Sugarcane Bagasse: Challenges and Opportunities for Waste Recycling
Abstract
:1. Introduction
2. Biofuels from Lignocellulosic Biomass
Biofuel Prospects
3. Sugarcane Bagasse Pyrolysis
3.1. Slow or Conventional Pyrolysis
3.2. Fast Pyrolysis
3.3. Flash or Ultrafast Pyrolysis
3.4. Comparative Studies
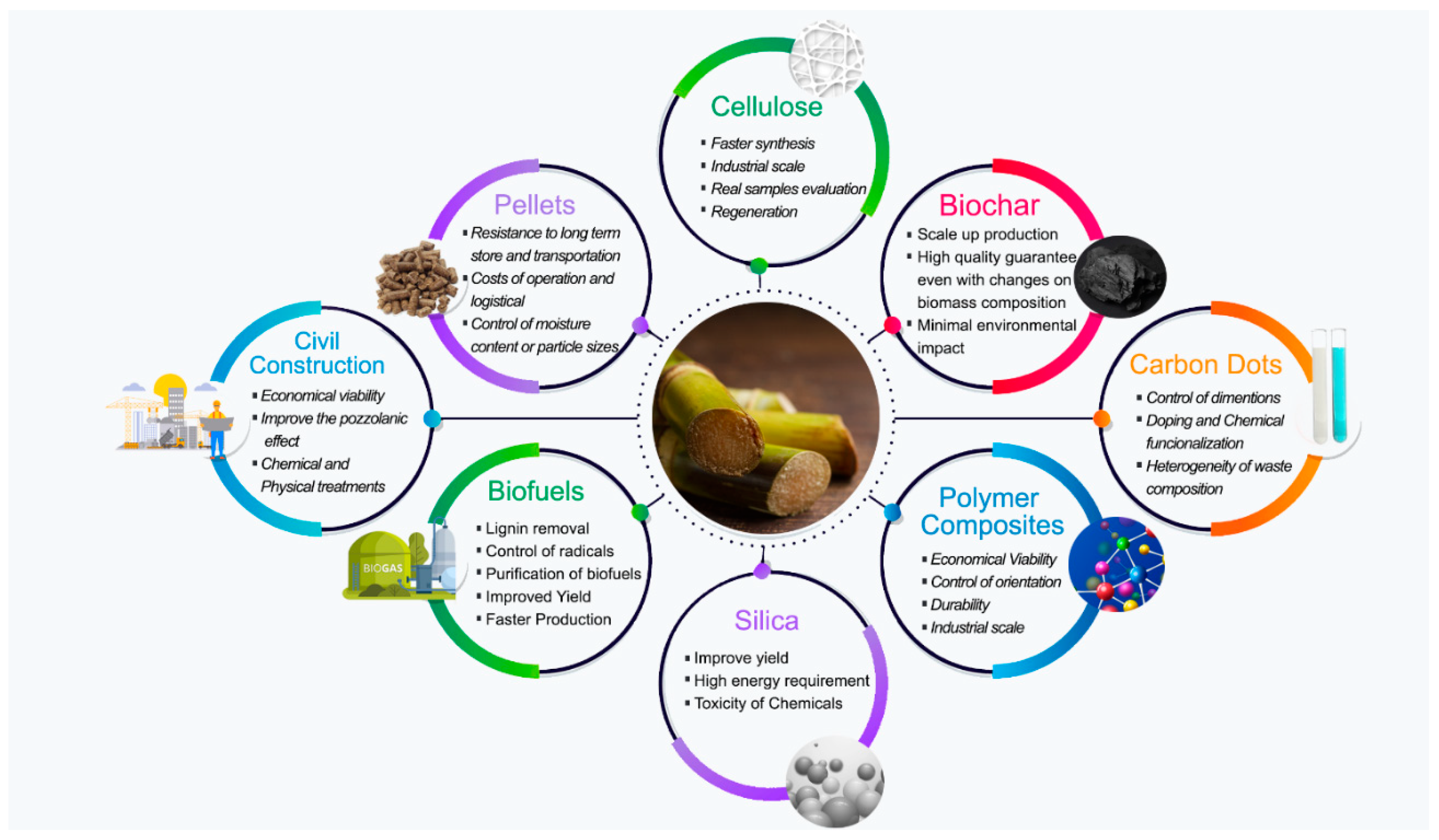
3.5. Pyrolysis Prospects
4. Biochar from Sugarcane
4.1. Remediation of Soil Pollutants and Gas Removal
4.2. Biochar as an Electrochemical Component
4.3. Biochar Prospects
5. Boosting Energy Efficiency Using Sugarcane Bagasse Pellets
5.1. Parameters Influencing Sugarcane Bagasse Pellets
5.2. Sugarcane Bagasse Pellets as Energy Sources
5.3. Other Applications of Sugarcane Bagasse Pallets
5.4. Pellets Prospects
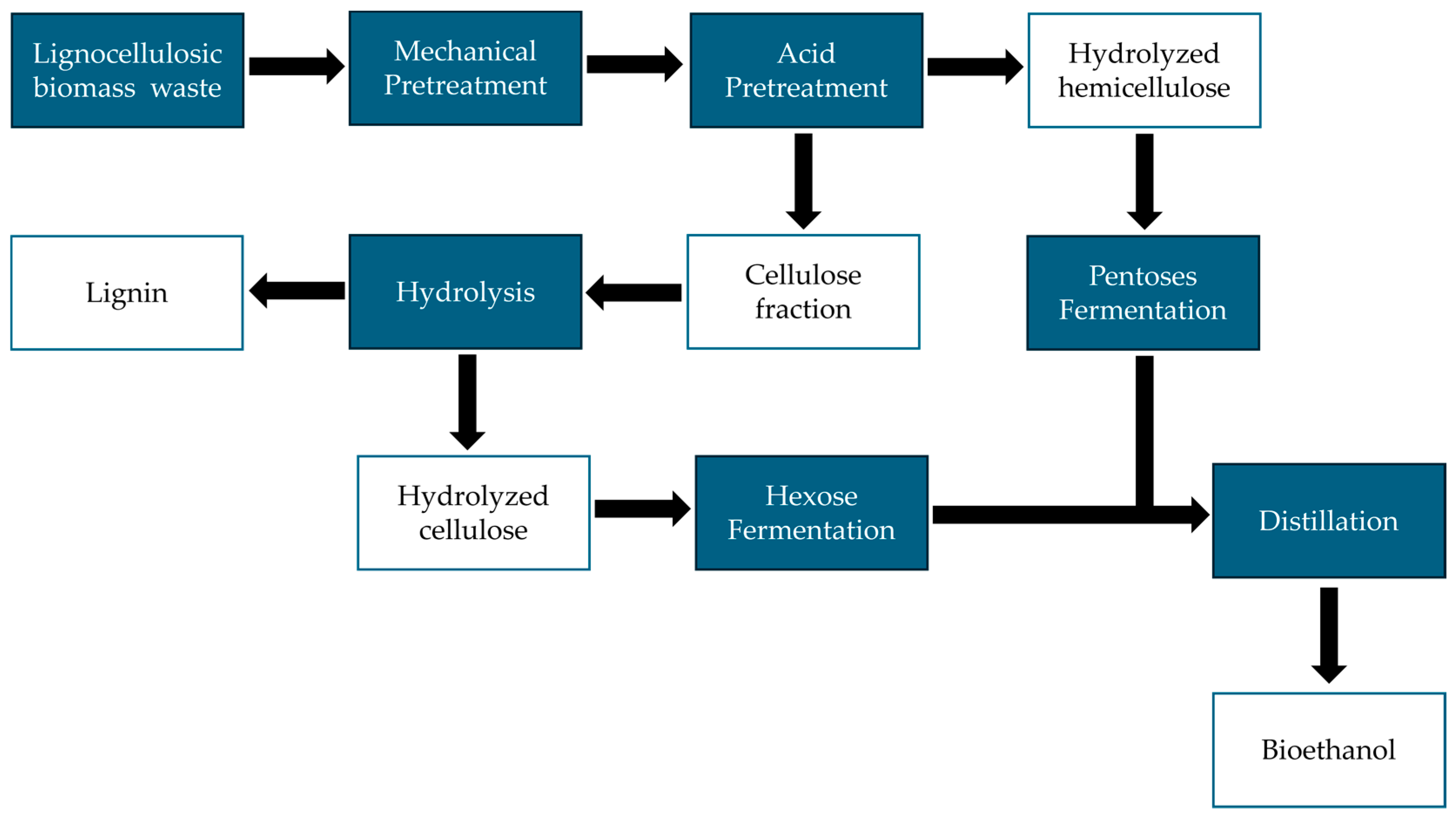
6. Second-Generation Ethanol (2G)
7. Sugarcane Bagasse: The Potential Cellulose Source for Biosorbents
7.1. Removal of Contaminants from Wastewater
