Understanding the Anomalous Corrosion Behaviour of 17% Chromium Martensitic Stainless Steel in Laboratory CCS-Environment—A Descriptive Approach
Abstract
:1. Introduction
- Temperature (60 °C is a severe damaging temperature region);
- CO2 partial pressure;
- alloy composition;
- heat treatment of steels (austenitizing temperature and durance as well as annealing
- element distribution in the corrosive media);
- purity of alloy and aquifer media;
- conditions of flow;
- pressure during injection and;
- protecting corrosion scales.
2. Materials and Methods
2.1. Steels
2.2. Aquifer Water
2.3. Heat Treatment and Static Corrosion Experiments
2.4. Analysis
3. Results and Discussion
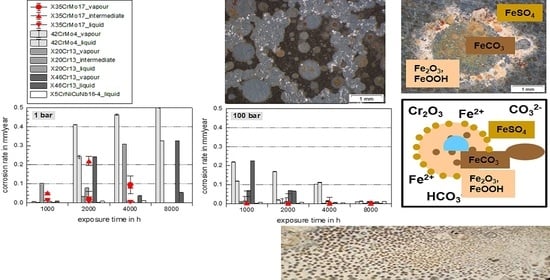
3.1. Comprehensive Demonstration of Corrosion Kinetics
- A possible final failure of the passivating layer exposes the newly formed metal surface to an electrolyte with high CO2 partial pressure that then accelerates the corrosion reactions.
