The Effect of Manure Application Rates on the Vertical Distribution of Antibiotic Resistance Genes in Farmland Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Antibiotic and Heavy Metal Determination
2.3. Soil Physical and Chemical Indicators
2.4. DNA Extraction and Metagenomic Sequencing
2.5. Statistical Analysis
3. Results and Discussion
3.1. Types and Abundance of ARGs in Manure
3.2. Diversity and Abundance of ARGs in Fertilized Farmland Soil
3.3. Soil Microbial Community in Fertilized Farmland Soil
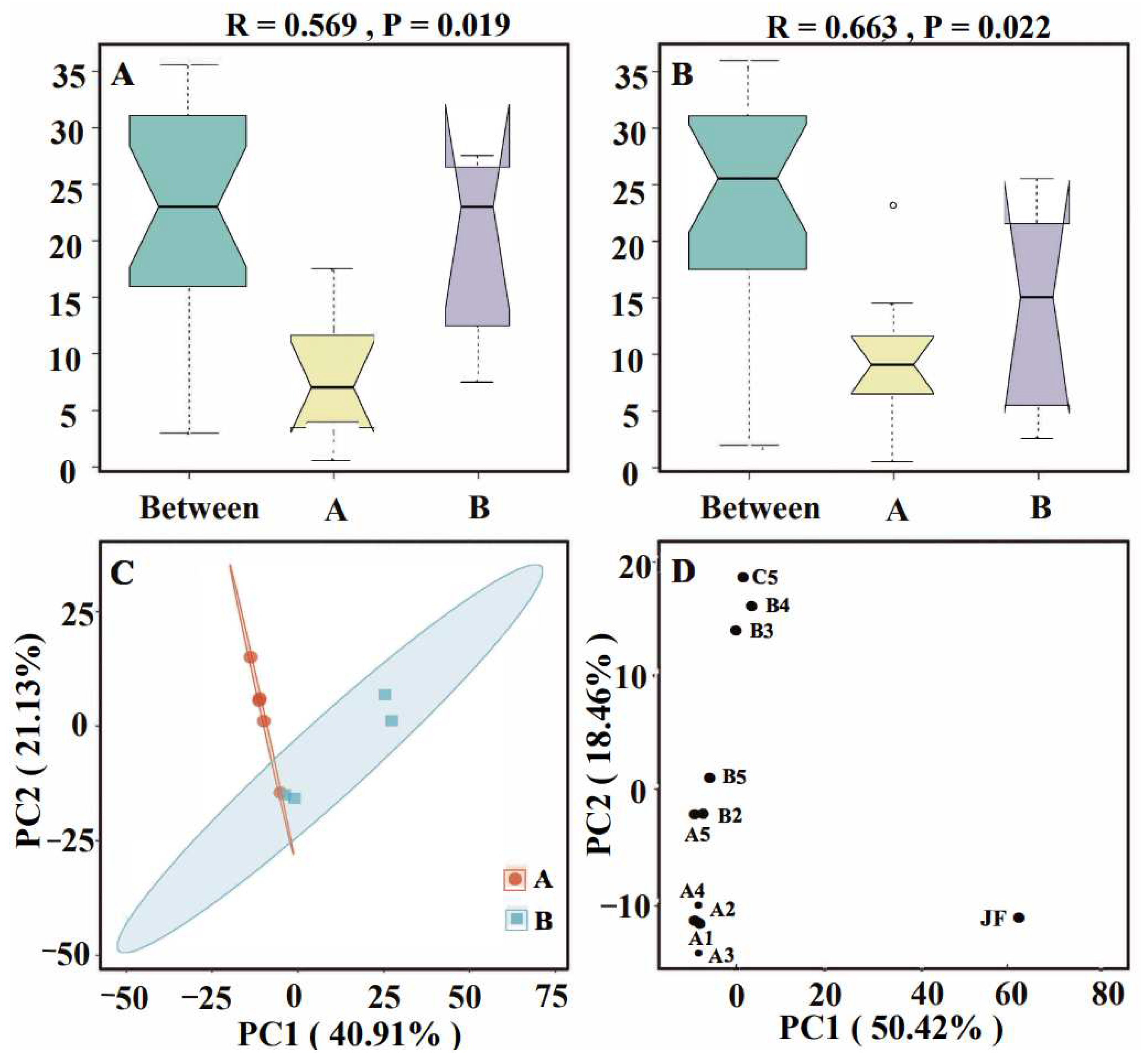
3.4. Correlation Analysis of ARGs with Microorganisms in Soil
3.5. Correlation Analysis of ARGs in Soil with Environmental Factors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fatondji, D.; Ibrahim, A. Improving nutrient use efficiency from decomposing manure and millet yield under Plinthosols in Niger. Nutr. Cycl. Agroecosys. 2018, 110, 485–499. [Google Scholar] [CrossRef]
- Mažeika, R.; Arbačiauskas, J.; Masevičienė, A.; Narutytė, I.; Šumskis, D.; Žičkienė, L.; Rainys, K.; Drapanauskaite, D.; Staugaitis, G.; Baltrusaitis, J. Nutrient Dynamics and Plant Response in Soil to Organic Chicken Manure-Based Fertilizers. Waste Biomass Valori. 2021, 12, 371–382. [Google Scholar] [CrossRef]
- Baxter, A.E.; Leytem, A.B.; Dungan, R.S.; Bjorneberg, D. Potential of winter double crops and tillage for managing manure-based nutrient loading. Plant Soil 2023, 11, 06072. [Google Scholar] [CrossRef]
- Keskinen, R.; Suojala-Ahlfors, T.; Sarvi, M.; Hagner, M.; Kaseva, J.; Salo, T.; Uusitalo, R.; Rasa, K. Granulated broiler manure based organic fertilizers as sources of plant available nitrogen. Environ. Technol. Innov. 2020, 18, 100734. [Google Scholar] [CrossRef]
- Samara, E.; Matsi, T.; Barbayiannis, N.; Lithourgidis, A. Liquid Cattle Manure Effect on Corn Yield and Nutrients’ Uptake and Soil Fertility, in Comparison to the Common and Recommended Inorganic Fertilization. J. Soil Sci. Plant Nutr. 2020, 20, 2283–2293. [Google Scholar] [CrossRef]
- Lehmann, J.; Hansel, C.M.; Kaiser, C.; Kleber, M.; Maher, K.; Manzoni, S.; Nunan, N.; Reichstein, M.; Schimel, J.P.; Torn, M.S.; et al. Persistence of soil organic carbon caused by functional complexity. Nat. Geosci. 2020, 13, 529–534. [Google Scholar] [CrossRef]
- Zhai, L.; Wang, Z.; Zhai, Y.; Zhang, L.; Zheng, M.; Yao, H.; Lv, L.; Shen, H.; Zhang, J.; Yao, Y.; et al. Partial substitution of chemical fertilizer by organic fertilizer benefits grain yield, water use efficiency, and economic return of summer maize. Soil Till. Res. 2022, 217, 105287. [Google Scholar] [CrossRef]