7.2. Heavy Metal Removal Using Sugarcane Bagasse
7.3. Dye Removal by Sugarcane Bagasse
7.4. Organic Compound Removal
7.5. Cellulose Hydrogel, Aerogel, and Xerogel
7.6. Gas Adsorption by Sugarcane Bagasse
7.7. Adsorption Prospects
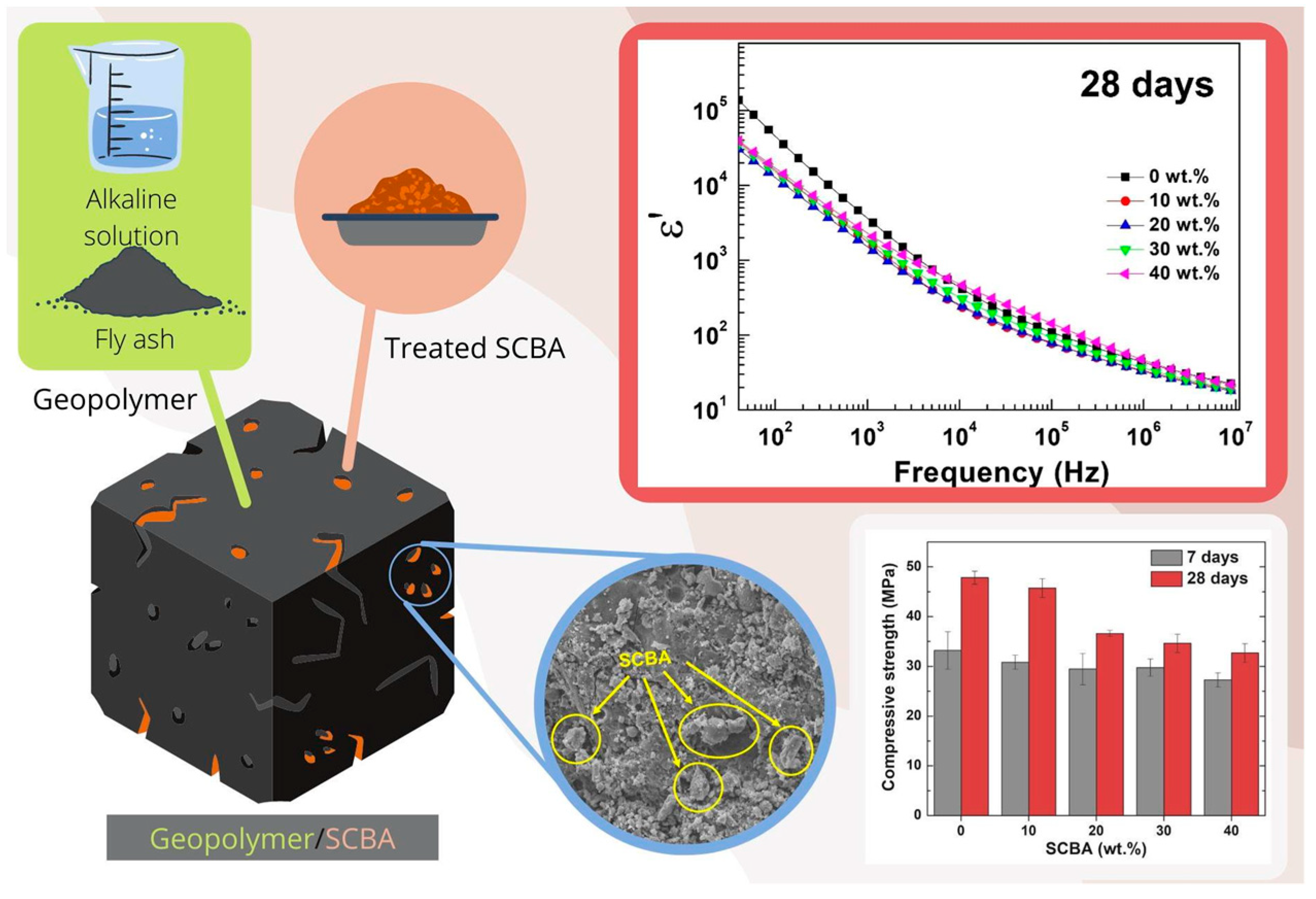
8. Sugarcane Bagasse in Civil Construction
Prospects for Sugarcane Bagasse in Civil Construction
9. Fiber-Based Filler in Polymer Composites
9.1. Sugarcane Bagasse Ash as Filler in Polymer Composites
9.2. Prospects for Sugarcane Bagasse Ash
10. Sugarcane-Based Silica
Prospects for Sugarcane Bagasse Silica
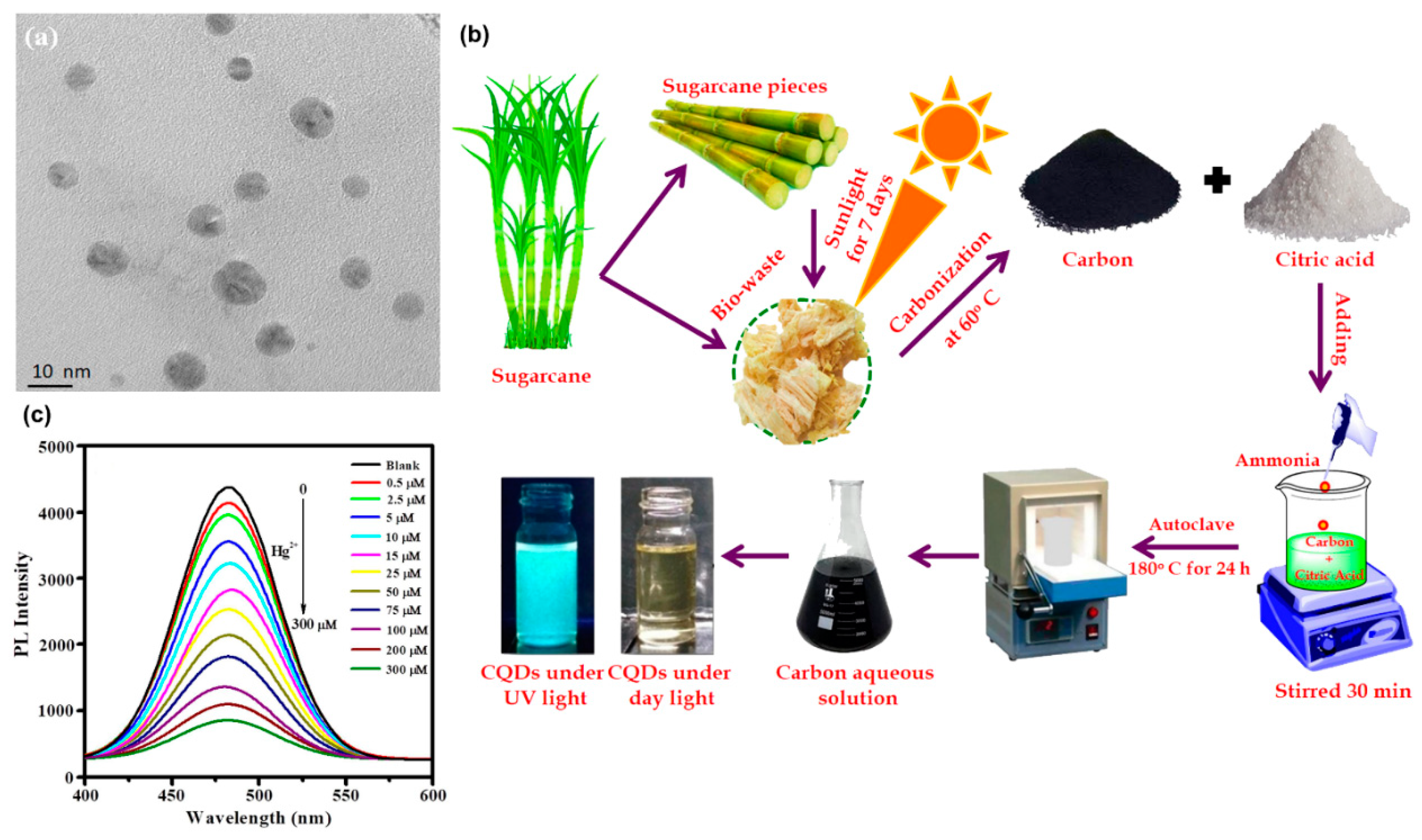
11. Carbon Dot Synthesis
Prospects for Carbon Dots
12. Dietary Products
13. Source of Biochemicals
14. Packaging
15. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAO—Food and Agriculture Organization of the United Nations Crops and Livestock Products. Available online: https://www.fao.org/faostat/en/#data/QCL/visualize (accessed on 2 March 2024).