- Long exposure times enhance carbide precipitation that depletes the surrounding metal matrix of chromium and prohibit surface passivation. Although independent of the pressure, the CO32− concentration remains the same [3], the higher corrosion rates in supercritical CO2 result in increased formation rate of Fe2+ ions, offering a high number of carbides precipitating on the steel’s surface. These are more susceptible to decomposing reactions, but carbides also affect the scale growth mechanism [3].
- At high pressure with lower CO2 supersaturation in the liquid phase than in the supercritical phase, nucleation reactions are slow and stable crystal growth of siderite dominates the kinetics. A stable and dense siderite layer is formed, giving low corrosion rates in water-saturated supercritical CO2 as shown in Figure 2 and Figure 3.
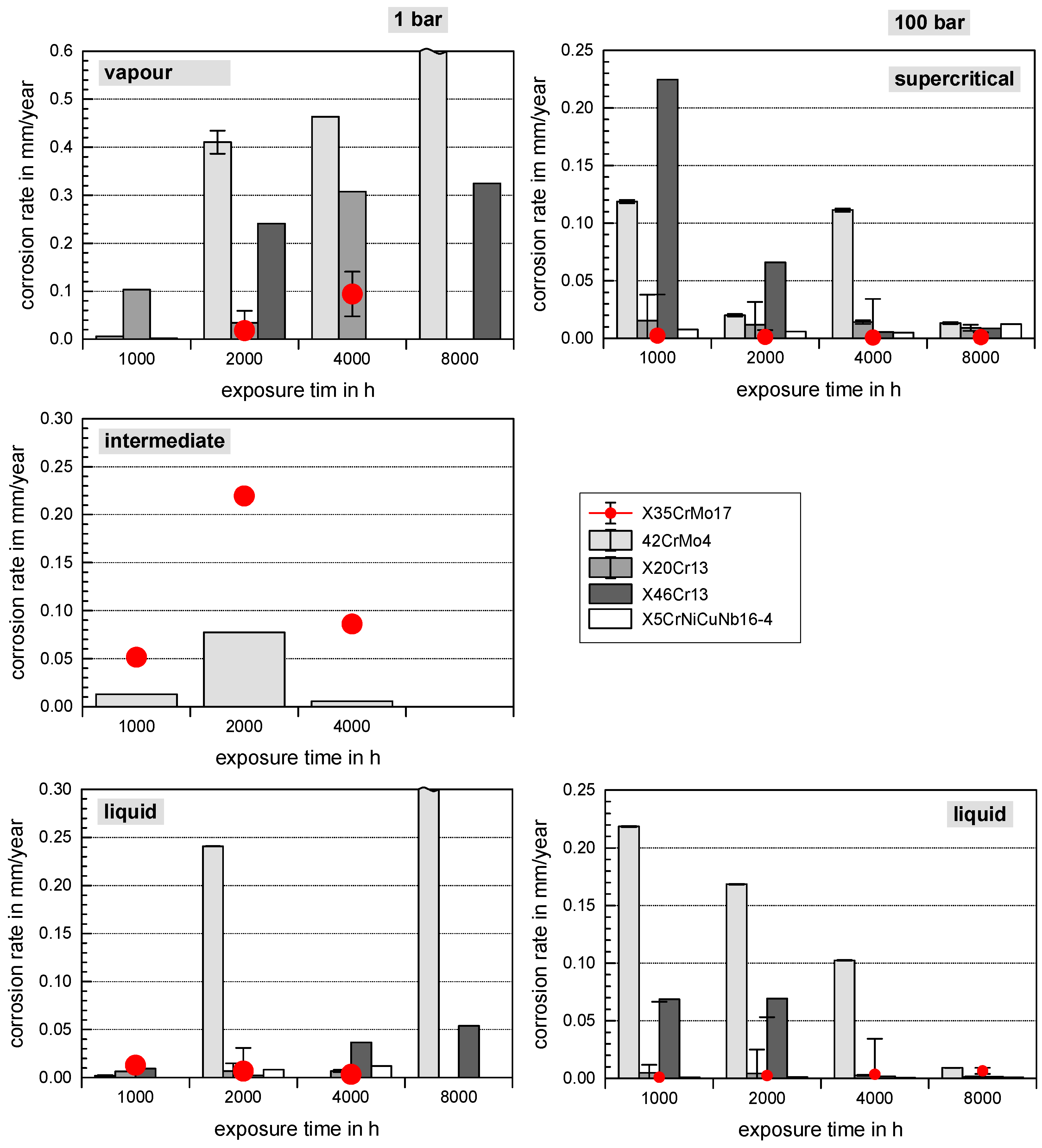
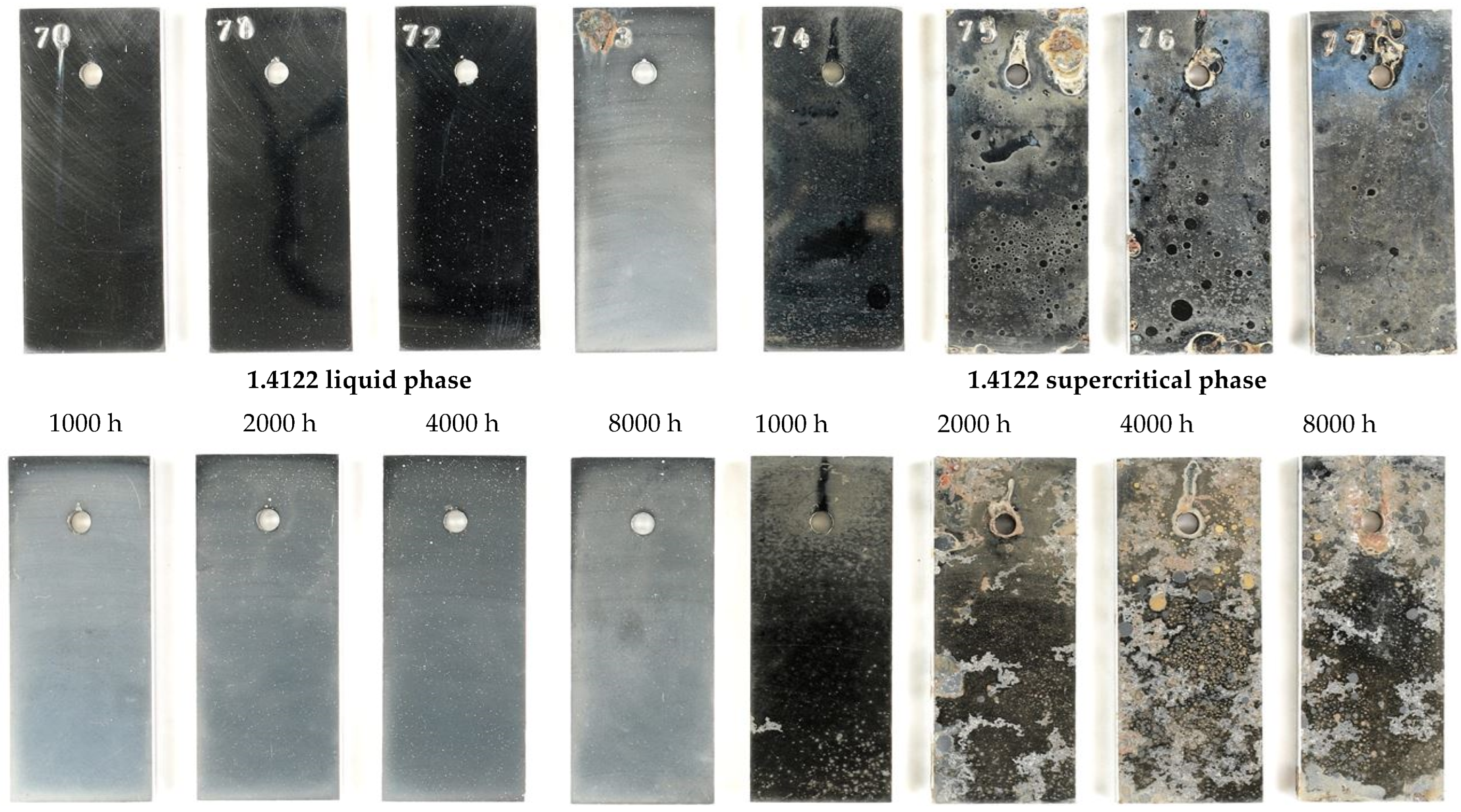
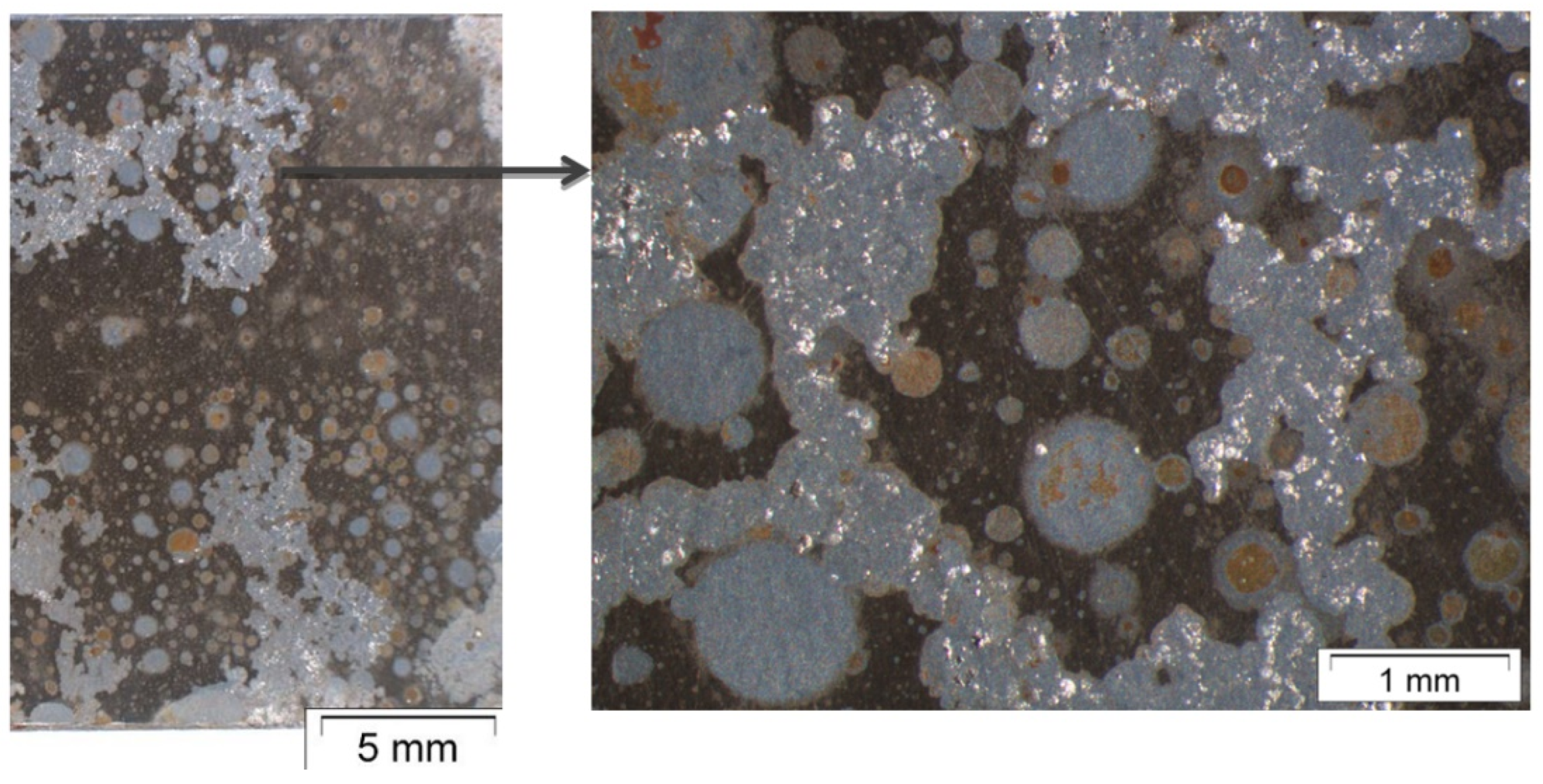
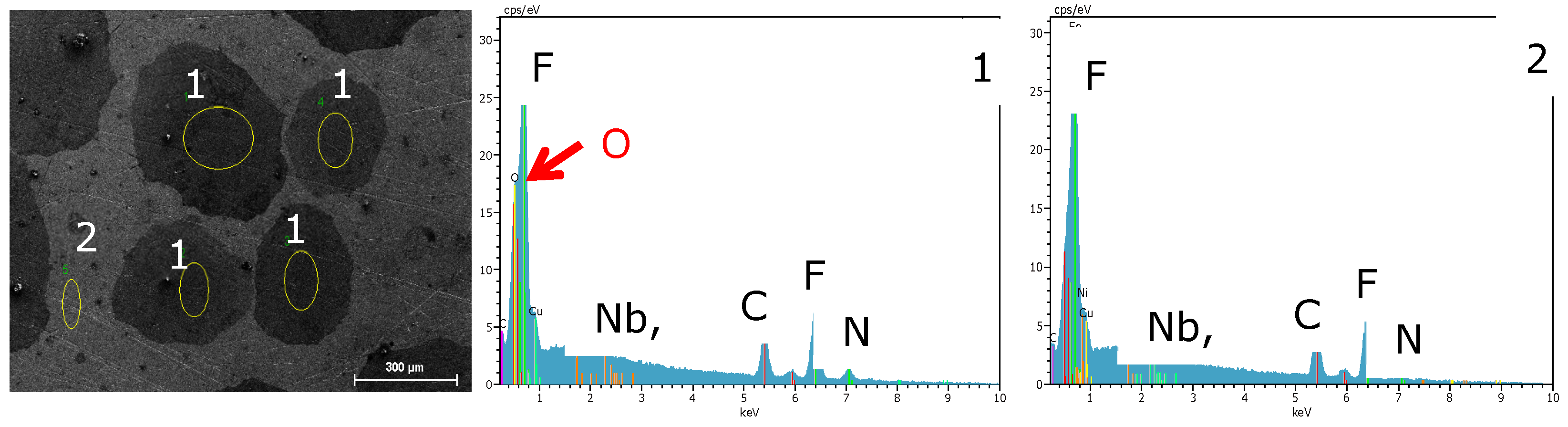
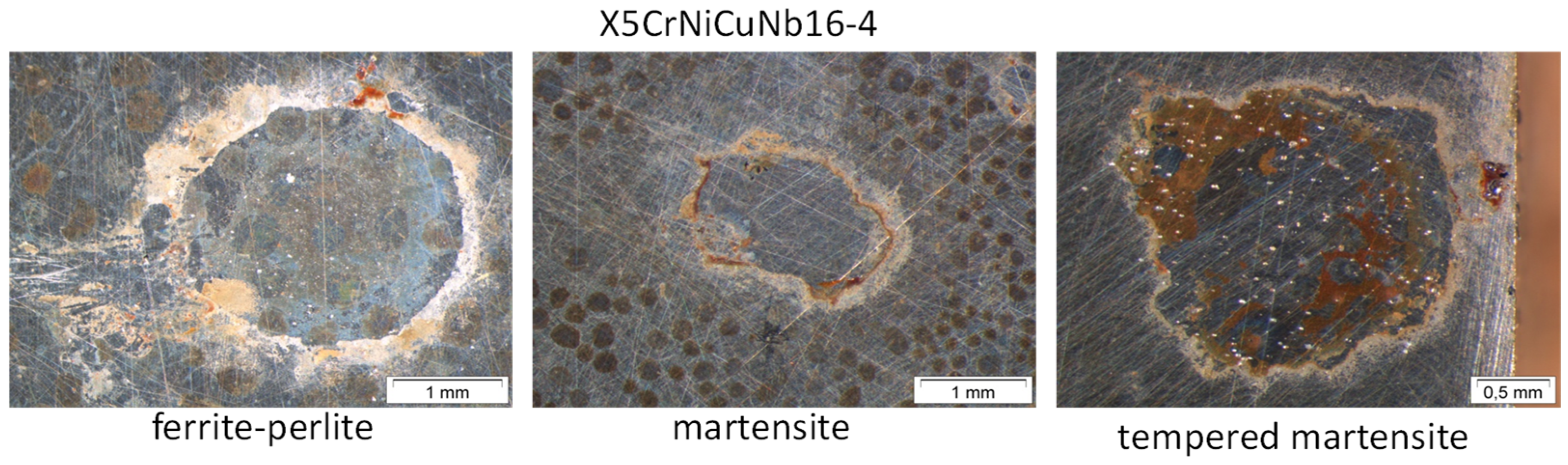
3.2. Surface Morphology and Scale Precipitation
3.3. Corrosion Initiation in Water Saturated Supercritical CO2 (SCC)
- (a)
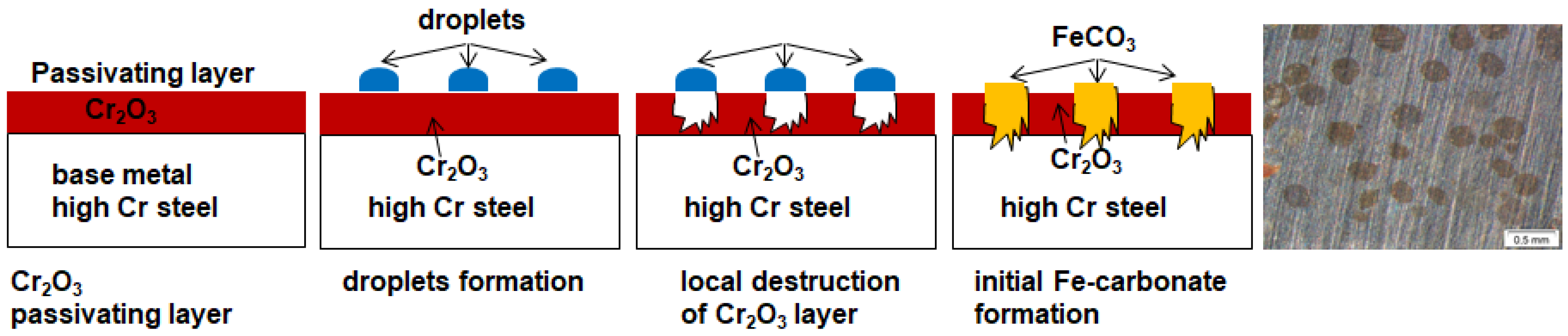
