An Integrated Approach to Remediate Saline Soils and Mining Waste Using Technosols and Pasture Development
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Areas
2.2. Experimental Setup
2.3. Sample Analysis
2.4. Data Analysis
3. Results and Discussion
3.1. Physicochemical Characterisations of Gossan Waste, Salt-Affected Fluvisol, and Technosols
3.2. Biological Characterisations of Gossan Waste, Satl-Affected Fluvisol, and Technosols
3.3. Ecotoxicological Characterisations of Gossan Waste, Salt-Affected Fluvisol, and Technosols
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Elements (mg kg−1) | G | VF |
|---|---|---|
| As | 9126.67 ± 238.77 | 15.6 ± 0.01 |
| Cd | 0.11 ± 0.08 | 0.08 ± 0.01 |
| Cu | 218.67 ± 5.81 | 27.33 ± 0.69 |
| Hg | 26.67 ± 6.67 | 0.07 ± 0.01 |
| Mn | 27.67 ± 1.76 | 598.32 ± 12.27 |
| Ni | 2.77 ± 0.43 | 27.50 ± 0.67 |
| Pb | 29,633.33 ± 554.78 | 37.73 ± 2.19 |
| Zn | 83.33 ± 6.62 | 103.67 ± 1.89 |
References
- Environment & Resources Authority. Soil Degradation Threats. Available online: https://era.org.mt/topic/soil-degradation-threats/ (accessed on 20 May 2024).
- Montanarella, L. Trends in Land Degradation in Europe. In Climate and Land Degradation. Environmental Science and Engineering; Sivakumar, M.V.K., Ndiang’ui, N., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 83–104. [Google Scholar]
- Khan, S.; Naushad, M.; Lima, E.C.; Zhang, S.; Shaheen, S.M.; Rinklebe, J. Global Soil Pollution by Toxic Elements: Current Status and Future Perspectives on the Risk Assessment and Remediation Strategies—A Review. J. Hazard. Mater. 2021, 417, 126039. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Chu, L.; Lu, H.; Qi, W.; Chen, X.; Liu, J.; Kuang, S.; Tang, B.; Wong, V. Towards Sustainable Agriculture for the Salt-Affected Soil. Land. Degrad. Dev. 2019, 30, 574–579. [Google Scholar] [CrossRef]
- Karimzadeh, S.; Hartman, S.; Chiarelli, D.D.; Rulli, M.C.; D’Odorico, P. The Tradeoff between Water Savings and Salinization Prevention in Dryland Irrigation. Adv. Water Resour. 2024, 183, 104604. [Google Scholar] [CrossRef]
- Srivastava, N. Reclamation of Saline and Sodic Soil Through Phytoremediation. In Environmental Concerns and Sustainable Development; Springer: Singapore, 2020; pp. 279–306. [Google Scholar] [CrossRef]
- Barbosa, B.; Fernando, A.L. Aided Phytostabilization of Mine Waste. Bio-Geotechnol. Mine Site Rehabil. 2018, 147–157. [Google Scholar] [CrossRef]
- Matanzas, N.; Afif, E.; Díaz, T.E.; Gallego, J.R. Phytoremediation Potential of Native Herbaceous Plant Species Growing on a Paradigmatic Brownfield Site. Water Air Soil Pollut. 2021, 232, 1–14. [Google Scholar] [CrossRef]
- Xie, L.; van Zyl, D. Identification of Grass Species Candidates for Phytostabilization and Enhanced Metal(Loid)s Immobilisation Using Cost-Effective Amendments on Sulfidic Mine Tailings. Int. J. Min. Reclam. Environ. 2023, 37, 489–503. [Google Scholar] [CrossRef]
- Santos, E.S.; Arán, D.; Abreu, M.M.; de Varennes, A. Engineered Soils Using Amendments for in Situ Rehabilitation of Mine Lands. In Bio-Geotechnologies for Mine Site Rehabilitation; Prasad, M.N.V., Favas, P.J.d.C., Maiti, S.K., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 131–146. ISBN 9780128129876. [Google Scholar]
- Rodríguez-Vila, A.; Covelo, E.F.; Forján, R.; Asensio, V. Phytoremediating a Copper Mine Soil with Brassica Juncea L., Compost and Biochar. Environ. Sci. Pollut. Res. 2014, 21, 11293–11304. [Google Scholar] [CrossRef]