- CONAB—Companhia Nacional de Abastecimento. Acompanhamento Da Safra Brasileira de Cana-de-Açucar, 3rd ed.; CONAB—Companhia Nacional de Abastecimento: Brasília, DF, Brazil, 2022; Volume 9. [Google Scholar]
- MME—Ministério de Minas e Energia; EPE—Empresa de Pesquisa Energética. BEN: Balanço Energético Nacional—Relatório Síntese 2022: Ano Base 2021; MME—Ministério de Minas e Energia: Brasília, Brazil; EPE—Empresa de Pesquisa Energética: Rio de Janeiro, Brazil, 2022. [Google Scholar]
- Federal Government of Brazil. LEI No 13.576. Available online: https://www2.camara.leg.br/legin/fed/lei/2017/lei-13576-26-dezembro-2017-786013-publicacaooriginal-154631-pl.html (accessed on 6 December 2023).
- Federal Government of Brazil. DECRETO No 9.888. Available online: https://www.planalto.gov.br/ccivil_03/_ato2019-2022/2019/decreto/d9888.htm (accessed on 6 December 2023).
- Negrão, D.R.; Grandis, A.; Buckeridge, M.S.; Rocha, G.J.M.; Leal, M.R.L.V.; Driemeier, C. Inorganics in Sugarcane Bagasse and Straw and Their Impacts for Bioenergy and Biorefining: A Review. Renew. Sustain. Energy Rev. 2021, 148, 111268. [Google Scholar] [CrossRef]
- Shabbirahmed, A.M.; Haldar, D.; Dey, P.; Patel, A.K.; Singhania, R.R.; Dong, C.-D.; Purkait, M.K. Sugarcane Bagasse into Value-Added Products: A Review. Environ. Sci. Pollut. Res. 2022, 29, 62785–62806. [Google Scholar] [CrossRef]
- Khatri, P.; Pandit, A.B. Systematic Review of Life Cycle Assessments Applied to Sugarcane Bagasse Utilization Alternatives. Biomass Bioenergy 2022, 158, 106365. [Google Scholar] [CrossRef]
- Iwuozor, K.O.; Chizitere Emenike, E.; Ighalo, J.O.; Omoarukhe, F.O.; Omuku, P.E.; George Adeniyi, A. A Review on the Thermochemical Conversion of Sugarcane Bagasse into Biochar. Clean. Mater. 2022, 6, 100162. [Google Scholar] [CrossRef]
- Gond, R.K.; Gupta, M.K.; Jawaid, M. Extraction of Nanocellulose from Sugarcane Bagasse and Its Characterization for Potential Applications. Polym. Compos. 2021, 42, 5400–5412. [Google Scholar] [CrossRef]
- Cardozo, E.; Malmquist, A. Performance Comparison between the Use of Wood and Sugarcane Bagasse Pellets in a Stirling Engine Micro-CHP System. Appl. Therm. Eng. 2019, 159, 113945. [Google Scholar] [CrossRef]
- de Palma, K.R.; García-Hernando, N.; Silva, M.A.; Tomaz, E.; Soria-Verdugo, A. Pyrolysis and Combustion Kinetic Study and Complementary Study of Ash Fusibility Behavior of Sugarcane Bagasse, Sugarcane Straw, and Their Pellets—Case Study of Agro-Industrial Residues. Energy Fuels 2019, 33, 3227–3238. [Google Scholar] [CrossRef]
- Yan, X.; Zhu, M.-J. Enhanced Bioelectricity Generation in Thermophilic Microbial Fuel Cell with Lignocellulose as an Electron Donor by Resazurin-Mediated Electron Transfer. Bioresour. Technol. 2023, 388, 129764. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.F.K.; Ali, U.F.M.; Isa, K.M.; Gopinath, S.C.B. Study on Characterization of Bio-Oil Derived from Sugarcane Bagasse (Saccharum barberi) for Application as Biofuel. Clean Energy 2022, 6, 297–304. [Google Scholar] [CrossRef]
- Mohapatra, S.S.; Gouda, N.; Singh, R.K. Investigation on Thermokinetic Study and Optimization of Sugarcane Bagasse Thermal Pyrolysis. Sugar Tech 2023, 25, 198–209. [Google Scholar] [CrossRef]
- Yerrayya, A.; Nikunj, A.; Prashanth, P.F.; Chakravarthy, S.R.; Natarajan, U.; Vinu, R. Optimization of Bio-Crude Yield and Its Calorific Value from Hydrothermal Liquefaction of Bagasse Using Methanol as Co-Solvent. Energy 2022, 244, 123192. [Google Scholar] [CrossRef]
- Agarwal, N.K.; Kumar, M.; Ghosh, P.; Kumar, S.S.; Singh, L.; Vijay, V.K.; Kumar, V. Anaerobic Digestion of Sugarcane Bagasse for Biogas Production and Digestate Valorization. Chemosphere 2022, 295, 133893. [Google Scholar] [CrossRef]
- de Almeida, S.G.C.; Tarelho, L.A.C.; Hauschild, T.; Costa, M.A.M.; Dussán, K.J. Biochar Production from Sugarcane Biomass Using Slow Pyrolysis: Characterization of the Solid Fraction. Chem. Eng. Process.—Process Intensif. 2022, 179, 109054. [Google Scholar] [CrossRef]
- Aruna; Bagotia, N.; Sharma, A.K.; Kumar, S. A Review on Modified Sugarcane Bagasse Biosorbent for Removal of Dyes. Chemosphere 2021, 268, 129309. [Google Scholar] [CrossRef]
- Iwuozor, K.O.; Emenike, E.C.; Ighalo, J.O.; Eshiemogie, S.; Omuku, P.E.; Adeniyi, A.G. Valorization of Sugar Industry’s By-Products: A Perspective. Sugar Tech 2022, 24, 1052–1078. [Google Scholar] [CrossRef]
- Guo, Y.; Tan, C.; Sun, J.; Li, W.; Zhang, J.; Zhao, C. Porous Activated Carbons Derived from Waste Sugarcane Bagasse for CO2 Adsorption. Chem. Eng. J. 2020, 381, 122736. [Google Scholar] [CrossRef]
- Katakojwala, R.; Mohan, S.V. Multi-Product Biorefinery with Sugarcane Bagasse: Process Development for Nanocellulose, Lignin and Biohydrogen Production and Lifecycle Analysis. Chem. Eng. J. 2022, 446, 137233. [Google Scholar] [CrossRef]
- Khalil, M.J.; Aslam, M.; Ahmad, S. Utilization of Sugarcane Bagasse Ash as Cement Replacement for the Production of Sustainable Concrete—A Review. Constr. Build. Mater. 2021, 270, 121371. [Google Scholar] [CrossRef]
- Neto, J.d.S.A.; de França, M.J.S.; de Amorim Júnior, N.S.; Ribeiro, D.V. Effects of Adding Sugarcane Bagasse Ash on the Properties and Durability of Concrete. Constr. Build. Mater. 2021, 266, 120959. [Google Scholar] [CrossRef]
- Falk, G.; Shinhe, G.P.; Teixeira, L.B.; Moraes, E.G.; de Oliveira, A.P.N. Synthesis of Silica Nanoparticles from Sugarcane Bagasse Ash and Nano-Silicon via Magnesiothermic Reactions. Ceram. Int. 2019, 45, 21618–21624. [Google Scholar] [CrossRef]
- Paone, E.; Mauriello, F. Sustainable Production of Textile Fibers, Biofuels, and Chemicals from Poplar Wood. Trends Chem. 2023, 5, 1–2. [Google Scholar] [CrossRef]
