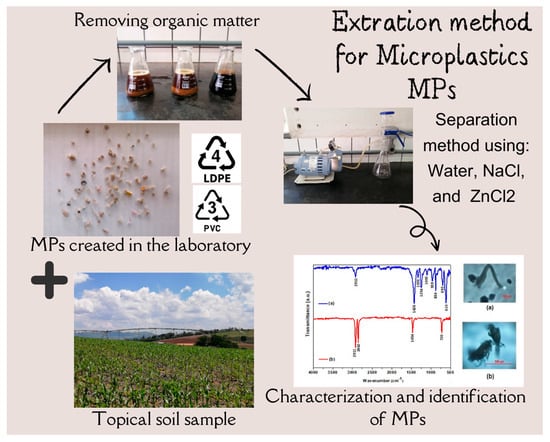
A Method for the Extraction and Analysis of Microplastics from Tropical Agricultural Soils in Southeastern Brazil
Abstract
1. Introduction
2. Materials and Methods
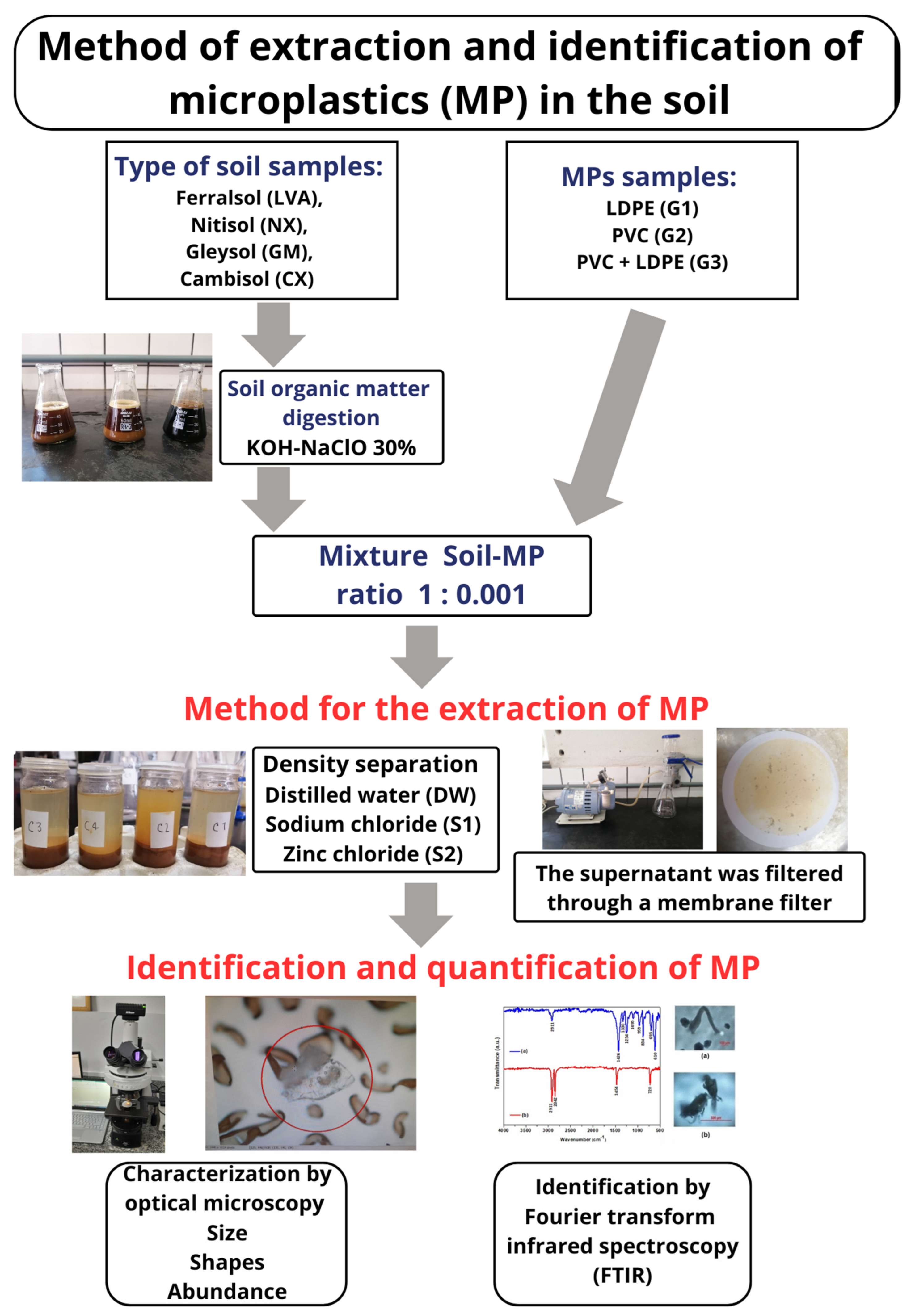
2.1. Sample Preparing
2.1.1. Microplastic Samples
2.1.2. Soil Samples
2.1.3. Microplastic/Soil Samples
2.2. Extraction of Microplastics: Density Separation, Centrifugation, and Filtration
2.3. Evaluation of Organic Matter Removal from the Soil Samples
2.4. Microplastic Recovery Rate
2.5. Identification and Quantification of Microplastics
2.6. Application of Microplastic Extraction Method in Raw Agricultural Soils
2.7. Statistical Analyses
3. Results and Discussion
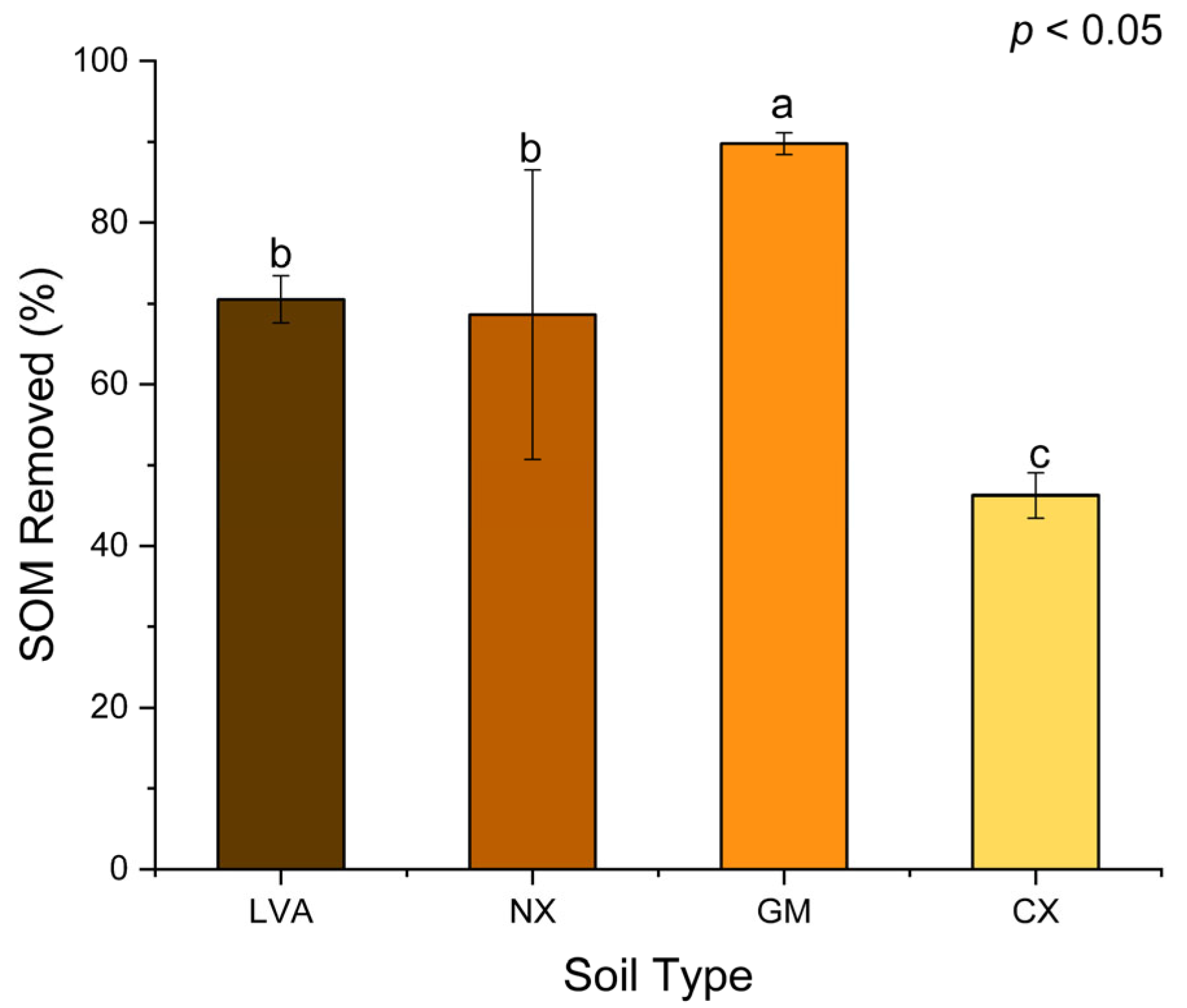
3.1. Efficiency of Digestion Method for Organic Matter Removal in Soil Samples
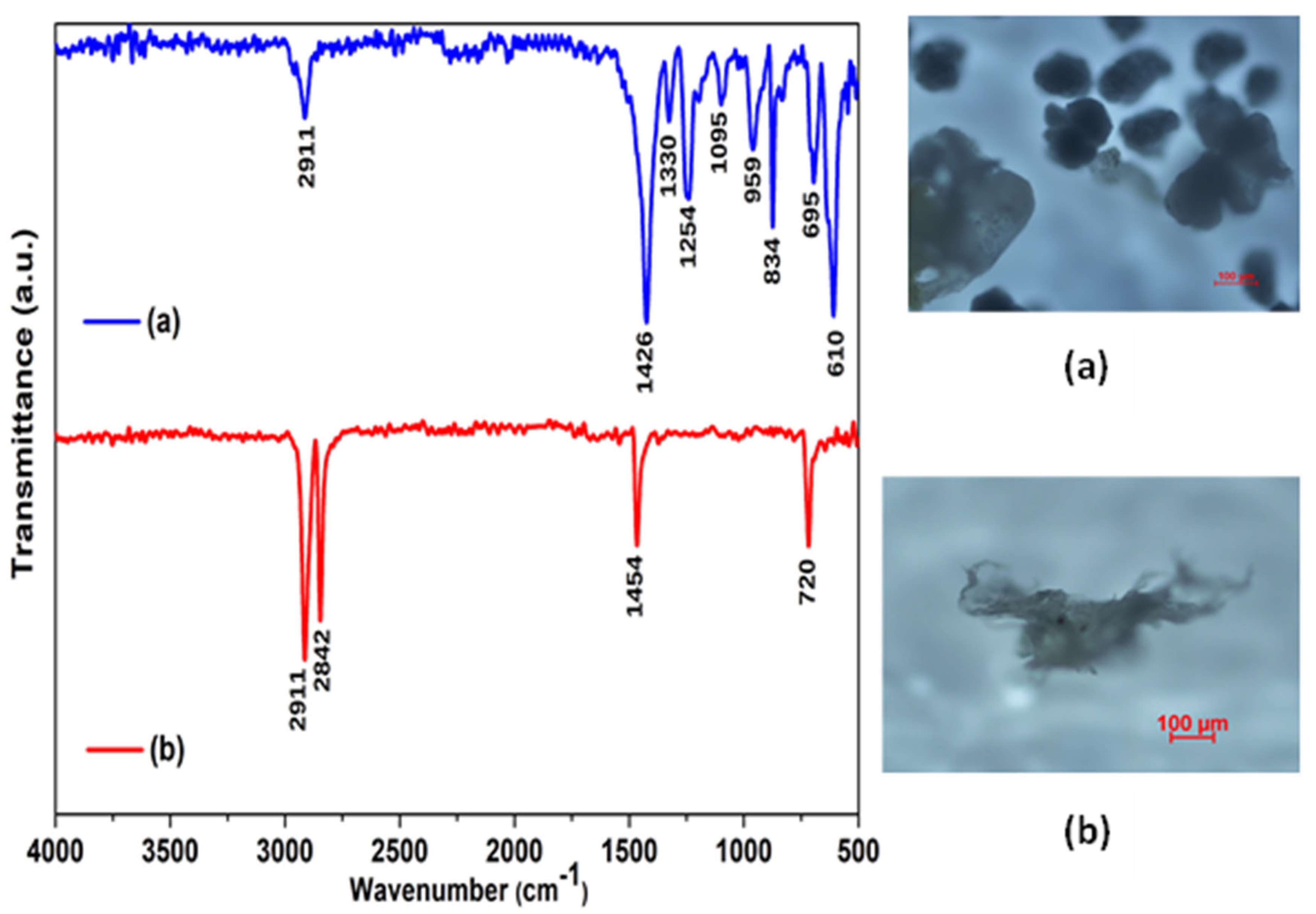
3.2. Characterization of the Microplastics Before and After the Extraction Method
3.3. Recovery Efficiency of Microplastics Through Extraction Method
3.4. Testing the Recovery of Microplastics from Raw Agricultural Soils
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MP | microplastics |
| LDPE | low-density polyethylene |
| PVC | poly(vinyl chloride) |
| GM | gleysols |
| LVA | ferralsols |
| NX | nitisols |
| CX | cambisols |
| NaClO | sodium hypochlorite |
| KOH | potassium hydroxide |
| NaCl | sodium chloride |
| ZnCl2 | zinc chloride |
| FTIR | Fourier transform infrared spectroscopy |
| LOI | loss-on-ignition |
| SOM | soil organic matter |
References
- Ju, T.; Yang, K.; Chang, L.; Zhang, K.; Wang, X.; Zhang, J.; Xu, B.; Li, Y. Microplastics sequestered in the soil affect the turnover and stability of soil aggregates: A review. Sci. Total Environ. 2023, 904, 166776. [Google Scholar] [CrossRef] [PubMed]
- O’Kelly, B.C.; El-Zein, A.; Liu, X.; Patel, A.; Fei, X.; Sharma, S.; Mohammad, A.; Goli, V.S.N.S.; Wang, J.J.; Li, D.; et al. Microplastics in soils: An environmental geotechnics perspective. Environ. Geotech. 2021, 8, 586–618. [Google Scholar] [CrossRef]
- He, D.; Luo, Y.; Lu, S.; Liu, M.; Song, Y.; Lei, L. Microplastics in soils: Analytical methods, pollution characteristics and ecological risks. TrAC Trends Anal. Chem. 2018, 109, 163–172. [Google Scholar] [CrossRef]
- Grause, G.; Kuniyasu, Y.; Chien, M.-F.; Inoue, C. Separation of microplastic from soil by centrifugation and its application to agricultural soil. Chemosphere 2022, 288, 132654. [Google Scholar] [CrossRef]
- Rychter, P.; Kot, M.; Bajer, K.; Rogacz, D.; Šišková, A.; Kapuśniak, J. Utilization of starch films plasticized with urea as fertilizer for improvement of plant growth. Carbohydr. Polym. 2016, 137, 127–138. [Google Scholar] [CrossRef]
- Zhang, S.; Shen, T.; Yang, Y.; Li, Y.C.; Wan, Y.; Zhang, M.; Tang, Y.; Allen, S.C. Controlled-release urea reduced nitrogen leaching and improved nitrogen use efficiency and yield of direct-seeded rice. J. Environ. Manag. 2018, 220, 191–197. [Google Scholar] [CrossRef]