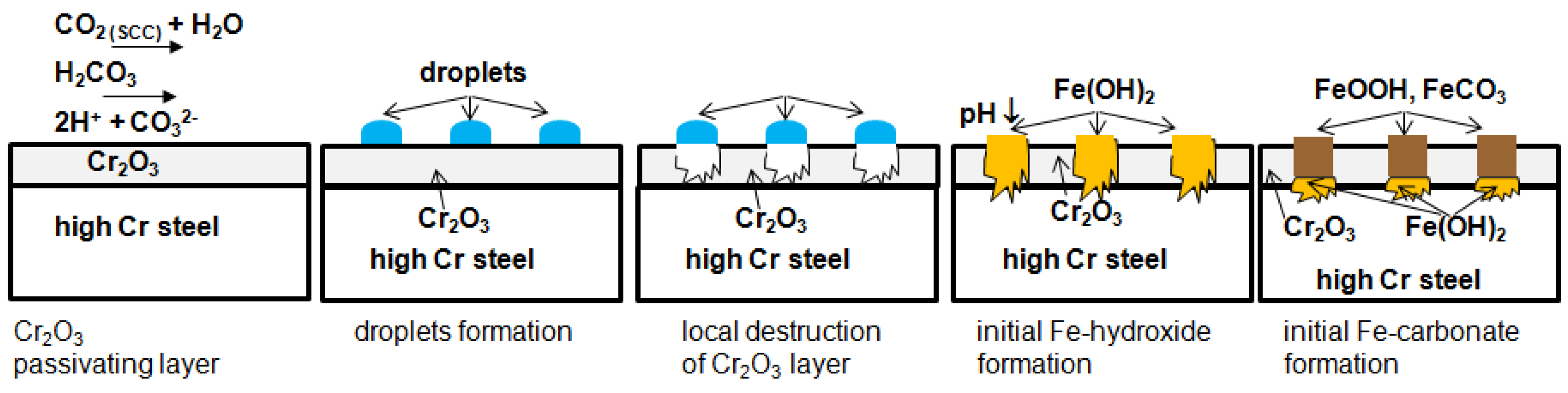
- The passivating layer is locally destroyed, possibly due to locally very low pH as a consequence of the formation of carbonic acid in water-saturated supercritical CO2 leading to anodic dissolution.
- (b)
- The carbide distribution within the steels’ microstructure is not homogeneous. Carbides located at the metal surface corrode locally because carbides are more susceptible to anodic dissolution [20]. Consequently, ellipsoids grow from the initial carbide dissolution leaving a newly exposed metal surface that is highly susceptible to the corrosive environment.
- (c)
- Carbonic acid H2CO3 (as a reaction product from water and CO2) is not soluted equally along the entire sample surfaces. Hence, a thin passivating layer is formed in the initial corrosion stage that then starts growing locally. Once a sufficient thickness of these corrosion islands is achieved, it detaches laterally, causing corrosion reactions.
- (d)
- In general, raising the temperature accelerates the water solubility in supercritical CO2. Choi et al. reported that the solubility of water in CO2 decreases in the region 0 bar—50 bar and then slightly raises again [62]. Because the temperature was kept constant (60 °C) and the pressure was at a constant 100 bar, both, neither the temperature nor pressure influence the solubility of water in supercritical CO2 over time. Furthermore, in this particular CCS environment, the solubility decreases overall. Consequently, at 100 bar and 60 °C, the metal surface that precipitated a passivating layer consisting of Cr2O3 and (Fex(Cr1−x))3O4 is wetted by very thin and small water droplets. Distinct “leopard”-shaped corrosion layers form associated with initial droplets condensed on the surface. The residual water droplets can be seen in Figure 8, with bigger droplets in the middle and the small former droplets now being the “leopard” ellipsoids. At the metal–water–supercritical CO2 phase boundary, the surface is locally depassivated, whereas the remaining surface is covered by thin passivating corrosion layers. This formation model will be described in detail below.