- Brenelli, L.B.; Bhatia, R.; Djajadi, D.T.; Thygesen, L.G.; Rabelo, S.C.; Leak, D.J.; Franco, T.T.; Gallagher, J.A. Xylo-Oligosaccharides, Fermentable Sugars, and Bioenergy Production from Sugarcane Straw Using Steam Explosion Pretreatment at Pilot-Scale. Bioresour. Technol. 2022, 357, 127093. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.M.; Crow, S.E.; Khanal, S.K.; Turn, S.Q. Lignin Chemical Controls on Bioconversion of Tropically Grown C4 Bioenergy Grasses to Biofuels and Biobased Products. Bioresour. Technol. Rep. 2022, 18, 101015. [Google Scholar] [CrossRef]
- Bhatia, L.; Sarangi, P.K.; Singh, A.K.; Prakash, A.; Shadangi, K.P. Lignocellulosic Waste Biomass for Biohydrogen Production: Future Challenges and Bio-economic Perspectives. Biofuels Bioprod. Biorefin. 2022, 16, 838–858. [Google Scholar] [CrossRef]
- Saravanan, A.; Senthil Kumar, P.; Jeevanantham, S.; Karishma, S.; Vo, D.-V.N. Recent Advances and Sustainable Development of Biofuels Production from Lignocellulosic Biomass. Bioresour. Technol. 2022, 344, 126203. [Google Scholar] [CrossRef]
- Ashokkumar, V.; Venkatkarthick, R.; Jayashree, S.; Chuetor, S.; Dharmaraj, S.; Kumar, G.; Chen, W.-H.; Ngamcharussrivichai, C. Recent Advances in Lignocellulosic Biomass for Biofuels and Value-Added Bioproducts—A Critical Review. Bioresour. Technol. 2022, 344, 126195. [Google Scholar] [CrossRef]
- Van Meerbeek, K.; Muys, B.; Hermy, M. Lignocellulosic Biomass for Bioenergy beyond Intensive Cropland and Forests. Renew. Sustain. Energy Rev. 2019, 102, 139–149. [Google Scholar] [CrossRef]
- Velvizhi, G.; Goswami, C.; Shetti, N.P.; Ahmad, E.; Pant, K.K.; Aminabhavi, T.M. Valorisation of Lignocellulosic Biomass to Value-Added Products: Paving the Pathway towards Low-Carbon Footprint. Fuel 2022, 313, 122678. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Lu, C. Development Perspectives of Promising Lignocellulose Feedstocks for Production of Advanced Generation Biofuels: A Review. Renew. Sustain. Energy Rev. 2021, 136, 110445. [Google Scholar] [CrossRef]
- Usmani, Z.; Sharma, M.; Awasthi, A.K.; Sivakumar, N.; Lukk, T.; Pecoraro, L.; Thakur, V.K.; Roberts, D.; Newbold, J.; Gupta, V.K. Bioprocessing of Waste Biomass for Sustainable Product Development and Minimizing Environmental Impact. Bioresour. Technol. 2021, 322, 124548. [Google Scholar] [CrossRef]
- Ingle, A.P.; Ingle, P.; Gupta, I.; Rai, M. Socioeconomic Impacts of Biofuel Production from Lignocellulosic Biomass. In Sustainable Bioenergy; Elsevier: Amsterdam, The Netherlands, 2019; pp. 347–366. [Google Scholar]
- Sarwer, A.; Hussain, M.; Al-Muhtaseb, A.H.; Inayat, A.; Rafiq, S.; Khurram, M.S.; Ul-Haq, N.; Shah, N.S.; Din, A.A.; Ahmad, I.; et al. Suitability of Biofuels Production on Commercial Scale from Various Feedstocks: A Critical Review. ChemBioEng Rev. 2022, 9, 423–441. [Google Scholar] [CrossRef]
- Sidana, A.; Yadav, S.K. Recent Developments in Lignocellulosic Biomass Pretreatment with a Focus on Eco-Friendly, Non-Conventional Methods. J. Clean. Prod. 2022, 335, 130286. [Google Scholar] [CrossRef]
- de Moraes Dutenkefer, R.; Machado, P.G.; de Oliveira Ribeiro, C. Lignin Chemical Derivatives in Brazilian Sugarcane Sector: An Alternative to Make 2G Ethanol Viable? J. Clean. Prod. 2022, 369, 133286. [Google Scholar] [CrossRef]
- Curran, L.M.L.K.; Pham, L.T.M.; Sale, K.L.; Simmons, B.A. Review of Advances in the Development of Laccases for the Valorization of Lignin to Enable the Production of Lignocellulosic Biofuels and Bioproducts. Biotechnol. Adv. 2022, 54, 107809. [Google Scholar] [CrossRef]
- Radhika, N.L.; Sachdeva, S.; Kumar, M. Lignin Depolymerization and Biotransformation to Industrially Important Chemicals/Biofuels. Fuel 2022, 312, 122935. [Google Scholar] [CrossRef]
- Romanenko, I.; Kurz, F.; Baumgarten, R.; Jevtovikj, I.; Lindner, J.-P.; Kundu, A.; Kindler, A.; Schunk, S.A. Lignin Depolymerization in the Presence of Base, Hydrogenation Catalysts, and Ethanol. Catalysts 2022, 12, 158. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, H.; Li, H.; Han, X.; Zhang, M.; Sun, Y.; Fan, X.; Tu, R.; Zeng, Y.; Xu, C.C.; et al. Applications of Catalysts in Thermochemical Conversion of Biomass (Pyrolysis, Hydrothermal Liquefaction and Gasification): A Critical Review. Renew. Energy 2022, 196, 462–481. [Google Scholar] [CrossRef]
- Zeilerbauer, L.; Lindorfer, J.; Süss, R.; Kamm, B. Techno-economic and Life-cycle Assessment of a Wood Chips-based Organosolv Biorefinery Concept for Production of Lignin Monomers and Oligomers by Base-catalyzed Depolymerization. Biofuels Bioprod. Biorefin. 2022, 16, 370–388. [Google Scholar] [CrossRef]
- Brown, E.E. Minireview: Recent Efforts toward Upgrading Lignin-Derived Phenols in Continuous Flow. J. Flow Chem. 2023, 13, 91–102. [Google Scholar] [CrossRef]
- Sarker, T.C.; Azam, S.M.G.G.; Bonanomi, G. Recent Advances in Sugarcane Industry Solid By-Products Valorization. Waste Biomass Valoriz. 2017, 8, 241–266. [Google Scholar] [CrossRef]
- Mota, I.F.; da Silva Burgal, J.; Antunes, F.; Pintado, M.E.; Costa, P.S. High Value-Added Lignin Extracts from Sugarcane by-Products. Int. J. Biol. Macromol. 2023, 230, 123144. [Google Scholar] [CrossRef]
- Silva, J.C.d.A.; Gonçalves, E.P.; Viana, J.S.; Souza, C.M.P.G.; Borges, J.P.G.d.S.; Cavalcante, W.F. Growth and Physiology of Two Sunflower Cultivars Fertilized with Sugarcane Bagasse Ash. Acta Sci. Agron. 2022, 44, e54392. [Google Scholar] [CrossRef]
- Jayamani, E.; Rahman, M.R.; Benhur, D.A.; Bakri, M.K.B.; Kakar, A.; Khan, A. Comparative Study of Fly Ash/Sugarcane Fiber Reinforced Polymer Composites Properties. Bioresources 2020, 15, 5514–5531. [Google Scholar] [CrossRef]
- Niju, S.; Swathika, M. Delignification of Sugarcane Bagasse Using Pretreatment Strategies for Bioethanol Production. Biocatal. Agric. Biotechnol. 2019, 20, 101263. [Google Scholar] [CrossRef]
- Tantayotai, P.; Gundupalli, M.P.; Katam, K.; Rattanaporn, K.; Cheenkachorn, K.; Sriariyanun, M. In-Depth Investigation of the Bioethanol and Biogas Production from Organic and Mineral Acid Pretreated Sugarcane Bagasse: Comparative and Optimization Studies. Biocatal. Agric. Biotechnol. 2022, 45, 102499. [Google Scholar] [CrossRef]