- Oldfield, T.L.; Sikirica, N.; Mondini, C.; López, G.; Kuikman, P.J.; Holden, N.M. Biochar, Compost and Biochar-Compost Blend as Options to Recover Nutrients and Sequester Carbon. J. Environ. Manag. 2018, 218, 465–476. [Google Scholar] [CrossRef]
- Macías, F. Recuperación de Suelos Degradados, Reutilización de Residuos y Secuestro de Carbono. Una Alternativa Integral de Mejora de La Calidad Ambiental. Recur. Rurais 2004, 1, 49–56. [Google Scholar]
- Macías, F.; Macías-García, F.; Nieto, C.; Verde, J.R.; Pérez, C.; Bao, M.; Camps-Arbestain, M. Gestión de Residuos y Cambio Climático. In Gestión de Residuos Orgánicos de uso Agrícola; Mosquera, M.E.L., Osés, M.J.S., Eds.; Servizo de Publicacións e Intercambio Científico de la Universidade de Santiago de Compostela: Santiago de Compostela, Spain, 2011; pp. 11–24. ISBN 9788498878226. [Google Scholar]
- Pérez-De-Mora, A.; Madejón, P.; Burgos, P.; Cabrera, F.; Lepp, N.W.; Madejón, E. Phytostabilization of Semiarid Soils Residually Contaminated with Trace Elements Using By-Products: Sustainability and Risks. Environ. Pollut. 2011, 159, 3018–3027. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources. In International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Sciences (IUSS): Vienna, Austria, 2022; ISBN 9798986245119. [Google Scholar]
- Santos, E.S.; Abreu, M.M.; Macías, F. Rehabilitation of Mining Areas through Integrated Biotechnological Approach: Technosols Derived from Organic/Inorganic Wastes and Autochthonous Plant Development. Chemosphere 2019, 224, 765–775. [Google Scholar] [CrossRef] [PubMed]
- Macías, F.; Bao, M.; Macías-García, F.; Camps Arbestain, M. Valorización Biogeoquímica de Residuos Por Medio de La Elaboración de Tecnosoles Con Diferentes Aplicaciones Ambientales. Agua Residuos 2007, 5, 12–25. [Google Scholar]
- Santos, E.S.; Abreu, M.M.; Macías, F.; de Varennes, A. Chemical Quality of Leachates and Enzymatic Activities in Technosols with Gossan and Sulfide Wastes from the São Domingos Mine. J. Soils Sediments 2016, 16, 1366–1382. [Google Scholar] [CrossRef]
- Asensio, V.; Flórido, F.G.; Ruiz, F.; Perlatti, F.; Otero, X.L.; Oliveira, D.P.; Ferreira, T.O. The Potential of a Technosol and Tropical Native Trees for Reclamation of Copper-Polluted Soils. Chemosphere 2019, 220, 892–899. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Vila, A.; Asensio, V.; Forján, R.; Covelo, E.F. Assessing the Influence of Technosol and Biochar Amendments Combined with Brassica Juncea L. on the Fractionation of Cu, Ni, Pb and Zn in a Polluted Mine Soil. J. Soils Sediments 2016, 16, 339–348. [Google Scholar] [CrossRef]
- Asemaninejad, A.; Arteaga, J.; Spiers, G.; Beckett, P.; McGarry, S.; Mykytczuk, N.; Basiliko, N. Blended Pulp Mill, Forest Humus and Mine Residual Material Technosols for Mine Reclamation: A Growth-Chamber Study to Explore the Role of Physiochemical Properties of Substrates and Microbial Inoculation on Plant Growth. J. Environ. Manag. 2018, 228, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Jordán, M.M.; García-Sánchez, E.; Almendro-Candel, M.B.; Pardo, F.; Vicente, A.B.; Sanfeliu, T.; Bech, J. Technosols Designed for Rehabilitation of Mining Activities Using Mine Spoils and Biosolids. Ion Mobility and Correlations Using Percolation Columns. Catena 2017, 148, 74–80. [Google Scholar] [CrossRef]
- Cortinhas, A.; Caperta, A.D.; Teixeira, G.; Carvalho, L.; Abreu, M.M. Harnessing Sediments of Coastal Aquaculture Ponds through Technosols Construction for Halophyte Cultivation Using Saline Water Irrigation. J. Environ. Manag. 2020, 261, 109907. [Google Scholar] [CrossRef]