- Hu, B.; Ni, H.; Xie, M.; Li, H.; Wen, Y.; Chen, S.; Zhou, Y.; Teng, H.; Bourennane, H.; Shi, Z. Mapping soil organic matter and identifying potential controls in the farmland of Southern China: Integration of multi-source data, machine learning and geostatistics. Land Degrad. Dev. 2023, 34, 5468–5485. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, C.; Wang, H.; Li, F.; Pan, H.; Yang, Q.; Li, J.; Zhang, J. The Effects of Natural Humus Material Amendment on Soil Organic Matter and Integrated Fertility in the Black Soil of Northeast China: Preliminary Results. Agronomy 2023, 13, 794. [Google Scholar] [CrossRef]
- Usharani, K.V.; Roopashree, K.M.; Naik, D. Role of soil physical, chemical and biological properties for soil health improvement and sustainable agriculture. J. Pharmacog. Phytoch. 2019, 8, 1256–1267. [Google Scholar]
- Li, S.; Zhu, Y.; Zhong, G.; Huang, Y.; Jones, K.C. Comprehensive Assessment of Environmental Emissions, Fate, and Risks of Veterinary Antibiotics in China: An Environmental Fate Modeling Approach. Environ. Sci. Technol. 2024, 58, 5534–5547. [Google Scholar] [CrossRef] [PubMed]
- Chee-Sanford, J.C.; Mackie, R.I.; Koike, S.; Krapac, I.G.; Lin, Y.F.; Yannarell, A.C.; Maxwell, S.; Aminov, R.I. Fate and Transport of Antibiotic Residues and Antibiotic Resistance Genes following Land Application of Manure Waste. J. Environ. Qual. 2009, 38, 1086–1108. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, I.R.; Watanabe, N.; Harter, T.; Glaser, B.; Radke, M. Effect of sulfonamide antibiotics on microbial diversity and activity in a Californian Mollic Haploxeralf. J. Soil Sediment. 2010, 10, 537–544. [Google Scholar] [CrossRef]
- Munk, P.; Knudsen, B.E.; Lukjancenko, O.; Duarte, A.S.R.; Van Gompel, L.; Luiken, R.E.C.; Smit, L.A.M.; Schmitt, H.; Garcia, A.D.; Hansen, R.B.; et al. Abundance and diversity of the faecal resistome in slaughter pigs and broilers in nine European countries. Nat. Microbiol. 2018, 3, 898–908. [Google Scholar] [CrossRef]
- Gaballah, M.S.; Guo, J.; Sun, H.; Aboagye, D.; Sobhi, M.; Muhmood, A.; Dong, R. A review targeting veterinary antibiotics removal from livestock manure management systems and future outlook. Bioresour. Technol. 2021, 333, 125069. [Google Scholar] [CrossRef] [PubMed]
- Al-Wabel, M.I.; Ahmad, M.; Ahmad, J.; Lubis, N.M.A.; Usman, A.R.A.; Al-Farraj, A.S.F. Assessing the prevalence of veterinary antibiotics and associated potential ecological risk in dryland soil, manure, and compost: A case study from Saudi Arabia. J. King Saud Univ. Sci. 2021, 33, 101558. [Google Scholar] [CrossRef]
- Pan, Z.; Yang, S.; Zhao, L.; Li, X.; Weng, L.; Sun, Y.; Li, Y. Temporal and spatial variability of antibiotics in agricultural soils from Huang-Huai-Hai Plain, northern China. Chemosphere 2021, 272, 129803. [Google Scholar] [CrossRef]
- Gu, J.; Chen, C.; Huang, X.; Mo, J.; Xie, Q.; Zeng, Q. Occurrence and risk assessment of tetracycline antibiotics in soils and vegetables from vegetable fields in Pearl River Delta, South China. Sci. Total Environ. 2021, 776, 145959. [Google Scholar] [CrossRef]