- Gomes, M.G.; Paranhos, A.G.d.O.; Camargos, A.B.; Baêta, B.E.L.; Baffi, M.A.; Gurgel, L.V.A.; Pasquini, D. Pretreatment of Sugarcane Bagasse with Dilute Citric Acid and Enzymatic Hydrolysis: Use of Black Liquor and Solid Fraction for Biogas Production. Renew. Energy 2022, 191, 428–438. [Google Scholar] [CrossRef]
- Alino, J.H.L.; Bastos, J.A.; Remor, P.V.; Frare, L.M.; Orssatto, F.; Damaceno, F.M.; Edwiges, T. Alkaline Pretreatment and Pre-Hydrolysis Using Acidic Biowastes to Increase Methane Production from Sugarcane Bagasse. Methane 2022, 1, 189–200. [Google Scholar] [CrossRef]
- Monroy Vázquez, F.J.; González Uribe, E.E.; García Enríquez, S.; Fernández-Escamilla, V.V.A.; González Núñez, R.; Canche Escamilla, G.; Moscoso Sanchez, F.J. Influence of Pretreated Sugarcane Bagasse Fiber by Steam Explosion, Soaking with Caustic Soda, and Addition of Coupling Agent into Polylactic Acid Biocomposites. J. Compos. Mater. 2022, 56, 4621–4633. [Google Scholar] [CrossRef]
- Hermsdorff, G.B.; Escobar, E.L.N.; da Silva, T.A.; Filho, A.Z.; Corazza, M.L.; Ramos, L.P. Ethanol Organosolv Pretreatment of Sugarcane Bagasse Assisted by Organic Acids and Supercritical Carbon Dioxide. Carbohydr. Polym. 2023, 300, 120263. [Google Scholar] [CrossRef]
- Patel, A.; Shah, A.R. Integrated Lignocellulosic Biorefinery: Gateway for Production of Second Generation Ethanol and Value Added Products. J. Bioresour. Bioprod. 2021, 6, 108–128. [Google Scholar] [CrossRef]
- Gan, J.; Iqbal, H.M.N.; Show, P.L.; Rahdar, A.; Bilal, M. Upgrading Recalcitrant Lignocellulosic Biomass Hydrolysis by Immobilized Cellulolytic Enzyme–Based Nanobiocatalytic Systems: A Review. Biomass Convers. Biorefin. 2024, 14, 4485–4509. [Google Scholar] [CrossRef]
- Lin, S.-P.; Huang, S.-H.; Ting, Y.; Hsu, H.-Y.; Cheng, K.-C. Evaluation of Detoxified Sugarcane Bagasse Hydrolysate by Atmospheric Cold Plasma for Bacterial Cellulose Production. Int. J. Biol. Macromol. 2022, 204, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Ingle, A.P.; Philippini, R.R.; da Silva, S.S. Pretreatment of Sugarcane Bagasse Using Two Different Acid-Functionalized Magnetic Nanoparticles: A Novel Approach for High Sugar Recovery. Renew. Energy 2020, 150, 957–964. [Google Scholar] [CrossRef]
- de Oliveira, B.P.; da Silva Abreu, F.O.M. Carbon Quantum Dots Synthesis from Waste and By-Products: Perspectives and Challenges. Mater. Lett. 2021, 282, 128764. [Google Scholar] [CrossRef]
- Zhuang, J.; Kim, K.H.; Jia, L.; Meng, X.; Kumar, D.; Leem, G.; Kang, S.B.; Li, Y.; Ragauskas, A.J.; Hou, Y.; et al. Ferric Chloride Aided Peracetic Acid Pretreatment for Effective Utilization of Sugarcane Bagasse. Fuel 2022, 319, 123739. [Google Scholar] [CrossRef]
- Hoang, A.T.; Ong, H.C.; Fattah, I.M.R.; Chong, C.T.; Cheng, C.K.; Sakthivel, R.; Ok, Y.S. Progress on the Lignocellulosic Biomass Pyrolysis for Biofuel Production toward Environmental Sustainability. Fuel Process. Technol. 2021, 223, 106997. [Google Scholar] [CrossRef]
- Liu, W.-J.; Yu, H.-Q. Thermochemical Conversion of Lignocellulosic Biomass into Mass-Producible Fuels: Emerging Technology Progress and Environmental Sustainability Evaluation. ACS Environ. Au 2022, 2, 98–114. [Google Scholar] [CrossRef] [PubMed]
- Ong, H.C.; Chen, W.-H.; Farooq, A.; Gan, Y.Y.; Lee, K.T.; Ashokkumar, V. Catalytic Thermochemical Conversion of Biomass for Biofuel Production: A Comprehensive Review. Renew. Sustain. Energy Rev. 2019, 113, 109266. [Google Scholar] [CrossRef]
- Naqvi, S.R.; Ullah, Z.; Taqvi, S.A.A.; Khan, M.N.A.; Farooq, W.; Mehran, M.T.; Juchelková; D; Štěpanec, L. Applications of Machine Learning in Thermochemical Conversion of Biomass—A Review. Fuel 2023, 332, 126055. [Google Scholar] [CrossRef]
- Dou, B.; Zhang, H.; Song, Y.; Zhao, L.; Jiang, B.; He, M.; Ruan, C.; Chen, H.; Xu, Y. Hydrogen Production from the Thermochemical Conversion of Biomass: Issues and Challenges. Sustain. Energy Fuels 2019, 3, 314–342. [Google Scholar] [CrossRef]
- Nunes, L.J.R. Biomass Gasification as an Industrial Process with Effective Proof-of-Concept: A Comprehensive Review on Technologies, Processes and Future Developments. Results Eng. 2022, 14, 100408. [Google Scholar] [CrossRef]
- Osman, A.I.; Mehta, N.; Elgarahy, A.M.; Al-Hinai, A.; Al-Muhtaseb, A.H.; Rooney, D.W. Conversion of Biomass to Biofuels and Life Cycle Assessment: A Review. Environ. Chem. Lett. 2021, 19, 4075–4118. [Google Scholar] [CrossRef]
- Yogalakshmi, K.N.; Sivashanmugam, P.; Kavitha, S.; Kannah, Y.; Varjani, S.; AdishKumar, S.; Kumar, G. Lignocellulosic Biomass-Based Pyrolysis: A Comprehensive Review. Chemosphere 2022, 286, 131824. [Google Scholar] [CrossRef] [PubMed]
- Barros, J.A.S.; Schneider, J.K.; Farrapeira, R.O.; Andrade, Y.B.; Krause, L.C.; Bjerk, T.R.; Caramão, E.B. Recovery of Waste Biomass: Pyrolysis and Characterization of Sugarcane Residues and Their Bio-Oils. Biofuels 2022, 13, 843–852. [Google Scholar] [CrossRef]
- Saif, A.G.H.; Wahid, S.S.; Ali, M.R.O. Pyrolysis of Sugarcane Bagasse: The Effects of Process Parameters on the Product Yields. Mater. Sci. Forum 2020, 1008, 159–167. [Google Scholar] [CrossRef]
- Miranda, N.T.; Motta, I.L.; Filho, R.M.; Maciel, M.R.W. Sugarcane Bagasse Pyrolysis: A Review of Operating Conditions and Products Properties. Renew. Sustain. Energy Rev. 2021, 149, 111394. [Google Scholar] [CrossRef]
- Boer, F.D.; Valette, J.; Commandré, J.M.; Fournier, M.; Thévenon, M.F. Slow Pyrolysis of Sugarcane Bagasse for the Production of Char and the Potential of Its By-Product for Wood Protection. J. Renew. Mater. 2021, 9, 97–117. [Google Scholar] [CrossRef]
- Rodier, L.; Bilba, K.; Onésippe, C.; Arsène, M.-A. Utilization of Bio-Chars from Sugarcane Bagasse Pyrolysis in Cement-Based Composites. Ind. Crops Prod. 2019, 141, 111731. [Google Scholar] [CrossRef]