- Sutherland, W.J.; Dias, M.P.; Dicks, L.V.; Doran, H.; Entwistle, A.C.; Fleishman, E.; Gibbons, D.W.; Hails, R.; Hughes, A.C.; Hughes, J.; et al. A Horizon Scan of Emerging Global Biological Conservation Issues for 2020. Trends Ecol. Evol. 2020, 35, 1–7. [Google Scholar] [CrossRef]
- Junhao, C.; Xining, Z.; Xiaodong, G.; Li, Z.; Qi, H.; Siddique, K.H. Extraction and identification methods of microplastics and nanoplastics in agricultural soil: A review. J. Environ. Manag. 2021, 294, 112997. [Google Scholar] [CrossRef]
- Möller, J.N.; Löder, M.G.J.; Laforsch, C. Finding Microplastics in Soils: A Review of Analytical Methods. Environ. Sci. Technol. 2020, 54, 2078–2090. [Google Scholar] [CrossRef]
- Kumar, P.; Lee, J.; Brown, R.J.C.; Kim, K. Micro- and nano-plastic pollution: Behavior, microbial ecology, and remediation technologies. J. Clean. Prod. 2021, 291, 125240. [Google Scholar] [CrossRef]
- Crichton, E.M.; Noël, M.; Gies, E.A.; Ross, P.S. A novel, density-independent and FTIR-compatible approach for the rapid extraction of microplastics from aquatic sediments. Anal. Methods 2017, 9, 1419–1428. [Google Scholar] [CrossRef]
- Yang, X.; Man, Y.B.; Wong, M.H.; Owen, R.B.; Chow, K.L. Environmental health impacts of microplastics exposure on structural organization levels in the human body. Sci. Total Environ. 2022, 825, 154025. [Google Scholar] [CrossRef] [PubMed]
- Bhavsar, P.S.; Chovatiya, B.V.; Kamble, S.B.; Gore, A.H. Extraction and Analysis of Microplastics in the Soil of Diamond City, Surat (Gujarat, India): Ecological Risk, Pollution Indices, and Greenness Evaluation. ACS Agric. Sci. Technol. 2024, 4, 614–625. [Google Scholar] [CrossRef]
- Fan, W.; Qiu, C.; Qu, Q.; Hu, X.; Mu, L.; Gao, Z.; Tang, X. Sources and identification of microplastics in soils. Soil Environ. Health 2023, 1, 100019. [Google Scholar] [CrossRef]
- Kononov, A.; Hishida, M.; Suzuki, K.; Harada, N. Microplastic Extraction from Agricultural Soils Using Canola Oil and Unsaturated Sodium Chloride Solution and Evaluation by Incineration Method. Soil Syst. 2022, 6, 54. [Google Scholar] [CrossRef]
- Thomas, D.; Schütze, B.; Heinze, W.M.; Steinmetz, Z. Sample Preparation Techniques for the Analysis of Microplastics in Soil—A Review. Sustainability 2020, 12, 9074. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, X.; Gertsen, H.; Peters, P.; Salánki, T.; Geissen, V. A simple method for the extraction and identification of light density microplastics from soil. Sci. Total Environ. 2018, 616–617, 1056–1065. [Google Scholar] [CrossRef]
- Quilez, M.A.I.; Uttam, C.P.; Merino, D.; Athanassiou, A. Composites of Thermoplastic Starch and Lignin-Rich Agricultural Waste for the Packaging of Fatty Foods. ACS Sustain. Chem. Eng. 2022, 10, 15402–15413. [Google Scholar] [CrossRef]
- Yan, P.; Zhang, S.; Wang, J.; Xiao, Z.; Yan, S.; Wang, W.; Aurangzeib, M. Heterogeneity of plastic residue was determined by both mulch film and external plastic pollutants in the farmland of Northeast China. Sci. Total Environ. 2022, 853, 158681. [Google Scholar] [CrossRef]
