prey/predator ratio favoring natural control. However, it is worth noting that the population of some plant-parasitic nematode species, such as P. vulnus found in C. leylandii, showed a strong increase in abundance. This pest, listed in Annex IV, Teg. (EU) 2072/2019 among regulated non-quarantine pests, was the most numerous species representing more than 70% of the entire plant-parasitic nematode population. Further studies are required to confirm these findings. Additional research is recommended to investigate the mechanisms determining differences in plant-parasitic nematodes between plant species and to explore the effectiveness of combining pot cultivation with other control methods.
Author Contributions
Conceptualization, S.L. and E.R.; methodology, B.C., S.C. and B.N.; investigation, B.C., F.B., S.C. and B.N.; data curation, S.L. and F.B.; writing—original draft preparation, S.L.; writing—review and editing, F.B., B.C., S.C., B.N., E.R. and S.S.; visualization, S.L. and F.B.; supervision, S.L., P.F.R. and S.S.; funding acquisition, P.F.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Rural Development Program, Tuscany Region, Italy, UE, grant number 2014IT6RDRP010 under the project AUTOFITOVIV “Good practices for a self-controlled and sustainable management in ornamental nurseries”. The APC was not due as this paper was presented upon invitation, and was therefore published free of charge.
Institutional Review Board Statement
No ethical approval was required for the sample types collected in this study.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
We wish to thank “Azienda Agricola Vannucci Piante di Vannino Vannucci” for their support and field logistics.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Howland, A.D. Plant-Parasitic Nematodes and their Effects on Ornamental Plants: A Review. J. Nematol. 2023, 55, 20230007. [Google Scholar] [CrossRef]
- Sas Paszt, L.; Trzciński, P.; Bakalarska, M.; Holownicki, R.; Konopacki, P.; Treder, W. The influence of heated soil in crop of “Tamaris” tomato plants on the biological activity of the rhizosphere soil. Adv. Microbiol. 2014, 4, 191–201. [Google Scholar] [CrossRef]
- Landi, S.; Valboa, G.; Vignozzi, N.; d’Errico, G.; Pellegrini, S.; Simoncini, S.; Torrini, G.; Roversi, P.F.; Priori, S. Response of nematode community structure to different restoration practices in two vineyard soils in Tuscany (Italy). Biol. Agric. Hortic. 2023, 39, 149–169. [Google Scholar] [CrossRef]
- Barker, K.R.; Koenning, S.R. Developing sustainable systems for nematode management. Annu. Rev. Phytopathol. 1998, 36, 165–205. [Google Scholar] [CrossRef]
- Landi, S.; Papini, R.; d’Errico, G.; Brandi, G.; Rocchini, A.; Roversi, P.F.; Bazzoffi, P.; Mocali, S. Effect of different set-aside management systems on soil nematode community and soil fertility in North, Central and South Italy. Agric. Ecosyst. Environ. 2018, 261, 251–260. [Google Scholar] [CrossRef]
- Phani, V.; Khan, M.R.; Dutta, T.K. Plant-parasitic nematodes as a potential threat to protected agriculture: Current status and management options. Crop Prot. 2021, 144, 105573. [Google Scholar] [CrossRef]
- Hallmann, J.; Hänisch, D.; Braunsmann, J.; Klenner, M. Plant-parasitic nematodes in soil-less culture systems. Nematology 2005, 7, 1–4. [Google Scholar] [CrossRef]
- Sabir, N.; Walia, R.K. Management of nematodes in protected cultivation with shortnotes on key pests. In All Indian Coordinated Research Project on Nematodes in Cropping Systems, ICAR; Indian Agricultural Research Institute: New Delhi, India, 2017. [Google Scholar]
- Noling, J.W.; Rich, J.R. Greenhouse Nematode Management. University of Florida, IFAS Extension. Available online: https://hortintl.cals.nesu.udu/articles/greenhouse-nematode-management (accessed on 30 August 2020).