- Cortinhas, A.; Ferreira, T.C.; Abreu, M.M.; Caperta, A.D. Conservation of a Critically Endangered Endemic Halophyte of West Portugal: A Microcosm Assay to Assess the Potential of Soil Technology for Species Reintroduction. Front. Ecol. Evol. 2021, 9, 604509. [Google Scholar] [CrossRef]
- Cortinhas, A.; Ferreira, T.C.; Abreu, M.M.; Caperta, A.D. Germination and Sustainable Cultivation of Succulent Halophytes Using Resources from a Degraded Estuarine Area through Soil Technologies Approaches and Saline Irrigation Water. Land. Degrad. Dev. 2023, 34, 5029–5041. [Google Scholar] [CrossRef]
- Kong, C.; Camps-Arbestain, M.; Clothier, B.; Bishop, P.; Vázquez, F.M. Reclamation of Salt-Affected Soils Using Pumice and Algal Amendments: Impact on Soil Salinity and the Growth of Lucerne. Environ. Technol. Innov. 2021, 24, 101867. [Google Scholar] [CrossRef]
- Kong, C.; Camps-Arbestain, M.; Clothier, B.; Bishop, P.; Vázquez, F.M. Use of Either Pumice or Willow-Based Biochar Amendments to Decrease Soil Salinity under Arid Conditions. Environ. Technol. Innov. 2021, 24, 101849. [Google Scholar] [CrossRef]
- Asensio, V.; Vega, F.A.; Andrade, M.L.; Covelo, E.F. Technosols Made of Wastes to Improve Physico-Chemical Characteristics of a Copper Mine Soil. Pedosphere 2013, 23, 1–9. [Google Scholar] [CrossRef]
- Ruiz, F.; Perlatti, F.; Oliveira, D.P.; Ferreira, T.O. Revealing Tropical Technosols as an Alternative for Mine Reclamation and Waste Management. Minerals 2020, 10, 110. [Google Scholar] [CrossRef]
- Queiroz, H.M.; Ferreira, A.D.; Ruiz, F.; Bovi, R.C.; Deng, Y.; de Souza Júnior, V.S.; Otero, X.L.; Bernardino, A.F.; Cooper, M.; Ferreira, T.O. Early Pedogenesis of Anthropogenic Soils Produced by the World’s Largest Mining Disaster, the “Fundão” Dam Collapse, in Southeast Brazil. Catena 2022, 219, 106625. [Google Scholar] [CrossRef]
- Walmsley, A.; Mundodi, L.; Sederkenny, A.; Anderson, N.; Missen, J.; Yellishetty, M. From Spoil to Soil: Utilising Waste Materials to Create Soils for Mine Rehabilitation. In Proceedings of the International Conference on Mine Closure, Brisbane, Australia, 4–6 October 2022; Tibbett, M., Fourie, A.B., Boggs, G., Eds.; Mine Earth: Brisbane, Australia, 2022; Volume 1, pp. 1237–1248. [Google Scholar]
- Arán, D.; Santos, E.S.; Abreu, M.M.; Antelo, J.; Macías, F. Use of Combined Tools for Effectiveness Evaluation of Tailings Rehabilitated with Designed Technosol. Environ. Geochem. Health 2022, 44, 1857–1873. [Google Scholar] [CrossRef]
- Álvarez-Valero, A.M.; Pérez-López, R.; Matos, J.; Capitán, M.A.; Nieto, J.M.; Sáez, R.; Delgado, J.; Caraballo, M. Potential Environmental Impact at São Domingos Mining District (Iberian Pyrite Belt, SW Iberian Peninsula): Evidence from a Chemical and Mineralogical Characterization. Environ. Geol. 2008, 55, 1797–1809. [Google Scholar] [CrossRef]
- Quental, L.; Bourguignon, A.; Sousa, A.J.; Batista, M.J.; Brito, M.G.; Tavares, T.; Abreu, M.M.; Vairinho, M.M.; Cottard, F. MINEO Southern Europe Environment Test Site: Contamination Impact Mapping and Modelling: Final Report; MINEO Project-Assessing and Monitoring the Environmental Impact of Mining in Europe Using Advanced Earth Observation Techniques; Information Society Technologies, EU: Luxembourg, 2002. [Google Scholar]
- Pérez-López, R.; Álvarez-Valero, A.M.; Nieto, J.M.; Sáez, R.; Matos, J.X. Use of Sequential Extraction Procedure for Assessing the Environmental Impact at Regional Scale of the São Domingos Mine (Iberian Pyrite Belt). Appl. Geochem. 2008, 23, 3452–3463. [Google Scholar] [CrossRef]