3.4. Formation Mechanism in Water Saturated Supercritical CO2 (SCC)
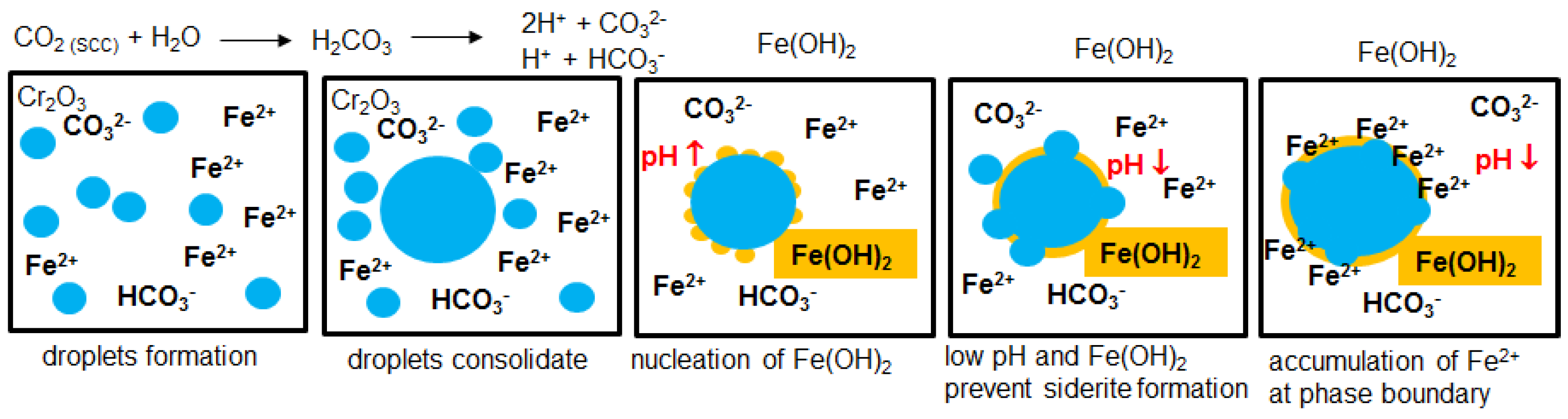
3.5. Degradation of Carbonate and Hydroxide Layer
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Thomas, C. Carbon Dioxide Capture for Storage in Deep Geologic Formations—Results from CO2 Capture Project; Elsevier Ltd.: London, UK, 2005; ISBN 0080445748. [Google Scholar]
- Broek, M.V.D.; Hoefnagels, R.; Rubin, E.; Turkenburg, W.; Faaij, A. Effects of technological learning on future cost and performance of power plants with CO2 capture. Prog. Energy Combust. Sci. 2009, 35, 457–480. [Google Scholar] [CrossRef]
- Nešić, S. Key issues related to modelling of internal corrosion of oil and gas pipelines—A review. Corros. Sci. 2007, 49, 4308–4338. [Google Scholar] [CrossRef]
- Hurter, S. Impact of Mutual Solubility of H2O and CO2 on Injection Operations for Geological Storage of CO2. In Proceedings of the International Conference of the Properties of Water and Steam ICPWS, Berlin, Germany, 8–11 September 2012. [Google Scholar]
- Zhang, L.; Yang, J.; Sun, J.S.; Lu, M. Effect of pressure on wet H2S/CO2 corrosion of pipeline steel. In Proceedings of the NACE Corrosion 2008 Conference and Expo, New Orleans, LA, USA, 16–20 March 2008. Paper No. 09565. [Google Scholar]
- Mu, L.J.; Zhao, W.Z. Investigation on Carbon Dioxide Corrosion Behaviors of 13Cr Stainless Steel in Simulated Strum Water. Corros. Sci. 2010, 2, 82–89. [Google Scholar] [CrossRef]
- Seiersten, M. Material selection for separation, transportation and disposal of CO2. In NACE International, Houston, CORROSION/2001; Paper No. 01042; NACE International: Houston, TX, USA, 2001. [Google Scholar]
- Cui, Z.D.; Wu, S.L.; Zhu, S.L.; Yang, X.J. Study on corrosion properties of pipelines in simulated produced water saturat-ed with supercritical CO2. Appl. Surf. Sci. 2006, 252, 2368–2374. [Google Scholar] [CrossRef]
- Pfennig, A.; Kranzmann, A. Reliability of pipe steels with different amounts of C and Cr during onshore carbon dioxide injection. Int. J. Greenh. Gas Control 2011, 5, 757–769. [Google Scholar]
- Zhang, H.; Zhao, Y.L.; Jiang, Z.D. Effects of temperature on the corrosion behavior of 13Cr martensitic stainless steel dur-ing exposure to CO2 and Cl environment. Mater. Lett. 2005, 59, 3370–3374. [Google Scholar] [CrossRef]
- Alhajji, J.; Reda, M. The effect of alloying elements on the electrochemical corrosion of low residual carbon steels in stagnant CO2-saturated brine. Corros. Sci. 1993, 34, 1899–1911. [Google Scholar] [CrossRef]
- Choi, Y.-S.; Nešić, S. Corrosion behavior of carbon steel in supercritical CO2-water environments. In Proceedings of the NACE Corrosion 2008 Conference and Expo, New Orleans, LA, USA, 16–20 March 2008. Paper No. 09256. [Google Scholar]
- Jiang, X.; Nešić, S.; Huet, F. The Effect of Electrode Size on Electrochemical Noise Measurements and the Role of Chloride on Localized CO2 Corrosion of Mild Steel. In Proceedings of the NACE Corrosion 2008 Conference and Expo, New Orleans, LA, USA, 16–20 March 2008; p. 09575. [Google Scholar]
- Ahmad, Z.; Allam, I.M.; Abdul, B.; Aleem, J. Effect of environmental factors on the atmospheric corrosion of mild steel in aggressive sea coastal environment. Anti Corros. Methods Mater. 2000, 47, 215–225. [Google Scholar] [CrossRef]
- Pfennig, A.L.; Bäßler, R. Effect of CO2 on the stability of steels with 1% and 13% Cr in saline water. Corros. Sci. 2009, 51, 931–940. [Google Scholar] [CrossRef]
- Pfennig, A.; Wolf, M.; Kranzmann, A. Corrosion and Corrosion Fatigue of Steels in Downhole CCS Environment-A Summary. Processes 2021, 9, 594. [Google Scholar] [CrossRef]
- Pfennig, A.; Zastrow, P.; Kranzmann, A. Influence of heat treatment on the corrosion behavior of stainless steels during CO2-sequestration into saline aquifer. Int. J. Greenh. Gas Control 2013, 15, 213–224. [Google Scholar] [CrossRef]
- Nyborg, R. Controlling Internal Corrosion in Oil and Gas Pipelines. Business Briefing: Exploration & Production. Oil Gas Rev. 2005, 2, 70–74. [Google Scholar]
- Carvalho, D.S.; Joia, C.; Mattos, O. Corrosion rate of iron and iron–chromium alloys in CO2 medium. Corros. Sci. 2005, 47, 2974–2986. [Google Scholar] [CrossRef]
- Linter, B.R.; Burstein, G.T. Reactions of pipeline steels in carbon dioxide solutions. Corros. Sci. 1999, 41, 117–139. [Google Scholar] [CrossRef]