- Okonkwo, C.A.; Menkiti, M.C.; Obiora-Okafo, I.A.; Ezenwa, O.N. Controlled Pyrolysis of Sugarcane Bagasse Enhanced Mesoporous Carbon for Improving Capacitance of Supercapacitor Electrode. Biomass Bioenergy 2021, 146, 105996. [Google Scholar] [CrossRef]
- Ghorbannezhad, P.; Firouzabadi, M.D.; Ghasemian, A.; de Wild, P.J.; Heeres, H.J. Sugarcane Bagasse Ex-Situ Catalytic Fast Pyrolysis for the Production of Benzene, Toluene and Xylenes (BTX). J. Anal. Appl. Pyrolysis 2018, 131, 1–8. [Google Scholar] [CrossRef]
- Lu, Q.; Ye, X.; Zhang, Z.; Wang, Z.; Cui, M.; Yang, Y. Catalytic Fast Pyrolysis of Sugarcane Bagasse Using Activated Carbon Catalyst in a Hydrogen Atmosphere to Selectively Produce 4-Ethyl Phenol. J. Anal. Appl. Pyrolysis 2018, 136, 125–131. [Google Scholar] [CrossRef]
- Soongprasit, K.; Sricharoenchaikul, V.; Atong, D. Selective Aromatic Production from Fast Pyrolysis of Sugarcane Bagasse Lignin over ZSM-5 Catalyst. Energy Rep. 2021, 7, 830–843. [Google Scholar] [CrossRef]
- Inocente, J.M.; Elyseu, F.; Nieves, L.J.J.; Jiusti, J.; Cargnin, M.; Peterson, M. Production and Characterization of High-Reactivity Metakaolins Calcined in Flash Reactor. Appl. Clay Sci. 2021, 213, 106247. [Google Scholar] [CrossRef]
- Ighalo, J.O.; Iwuchukwu, F.U.; Eyankware, O.E.; Iwuozor, K.O.; Olotu, K.; Bright, O.C.; Igwegbe, C.A. Flash Pyrolysis of Biomass: A Review of Recent Advances. Clean Technol. Environ. Policy 2022, 24, 2349–2363. [Google Scholar] [CrossRef]
- Ayyadurai, S.; Arunachalam, K.D. Experimental Investigations on Sugarcane Bagasse Pyrolytic Oil Production from Flash Pyrolysis Using a Rotary Screw Reactor. Biofuels Bioprod. Biorefin. 2022, 16, 576–586. [Google Scholar] [CrossRef]
- Al Arni, S. Comparison of Slow and Fast Pyrolysis for Converting Biomass into Fuel. Renew. Energy 2018, 124, 197–201. [Google Scholar] [CrossRef]
- Pradana, Y.S.; Daniyanto; Hartono, M.; Prasakti, L.; Budiman, A. Effect of Calcium and Magnesium Catalyst on Pyrolysis Kinetic of Indonesian Sugarcane Bagasse for Biofuel Production. Energy Procedia 2019, 158, 431–439. [Google Scholar] [CrossRef]
- Hass, A.; Lima, I.M. Effect of Feed Source and Pyrolysis Conditions on Properties and Metal Sorption by Sugarcane Biochar. Environ. Technol. Innov. 2018, 10, 16–26. [Google Scholar] [CrossRef]
- Monisha, R.S.; Mani, R.L.; Sivaprakash, B.; Rajamohan, N.; Vo, D.-V.N. Green Remediation of Pharmaceutical Wastes Using Biochar: A Review. Environ. Chem. Lett. 2022, 20, 681–704. [Google Scholar] [CrossRef]
- Jacob, M.M.; Ponnuchamy, M.; Kapoor, A.; Sivaraman, P. Adsorptive Decontamination of Organophosphate Pesticide Chlorpyrifos from Aqueous Systems Using Bagasse-Derived Biochar Alginate Beads: Thermodynamic, Equilibrium, and Kinetic Studies. Chem. Eng. Res. Des. 2022, 186, 241–251. [Google Scholar] [CrossRef]
- Khan, A.Z.; Khan, S.; Ayaz, T.; Brusseau, M.L.; Khan, M.A.; Nawab, J.; Muhammad, S. Popular Wood and Sugarcane Bagasse Biochars Reduced Uptake of Chromium and Lead by Lettuce from Mine-Contaminated Soil. Environ. Pollut. 2020, 263, 114446. [Google Scholar] [CrossRef] [PubMed]
- Bashir, M.A.; Naveed, M.; Ahmad, Z.; Gao, B.; Mustafa, A.; Núñez-Delgado, A. Combined Application of Biochar and Sulfur Regulated Growth, Physiological, Antioxidant Responses and Cr Removal Capacity of Maize (Zea mays L.) in Tannery Polluted Soils. J. Environ. Manag. 2020, 259, 110051. [Google Scholar] [CrossRef] [PubMed]
- Emenike, E.C.; Iwuozor, K.O.; Ighalo, J.O.; Bamigbola, J.O.; Omonayin, E.O.; Ojo, H.T.; Adeleke, J.; Adeniyi, A.G. Advancing the Circular Economy through the Thermochemical Conversion of Waste to Biochar: A Review on Sawdust Waste-Derived Fuel. Biofuels 2024, 15, 433–447. [Google Scholar] [CrossRef]
- Deng, C.; Peng, L.; Ling, X.; Wang, T.; Xu, R.; Zhu, Y.; Wang, C.; Qian, X.; Wang, L.; Wu, Y.; et al. Construction of S-Scheme Zn0.2Cd0.8S/Biochar Aerogel Architectures for Boosting Photocatalytic Hydrogen Production under Sunlight Irradiation. J. Clean. Prod. 2023, 414, 137616. [Google Scholar] [CrossRef]
- Hsiao, C.-H.; Gupta, S.; Lee, C.-Y.; Tai, N.-H. Effects of Physical and Chemical Activations on the Performance of Biochar Applied in Supercapacitors. Appl. Surf. Sci. 2023, 610, 155560. [Google Scholar] [CrossRef]
- Sang, J.; Sun, C.; Pan, J.; Gao, C.; Zhang, R.; Jia, F.; Wang, F.; Wang, Q. Seaweed—Modification of Si by Natural Nitrogen-Doped Porous Biochar for High-Efficiency Lithium Batteries. ACS Appl. Mater. Interfaces 2024, 16, 11389–11399. [Google Scholar] [CrossRef] [PubMed]
- Chi, N.T.L.; Anto, S.; Ahamed, T.S.; Kumar, S.S.; Shanmugam, S.; Samuel, M.S.; Mathimani, T.; Brindhadevi, K.; Pugazhendhi, A. A Review on Biochar Production Techniques and Biochar Based Catalyst for Biofuel Production from Algae. Fuel 2021, 287, 119411. [Google Scholar] [CrossRef]
- Haider, F.U.; Coulter, J.A.; Cai, L.; Hussain, S.; Cheema, S.A.; Wu, J.; Zhang, R. An Overview on Biochar Production, Its Implications, and Mechanisms of Biochar-Induced Amelioration of Soil and Plant Characteristics. Pedosphere 2022, 32, 107–130. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, E.; Khapre, A.; Bordoloi, N.; Kumar, S. Sorption of Volatile Organic Compounds on Non-Activated Biochar. Bioresour. Technol. 2020, 297, 122469. [Google Scholar] [CrossRef]
- Moharm, A.E.; El Naeem, G.A.; Soliman, H.M.A.; Abd-Elhamid, A.I.; El-Bardan, A.A.; Kassem, T.S.; Nayl, A.A.; Bräse, S. Fabrication and Characterization of Effective Biochar Biosorbent Derived from Agricultural Waste to Remove Cationic Dyes from Wastewater. Polymers 2022, 14, 2587. [Google Scholar] [CrossRef]
- Yaashikaa, P.R.; Kumar, P.S.; Varjani, S.; Saravanan, A. A Critical Review on the Biochar Production Techniques, Characterization, Stability and Applications for Circular Bioeconomy. Biotechnol. Rep. 2020, 28, e00570. [Google Scholar] [CrossRef] [PubMed]