- Póvoas, I.; Barral, M.F. Métodos de Análise de Solos. Comunicações Do Instituto de Investigação Científica Tropical. In Série de Ciências Agrárias N.o 10; Instituto de Investigação Científica Tropical: Lisbon, Portugal, 1992. [Google Scholar]
- Lakanen, E.; Erviö, R. A Comparison of Eight Extractants for the Determination of Plant Available Micronutrients in Soils. Acta Agric. Fenn. 1971, 123, 223–232. [Google Scholar]
- Activation Laboratories Ltd. Code 6—Total Recoverable Natural Waters with Low TDS (<0.05%), Analysed by ICP-MS. Available online: https://actlabs.com/wp-content/uploads/2021/07/Actlabs-Schedule-of-Services-Euro-2021.pdf (accessed on 16 July 2024).
- Activation Laboratories Ltd. Code 6—Overrange Elements in Code 6 MB Reanalyzed by ICP-MS If Required. Available online: https://actlabs.com/wp-content/uploads/2021/07/Actlabs-Schedule-of-Services-Euro-2021.pdf (accessed on 16 July 2024).
- Feng, M.H.; Shan, X.Q.; Zhang, S.; Wen, B. A Comparison of the Rhizosphere-Based Method with DTPA, EDTA, CaCl2, and NaNO3 Extraction Methods for Prediction of Bioavailability of Metals in Soil to Barley. Environ. Pollut. 2005, 137, 231–240. [Google Scholar] [CrossRef]
- Le Bissonnais, Y.Y. Analyse Des Mécanismes de Désagrégation et de La Mobilisation Des Particules de Terre Sous l’action Des Pluies. Ph.D. Thesis, Université d’Orléans, Orléans, France, 1988. [Google Scholar]
- Tabatabai, M.A. Soil Enzymes. In Methods of Soil Analysis, Part 2. Microbiological and Biochemical Properties; Mickelson, S.H., Bigham, J.M., Eds.; SSSA Book Series; Soil Science Society of America: Madison, WI, USA, 1994; pp. 775–833. ISBN 9780891188650. [Google Scholar]
- Dotaniya, M.L.; Aparna, K.; Dotaniya, C.K.; Singh, M.; Regar, K.L. Role of Soil Enzymes in Sustainable Crop Production. In Enzymes in Food Biotechnology: Production, Applications, and Future Prospects; Kuddus, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 569–589. ISBN 9780128132807. [Google Scholar]
- Kumar, S.; Chaudhuri, S.; Maiti, S.K. Soil Dehydrogenase Enzyme Activity in Natural and Mine Soil—A Review. Middle-East. J. Sci. Res. 2013, 13, 898–906. [Google Scholar] [CrossRef]
- Eivazi, F.; Tabatabai, M.A. Glucosidases and Galactosidases in Soils. Soil. Biol. Biochem. 1988, 20, 601–606. [Google Scholar] [CrossRef]
- Eivazi, F.; Tabatabai, M.A. Phosphatases in Soils. Soil. Biol. Biochem. 1977, 9, 167–172. [Google Scholar] [CrossRef]
- Kandeler, E.; Gerber, H. Short-Term Assay of Soil Urease Activity Using Colorimetric Determination of Ammonium. Biol. Fertil. Soils 1988, 6, 68–72. [Google Scholar] [CrossRef]
- Bai, H.; Wang, H.; Sun, J.; Irfan, M.; Han, M.; Huang, Y.; Han, X.; Yang, Q. Production, Purification and Characterization of Novel Beta Glucosidase from Newly Isolated Penicillium Simplicissimum h-11 in Submerged Fermentation. EXCLI J. 2013, 12, 528–540. [Google Scholar]
- Krämer, S.; Green, D.M. Acid and Alkaline Phosphatase Dynamics and Their Relationship to Soil Microclimate in a Semiarid Woodland. Soil Biol. Biochem. 2000, 32, 179–188. [Google Scholar] [CrossRef]
- Liang, Y.; Yang, Y.; Yang, C.; Shen, Q.; Zhou, J.; Yang, L. Soil Enzymatic Activity and Growth of Rice and Barley as Influenced by Organic Manure in an Anthropogenic Soil. Geoderma 2003, 115, 149–160. [Google Scholar] [CrossRef]