- Wu, M.; Yang, C.; Du, C.; Liu, H. Microplastics in waters and soils: Occurrence, analytical methods and ecotoxicological effects. Ecotoxicol. Environ. Saf. 2020, 202, 110910. [Google Scholar] [CrossRef]
- Monikh, F.A.; Doornhein, N.; Romeijn, S.; Vijver, M.G.; Peijnenburg, W.J.G.M. Method for extraction of nanoscale plastic debris from soil. Anal. Methods 2021, 13, 1576–1583. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Song, Y.; Lu, S.; Qiu, R.; Hu, J.; Li, X.; Bigalke, M.; Shi, H.; He, D. A method for extracting soil microplastics through circulation of sodium bromide solutions. Sci. Total Environ. 2019, 691, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Surendran, U.; Jayakumar, M.; Raja, P.; Gopinath, G.; Chellam, P.V. Microplastics in terrestrial ecosystem: Sources and migration in soil environment. Chemosphere 2023, 318, 137946. [Google Scholar] [CrossRef] [PubMed]
- Kitahara, K.-I.; Nakata, H. Plastic additives as tracers of microplastic sources in Japanese road dusts. Sci. Total Environ. 2020, 736, 139694. [Google Scholar] [CrossRef]
- Perez, C.N.; Carr, F.; Hoarau, A.B.; Joris, A.; Leonards, G.P.E.; Lamoree, M.H. Innovations in analytical methods to assess the occurrence of microplastics in soil. J. Environ. Chem. Eng. 2022, 10, 107421. [Google Scholar] [CrossRef]
- Cai, L.; Zhao, X.; Liu, Z.; Han, J. The abundance, characteristics and distribution of microplastics (MPs) in farmland soil—Based on research in China. Sci. Total Environ. 2023, 876, 162782. [Google Scholar] [CrossRef]
- Zhou, J.; Gui, H.; Banfield, C.C.; Wen, Y.; Zang, H.; Dippold, M.A.; Charlton, A.; Jones, D.L. The microplastisphere: Biodegradable microplastics addition alters soil microbial community structure and function. Soil Biol. Biochem. 2021, 156, 108211. [Google Scholar] [CrossRef]
- Hossain, A.; Adham, I.; Hasan, M.; Ali, M.M.; Siddique, A.B.; Senapathi, V.; Islam, A.R.M.T. Analysis and risk evaluation of soil microplastics in the Rohingya refugee camp area, Bangladesh: A comprehensive study. Reg. Stud. Mar. Sci. 2024, 76, 103578. [Google Scholar] [CrossRef]
- Fernandes, A.; Bertoldi, C.; Lara, L.; Stival, J.; Alves, N.; Cabrera, P.; Grassi, M. Microplastics in Latin America Ecosystems: A Critical Review of the Current Stage and Research Needs. J. Braz. Chem. Soc. 2022, 33, 303–326. [Google Scholar] [CrossRef]
- Rani-Borges, B.; Martins, T.F.G.; Pompêo, M. Status of Brazilian research on microplastics present in aquatic ecosystems: Freshwater. Panam J. Aquat. Sci. 2021, 16, 106–117. [Google Scholar]
- Bläsing, M.; Amelung, W. Plastics in soil: Analytical methods and possible sources. Sci. Total Environ. 2018, 612, 422–435. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.M.; Santos, W.J.R.; Marques, J.J. Detailed Soil Survey of Muquém Farm/UFLA, Lavras-MG; UFLA: Lavras, Brazil, 2014; p. 76. Available online: https://www.researchgate.net/publication/270750713_Levantamento_Detalhado_dos_Solos_da_Fazenda_Muquem_UFLA_Lavras_-_MG (accessed on 30 August 2022).
- Wang, J.; Huang, M.; Wang, Q.; Sun, Y.; Zhao, Y.; Huang, Y. LDPE microplastics significantly alter the temporal turnover of soil microbial communities. Sci. Total Environ. 2020, 726, 138682. [Google Scholar] [CrossRef] [PubMed]