- Dahlquist, E. Technologies for Converting Biomass to Useful Energy: Combustion, Gasification, Pyrolysis, Torrefaction and Fermentation; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Basu, P. Biomass Gasification and Pyrolysis: Practical Design and Theory; Academic Press: Cambridge, MA, USA, 2010. [Google Scholar]
- Nizamuddin, S.; Baloch, H.A.; Griffin, G.J.; Mubarak, N.M.; Bhutto, A.W.; Abro, R.; Mazari, S.A.; Ali, B.S. An Overview of Effect of Process Parameters on Hydrothermal Carbonization of Biomass. Renew. Sustain. Energy Rev. 2017, 73, 1289–1299. [Google Scholar] [CrossRef]
- dos Santos, J.V.; Fregolente, L.G.; Laranja, M.J.; Moreira, A.B.; Ferreira, O.P.; Bisinoti, M.C. Hydrothermal Carbonization of Sugarcane Industry By-Products and Process Water Reuse: Structural, Morphological, and Fuel Properties of Hydrochars. Biomass Convers. Biorefin. 2022, 12, 153–161. [Google Scholar] [CrossRef]
- Briongos, J.V.; Taramona, S.; Gómez-Hernández, J.; Mulone, V.; Santana, D. Solar and Biomass Hybridization through Hydrothermal Carbonization. Renew. Energy 2021, 177, 268–279. [Google Scholar] [CrossRef]
- Raheem, A.; Zhao, M.; Dastyar, W.; Channa, A.Q.; Ji, G.; Zhang, Y. Parametric Gasification Process of Sugarcane Bagasse for Syngas Production. Int. J. Hydrogen Energy 2019, 44, 16234–16247. [Google Scholar] [CrossRef]
- Cao, W.; Guo, L.; Yan, X.; Zhang, D.; Yao, X. Assessment of Sugarcane Bagasse Gasification in Supercritical Water for Hydrogen Production. Int. J. Hydrogen Energy 2018, 43, 13711–13719. [Google Scholar] [CrossRef]
- Higman, C.; van der Burgt, M.; Higman, C.; van der Burgt, M. Chapter 3—The Kinetics of Gasification and Reactor Theory. In Gasification; Gulf Professional Publishing: Burlington, NJ, USA, 2008; pp. 33–45. [Google Scholar]
- Niu, Y.; Lv, Y.; Lei, Y.; Liu, S.; Liang, Y.; Wang, D.; Hui, S. Biomass Torrefaction: Properties, Applications, Challenges, and Economy. Renew. Sustain. Energy Rev. 2019, 115, 109395. [Google Scholar] [CrossRef]
- Pimchuai, A.; Dutta, A.; Basu, P. Torrefaction of Agriculture Residue To Enhance Combustible Properties. Energy Fuels 2010, 24, 4638–4645. [Google Scholar] [CrossRef]
- Stegen, S.; Kaparaju, P. Effect of Temperature on Oil Quality Obtained through Pyrolysis of Sugarcane Bagasse. Fuel 2020, 276, 118112. [Google Scholar] [CrossRef]
- Guida, M.Y.; Hannioui, A. Properties of Bio-Oil and Bio-Char Produced by Sugar Cane Bagasse Pyrolysis in a Stainless Steel Tubular Reactor. Prog. Agric. Eng. Sci. 2017, 13, 13–33. [Google Scholar] [CrossRef]
- Amenaghawon, A.N.; Anyalewechi, C.L.; Okieimen, C.O.; Kusuma, H.S. Biomass Pyrolysis Technologies for Value-Added Products: A State-of-the-Art Review. Environ. Dev. Sustain. 2021, 23, 14324–14378. [Google Scholar] [CrossRef]
- Allohverdi, T.; Mohanty, A.K.; Roy, P.; Misra, M. A Review on Current Status of Biochar Uses in Agriculture. Molecules 2021, 26, 5584. [Google Scholar] [CrossRef] [PubMed]
- Oni, B.A.; Oziegbe, O.; Olawole, O.O. Significance of Biochar Application to the Environment and Economy. Ann. Agric. Sci. 2019, 64, 222–236. [Google Scholar] [CrossRef]
- Matos, T.T.S.; Fornari, M.R.; Mangrich, A.S.; Schultz, J.; Cardoso Batista, E.M.C.; Ribeiro, R.O.C.; Romão, L.P.C.; Yamamoto, C.I.; Grasel, F.S.; Bayer, C.; et al. Low Temperature Production of Biochars from Different Biomasses: Effect of Static and Rotary Lab Reactors and Application as Soil Conditioners. J. Environ. Chem. Eng. 2021, 9, 105472. [Google Scholar] [CrossRef]
- Wei, Z.; Wang, J.J.; Gaston, L.A.; Li, J.; Fultz, L.M.; DeLaune, R.D.; Dodla, S.K. Remediation of Crude Oil-Contaminated Coastal Marsh Soil: Integrated Effect of Biochar, Rhamnolipid Biosurfactant and Nitrogen Application. J. Hazard. Mater. 2020, 396, 122595. [Google Scholar] [CrossRef] [PubMed]
- Wuri, M.A.; Pertiwiningrum, A.; Budiarto, R.; Gozan, M.; Harto, A.W. The Waste Recycling of Sugarcane Bagasse-Based Biochar for Biogas Purification. IOP Conf. Ser. Earth Environ. Sci. 2021, 940, 012029. [Google Scholar] [CrossRef]
- Bundschuh, J.; Kaczmarczyk, M.; Ghaffour, N.; Tomaszewska, B. State-of-the-Art of Renewable Energy Sources Used in Water Desalination: Present and Future Prospects. Desalination 2021, 508, 115035. [Google Scholar] [CrossRef]
- Tang, Y.-H.; Liu, S.-H.; Tsang, D.C.W. Microwave-Assisted Production of CO2-Activated Biochar from Sugarcane Bagasse for Electrochemical Desalination. J. Hazard. Mater. 2020, 383, 121192. [Google Scholar] [CrossRef]
- Valenga, M.G.P.; Martins, G.; Martins, T.A.C.; Didek, L.K.; Gevaerd, A.; Marcolino-Junior, L.H.; Bergamini, M.F. Biochar: An Environmentally Friendly Platform for Construction of a SARS-CoV-2 Electrochemical Immunosensor. Sci. Total Environ. 2023, 858, 159797. [Google Scholar] [CrossRef]
- Iftikhar, M.; Asghar, A.; Ramzan, N.; Sajjadi, B.; Chen, W. Biomass Densification: Effect of Cow Dung on the Physicochemical Properties of Wheat Straw and Rice Husk Based Biomass Pellets. Biomass Bioenergy 2019, 122, 1–16. [Google Scholar] [CrossRef]
- Bot, B.V.; Sosso, O.T.; Tamba, J.G.; Lekane, E.; Bikai, J.; Ndame, M.K. Preparation and Characterization of Biomass Briquettes Made from Banana Peels, Sugarcane Bagasse, Coconut Shells and Rattan Waste. Biomass Convers. Biorefin. 2023, 13, 7937–7946. [Google Scholar] [CrossRef]
- Gautam, N.; Chaurasia, A. Study on Kinetics and Bio-Oil Production from Rice Husk, Rice Straw, Bamboo, Sugarcane Bagasse and Neem Bark in a Fixed-Bed Pyrolysis Process. Energy 2020, 190, 116434. [Google Scholar] [CrossRef]
- Sagani, A.; Hagidimitriou, M.; Dedoussis, V. Perennial Tree Pruning Biomass Waste Exploitation for Electricity Generation: The Perspective of Greece. Sustain. Energy Technol. Assess. 2019, 31, 77–85. [Google Scholar] [CrossRef]