- Abbas, F.; Thomas, P.; Cully-Duse, B.; Andronicos, N.M.; Winter, G. Cattle-compost-soil: The transfer of antibiotic resistance in livestock agriculture. Microbiologyopen 2023, 12, e1375. [Google Scholar] [CrossRef]
- Li, Y.; Kong, F.; Li, S.; Wang, J.; Hu, J.; Chen, S.; Chen, Q.; Li, Y.; Ha, X.; Sun, W. Insights into the driving factors of vertical distribution of antibiotic resistance genes in long-term fertilized soils. J. Hazard. Mater. 2023, 456, 131706. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, X.; Xu, Y.; Jin, M.; Thompson, M.L. Soil labile organic carbon fractions and soil enzyme activities after 10 years of continuous fertilization and wheat residue incorporation. Sci. Rep. 2020, 10, 11318. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; He, X.; Zhang, Z.; Li, M.; Zhang, Q.; Zhu, H.; Xu, S.; Yang, P. Eight years of manure fertilization favor copiotrophic traits in paddy soil microbiomes. Eur. J. Soil Biol. 2021, 106, 103352. [Google Scholar] [CrossRef]
- Caban, J.R.; Kuppusamy, S.; Kim, J.H.; Yoon, Y.E.; Kim, S.Y.; Lee, Y.B. Green manure amendment enhances microbial activity and diversity in antibiotic-contaminated soil. Appl. Soil Ecol. 2018, 129, 72–76. [Google Scholar] [CrossRef]
- Sayre, J.M.; Wang, D.; Lin, J.; Danielson, R.E.; Scow, K.M.; Rodrigues, J.L.M. Repeated manure inputs to a forage production soil increase microbial biomass and diversity and select for lower abundance genera. Agr. Ecosyst. Environ. 2023, 354, 108567. [Google Scholar] [CrossRef]
- Gautam, A.; Sekaran, U.; Guzman, J.; Kovács, P.; Hernandez, J.L.G.; Kumar, S. Responses of soil microbial community structure and enzymatic activities to long-term application of mineral fertilizer and beef manure. Environ. Sustain. Indic. 2020, 8, 100073. [Google Scholar] [CrossRef]
- Ren, F.; Sun, N.; Xu, M.; Zhang, X.; Wu, L.; Xu, M. Changes in soil microbial biomass with manure application in cropping systems: A meta-analysis. Soil Till. Res. 2019, 194, 104291. [Google Scholar] [CrossRef]
- Peng, S.; Wang, Y.; Chen, R.; Lin, X. Chicken Manure and Mushroom Residues Affect Soil Bacterial Community Structure but Not the Bacterial Resistome When Applied at the Same Rate of Nitrogen for 3 Years. Front. Microbiol. 2021, 12, 618693. [Google Scholar] [CrossRef] [PubMed]
- Garbini, G.L.; Grenni, P.; Rauseo, J.; Patrolecco, L.; Pescatore, T.; Spataro, F.; Barra Caracciolo, A. Insights into structure and functioning of a soil microbial community amended with cattle manure digestate and sulfamethoxazole. J. Soil Sediment 2022, 22, 2158–2173. [Google Scholar] [CrossRef]
- Cheng, S.; Shi, M.; Xing, L.; Wang, X.; Gao, H.; Sun, Y. Sulfamethoxazole affects the microbial composition and antibiotic resistance gene abundance in soil and accumulates in lettuce. Environ Sci Pollut. 2020, 27, 29257–29265. [Google Scholar] [CrossRef]
- Seiler, C.; Berendonk, T.U. Heavy metal driven co-selection of antibiotic resistance in soil and water bodies impacted by agriculture and aquaculture. Front. Microbiol. 2012, 3, 399. [Google Scholar] [CrossRef]