- Weithmann, N.; Möller, J.N.; Löder, M.G.J.; Piehl, S.; Laforsch, C.; Freitag, R. Organic fertilizer as a vehicle for the entry of microplastic into the environment. Sci. Adv. 2018, 4, eaap8060. [Google Scholar] [CrossRef] [PubMed]
- IUSS Working Group WRB. World Reference Base for Soil Resources. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Sciences (IUSS): Vienna, Austria, 2022; pp. 1–236. [Google Scholar]
- Zhou, Y.; Wang, J.; Zou, M.; Jia, Z.; Zhou, S.; Li, Y. Microplastics in soils: A review of methods, occurrence, fate, transport, ecological and environmental risks. Sci. Total Environ. 2020, 748, 141368. [Google Scholar] [CrossRef]
- Wrigley, O.; Braun, M.; Amelung, W. Global soil microplastic assessment in different land-use systems is largely determined by the method of analysis: A meta-analysis. Sci. Total Environ. 2024, 957, 177226. [Google Scholar] [CrossRef]
- Teixeira, P.C.; Donagemma, G.K.; Fontana, A.; Teixeira, W. (Eds.) Manual de Métodos de Análise de Solo; Embrapa: Brazilia, Brazil, 2017. [Google Scholar]
- Oden, C.P.; Werth, C.J.; Kienzle, B.A.; Katz, L.E. Impact of organic matter on transformation during thermal remediation of pyrene-contaminated substrates. Sci. Total Environ. 2024, 906, 167569. [Google Scholar] [CrossRef]
- Wang, J.-P.; Wang, X.-J.; Zhang, J. Evaluating Loss-on-Ignition Method for Determinations of Soil Organic and Inorganic Carbon in Arid Soils of Northwestern China. Pedosphere 2013, 23, 593–599. [Google Scholar] [CrossRef]
- Maw, M.M.; Boontanon, N.; Fujii, S.; Boontanon, S.K. Rapid and efficient removal of organic matter from sewage sludge for extraction of microplastics. Sci. Total Environ. 2022, 853, 158642. [Google Scholar] [CrossRef]
- Al-Azzawi, M.S.M.; Kefer, S.; Weißer, J.; Reichel, J.; Schwaller, C.; Glas, K.; Knoop, O.; Drewes, J.E. Validation of Sample Preparation Methods for Microplastic Analysis in Wastewater Matrices—Reproducibility and Standardization. Water 2020, 12, 2445. [Google Scholar] [CrossRef]
- Enders, K.; Lenz, R.; Beer, S.; Stedmon, C.A. Extraction of microplastic from biota: Recommended acidic digestion destroys common plastic polymers. ICES J. Mar. Sci. 2016, 74, 326–331. [Google Scholar] [CrossRef]
- Vermeiren, P.; Muñoz, C.; Ikejima, K. Microplastic identification and quantification from organic rich sediments: A validated laboratory protocol. Environ. Pollut. 2020, 262, 114298. [Google Scholar] [CrossRef] [PubMed]
- Radford, F.; Zapata-Restrepo, L.M.; Horton, A.A.; Hudson, M.D.; Shaw, P.J.; Williams, I.D. Developing a systematic method for extraction of microplastics in soils. Anal. Methods 2021, 13, 1695–1705. [Google Scholar] [CrossRef] [PubMed]
- Scopetani, C.; Chelazzi, D.; Mikola, J.; Leiniö, V.; Heikkinen, R.; Cincinelli, A.; Pellinen, J. Olive oil-based method for the extraction, quantification and identification of microplastics in soil and compost samples. Sci. Total Environ. 2020, 733, 139338. [Google Scholar] [CrossRef] [PubMed]