- Wu, S.L.; Cui, Z.D.; Zhao, G.X.; Yan, M.L.; Zhu, S.L.; Yang, X.J. EIS study of the surface film on the surface of carbon steel form supercritical carbon dioxide corrosion. Appl. Surf. Sci. 2004, 228, 17–25. [Google Scholar] [CrossRef]
- Bülbül, Ş.; Sun, Y. Corrosion behavior of high Cr-Ni cast steels in the HCl solution. J. Alloys Compd. 2010, 598, 143–147. [Google Scholar] [CrossRef]
- Hou, B.; Li, Y.; Li, Y.; Zhang, J. Effect of alloy elements on the anti-corrosion properties of low alloy steel. Bull. Mater. Sci 2000, 23, 189–192. [Google Scholar] [CrossRef]
- Cvijović, Z.; Radenković, G. Microstructure and pitting corrosion resistance of annealed duplex stainless steel. Corros. Sci. 2006, 48, 3887–3906. [Google Scholar] [CrossRef]
- Park, J.-Y.; Park, Y.-S. The effects of heat-treatment parameters on corrosion resistance and phase transformations of 14Cr–3Mo martensitic stainless steel. Mater. Sci. Eng. A 2007, 449–451, 1131–1134. [Google Scholar] [CrossRef]
- Banaś, J.; Lelek-Borkowska, U.; Mazurkiewicz, B.; Solarski, W. Effect of CO2 and H2S on the composition and stability of passive film on iron alloys in geothermal water. Electrochim. Acta 2007, 52, 5704–5714. [Google Scholar] [CrossRef]
- Bilmes, P.; Llorente, C.; Méndez, C.; Gervasi, C. Microstructure, heat treatment and pitting corrosion of 13CrNiMo plate and weld metals. Corros. Sci. 2009, 51, 876–881. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, W.; Jiang, Y.; Deng, B.; Sun, D.; Li, J. Influence of annealing treatment on the corrosion resistance of lean duplex stainless steel 2101. Electrochimica Acta 2009, 54, 5387–5392. [Google Scholar] [CrossRef]
- Brown, B.; Parakala, S.R.; Nešić, S. CO2 corrosion in the presence of trace amounts of H2S. In Proceedings of the NACE International Corrosion Conference Series: Corrosion 2004, New Orleans, LA, USA, 28 March–1 April 2004. Paper No. 04736. [Google Scholar]
- Isfahany, A.N.; Saghafian, H.; Borhani, G. The effect of heat treatment on mechanical properties and corrosion behavior of AISI420 martensitic stainless steel. J. Alloys Compd. 2011, 509, 3931–3936. [Google Scholar] [CrossRef]
- Lucio-Garcia, M.; Gonzalez-Rodriguez, J.; Casales, M.; Martinez, L.; Chacon-Nava, J.; Neri-Flores, M.; Martinez-Villafañe, A. Effect of heat treatment on H2S corrosion of a micro-alloyed C–Mn steel. Corros. Sci. 2009, 51, 2380–2386. [Google Scholar] [CrossRef]
- Pfennig, A.; Wolthusen, H.; Wolf, M.; Kranzmann, A. Effect of heat Treatment of Injection Pipe Steels on the Reliability of a Saline Aquifer Water CCS-site in the Northern German Basin. Energy Procedia 2014, 63, 5762–5772. [Google Scholar] [CrossRef] [Green Version]
- Thorbjörnsson, I. Corrosion fatigue testing of eight different steels in an Icelandic geothermal environment. Mater. Des. 1995, 16, 97–102. [Google Scholar] [CrossRef]
- Pfennig, A.; Kranzmann, A. Borehole Integrity of Austenitized and Annealed Pipe Steels Suitable for Carbon Capture and Storage (CCS). Int. J. Mater. Mech. Manuf. 2017, 5, 213–218. [Google Scholar] [CrossRef] [Green Version]
- Maranhão, J.P. Davim, finite element modelling of machining of AISI 316steel: Numerical simulation and experimental validation. Simul. Modell. Pract. Theory 2010, 18, 139–156. [Google Scholar]
- Martin, M.; Weber, S.; Izawa, C.; Wagner, S.; Pundt, A.; Theisen, W. Influence of machining-induced martensite on hydrogen-assisted fracture of AISI type 304 austenitic stainless steel. Int. J. Hydrog. Energy 2011, 36, 11195–11206. [Google Scholar] [CrossRef]
- Evgenya, B.; Hughesa, T.; Eskinba, D. Effect of surface roughness on corrosion behavior of low carbon steelin inhibited 4 M hydrochloric acid under laminar and turbulent flow conditions. Corros. Sci. 2016, 103, 196–205. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, Q.; Yang, X.X.; Wanga, Z.J.; Liub, J.; Li, Z. Impact of surface roughness and humidity on X70 steel corrosion in supercritical CO2mixture with SO2, H2O, and O2. J. Supercrit. Fluids 2016, 107, 286–297. [Google Scholar] [CrossRef]
- Llaneza, V.; Belzunce, F.J. Study of the effects produced by shot peening on the surface of quenched and tempered steels: Roughness, residual stresses and work. Appl. Surf. Sci. 2015, 356, 475–485. [Google Scholar] [CrossRef]
- Lee, S.M.; Lee, W.G.; Kim, Y.H.; Jang, H. Surface roughness and the corrosion resistance of 21Cr ferritic stainless steel. Corros. Sci. 2012, 63, 404–409. [Google Scholar] [CrossRef]