- Jecu, L. Solid State Fermentation of Agricultural Wastes for Endoglucanase Production. Ind. Crops Prod. 2000, 11, 1–5. [Google Scholar] [CrossRef]
- Hope, C.F.A.; Burns, R.G. Activity, Origins and Location of Cellulases in a Silt Loam Soil. Biol. Fertil. Soils 1987, 5, 164–170. [Google Scholar] [CrossRef]
- Ladd, J.N.; Butler, J.H.A. Short-Term Assays of Soil Proteolytic Enzyme Activities Using Proteins and Dipeptide Derivatives as Substrates. Soil. Biol. Biochem. 1972, 4, 19–30. [Google Scholar] [CrossRef]
- Adetunji, A.T.; Lewu, F.B.; Mulidzi, R.; Ncube, B. The Biological Activities of β-Glucosidase, Phosphatase and Urease as Soil Quality Indicators: A Review. J. Soil. Sci. Plant Nutr. 2017, 17, 794–807. [Google Scholar] [CrossRef]
- ISO/IEC 17025; General Requirements for the Competence of Testing and Calibration Laboratories. ISO (International Organization for Standardization) and IEC (International Electrotechnical Commission): Geneva, Switzerland, 2017.
- Veloso, A.; Sempiterno, C.; Calouro, F.; Rebelo, F.; Pedra, F.; Castro, I.V.; da Conceição Gonçalves, M.; da Encarnação Marcelo, M.; Pereira, P.; Fareleira, P.; et al. Manual de Fertilização Das Culturas, 3rd ed.; Calouro, F., Ed.; INIAV (Instituto Nacional de Investigação Agrária e Veterinária, I.P.): Lisbon, Portugal, 2022; ISBN 978-972-579-063-2. [Google Scholar]
- Aguilar-Garrido, A.; Reyes-Martín, M.P.; Vidigal, P.; Abreu, M.M. A Green Solution for the Rehabilitation of Marginal Lands: The Case of Lablab Purpureus (L.) Sweet Grown in Technosols. Plants 2023, 12, 2682. [Google Scholar] [CrossRef] [PubMed]
- APA (Agência Portuguesa do Ambiente). Solos Contaminados—Guia Técnico—Valores de Referência Para Solo. Revisão 3—Setembro de 2022; Agência Portuguesa do Ambiente: Lisbon, Portugal, 2019. [Google Scholar]
- CCME (Canada Council of Ministers of the Environment). Canadian Soil Quality Guidelines for the Protection of Environmental and Human Health: Summary Tables (Updated September, 2007); Canada Council of Ministers of the Environment: Winnipeg, MB, Canada, 2007. [Google Scholar]
- Kiran; Bharti, R.; Sharma, R. Effect of Heavy Metals: An Overview. Mater. Today Proc. 2022, 51, 880–885. [Google Scholar] [CrossRef]
- Hamad, R.; Balzter, H.; Kolo, K. Assessment of Heavy Metal Release into the Soil after Mine Clearing in Halgurd-Sakran National Park, Kurdistan, Iraq. Environ. Sci. Pollut. Res. 2019, 26, 1517–1536. [Google Scholar] [CrossRef] [PubMed]
- Madejón, P.; Caro-Moreno, D.; Navarro-Fernández, C.M.; Rossini-Oliva, S.; Marañón, T. Rehabilitation of Waste Rock Piles: Impact of Acid Drainage on Potential Toxicity by Trace Elements in Plants and Soil. J. Environ. Manag. 2021, 280, 111848. [Google Scholar] [CrossRef]
- Weil, R.R.; Brady, N.C. The Nature and Properties of Soils, 15th ed.; Pearson Education: London, UK, 2017; ISBN 9780133254488. [Google Scholar]
- Oster, J.D. Sodic Soil Reclamation. In Towards the Rational Use of High Salinity Tolerant Plants. Tasks for Vegetation Science; Lieth, H., Al Masoom, A.A., Eds.; Springer: Dordrecht, The Netherlands, 1993; Volume 27, pp. 485–490. ISBN 978-94-011-1858-3. [Google Scholar]
- Macía, P.; Fernández-Costas, C.; Rodríguez, E.; Sieiro, P.; Pazos, M.; Sanromán, M.A. Technosols as a Novel Valorization Strategy for an Ecological Management of Dredged Marine Sediments. Ecol. Eng. 2014, 67, 182–189. [Google Scholar] [CrossRef]