- Corradini, F.; Meza, P.; Eguiluz, R.; Casado, F.; Huerta-Lwanga, E.; Geissen, V. Evidence of microplastic accumulation in agricultural soils from sewage sludge disposal. Sci. Total Environ. 2019, 671, 411–420. [Google Scholar] [CrossRef]
- da Silva Paes, E.; Gloaguen, T.V.; da Conceição Silva, H.D.A.; Duarte, T.S.; de Almeida, M.D.C.; Costa, O.D.A.V.; Bomfim, M.R.; Santos, J.A.G. Widespread microplastic pollution in mangrove soils of Todos os Santos. Environ. Res. 2022, 210, 112952. [Google Scholar] [CrossRef]
- Izquierdo, B.J.; Arévalo, H.J.J. Determinación del carbono orgánico por el método químico y por calcinación. Ing. Región 2021, 26, 20–28. [Google Scholar] [CrossRef]
- Quinn, B.; Murphy, F.; Ewins, C. Validation of density separation for the rapid recovery of microplastics from sediment. Anal. Methods 2016, 9, 1491–1498. [Google Scholar] [CrossRef]
- OriginPro Lab Corporation. OriginPro, Version 2024b; OriginPro Lab Corporation: Northampton, MA, USA, 2024. [Google Scholar]
- Wang, J.; Li, J.; Liu, S.; Li, H.; Chen, X.; Peng, C.; Zhang, P.; Liu, X. Distinct microplastic distributions in soils of different land-use types: A case study of Chinese farmlands. Environ. Pollut. 2021, 269, 116199. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Y.; Kang, S.; Wang, Z.; Wu, C. Microplastics in soil: A review on methods, occurrence, sources, and potential risk. Sci. Total Environ. 2021, 780, 146546. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 11 September 2024).
- Liu, Y.; Zhong, Y.; Hu, C.; Xiao, M.; Ding, F.; Yu, Y.; Yao, H.; Zhu, Z.; Chen, J.; Ge, T.; et al. Distribution of microplastics in soil aggregates after film mulching. Soil Ecol. Lett. 2023, 5, 230171. [Google Scholar] [CrossRef]
- Prata, J.C.; da Costa, J.P.; Girão, A.V.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Identifying a quick and efficient method of removing organic matter without damaging microplastic samples. Sci. Total Environ. 2019, 686, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Pfohl, P.; Roth, C.; Meyer, L.; Heinemeyer, U.; Gruendling, T.; Lang, C.; Nestle, N.; Hofmann, T.; Wohlleben, W.; Jessl, S. Microplastic extraction protocols can impact the polymer structure. Microplast. Nanoplast. 2021, 1, 8. [Google Scholar] [CrossRef]
- Guimarães, D.V.; Silva, M.L.N.; Beniaich, A.; Pio, R.; Gonzaga, M.I.S.; Avanzi, J.C.; Bispo, D.F.A.; Curi, N. Dynamics and losses of soil organic matter and nutrients by water erosion in cover crop management systems in olive groves, in tropical regions. Soil Tillage Res. 2021, 209, 104863. [Google Scholar] [CrossRef]
- Thiele, C.J.; Hudson, M.D.; Russell, A.E. Evaluation of existing methods to extract microplastics from bivalve tissue: Adapted KOH digestion protocol improves filtration at single-digit pore size. Mar. Pollut. Bull. 2019, 142, 384–393. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Sierra, I.; Chialanza, M.R.; Faccio, R.; Carrizo, D.; Fornaro, L.; Pérez-Parada, A. Identification of microplastics in wastewater samples by means of polarized light optical microscopy. Environ. Sci. Pollut. Res. 2019, 27, 7409–7419. [Google Scholar] [CrossRef]