- Lin, Y.; Yu, J.; Wu, L. The status and ecological effects of antibiotics and heavy metals combined pollution:A review. Appl. Chem. Ind. 2023, 52, 504–510. [Google Scholar]
- Wang, X.; Lan, B.; Fei, H.; Wang, S.; Zhu, G. Heavy metal could drive co-selection of antibiotic resistance in terrestrial subsurface soils. J. Hazard. Mater. 2021, 411, 124848. [Google Scholar] [CrossRef] [PubMed]
- Kuppusamy, S.; Venkateswarlu, K.; Megharaj, M.; Sellappa, K.; Lee, Y.B. Contamination of long-term manure-fertilized Indian paddy soils with veterinary antibiotics: Impact on bacterial communities and antibiotics resistance genes. Appl. Soil Ecol. 2023, 192, 105106. [Google Scholar] [CrossRef]
- De Oliveira Paranhos, A.G.; Pereira, A.R.; da Fonseca, I.C.; Sanson, A.L.; de Cássia Franco Afonso, R.J.; de Aquino, S.F. Analysis of tylosin in poultry litter by HPLC-UV and HPLC-MS/MS after LTPE. Int. J. Environ. Anal. Chem. 2020, 101, 2568–2585. [Google Scholar] [CrossRef]
- Acaroz, U.; Kucukkurt, I.; Ince, S.; Arslan-Acaroz, D.; Gurler, Z.; Eryavuz, A. Assessment for the passage of tylosin into the milk of Anatolian buffaloes. J. Hell. Vet. Med. Soc. 2021, 72, 3127–3132. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, D.; Xie, J.; Zhang, Y.; Wan, Y.; Zhang, Y.; Shi, X. Impacts of farmland application of antibiotic-contaminated manures on the occurrence of antibiotic residues and antibiotic resistance genes in soil: A meta-analysis study. Chemosphere 2022, 300, 134529. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chen, J.; Wang, J.; Ma, Z.; Han, P.; Luan, Y.; Lu, A. Occurrence of antibiotics in soils and manures from greenhouse vegetable production bases of Beijing, China and an associated risk assessment. Sci. Total Environ. 2015, 521–522, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, M.; Ellerbrock, R.H.; Gerke, H.H. Cation Exchange Capacity and Composition of Soluble Soil Organic Matter Fractions. Soil Sci. Soc. Am. J. 2008, 72, 1278–1285. [Google Scholar] [CrossRef]
- Li, D.; Liu, C.; Luo, R.; Sadakane, K.; Lam, T.W. MEGAHIT: An ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics 2015, 31, 1674–1676. [Google Scholar] [CrossRef]
- Hyatt, D.; Chen, G.; LoCascio, P.F.; Land, M.L.; Larimer, F.W.; Hause, L.J. Prodigal: Prokaryotic gene recognition and translation initiation site identification. BMC Bioinform. 2010, 11, 119. [Google Scholar] [CrossRef]
- Qian, X.; Gu, J.; Sun, W.; Wang, X.; Su, J.; Stedfeld, R. Diversity, abundance, and persistence of antibiotic resistance genes in various types of animal manure following industrial composting. J. Hazard. Mater. 2018, 344, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Pang, L.; Chatzisymeon, E.; Liu, X.; Xu, K.; Yang, P.; Gou, M. Copper in different forms and tetracycline affect behavior and risk of antibiotic resistome in thermophilic anaerobic digestion of cattle manure. Environ. Sci. Pollut. R. 2023, 30, 108162–108175. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Yuan, Y.; Wang, Y.; Liu, D.; Shen, Q.; Zhao, F. Hazard reduction and persistence of risk of antibiotic resistance during thermophilic composting of animal waste. J. Environ. Manag. 2023, 330, 117249. [Google Scholar] [CrossRef]