- Navarro-Torre, S.; Garcia-Caparrós, P.; Nogales, A.; Abreu, M.M.; Santos, E.; Cortinhas, A.L.; Caperta, A.D. Sustainable Agricultural Management of Saline Soils in Arid and Semi-Arid Mediterranean Regions through Halophytes, Microbial and Soil-Based Technologies. Environ. Exp. Bot. 2023, 212, 105397. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; CRC Press (Taylor & Francis Group): Boca Raton, FL, USA, 2010; ISBN 9781420093704. [Google Scholar]
- Alloway, B.J. Heavy Metals in Soils. Trace Metals and Metalloids in Soils and Their Bioavailability, 3rd ed.; Springer: Dordrecht, The Netherlands, 2012; ISBN 9789400744691. [Google Scholar]
- Beesley, L.; Moreno-Jiménez, E.; Gomez-Eyles, J.L. Effects of Biochar and Greenwaste Compost Amendments on Mobility, Bioavailability and Toxicity of Inorganic and Organic Contaminants in a Multi-Element Polluted Soil. Environ. Pollut. 2010, 158, 2282–2287. [Google Scholar] [CrossRef]
- Paradelo, R.; Villada, A.; Barral, M.T. Reduction of the Short-Term Availability of Copper, Lead and Zinc in a Contaminated Soil Amended with Municipal Solid Waste Compost. J. Hazard. Mater. 2011, 188, 98–104. [Google Scholar] [CrossRef]
- Wang, S.; Mulligan, C.N. Occurrence of Arsenic Contamination in Canada: Sources, Behavior and Distribution. Sci. Total Environ. 2006, 366, 701–721. [Google Scholar] [CrossRef]
- Paniagua-López, M.; Vela-Cano, M.; Correa-Galeote, D.; Martín-Peinado, F.; Marínez Garzón, F.J.; Pozo, C.; González-López, J.; Sierra Aragón, M. Soil Remediation Approach and Bacterial Community Structure in a Long-Term Contaminated Soil by a Mining Spill (Aznalcóllar, Spain). Sci. Total Environ. 2021, 777, 145128. [Google Scholar] [CrossRef]
- Aguilar-Garrido, A.; Romero-Freire, A.; García-Carmona, M.; Martín Peinado, F.J.; Sierra Aragón, M.; Martínez Garzón, F.J. Arsenic Fixation in Polluted Soils by Peat Applications. Minerals 2020, 10, 968. [Google Scholar] [CrossRef]
- Bolan, N.; Kunhikrishnan, A.; Thangarajan, R.; Kumpiene, J.; Park, J.; Makino, T.; Kirkham, M.B.; Scheckel, K. Remediation of Heavy Metal(Loid)s Contaminated Soils—To Mobilize or to Immobilize? J. Hazard. Mater. 2014, 266, 141–166. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Salgado, M.M.; Gutiérrez-Romero, V.; Jannsens, M.; Ortega-Blu, R. Biological Soil Quality Indicators: A Review. In Current Research, Technology and Education Topics in Applied Microbiology and Microbial Biotechnology; Méndez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2010; pp. 319–328. ISBN 9788461461950. [Google Scholar]
- Kandeler, E.; Kampichler, C.; Horak, O. Influence of Heavy Metals on the Functional Diversity of Soil Microbial Communities. Biol. Fertil. Soils 1996, 23, 299–306. [Google Scholar] [CrossRef]
- Aponte, H.; Meli, P.; Butler, B.; Paolini, J.; Matus, F.; Merino, C.; Cornejo, P.; Kuzyakov, Y. Meta-Analysis of Heavy Metal Effects on Soil Enzyme Activities. Sci. Total Environ. 2020, 737, 139744. [Google Scholar] [CrossRef]
- Aguilar-Garrido, A.; Romero-Freire, A.; Paniagua-López, M.; Martínez-Garzón, F.J.; Martín-Peinado, F.J.; Sierra-Aragón, M. Technosols Derived from Mining, Urban, and Agro-Industrial Waste for the Remediation of Metal(Loid)-Polluted Soils: A Microcosm Assay. Toxics 2023, 11, 854. [Google Scholar] [CrossRef]
- National Research Council. Mineral Tolerance of Animals: Second Revised Edition, 2005; National Academies Press: Washington, DC, USA, 2005; ISBN 0309096545. [Google Scholar]
- de Varennes, A. Produtividade Dos Solos e Ambiente; Escolar Editora: Lisbon, Portugal, 2003; ISBN 978-972-592-156-2. [Google Scholar]