- Pfennig, A.; Kranzmann, A. Effect of CO2 and pressure on the stability of steels with different amounts of chromium in saline water. Corros. Sci. 2012, 65, 441–452. [Google Scholar] [CrossRef]
- Pfennig, A.; Kranzmann, A. Effect of CO2, Atmosphere and Pressure on the Stability of X35CrMo17 Stainless Steel in Laboratory CCS-Environment. In Proceedings of the 14th Greenhouse Gas Control Technologies Conference, Melbourne, VIC, Australia, 21–26 October 2018. [Google Scholar]
- Lopez, D.A.; Schreiner, W.H.; de Sánchez, S.R.; Simison, S.N. The influence of carbon steel microstructure on corrosion layers an XRS and SEM characterization. Appl. Surf. Sci. 2003, 207, 69–85. [Google Scholar] [CrossRef]
- Akbari Mousavi, S.A.A.; Sufizadeh, A.R. Metallurgical investigations of pulsed Nd:YAG laser welding of AISI 321 and AISI 630 stainless steels. Mater. Des. 2009, 30, 3150–3157. [Google Scholar] [CrossRef]
- Takemoto, M. Study on the Failure Threshold Stress Criteria for the Prevention and Mechanism of Stress Corrosion Cracking; Faculty of Science and Engineering, Aoyama Gakuin University: Tokyo, Japan, 1984. [Google Scholar]
- Nor Asma, R.B.A.; Yuli, P.A.; Mokhtar, C.I. Study on the effect of surface finish on corrosion of carbon steel in CO2 environment. J. Appl. Sci. 2011, 11, 2053–2057. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Zou, H. Relationship of microstructure transformation and hardening behavior of type 630 stainless steel. J. Univ. Sci. Technol. Beijing 2006, 3, 213–221. [Google Scholar] [CrossRef]
- Islam, A.W.; Sun, A.Y. Corrosion model of CO2 injection based on non-isothermal wellbore hydraulics. Int. J. Greenh. Gas Control 2016, 54, 219–227. [Google Scholar] [CrossRef] [Green Version]
- Pfennig, A.; Wolthusen, H.; Zastrow, P.; Kranzmann, A. Evaluation of heat treatment performance of potential pipe steels in CCS-environment. In Energy Technology 2015: Carbon Dioxide Management and Other Technologies; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; pp. 15–22. [Google Scholar] [CrossRef]
- Pfennig, A.; Trenner, S.; Wolf, M.; Bork, C. Vibration Tests for Determination of Mechanical Behavior in CO2-Containing Solutions in European Corrosion Congress EuroCorr 2013; Estoril Congress Center: Estoril, Portugal, 2013. [Google Scholar]
- Pfennig, A.; Wolthusen, H.; Kranzmann, A. Unusual Corrosion Behavior of 1.4542 Exposed a Laboratory Saline Aquifer Water CCS-environment. Energy Procedia 2017, 114, 5229–5240. [Google Scholar] [CrossRef]
- Pfennig, A.; Kranzmann, A. Potential of martensitic stainless steel X5CrNiCuNb 16-4 as pipe steel in corrosive CCS environment. Int. J. Environ. Sci. Dev. 2017, 8, 466–473. [Google Scholar] [CrossRef] [Green Version]
- Pfennig, A.; Kranzmann, A. Corrosion and Fatigue of Heat Treated Martensitic Stainless Steel 14542 Used For Geothermal Applications. Matter Int. J. Sci. Technol. 2019, 5, 138–158. [Google Scholar] [CrossRef]
- Han, J.; Yang, Y.; Nešić, S.; Brown, N.B. Roles of passivation and galvanic effects in localized CO2 corrosion of mild steel. In Proceedings of the NACE Corrosion 2008, New Orleans, LA, USA, 16–20 March 2008. Paper No. 08332. [Google Scholar]
- Förster, A.; Norden, B.; Zinck-Jørgensen, K.; Frykman, P.; Kulenkampff, J.; Spangenberg, E.; Erzinger, J.; Zimmer, M.; Kopp, J.; Borm, G.; et al. Baseline characterization of the CO2SINK geological storage site at Ketzin, Germany. Environ. Geosci. 2006, 13, 145–161. [Google Scholar] [CrossRef]
- Forster, A.; Schoner, R.; Förster, H.-J.; Norden, B.; Blaschke, A.-W.; Luckert, J.; Beutler, G.; Gaupp, R.; Rhede, D. Reservoir characterization of a CO2 storage aquifer: The Upper Triassic Stuttgart Formation in the Northeast German Basin. Mar. Pet. Geol. 2010, 27, 2156–2172. [Google Scholar] [CrossRef]
- Bäßler, R.; Sobetzki, J.; Klapper, H.S. Corrosion Resistance of High-Alloyed Materials in Artificial Geothermal Fluids. In Proceedings of the NACE International Corrosion Conference Series: Corrosion 2013, New Orleans, LA, USA, 17–21 March 2013. Paper No. 2327. [Google Scholar]
- Pfennig, A.; Wolf, M. Influence of geothermal environment on the corrosion fatigue behavior of standard duplex stainless steel X2CrNiMoN22-5-32. J. Phys. Conf. Ser. 2020, 1425, 012183. [Google Scholar]
- Pfennig, A.; Wolf, M.; Kranzmann, A. Evaluating corrosion and corrosion fatigue behavior via laboratory testing techniques in highly corrosive CCS-environment. In Proceedings of the 15th Greenhouse Gas Control Technologies Conference 2020, Abu Dhabi, United Arab Emirates, 15–18 March 2021. [Google Scholar]