- Wu, S.; Cui, L.; Han, Y.; Lin, F.; Huang, J.; Song, M.; Lan, Z.; Sun, S. Characteristics, Whole-Genome Sequencing and Pathogenicity Analysis of Escherichia coli from a White Feather Broiler Farm. Microorganisms 2023, 11, 2939. [Google Scholar] [CrossRef] [PubMed]
- Fatoba, D.O.; Amoako, D.G.; Akebe, A.L.K.; Ismail, A.; Essack, S.Y. Genomic analysis of antibiotic-resistant Enterococcus spp. reveals novel enterococci strains and the spread of plasmid-borne Tet(M), Tet(L) and Erm(B) genes from chicken litter to agricultural soil in South Africa. J. Environ. Manag. 2022, 302, 114101. [Google Scholar] [CrossRef] [PubMed]
- Chi, S.; Xu, W.; Han, Y. ARGs distribution and high-risk ARGs identification based on continuous application of manure in purple soil. Sci. Total Environ. 2022, 853, 158667. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, M.K.; Chen, H.; Awasthi, S.K.; Duan, Y.; Liu, T.; Pandey, A.; Varjani, S.; Zhang, Z. Application of metagenomic analysis for detection of the reduction in the antibiotic resistance genes (ARGs) by the addition of clay during poultry manure composting. Chemosphere 2019, 220, 137–145. [Google Scholar] [CrossRef]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Blazejewska, A.; Zalewska, M.; Grudniak, A.; Popowska, M. A Comprehensive Study of the Microbiome, Resistome, and Physical and Chemical Characteristics of Chicken Waste from Intensive Farms. Biomolecules 2022, 12, 1132. [Google Scholar] [CrossRef]
- Hadjadj, L.; Baron, S.A.; Diene, S.M.; Rolain, J.M. How to discover new antibiotic resistance genes? Expert Rev. Mol. Diagn. 2019, 9, 349–362. [Google Scholar] [CrossRef]
- Webber, M.A.; Piddock, L.J.V. The importance of efflux pumps in bacterial antibiotic resistance. J. Antimicrob. Chemother. 2003, 51, 9–11. [Google Scholar] [CrossRef] [PubMed]
- Lambert, P. Bacterial resistance to antibiotics: Modified target sites. Adv. Drug Deliv. Rev. 2005, 57, 1471–1485. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, K.; Ye, Y.; Zhang, Y.; Mi, H.; Li, C.; Li, Z.; Pei, Z.; Chen, F.; Yan, J.; et al. Reductive soil disinfestation attenuates antibiotic resistance genes in greenhouse vegetable soils. J. Hazard. Mater. 2021, 420, 126632. [Google Scholar] [CrossRef]
- Wang, B.; Song, L.; Li, W.; Hou, L.; Li, J.; Xu, X.; Sheng, G. Distribution and migration of antibiotic resistance genes, as well as their correlation with microbial communities in swine farm and its surrounding environments. Environ. Pollut. 2023, 316, 120618. [Google Scholar] [CrossRef]