- Balat, M.; Ayar, G. Biomass Energy in the World, Use of Biomass and Potential Trends. Energy Sources 2005, 27, 931–940. [Google Scholar] [CrossRef]


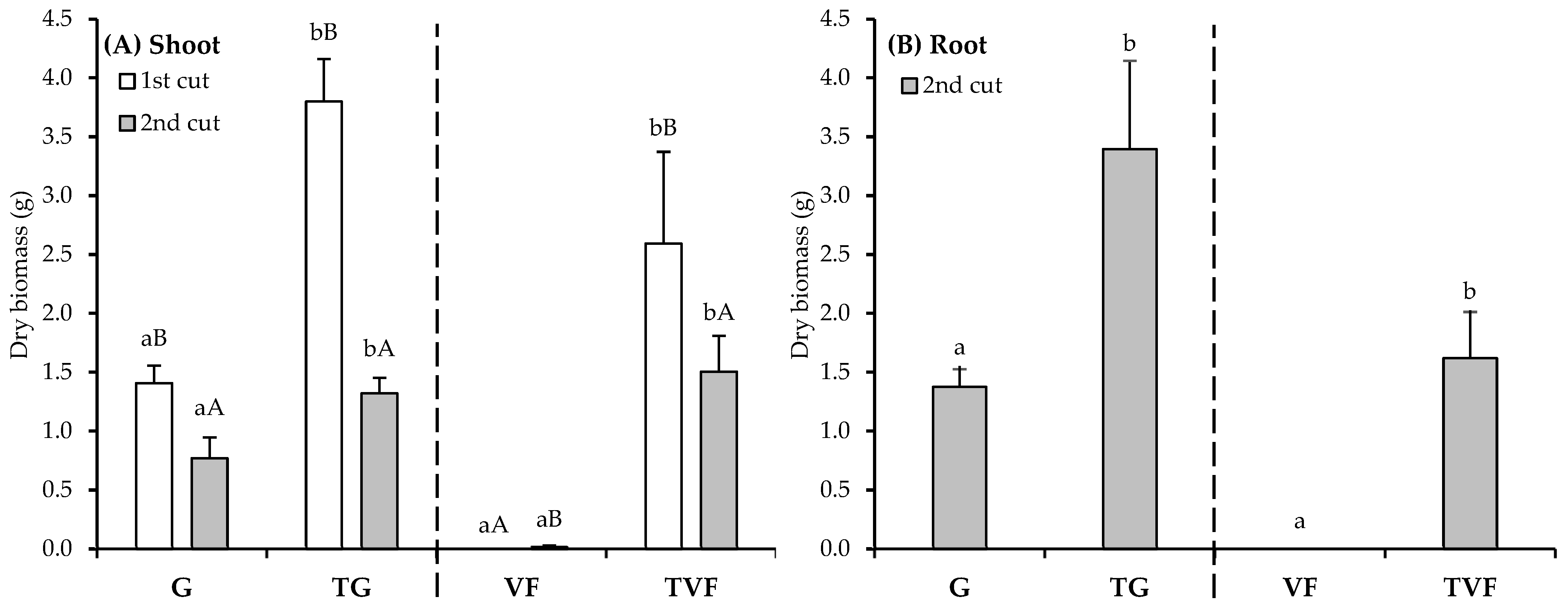
| Physicochemical Characteristics | G | TG | VF | TVF | ||||
|---|---|---|---|---|---|---|---|---|
| Initial | Final | Initial | Final | Initial | Final | Initial | Final | |
| pH (H2O) | 3.80 ± 0.04 aA | 4.08 ± 0.09 aB | 6.53 ± 0.23 b | 6.70 ± 0.13 b | 8.26 ± 0.07 A | 8.52 ± 0.05 B | 8.46 ± 0.13 A | 8.89 ± 0.11 B |
| EC (dS m−1) | 0.70 ± 0.17 aB | 0.12 ± 0.02 aA | 1.33 ± 0.30 bB | 0.32 ± 0.14 bA | 11.20 ± 1.52 bB | 0.83 ± 0.11 A | 3.96 ± 1.20 aB | 0.64 ± 0.45 A |
| Corg (g kg−1) | 4.22 ± 0.74 a | 5.41 ± 1.77 a | 9.23 ± 1.24 bA | 14.18 ± 2.50 bB | 18.87 ± 1.36 a | 19.43 ± 0.72 a | 28.15 ± 0.67 b | 28.13 ± 1.30 b |
| NT (g kg−1) | 0.16 ± 0.01 aA | 0.27 ± 0.02 aB | 1.18 ± 0.20 bB | 0.70 ± 0.22 bA | 1.65 ± 0.05 aA | 1.81 ± 0.10 aB | 2.73 ± 0.17 b | 2.48 ± 0.13 b |
| PExt (mg kg−1) | 0.56 ± 0.68 a | bdl a | 55.97 ± 8.02 b | 46.72 ± 9.94 b | 92.69 ± 2.19 a | 98.04 ± 2.77 a | 424.29 ± 27.35 b | 425.33 ± 43.02 b |
| KExt (mg kg−1) | 37.49 ± 17.30 a | 22.95 ± 6.21 | 153.81 ± 25.13 bB | 23.63 ± 2.50 A | 799.29 ± 29.89 a | 811.77 ± 24.34 | 942.85 ± 38.58 bB | 844.44 ± 54.21 A |
| Elements (g kg−1) | G | TG | VF | TVF | ||||
|---|---|---|---|---|---|---|---|---|
| Initial | Final | Initial | Final | Initial | Final | Initial | Final | |
| Ca | 0.38 ± 0.05 aB | 0.06 ± 0.02 aA | 2.25 ± 0.69 bB | 1.04 ± 0.54 bA | 4.04 ± 0.29 a | 3.23 ± 0.55 a | 8.61 ± 0.84 b | 7.85 ± 2.90 b |
| Mg | 0.03 ± 0.01 aB | 0.01 ± 0.01 aA | 0.07 ± 0.01 bB | 0.02 ± 0.01 bA | 2.25 ± 0.28 bB | 1.57 ± 0.07 A | 1.66 ± 0.09 aB | 1.49 ± 0.06 A |
| Na | 0.11 ± 0.04 B | 0.05 ± 0.01 A | 0.24 ± 0.17 | 0.09 ± 0.04 | 11.15 ± 2.64 bB | 5.36 ± 1.15 bA | 4.44 ± 2.08 aB | 2.25 ± 0.42 aA |
| K | 0.03 ± 0.01 a | 0.02 ± 0.01 | 0.16 ± 0.02 bB | 0.02 ± 0.00 A | 0.78 ± 0.03 a | 0.77 ± 0.02 | 0.99 ± 0.07 bB | 0.82 ± 0.05 A |
| Cu | 0.002 ± 0.001 aA | 0.006 ± 0.001 aB | 0.006 ± 0.001 bA | 0.009 ± 0.001 bB | 0.007 ± 0.000 aA | 0.011 ± 0.001 aB | 0.010 ± 0.000 bA | 0.014 ± 0.001 bB |
| Fe | 0.029 ± 0.002 aA | 0.044 ± 0.006 aB | 0.145 ± 0.015 bB | 0.125 ± 0.006 bA | 0.148 ± 0.003 aA | 0.175 ± 0.022 aB | 0.566 ± 0.025 bB | 0.495 ± 0.034 bA |
| Mn | 0.001 ± 0.001 a | 0.000 ± 0.000 a | 0.003 ± 0.001 bB | 0.001 ± 0.001 bA | 0.288 ± 0.009 b | 0.282 ± 0.017 b | 0.242 ± 0.006 aB | 0.221 ± 0.009 aA |
| Zn | 0.003 ± 0.001 a | 0.003 ± 0.001 a | 0.006 ± 0.001 b | 0.006 ± 0.001 b | 0.011 ± 0.001 aA | 0.014 ± 0.002 aB | 0.018 ± 0.001 bA | 0.020 ± 0.001 bB |
| Elements (mg kg−1) | G | TG | VF | TVF | ||||
|---|---|---|---|---|---|---|---|---|
| Initial | Final | Initial | Final | Initial | Final | Initial | Final | |
| As | 0.15 ± 0.17 a | bdl | 3.89 ± 0.16 b | 4.10 ± 0.24 | 0.15 ± 0.17 aA | 0.45 ± 0.06 B | 0.48 ± 0.05 b | 0.45 ± 0.13 |
| Cu | 1.05 ± 0.13 | 0.90 ± 0.07 b | 0.76 ± 0.29 | 0.71 ± 0.02 a | 0.21 ± 0.02 | 0.25 ± 0.03 | 0.26 ± 0.04 | 0.26 ± 0.03 |
| Ni | 0.14 ± 0.02 a | 0.15 ± 0.05 | 0.20 ± 0.04 bB | 0.11 ± 0.02 A | 0.18 ± 0.04 | 0.21 ± 0.07 | 0.16 ± 0.05 | 0.14 ± 0.03 |
| Pb | 5.48 ± 1.27 b | 6.01 ± 0.59 b | 0.95 ± 0.95 a | 0.88 ± 0.29 a | bdl | bdl | bdl | bdl |
| Sb | 0.23 ± 0.05 | 0.20 ± 0.00 | 0.20 ± 0.00 | 0.23 ± 0.05 | 0.10 ± 0.12 | 0.18 ± 0.13 | bdl | bdl |
| S | 647.56 ± 13.26 B | 162.08 ± 19.18 A | 907.58 ± 214.50 B | 197.21 ± 69.89 A | 792.24 ± 322.77 B | 239.53 ± 53.58 bA | 428.45 ± 191.97 B | 132.11 ± 35.52 aA |