- Smith, B.C. The Infrared Spectra of Polymers II: Polyethylene. Spectroscopy 2021, 36, 24–29. [Google Scholar] [CrossRef]
- Käppler, A.; Fischer, D.; Oberbeckmann, S.; Schernewski, G.; Labrenz, M.; Eichhorn, K.-J.; Voit, B. Analysis of environmental microplastics by vibrational microspectroscopy: FTIR, Raman or both? Anal. Bioanal. Chem. 2016, 408, 8377–8391. [Google Scholar] [CrossRef]
- Chouchene, K.; Nacci, T.; Modugno, F.; Castelvetro, V.; Ksibi, M. Soil contamination by microplastics in relation to local agricultural development as revealed by FTIR, ICP-MS and pyrolysis-GC/MS. Environ. Pollut. 2022, 303, 119016. [Google Scholar] [CrossRef]
- Zhang, G.S.; Liu, Y.F. The distribution of microplastics in soil aggregate fractions in southwestern China. Sci. Total Environ. 2018, 642, 12–20. [Google Scholar] [CrossRef]
- Monteiro, S.S.; da Costa, J.P. Methods for the extraction of microplastics in complex solid, water and biota samples. Trends Environ. Anal. Chem. 2022, 33, e00151. [Google Scholar] [CrossRef]
- La, Y.; Zhang, L.; Zhao, N.; Ye, H.; Zeng, Q.; Zhao, L.; Wang, Z.; Lin, D.; Wang, R. The microplastics distribution characteristics and their impact on soil physicochemical properties and bacterial communities in food legumes farmland in northern China. J. Hazard. Mater. 2024, 471, 134282. [Google Scholar] [CrossRef] [PubMed]
- Akca, H.; Albayrak, R.; Onur, M.; Gündo, S. An evaluation on microplastic accumulations in Turkish soils under different land uses. Sci Total Environ. 2024, 911, 168609. [Google Scholar] [CrossRef] [PubMed]
- Padha, S.; Kumar, R.; Dhar, A.; Sharma, P. Microplastic pollution in mountain terrains and foothills: A review on source, extraction, and distribution of microplastics in remote areas. Environ. Res. 2021, 207, 112232. [Google Scholar] [CrossRef]
- Büks, F.; Kaupenjohann, M. Global concentrations of microplastics in soils—A review. Soil Discuss. 2020, 6, 649–662. [Google Scholar] [CrossRef]
- Leitão, I.; van Schaik, L.; Ferreira, A.; Alexandre, N.; Geissen, V. The spatial distribution of microplastics in topsoils of an urban environment—Coimbra city case-study. Environ. Res. 2022, 218, 114961. [Google Scholar] [CrossRef]
- Rehm, R.; Zeyer, T.; Schmidt, A.; Fiener, P. Soil erosion as transport pathway of microplastic from agriculture soils to aquatic ecosystems. Sci. Total Environ. 2021, 795, 148774. [Google Scholar] [CrossRef]
- Han, N.; Zhao, Q.; Ao, H.; Hu, H.; Wu, C. Horizontal transport of macro- and microplastics on soil surface by rainfall induced surface runoff as affected by vegetations. Sci. Total Environ. 2022, 831, 154989. [Google Scholar] [CrossRef]
- Khan, M.A.; Huang, Q.; Khan, S.; Wang, Q.; Huang, J.; Fahad, S.; Sajjad, M.; Liu, Y.; Mašek, O.; Li, X.; et al. Abundance, spatial distribution, and characteristics of microplastics in agricultural soils and their relationship with contributing factors. J. Environ. Manag. 2022, 328, 117006. [Google Scholar] [CrossRef]