- Chen, Q.; An, X.; Li, H.; Zhu, Y.; Su, J.; Cui, L. Do manure-borne or indigenous soil microorganisms influence the spread of antibiotic resistance genes in manured soil? Soil Biol. Biochem. 2017, 114, 229–237. [Google Scholar] [CrossRef]
- Wang, W.; Shen, P.; Lu, Z.; Mo, F.; Liao, Y.; Wen, X. Metagenomics reveals the abundance and accumulation trend of antibiotic resistance gene profile under long-term no tillage in a rainfed agroecosystem. Front Microbiol. 2023, 14, 1238708. [Google Scholar] [CrossRef] [PubMed]
- Amarasekara, N.R.; Mafiz, A.I.; Qian, X.; Tiedje, J.M.; Hao, W.; Zhang, Y. Exploring the co-occurrence of antibiotic, metal, and biocide resistance genes in the urban agricultural environment. J. Agric. Food Res. 2023, 11, 100474. [Google Scholar] [CrossRef]
- Chen, C.; Pankow, C.A.; Oh, M.; Heath, L.S.; Zhang, L.; Du, P.; Xia, K.; Pruden, A. Effect of antibiotic use and composting on antibiotic resistance gene abundance and resistome risks of soils receiving manure-derived amendments. Environ Int. 2019, 128, 233–243. [Google Scholar] [CrossRef]
- Khalid, M.; Liu, X.; Zheng, B.; Su, L.; Kotze, D.J.; Setälä, H.; Ali, M.; Rehman, A.; Saeed-ur-Rahman; Hui, N. Distinct climatic regions drive antibiotic resistance genes dynamics across public parks and pristine soil ecosystems. J. Clean. Prod. 2023, 409, 137275. [Google Scholar] [CrossRef]
- Wang, F.; Xu, M.; Stedtfeld, R.D.; Sheng, H.; Fan, J.; Liu, M.; Chai, B.; de Carvalho, T.S.; Li, H.; Li, Z.; et al. Long-term effect of different fertilization and croping systems on the Soil Antibiotic Resistome. Environ. Sci. Technol. 2018; 52, 13037–13046. [Google Scholar] [CrossRef]
- Kang, M.; Yang, J.; Kim, S.; Park, J.; Kim, M.; Park, W. Occurrence of antibiotic resistance genes and multidrug-resistant bacteria during wastewater treatment processes. Sci. Total Environ. 2022, 811, 152331. [Google Scholar] [CrossRef]
- Deng, W.; Zhang, A.; Chen, S.; He, X.; Jin, L.; Yu, X.; Yang, S.; Li, B.; Fan, L.; Ji, L.; et al. Heavy metals, antibiotics and nutrients affect the bacterial community and resistance genes in chicken manure composting and fertilized soil. J. Environ. Manag. 2020, 257, 109980. [Google Scholar] [CrossRef]
- Shen, C.; He, M.; Zhang, J.; Liu, J.; Su, J.; Dai, J. Effects of the coexistence of antibiotics and heavy metals on the fate of antibiotic resistance genes in chicken manure and surrounding soils. Ecotox. Environ. Safe 2023, 263, 115367. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Wu, J.; Hu, Y.; Sha, C.; Yao, S.; Li, P.; Lin, K.; Cui, C. Occurrence of heavy metals, antibiotics, and antibiotic resistance genes in different kinds of land-applied manure in China. Environ. Sci. Pollut. Res. 2021, 28, 40011–40021. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Wang, J.; Han, Y.; Chen, J.; Liu, G.; Lu, H.; Yan, B.; Chen, S. Nutrients, heavy metals and microbial communities co-driven distribution of antibiotic resistance genes in adjacent environment ofmariculture. Environ. Pollut. 2017, 220 (B), 909–918. [Google Scholar] [CrossRef]