| Zn | 3.05 ± 0.52 B | 1.94 ± 0.21 aA | 3.70 ± 1.07 | 3.80 ± 0.23 b | 0.49 ± 0.08 b | 0.56 ± 0.04 b | 0.36 ± 0.06 a | 0.35 ± 0.05 a |
| Aggregates’ Distribution (%) | G | TG | VF | TVF |
|---|---|---|---|---|
| >2 mm | 34.53 ± 5.44 a | 67.98 ± 3.50 b | 73.76 ± 10.43 a | 93.56 ± 0.50 b |
| 1–2 mm | 15.05 ± 2.91 b | 7.69 ± 1.35 a | 10.01 ± 4.61 b | 1.04 ± 0.29 a |
| 0.5–1 mm | 15.19 ± 1.20 b | 7.78 ± 0.93 a | 6.81 ± 3.41 b | 0.68 ± 0.23 a |
| 0.2–0.5 mm | 13.32 ± 1.84 b | 6.56 ± 1.68 a | 3.94 ± 2.01 b | 0.41 ± 0.12 a |
| 0.1–0.2 mm | 10.79 ± 1.25 b | 5.15 ± 1.15 a | 1.84 ± 0.87 b | 0.20 ± 0.04 a |
| 0.05–0.1 mm | 5.78 ± 0.62 b | 2.56 ± 0.19 a | 0.50 ± 0.23 b | 0.05 ± 0.01 a |
| <0.05 mm | 5.34 ± 1.09 b | 2.29 ± 0.55 a | 3.15 ± 0.83 | 4.07 ± 0.19 |
| Elements | G | TG | VF | TVF | ||||
|---|---|---|---|---|---|---|---|---|
| 1st Cut | 2nd Cut | 1st Cut | 2nd Cut | 1st Cut | 2nd Cut | 1st Cut | 2nd Cut | |
| As (mg kg−1) | 62.55 ± 27.74 b | 78.33 ± 26.22 b | 16.07 ± 4.12 a | 17.65 ± 3.92 a | - | - | bdl | bdl |
| Cu (mg kg−1) | 25.12 ± 7.57 bA | 57.09 ± 8.02 B | 15.79 ± 2.55 aA | 55.04 ± 1.32 B | - | - | 46.74 ± 16.56 | 49.51 ± 6.31 |
| Ni (mg kg−1) | 10.63 ± 4.45 bB | 6.20 ± 0.32 bA | 4.15 ± 0.58 a | 4.44 ± 0.39 a | - | - | 5.00 ± 1.37 | 3.80 ± 0.67 |
| Pb (mg kg−1) | 160.39 ± 67.66 b | 216.52 ± 71.06 b | 27.99 ± 9.17 a | 40.50 ± 10.90 a | - | - | 0.98 ± 1.13 | 1.93 ± 2.23 |
| Sb (mg kg−1) | 2.78 ± 0.98 | 1.89 ± 1.52 | bdl | bdl | - | - | bdl | bdl |
| Zn (mg kg−1) | 46.75 ± 6.15 A | 65.00 ± 4.39 aB | 72.72 ± 27.08 | 85.82 ± 1.35 b | - | - | 56.00 ± 7.59 | 59.37 ± 3.48 |
| Ca (g kg−1) | 5.95 ± 0.74 aB | 4.43 ± 1.01 aA | 14.43 ± 1.70 b | 12.84 ± 0.94 b | - | - | 3.59 ± 0.50 | 3.99 ± 0.72 |
| Co (mg kg−1) | 0.55 ± 0.22 b | 0.48 ± 0.10 | 0.09 ± 0.18 a | bdl | - | - | bdl | bdl |
| Fe (g kg−1) | 1.31 ± 0.46 | 1.25 ± 0.34 b | 1.34 ± 1.86 | 0.49 ± 0.24 a | - | - | 0.37 ± 0.41 | 0.50 ± 0.34 |
| K (g kg−1) | 15.29 ± 2.98 a | 12.67 ± 2.08 a | 26.51 ± 2.29 bB | 21.47 ± 2.10 bA | - | - | 33.29 ± 2.94 B | 24.97 ± 4.76 A |
| Mn (mg kg−1) | 57.29 ± 4.96 a | 58.78 ± 14.24 a | 100.48 ± 22.03 bA | 207.89 ± 77.50 bB | - | - | 25.50 ± 5.15 | 35.97 ± 5.83 |
| Mg (g kg−1) | 1.95 ± 0.13 aB | 1.48 ± 0.38 aA | 3.65 ± 0.52 b | 3.47 ± 0.23 a | - | - | 3.06 ± 0.21 | 3.20 ± 0.28 |
| Mo (mg kg−1) | 4.56 ± 6.56 | bdl | 3.30 ± 1.43 A | 6.66 ± 1.38 B | - | - | 3.54 ± 0.79 | 3.31 ± 0.11 |
| Na (g kg−1) | 6.16 ± 1.03 A | 9.78 ± 0.48 B | 9.19 ± 3.62 | 7.31 ± 2.84 | - | - | 26.86 ± 2.91 | 24.54 ± 3.44 |
| P (g kg−1) | 3.12 ± 0.47 a | 2.74 ± 0.14 a | 5.29 ± 0.64 b | 5.76 ± 0.17 b | - | - | 6.76 ± 0.83 | 7.26 ± 0.55 |
| S (g kg−1) | 9.61 ± 1.34 b | 8.26 ± 1.03 | 7.90 ± 0.95 a | 8.29 ± 2.16 | - | - | 3.39 ± 0.05 | 3.76 ± 0.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguilar-Garrido, A.; Vidigal, P.; Caperta, A.D.; Abreu, M.M. An Integrated Approach to Remediate Saline Soils and Mining Waste Using Technosols and Pasture Development. Soil Syst. 2024, 8, 103. https://doi.org/10.3390/soilsystems8040103
Aguilar-Garrido A, Vidigal P, Caperta AD, Abreu MM. An Integrated Approach to Remediate Saline Soils and Mining Waste Using Technosols and Pasture Development. Soil Systems. 2024; 8(4):103. https://doi.org/10.3390/soilsystems8040103
Chicago/Turabian StyleAguilar-Garrido, Antonio, Patrícia Vidigal, Ana Delaunay Caperta, and Maria Manuela Abreu. 2024. "An Integrated Approach to Remediate Saline Soils and Mining Waste Using Technosols and Pasture Development" Soil Systems 8, no. 4: 103. https://doi.org/10.3390/soilsystems8040103