- Hossain, M.N.; Rahman, M.M.; Afrin, S.; Akbor, M.A.; Siddique, M.A.B.; Malafaia, G. Identification and quantification of microplastics in agricultural farmland soil and textile sludge in Bangladesh. Sci. Total Environ. 2023, 858, 160118. [Google Scholar] [CrossRef]
- Zhou, Y.; Jia, Z.; Zheng, G.; Chen, L.; Zhang, Q.; Su, B.; Zhou, S. Microplastics in agricultural soils on the coastal plain: Spatial characteristics, influencing factors and sources. Sci Total Environ. 2023, 901, 165948. [Google Scholar] [CrossRef] [PubMed]
- Zamprogno, G.C.; Caniçali, F.B.; Cozer, C.d.R.; Otegui, M.B.P.; Graceli, J.B.; da Costa, M.B. Spatial distribution of microplastics in the superficial sediment of a mangrove in Southeast Brazil: A comparison between fringe and basin. Sci. Total Environ. 2021, 784, 146963. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yen, H.; Wang, X.; Huang, C.-H.; Sun, J.; Hammac, A.; Wang, Y. Deposition- and transport-dominated erosion regime effects on the loss of dissolved and sediment-bound organic carbon: Evaluation in a cultivated soil with laboratory rainfall simulations. Sci. Total Environ. 2021, 750, 141717. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Zhang, J.; Liu, Y.; Chen, L.; Tao, S.; Liu, W. Distribution characteristics of microplastics in agricultural soils from the largest vegetable production base in China. Sci. Total Environ. 2021, 756, 143860. [Google Scholar] [CrossRef]
- Batista, P.V.; Laceby, J.P.; Davies, J.; Carvalho, T.S.; Tassinari, D.; Silva, M.L.; Curi, N.; Quinton, J.N. A framework for testing large-scale distributed soil erosion and sediment delivery models: Dealing with uncertainty in models and the observational data. Environ. Model. Softw. 2021, 137, 104961. [Google Scholar] [CrossRef]
- Chen, B. Current status and trends of research on microplastic fugacity characteristics and pollution levels in mangrove wetlands. Front. Environ. Sci. 2022, 10, 1021274. [Google Scholar] [CrossRef]
- Redondo-Hasselerharm, P.E.; Rico, A.; Koelmans, A.A. Risk assessment of microplastics in freshwater sediments guided by strict quality criteria and data alignment methods. J. Hazard. Mater. 2023, 441, 129814. [Google Scholar] [CrossRef]
- Guo, Z.; Li, P.; Yang, X.; Wang, Z.; Lu, B.; Chen, W.; Wu, Y.; Li, G.; Zhao, Z.; Liu, G.; et al. Soil texture is an important factor determining how microplastics affect soil hydraulic characteristics. Environ. Int. 2022, 165, 107293. [Google Scholar] [CrossRef]
- de Souza Machado, A.A.; Lau, C.W.; Till, J.; Kloas, W.; Lehmann, A.; Becker, R.; Rillig, M.C. Impacts of Microplastics on the Soil Biophysical Environment. Environ. Sci. Technol. 2018, 52, 9656–9665. [Google Scholar] [CrossRef]
- Yang, J.; Li, L.; Li, R.; Xu, L.; Shen, Y.; Li, S.; Tu, C.; Wu, L.; Christie, P.; Luo, Y. Microplastics in an agricultural soil following repeated application of three types of sewage sludge: A field study. Environ. Pollut. 2021, 289, 117943. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, Y.; An, S.; Zhao, J.; Xiao, L.; Li, H.; Huang, Q. Microplastics trapped in soil aggregates of different land-use types: A case study of Loess Plateau terraces, China. Environ. Pollut. 2022, 310, 119880. [Google Scholar] [CrossRef] [PubMed]