- Peng, H.; Gu, J.; Wang, X.; Wang, Q.; Sun, W.; Hu, T.; Guo, H.; Ma, J.; Bao, J. Insight into the fate of antibiotic resistance genes and bacterial community in co-composting green tea residues with swine manure. J. Environ. Manag. 2020, 266, 110581. [Google Scholar] [CrossRef]
- Liu, H.; Ye, X.; Chen, S.; Sun, A.; Duan, X.; Zhang, Y.; Zou, H.; Zhang, Y. Chitosan as additive affects the bacterial community, accelerates the removals of antibiotics and related resistance genes during chicken manure composting. Sci. Total Environ. 2021, 792, 148381. [Google Scholar] [CrossRef]
- Zhang, H.; Dong, M.; Zhou, Y.; Sun, J.; Chang, M.; Zhai, Z. Animal Manure Fertilization Promotes Antibiotic Resistance Gene Dissemination Among Manure, Soil, and Vegetables. Environ. Sci. 2021, 42, 2080–2088. [Google Scholar] [CrossRef]
- Kang, J.; Liu, Y.; Chen, X.; Xu, F.; Xiong, W.; Li, X. Shifts of Antibiotic Resistomes in Soil Following Amendments of Antibiotics-Contained Dairy Manure. Int. J. Environ. Res. Public Health 2022, 19, 10804. [Google Scholar] [CrossRef]
- Calleja-Cervantes, M.E.; Menéndez, S.; Fernández-González, A.J.; Irigoyen, I.; Cibriáin-Sabalza, J.F.; Toro, N.; Aparicio-Tejo, P.M.; Fernández-López, M. Changes in soil nutrient content and bacterial community after 12 years of organic amendment application to a vineyard. Eur. J. Soil Sci. 2015, 66, 802–812. [Google Scholar] [CrossRef]
- Zhang, X.; Meng, H.; Shen, Y.S.; Li, J.; Song, L.S. Survey on heavy metal concentrations and maturity indices of organic fertilizer in China. Int. J. Agric. Biol. Eng. 2018, 11, 172–179. [Google Scholar] [CrossRef]
- Byss, M.; Elhottová, D.; Tříska, J.; Baldrian, P. Fungal bioremediation of the creosote-contaminated soil: Influence of Pleurotus ostreatus and Irpex lacteus on polycyclic aromatic hydrocarbons removal and soil microbial community composition in the laboratory-scale study. Chemosphere 2008, 73, 1518–1523. [Google Scholar] [CrossRef]
- Lazcano, C.; Gómez-Brandón, M.; Revilla, P.; Domínguez, J. Short-term effects of organic and inorganic fertilizers on soil microbial community structure and function. Biol. Fertil. Soils 2012, 49, 723–733. [Google Scholar] [CrossRef]
- Kielak, A.M.; George, C.C.; Kowalchuk, G.A.; Veen, J.A.V.; Kuramae, E.E. The Ecology of Acidobacteria: Moving beyond Genes and Genomes. Front. Microbiol. 2016, 7, 171794. [Google Scholar] [CrossRef]
- Naylor, D.; McClure, R.; Jansson, J. Trends in Microbial Community Composition and Function by Soil Depth. Microorganisms 2022, 10, 540. [Google Scholar] [CrossRef]