- Eppo. Diagnostic. PM 7/119 (1) Nematode extraction. Bull. OEPP/EPPO Bull. 2013, 43, 471–495. [Google Scholar] [CrossRef]
- De Boodt, M.F.; Verdonck, O.F.; Cappaert, I.M. Method for measuring the water release curve of organic substrates. Acta Hortic. 1974, 37, 2054–2062. [Google Scholar] [CrossRef]
- Mai, W.F.; Lyon, H.H. Pictorial Key to Genera of Plant Parasitic Nematodes; Plates Reproduced by Art Craft of Ithaca, Inc.: Ithaca, NY, USA, 1962. [Google Scholar]
- Bongers, T. De Nematoden van Nederland; KNNV: Utrecht, The Nederlands, 1988. [Google Scholar]
- Marinari-Palmisano, A.; Vinciguerra, M. Classificazione dei nematodi. In Nematologia Agraria Generale e Applicata; Ambrogioni, L., d’Errico, F.P., Greco, N.A., Marinari-Palmisano, A., Roversi, P.F., Eds.; Società Italiana di Nematologia: Bari, Italy, 2014; pp. 23–42. [Google Scholar]
- Yeates, G.W.; Bongers, T.; De Goede, R.; Freckman, D.; Georgieva, S. Feeding habits in soil nematode families and genera in outline for soil ecologists. J. Nematol. 1993, 25, 315. [Google Scholar]
- Okada, H.; Harada, H.; Kadota, I. Fungal-feeding habits of six nematode isolates in the genus Filenchus. Soil Biol. Biochem. 2005, 37, 1113–1120. [Google Scholar] [CrossRef]
- Bongers, T. The maturity index: An ecological measure of environmental disturbance based on nematode species composition. Oecologia 1990, 83, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Ferris, H.; Bongers, T.; De Goede, R. A framework for soil food web diagnostics: Extension of the nematode faunal analysis concept. Appl. Soil Ecol. 2001, 18, 13–29. [Google Scholar] [CrossRef]
- Ferris, H. Form and function: Metabolic footprints of nematodes in the soil food web. Eur. J. Soil Biol. 2010, 46, 97–104. [Google Scholar] [CrossRef]
- Ferris, H.; Tuomisto, H. Unearthing the role of biological diversity in soil health. Soil Biol. Biochem. 2015, 85, 101–109. [Google Scholar] [CrossRef]
- Hammer, O.; Harper, D.A.T.; Ryan, P.D. Past version 1.95: Paleontological Statistical Software Package for Education and Data Analysis. Paleontol. Electron. 2001, 4, 9. [Google Scholar]
- Clarke, K.R. Non-parametric multivariate analysis of changes in community structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Loveland, P.; Legendre, L. Is there a critical level of organic production practices on soil quality indicators. J. Environ. Qual. 1998, 28, 1601–1609. [Google Scholar]
- Abad, M.; Noguera, P.; Bures, S. National inventory of organic wastes for use as growing media for ornamental potted plant production: Case study in Spain. Bioresour. Technol. 2001, 77, 197–200. [Google Scholar] [CrossRef]
- Carlile, W.R.; Raviv, M.; Prasad, M. Organic soilless media components. In Soilless Culture: Theory and Practice Theory and Practice; Raviv, M., Lieth, J.H., Bar-Tal, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 303–378. [Google Scholar] [CrossRef]
- Ferris, H.; Matute, M.M. Structural snf functional succession in the nematode fauna of a soil food web. Appl. Soil Ecol. 2003, 23, 93–110. [Google Scholar] [CrossRef]
- Rahman, L.; Chan, K.Y.; Heenan, D.P. Impact of tillage, stubble management and crop rotation on nematode populations in a long-term field experiment. Soil Till Res. 2007, 95, 110–119. [Google Scholar] [CrossRef]
- Georgieva, S.; Christensen, S.; Stevnbak, K. Nematode succession and microfauna-microorganism interactions during root residue decomposition. Soil Biol. Biochem. 2005, 37, 1763–1774. [Google Scholar] [CrossRef]
- Sohlenius, B.; Bostrom, S.; Sandor, A. Carbon and nitrogen budgets of nematodes in arable soil. Biol. Fertil. Soils 1988, 6, 1–8. [Google Scholar] [CrossRef]
- Palomares-Rius, J.E.; Castillo, P.; Montes-Borrego, M.; Müller, H.; Landa, B.B. Nematode community populations in the rhizosphere of cultivated olive differs according to the plant genotype. Soil Biol. Biochem. 2012, 45, 168–171. [Google Scholar] [CrossRef]
- Hugo, H.J.; Malan, A.P. Occurrence and Control of Plant-parasitic Nematodes in Irrigation Water—A Review. S. Afr. J. Enol. Vitic 2010, 31, 169–180. [Google Scholar] [CrossRef][Green Version]
- Ali, N.; Tavoillot, J.; Besnard, G.; Khadari, B.; Dmowska, E.; Winiszewska, G.; Fottati-Gaschignard, O.; Ater, M.; Hamza, M.A.; El Mousadik, A.; et al. How anthropogenic changes may affect soil-borne parasite diversity? Plant-parasitic nematode communities associated with olive trees in Morocco as a case study. BMC Ecol. 2017, 17, 4. [Google Scholar] [CrossRef]
- Landi, S.; d’Errico, G.; Papini, R.; Cutino, I.; Simoncini, S.; Rocchini, A.; Brandi, G.; Rizzo, R.; Gugliuzza, G.; Germinara, G.S.; et al. Impact of Super-High Density Olive Orchard Management System on Soil Free-Living and Plant-Parasitic Nematodes in Central and South Italy. Animals 2022, 12, 1551. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).