- Pfennig, A.; Wolf, M.; Kranzmann, A. Effects of saline aquifer water on the corrosion behavior of martensitic stainless steels during exposure to CO2 environment. In Proceedings of the 15th Greenhouse Gas Control Technologies Conference 2020, Abu Dhabi, United Arab Emirates, 15–18 March 2021. [Google Scholar]
- Kraus, S.W.; Nolze, G. POWDER CELL—A program for the representation and manipulation of crystal structures and calculation of the resulting X-ray powder patterns. J. Appl. Cryst. 1996, 29, 301–303. [Google Scholar] [CrossRef]
- Choi, Y.-Y.; Nešić, S. Determining the corrosive potential of CO2 transport pipline in high pCO2-water environments. Int. J. Greenh. Gas Control 2011, 5, 788–797. [Google Scholar] [CrossRef]
- Hassani, S.; Vu, T.N.; Rosli, N.R.; Esmaeely, S.N.; Choi, Y.S.; Young, D.; Nešić, S. Wellbore integrinanoty and corrosion of low alloy steel and stainless steels in high pressure CO2 geologic storage environment: An experimental study. Int. J. Greenh. Gas Control 2014, 23, 594–601. [Google Scholar] [CrossRef]
- Wei, L.; Pang, X.; Liu, C.; Gao, K. Formation mechanism and protective property of corrosion product scale on X70 steel under supercritical CO2 environment. Corros. Sci. 2015, 100, 404–420. [Google Scholar] [CrossRef]
- Liu, Z.-G.; Gao, S.-H.; Du, S.-X.; Li, J.-P.; Yu, C.; Wang, Y.-X.; Wang, X.-N. Comparison of corrosion mechanism of low alloy pipeline steel used for flexible pipes at vapor-saturated CO2 and CO2-saturated brine conditions. Mater. Corros. 2017, 68, 1200–1211. [Google Scholar] [CrossRef]
- Han, J.; Zhang, J.; Carey, J.W. Effect of bicarbonate on corrosion of carbon steel in CO2-saturated brines. Int. J. Greenh. Gas Control 2011, 5, 1680–1683. [Google Scholar] [CrossRef]




| Elements | C | Si | Mn | P | S | Cr | Mo | Ni | Co | Fe |
|---|---|---|---|---|---|---|---|---|---|---|
| acc standard a | 0.33–0.45 | <1.00 | ≤1.00 | ≤0.045 | ≤0.03 | 15.5–17.5 | 0.8–1.3 | ≤1.00 | 0.20–0.45 |
| Elements | C | Si | Mn | P | S | Cr | Mo | Ni | Cu | Nb |
|---|---|---|---|---|---|---|---|---|---|---|
| acc standard a | ≤0.07 | ≤0.70 | ≤1.50 | ≤0.04 | ≤0.015 | 15.0–17.0 | ≤0.60 | 3.00–5.00 | 3.00–5.00 | 0.20–0.45 |
| analysed b | 0.03 | 0.42 | 0.68 | 0.018 | 0.002 | 15.75 | 0.11 | 4.54 | 3.00 | 0.242 |
| According to the Northern German Basin or According to Stuttgart Formation | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NaCl | KCl | CaCl2 × 2H2O | MgCl2 × 6H2O | NH4Cl | ZnCl2 | SrCl2 × 6H2O | PbCl2 | Na2SO4 | pH value | |
| g/L | 98.22 | 5.93 | 207.24 | 4.18 | 0.59 | 0.33 | 4.72 | 0.30 | 0.07 | 5.4–6 |
| NaCl | KCl | CaCl2 × 2H2O | MgCl2 × 6H2O | Na2SO4 × 10H2O | KOH | NaHCO3 | ||||
| g/L | 224.6 | 0.39 | 6.45 | 10.62 | 12.07 | 0.321 | 0.048 | |||
| Ca+ | K2+ | Mg2+ | Na2+ | Cl− | SO42− | HCO3− | pH value | |||
| g/L | 1.76 | 0.43 | 1.27 | 90.1 | 14.33 | 3.6 | 0.04 | 8.2–9 | ||
| Heat Treatment | TAustenitizing/°C | TAnnealing/°C | Time | Cooling |
|---|---|---|---|---|
| Min | Medium | |||
| HT1 normalizing HT1 | 850 | 30 | oil | |
| HT2 hardening | 1040 | 30 | oil | |
| HT3 hardening plus tempering 1 | 100 | 655 | 30 | oil |
| HT4 hardening plus tempering 2 | 1000 | 670 | 30 | oil |
| HT5 hardening plus tempering 3 | 1000 | 755 | 30 | oil |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pfennig, A.; Kranzmann, A. Understanding the Anomalous Corrosion Behaviour of 17% Chromium Martensitic Stainless Steel in Laboratory CCS-Environment—A Descriptive Approach. Clean Technol. 2022, 4, 239-257. https://doi.org/10.3390/cleantechnol4020014
Pfennig A, Kranzmann A. Understanding the Anomalous Corrosion Behaviour of 17% Chromium Martensitic Stainless Steel in Laboratory CCS-Environment—A Descriptive Approach. Clean Technologies. 2022; 4(2):239-257. https://doi.org/10.3390/cleantechnol4020014
Chicago/Turabian StylePfennig, Anja, and Axel Kranzmann. 2022. "Understanding the Anomalous Corrosion Behaviour of 17% Chromium Martensitic Stainless Steel in Laboratory CCS-Environment—A Descriptive Approach" Clean Technologies 4, no. 2: 239-257. https://doi.org/10.3390/cleantechnol4020014
APA StylePfennig, A., & Kranzmann, A. (2022). Understanding the Anomalous Corrosion Behaviour of 17% Chromium Martensitic Stainless Steel in Laboratory CCS-Environment—A Descriptive Approach. Clean Technologies, 4(2), 239-257. https://doi.org/10.3390/cleantechnol4020014