- Wei, R.; Ge, F.; Zhang, L.; Hou, X.; Cao, Y.; Gong, L.; Chen, M.; Wang, R.; Bao, E. Occurrence of 13 veterinary drugs in animal manure-amended soils in Eastern China. Chemosphere 2016, 144, 2377–2383. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Han, L.; Zhang, H.; Long, Z.; Cai, L.; Yu, Y. Dissemination of antibiotic resistance genes and human pathogenic bacteria from a pig feedlot to the surrounding stream and agricultural soils. J. Hazard. Mater. 2018, 357, 53–62. [Google Scholar] [CrossRef]
- Zhao, B.; Xu, J.; Zhang, G.; Lu, S.; Liu, X.; Li, L.; Li, M. Occurrence of antibiotics and antibiotic resistance genes in the Fuxian Lake and antibiotic source analysis based on principal component analysis-multiple linear regression model. Chemosphere 2021, 262, 127741. [Google Scholar] [CrossRef] [PubMed]
- Baquero, F.; Lanza, V.F.; Duval, M.; Coque, T.M. Ecogenetics of antibiotic resistance in Listeria monocytogenes. Mol. Microbiol. 2020, 113, 570–579. [Google Scholar] [CrossRef]
- Agga, G.E.; Galloway, H.O.; Appala, K.; Mahmoudi, F.; Kasumba, J.; Loughrin, J.H.; Conte, E. Effect of continuous in-feed administration of tylosin to feedlot cattle on macrolide and tetracycline resistant enterococci in a randomized field trial. Prev. Vet. Med. 2023, 215, 105930. [Google Scholar] [CrossRef]
- Tyson, G.H.; Nyirabahizi, E.; Crarey, E.; Kabera, C.; Lam, C.; Rice-Trujillo, C.; McDermott, P.F.; Tate, H. Prevalence and antimicrobial resistance of enterococci isolated from retail meats in the United States, 2002 to 2014. Appl. Environ. Microb. 2018, 84, e01902–e01917. [Google Scholar] [CrossRef]
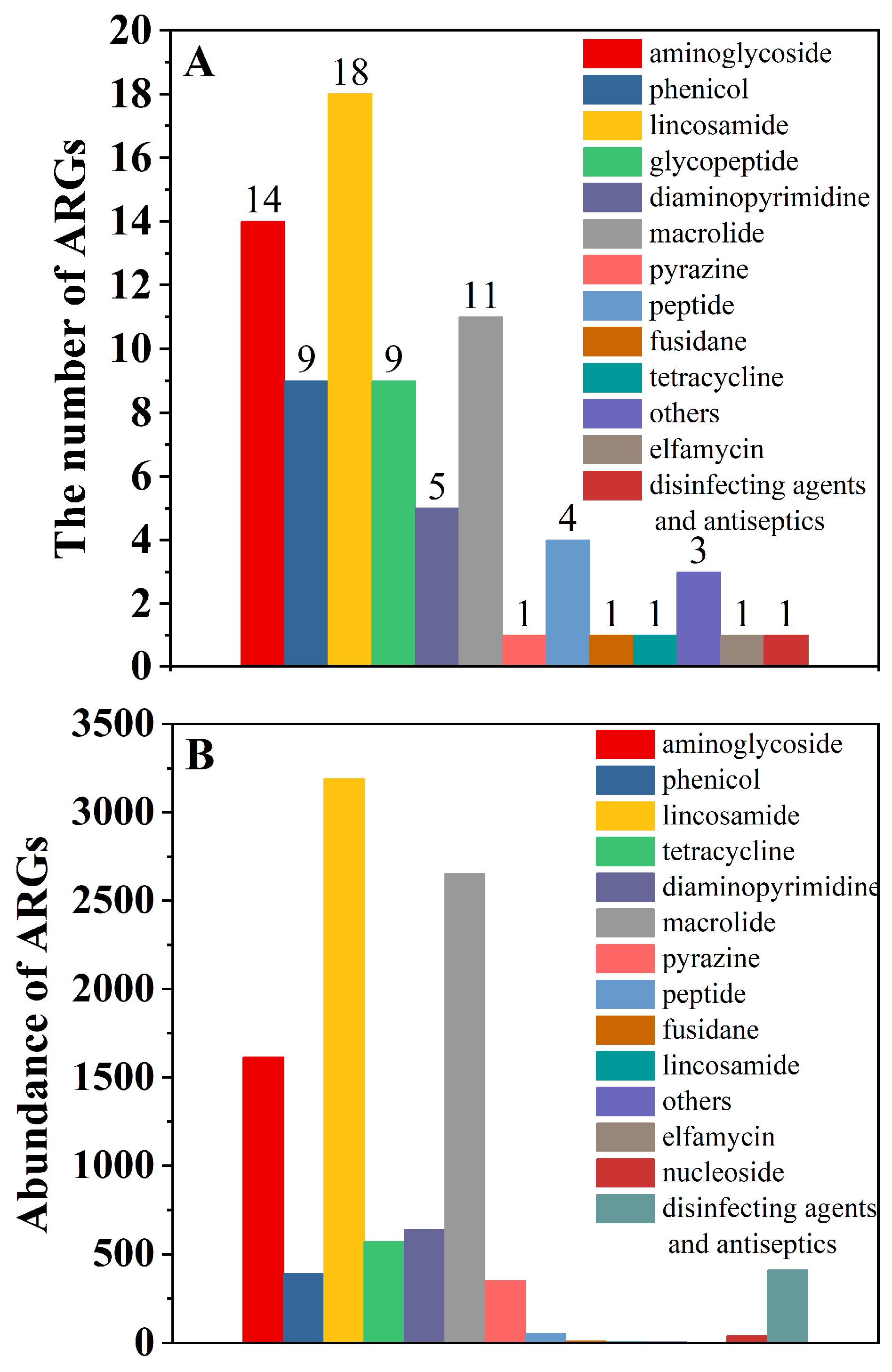

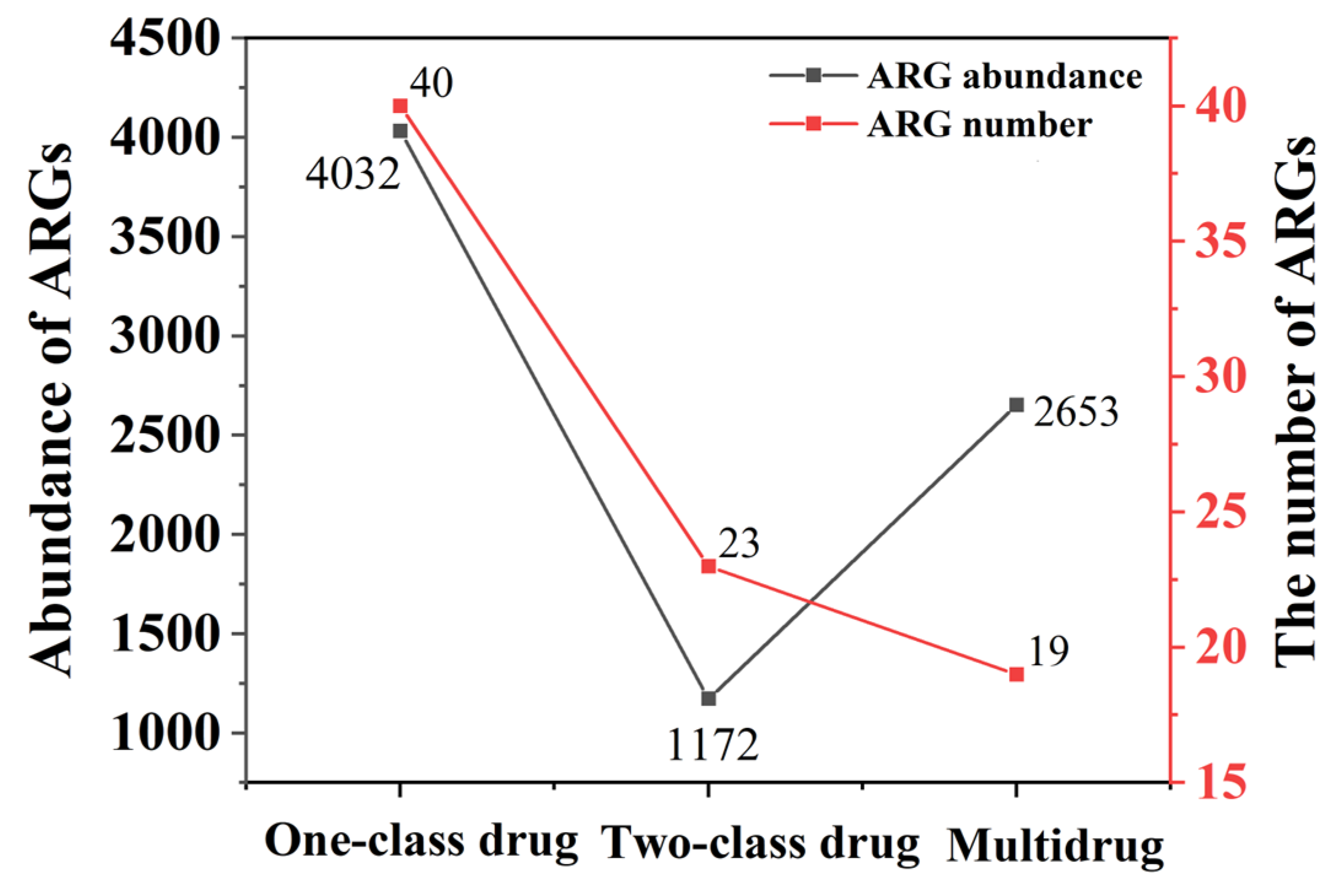
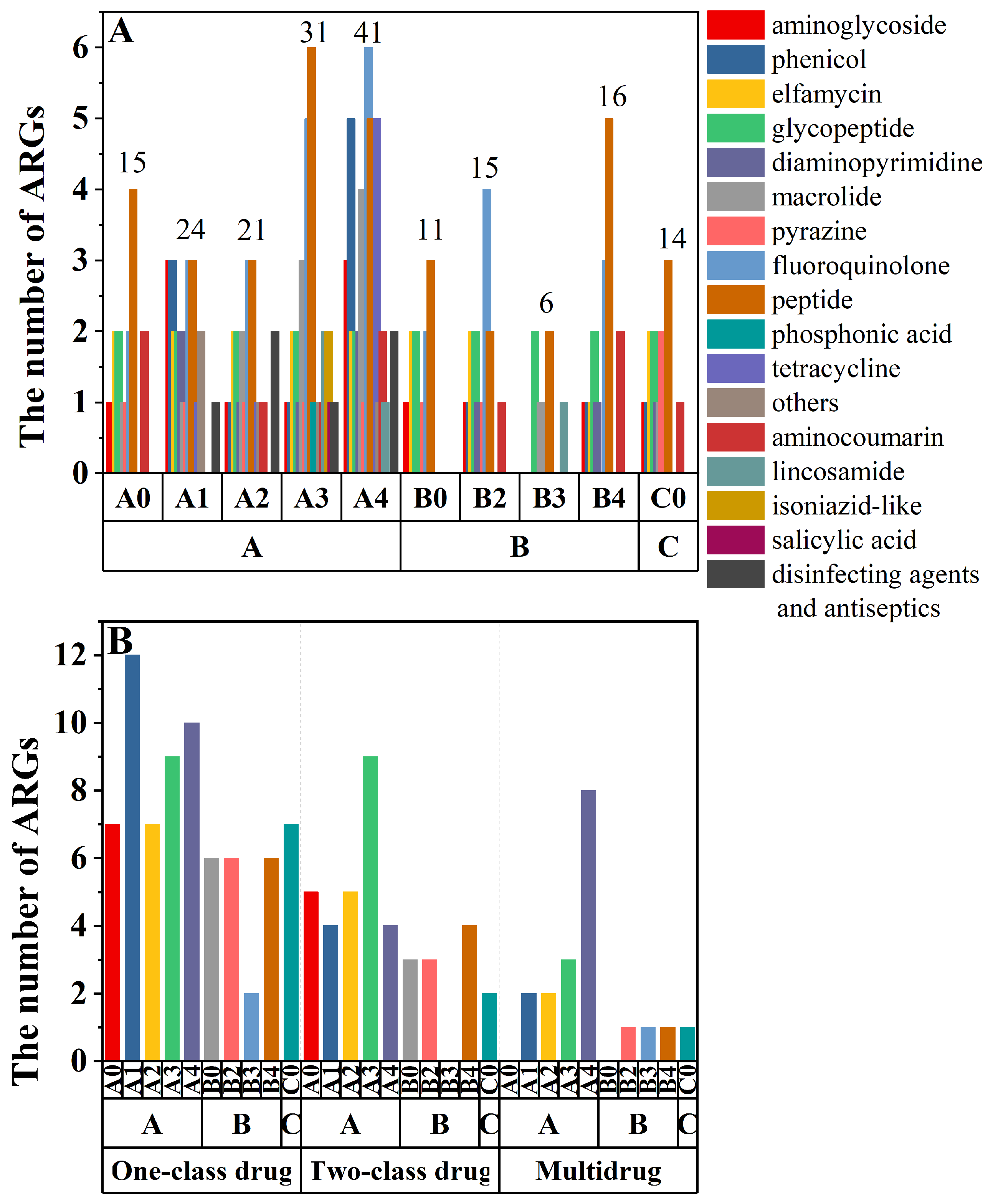
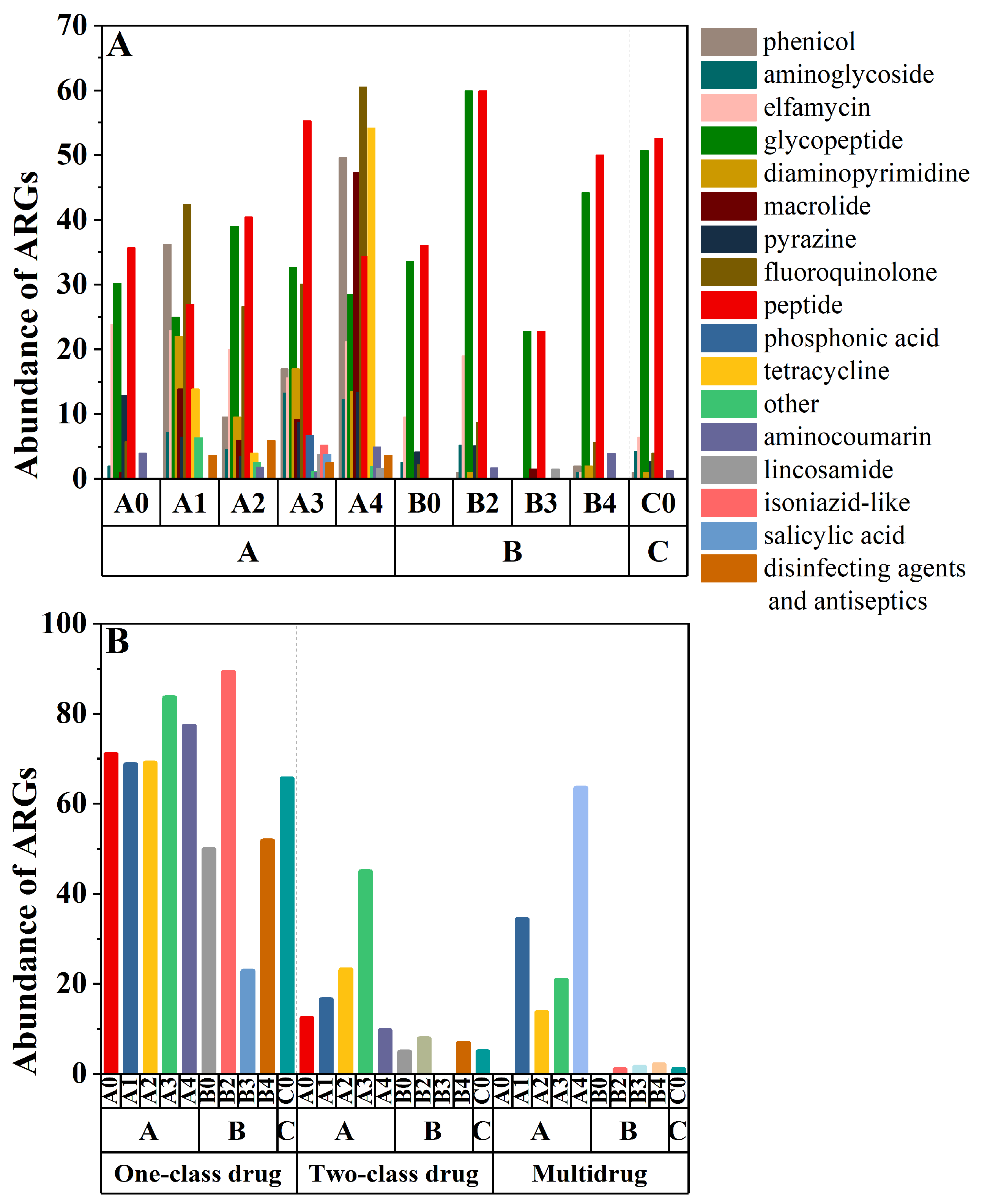

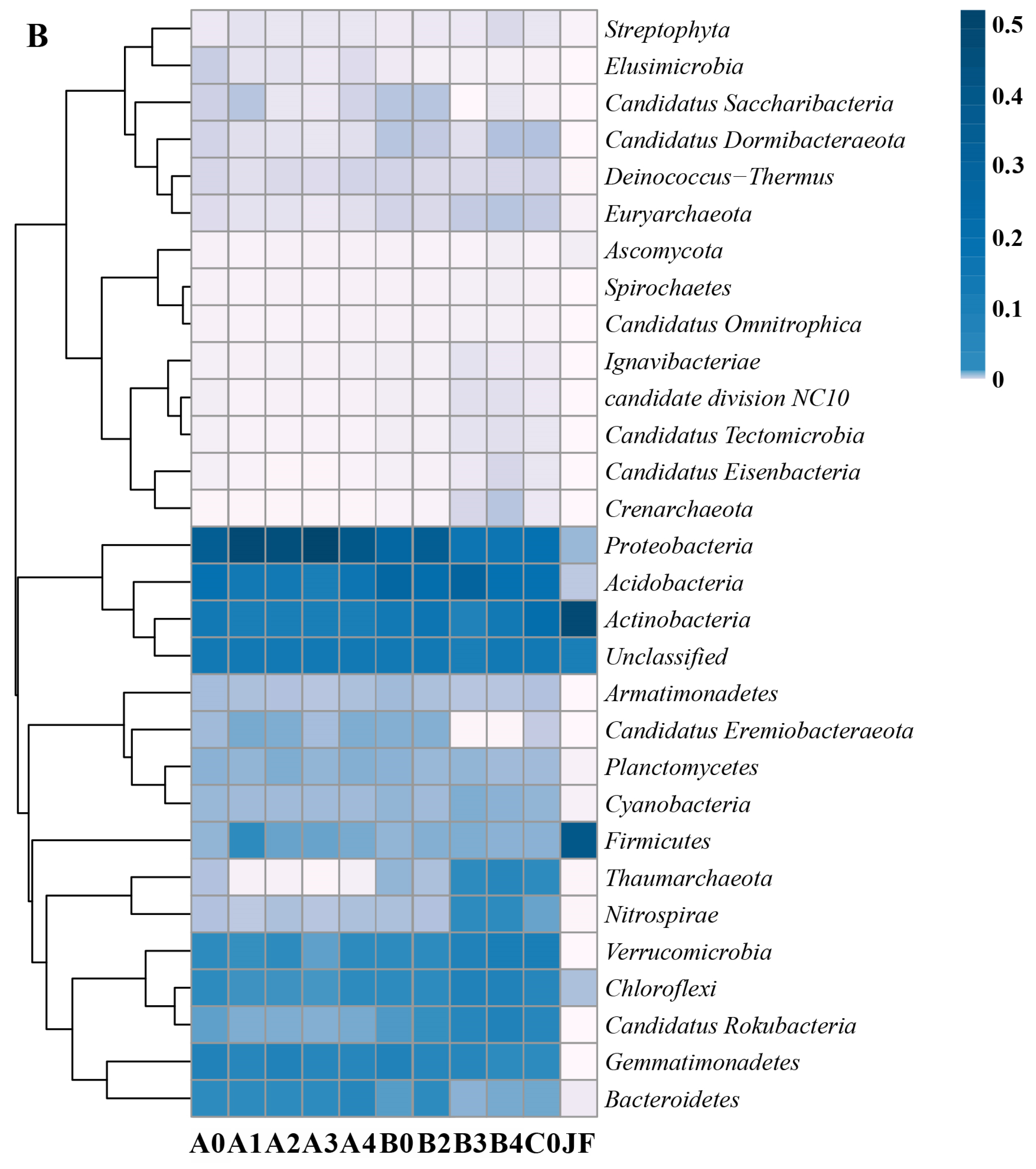


| Soil Layers | Control Group (100% Chemical Fertilizer) | Treatment 1 (25% Organic Fertilizer Replaced Chemical Fertilizer) | Treatment 2 (50% Organic Fertilizer Replaced Chemical Fertilizer) | Treatment 3 (75% Organic Fertilizer Replaced Chemical Fertilizer) | Treatment 4 (100% Organic Fertilizer) |
|---|---|---|---|---|---|
| A (0–20 cm) | A0 | A1 | A2 | A3 | A4 |
| B (20–40 cm) | B0 | B1 | B2 | B3 | B4 |
| C (40–60 cm) | C0 | C1 | C2 | C3 | C4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Yang, L.; Liu, W.; Zhuang, J. The Effect of Manure Application Rates on the Vertical Distribution of Antibiotic Resistance Genes in Farmland Soil. Soil Syst. 2024, 8, 89. https://doi.org/10.3390/soilsystems8030089
Wang Y, Yang L, Liu W, Zhuang J. The Effect of Manure Application Rates on the Vertical Distribution of Antibiotic Resistance Genes in Farmland Soil. Soil Systems. 2024; 8(3):89. https://doi.org/10.3390/soilsystems8030089
Chicago/Turabian StyleWang, Yuqian, Liqiong Yang, Weipeng Liu, and Jie Zhuang. 2024. "The Effect of Manure Application Rates on the Vertical Distribution of Antibiotic Resistance Genes in Farmland Soil" Soil Systems 8, no. 3: 89. https://doi.org/10.3390/soilsystems8030089