- Maddela, N.R.; Ramakrishnan, B.; Kadiyala, T.; Venkateswarlu, K.; Megharaj, M. Do Microplastics and Nanoplastics Pose Risks to Biota in Agricultural Ecosystems? Soil Syst. 2023, 7, 19. [Google Scholar] [CrossRef]
- Su, X.; Liu, M.; Dou, J.; Yuan, J.; Cheng, J.; Lu, Z.; He, Y. A review on enriched microplastics in environment: From the perspective of their aging impact and associate risk. Earth Crit. Zone 2024, 1, 100008. [Google Scholar] [CrossRef]
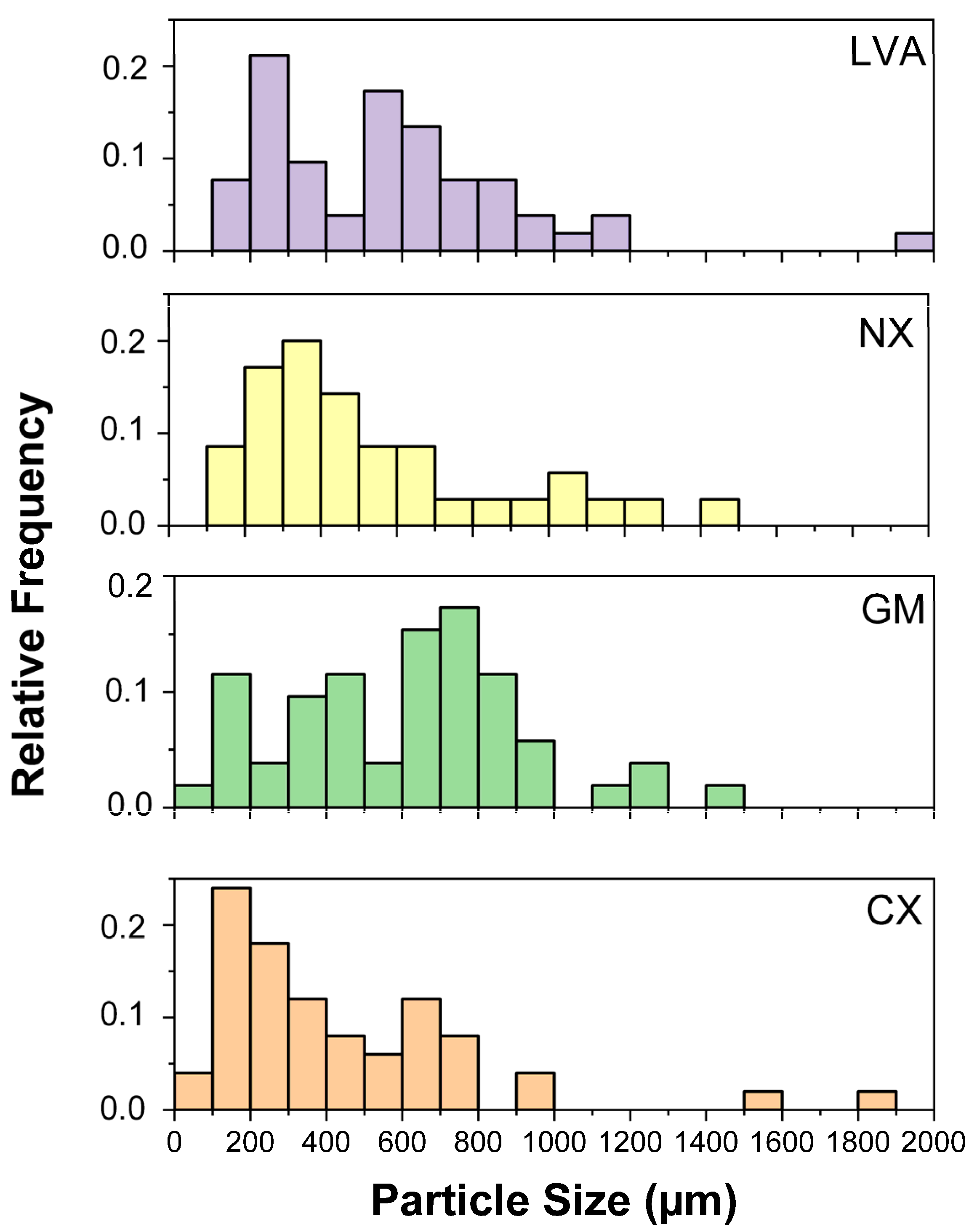
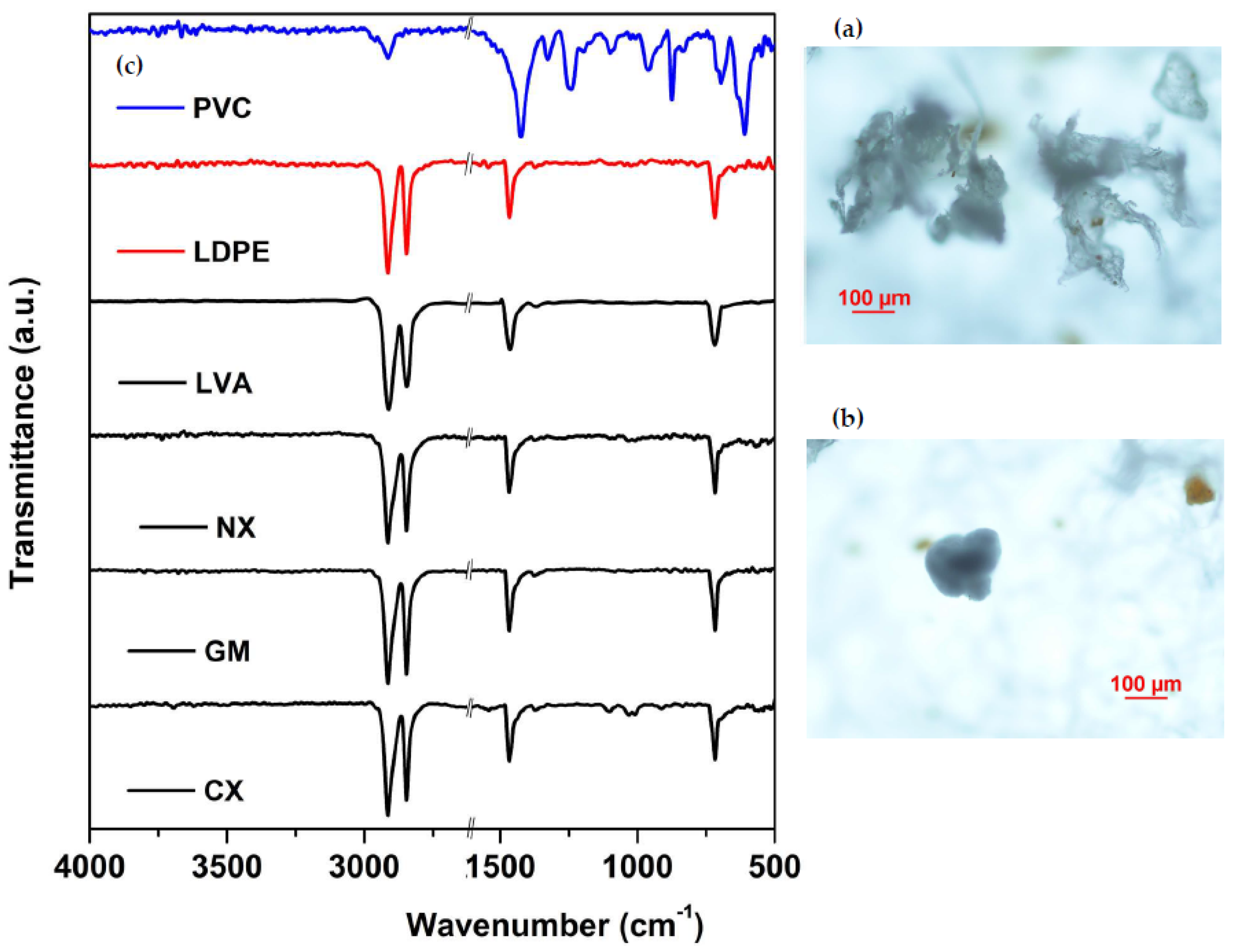
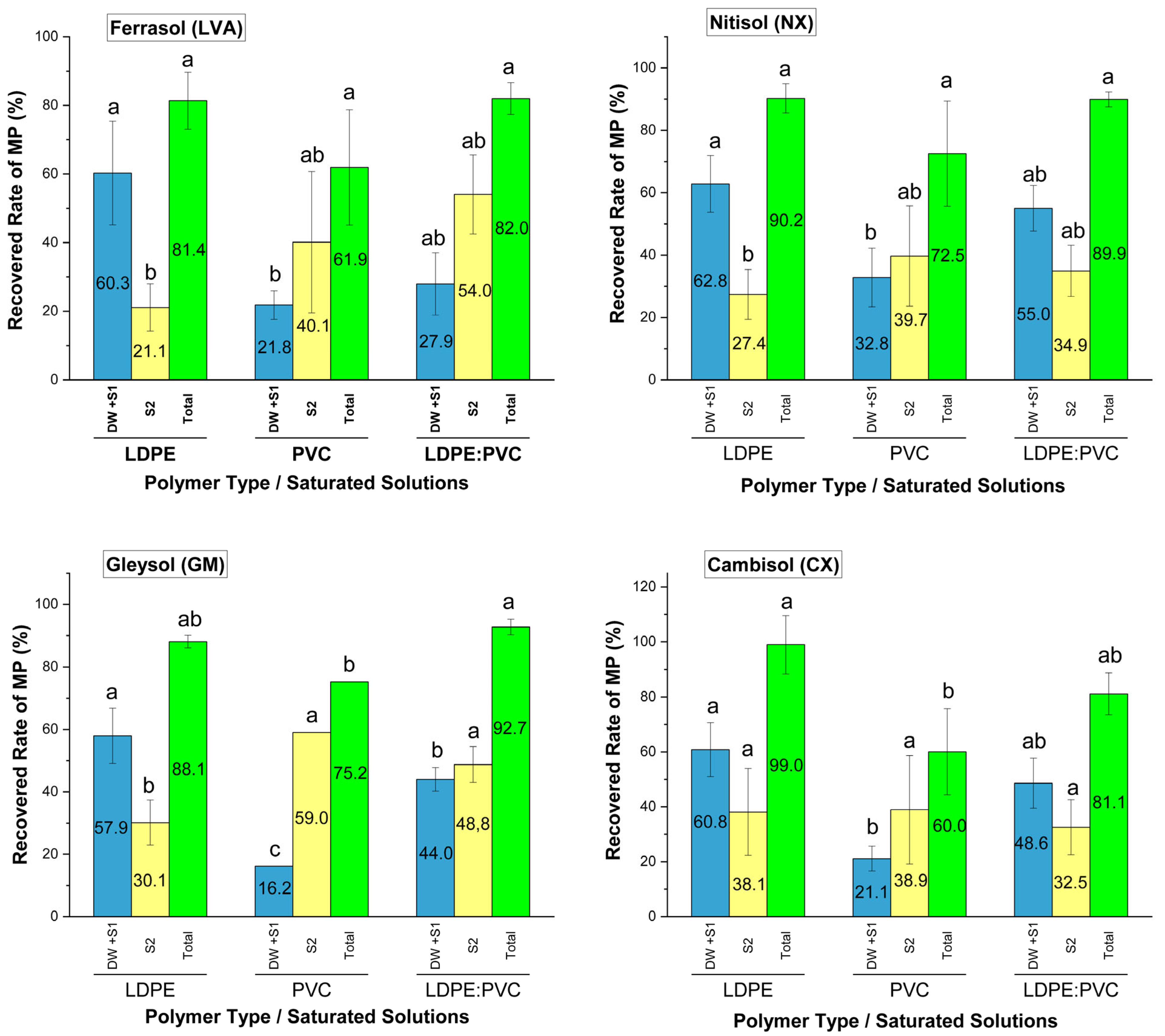

| Characteristics | Soil Class | |||
|---|---|---|---|---|
| LVA | NX | GM | CX | |
| Location | 21°12′16.62″ S 44°58′55.14″ W | 21°11′56.09″ S 44°58′51.02″ W | 21°12′4.23″ S 44°58′44.48″ W | 21°13′49.59″ S 44°59′0.44″ W |
| Altitude (m) | 996 | 978 | 960 | 918 |
| Slope (%) | 8–20 | 3–8 | 3–8 | 20–45 |
| Current crop | Soybean | Soybean | Rice-Beans | Olive trees |
| Clay (g kg−1) | 490 | 530 | 310 | 550 |
| Silt (g kg−1) | 100 | 60 | 220 | 170 |
| Sand (g kg−1) | 410 | 410 | 470 | 280 |
| Bulk density (g cm−3) | 1.36 | 1.29 | 0.75 | 0.92 |
| Macroporosity (cm3 cm−3) | 0.13 | 0.15 | 0.27 | 0.13 |
| Microporosity (cm3 cm−3) | 0.39 | 0.38 | 0.46 | 0.27 |
| Total Porosity (cm3 cm−3) | 0.52 | 0.52 | 0.73 | 0.40 |
| pH (H2O) | 5.6 | 6.9 | 5.0 | 5.0 |
| Al3+ (cmolc dm−3) | 0.10 | 0.10 | 0.20 | 0.00 |
| H +Al (cmolc dm−3) | 2.70 | 1.20 | 4.00 | 1.70 |
| SB (cmolc dm−3) | 5.68 | 8.69 | 9.15 | 1.86 |
| eCEC (cmolc dm−3) | 5.78 | 8.79 | 9.35 | 1.86 |
| tCEC (cmolc dm−3) | 8.38 | 9.89 | 13.15 | 3.56 |
| BS (%) | 67.78 | 87.90 | 69.55 | 52.28 |
| m (%) | 1.73 | 1.14 | 2.14 | 0.00 |
| SOM (g kg−1) | 30.8 | 26.3 | 172.9 | 4.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arevalo-Hernandez, J.J.; Barrera de Brito, A.D.; Curi, N.; Avanzi, J.C.; Silva, M.L.N. A Method for the Extraction and Analysis of Microplastics from Tropical Agricultural Soils in Southeastern Brazil. Soil Syst. 2025, 9, 34. https://doi.org/10.3390/soilsystems9020034
Arevalo-Hernandez JJ, Barrera de Brito AD, Curi N, Avanzi JC, Silva MLN. A Method for the Extraction and Analysis of Microplastics from Tropical Agricultural Soils in Southeastern Brazil. Soil Systems. 2025; 9(2):34. https://doi.org/10.3390/soilsystems9020034
Chicago/Turabian StyleArevalo-Hernandez, John Jairo, Angela Dayana Barrera de Brito, Nilton Curi, Junior Cesar Avanzi, and Marx Leandro Naves Silva. 2025. "A Method for the Extraction and Analysis of Microplastics from Tropical Agricultural Soils in Southeastern Brazil" Soil Systems 9, no. 2: 34. https://doi.org/10.3390/soilsystems9020034
APA StyleArevalo-Hernandez, J. J., Barrera de Brito, A. D., Curi, N., Avanzi, J. C., & Silva, M. L. N. (2025). A Method for the Extraction and Analysis of Microplastics from Tropical Agricultural Soils in Southeastern Brazil. Soil Systems, 9(2), 34. https://doi.org/10.3390/soilsystems9020034