- Antar, M.; Lyu, D.; Nazari, M.; Shah, A.; Zhou, X.; Smith, D.L. Biomass for a Sustainable Bioeconomy: An Overview of World Biomass Production and Utilization. Renew. Sustain. Energy Rev. 2021, 139, 110691. [Google Scholar] [CrossRef]
- Silva, D.A.L.; Filleti, R.A.P.; Musule, R.; Matheus, T.T.; Freire, F. A Systematic Review and Life Cycle Assessment of Biomass Pellets and Briquettes Production in Latin America. Renew. Sustain. Energy Rev. 2022, 157, 112042. [Google Scholar] [CrossRef]
- Nunes, L.J.R.; Causer, T.P.; Ciolkosz, D. Biomass for Energy: A Review on Supply Chain Management Models. Renew. Sustain. Energy Rev. 2020, 120, 109658. [Google Scholar] [CrossRef]
- Albashabsheh, N.T.; Heier Stamm, J.L. Optimization of Lignocellulosic Biomass-to-Biofuel Supply Chains with Densification: Literature Review. Biomass Bioenergy 2021, 144, 105888. [Google Scholar] [CrossRef]
- Martinez-Valencia, L.; Camenzind, D.; Wigmosta, M.; Garcia-Perez, M.; Wolcott, M. Biomass Supply Chain Equipment for Renewable Fuels Production: A Review. Biomass Bioenergy 2021, 148, 106054. [Google Scholar] [CrossRef]
- Varshney, D.; Mandade, P.; Shastri, Y. Multi-Objective Optimization of Sugarcane Bagasse Utilization in an Indian Sugar Mill. Sustain. Prod. Consum. 2019, 18, 96–114. [Google Scholar] [CrossRef]
- Lee, J.S.; Sokhansanj, S.; Lau, A.K.; Lim, C.J. Heats of Wetting and Sorption for Wood Pellets. Biomass Bioenergy 2020, 142, 105791. [Google Scholar] [CrossRef]
- Alan Sherrard Pellets Remain a Competitive Renewable Alternative. Available online: https://bioenergyinternational.com/pellets-remain-a-competitive-renewable-alternative/ (accessed on 2 March 2024).
- Setter, C.; Costa, K.L.S.; de Oliveira, T.J.P.; Mendes, R.F. The Effects of Kraft Lignin on the Physicomechanical Quality of Briquettes Produced with Sugarcane Bagasse and on the Characteristics of the Bio-Oil Obtained via Slow Pyrolysis. Fuel Process. Technol. 2020, 210, 106561. [Google Scholar] [CrossRef]
- Varma, A.K.; Mondal, P. Pyrolysis of Sugarcane Bagasse in Semi Batch Reactor: Effects of Process Parameters on Product Yields and Characterization of Products. Ind. Crops Prod. 2017, 95, 704–717. [Google Scholar] [CrossRef]
- Scatolino, M.V.; Neto, L.F.C.; de Paula Protásio, T.; de Cássia Oliveira Carneiro, A.; Andrade, C.R.; Guimarães Júnior, J.B.; Mendes, L.M. Options for Generation of Sustainable Energy: Production of Pellets Based on Combinations Between Lignocellulosic Biomasses. Waste Biomass Valoriz. 2018, 9, 479–489. [Google Scholar] [CrossRef]
- Sarker, T.R.; Nanda, S.; Meda, V.; Dalai, A.K. Densification of Waste Biomass for Manufacturing Solid Biofuel Pellets: A Review. Environ. Chem. Lett. 2023, 21, 231–264. [Google Scholar] [CrossRef]
- Chen, X.; Liang, J.; Liao, P.; Huang, W.; He, J.; Chen, J. Effect of Process Parameters and Raw Material Characteristics on the Physical and Mechanical Quality of Sugarcane Bagasse Pellets. Biomass Bioenergy 2021, 154, 106242. [Google Scholar] [CrossRef]
- da Silva, S.B.; Arantes, M.D.C.; de Andrade, J.K.B.; Andrade, C.R.; Carneiro, A.d.C.O.; Protásio, T.d.P. Influence of Physical and Chemical Compositions on the Properties and Energy Use of Lignocellulosic Biomass Pellets in Brazil. Renew. Energy 2020, 147, 1870–1879. [Google Scholar] [CrossRef]
- Akbar, A.; Aslam, U.; Asghar, A.; Aslam, Z. Effect of Binding Materials on Physical and Fuel Characteristics of Bagasse Based Pellets. Biomass Bioenergy 2021, 150, 106118. [Google Scholar] [CrossRef]
- de Almeida Moreira, B.R.; Barbosa Júnior, M.R.; de Brito Filho, A.L.; da Silva, R.P. Production of High-Quality Biogenic Fuels by Co-Pelletization of Sugarcane Bagasse with Pinewood Sawdust and Peanut Shell. Biomass Convers. Biorefin. 2024, 14, 6797–6820. [Google Scholar] [CrossRef]
- Santana, D.A.R.; Scatolino, M.V.; Lima, M.D.R.; de Oliveira Barros Junior, U.; Garcia, D.P.; Andrade, C.R.; de Cássia Oliveira Carneiro, A.; Trugilho, P.F.; de Paula Protásio, T. Pelletizing of Lignocellulosic Wastes as an Environmentally Friendly Solution for the Energy Supply: Insights on the Properties of Pellets from Brazilian Biomasses. Environ. Sci. Pollut. Res. 2021, 28, 11598–11617. [Google Scholar] [CrossRef]
- Kulkarni, R.M.; Dhanyashree, J.K.; Varma, E.; Sirivibha, S.P. Batch and Continuous Packed Bed Column Studies on Biosorption of Nickel (II) by Sugarcane Bagasse. Results Chem. 2022, 4, 100328. [Google Scholar] [CrossRef]
- Promnuan, K.; Sittijunda, S.; Reungsang, A. Evaluation of Commercial Moving Bed Media and Sugarcane Bagasse as Packing Material in Biotrickling Filter for Hydrogen Sulfide Removal. Bioresour. Technol. 2023, 388, 129788. [Google Scholar] [CrossRef]
- Shahbaz, M.; AlNouss, A.; Ghiat, I.; Mckay, G.; Mackey, H.; Elkhalifa, S.; Al-Ansari, T. A Comprehensive Review of Biomass Based Thermochemical Conversion Technologies Integrated with CO2 Capture and Utilisation within BECCS Networks. Resour Conserv. Recycl. 2021, 173, 105734. [Google Scholar] [CrossRef]
- Awan, A.T.; Tsukamoto, J.; Tasic, L. Orange Waste as a Biomass for 2G-Ethanol Production Using Low Cost Enzymes and Co-Culture Fermentation. RSC Adv. 2013, 3, 25071. [Google Scholar] [CrossRef]
- Pereira, S.C.; Maehara, L.; Machado, C.M.M.; Farinas, C.S. 2G Ethanol from the Whole Sugarcane Lignocellulosic Biomass. Biotechnol. Biofuels 2015, 8, 44. [Google Scholar] [CrossRef]
- Macrelli, S.; Mogensen, J.; Zacchi, G. Techno-Economic Evaluation of 2nd Generation Bioethanol Production from Sugar Cane Bagasse and Leaves Integrated with the Sugar-Based Ethanol Process. Biotechnol. Biofuels 2012, 5, 22. [Google Scholar] [CrossRef]
- Hans, M.; Lugani, Y.; Chandel, A.K.; Rai, R.; Kumar, S. Production of First- and Second-Generation Ethanol for Use in Alcohol-Based Hand Sanitizers and Disinfectants in India. Biomass Convers. Biorefin. 2023, 13, 7423–7440. [Google Scholar] [CrossRef]
- Oh, Y.-K.; Hwang, K.-R.; Kim, C.; Kim, J.R.; Lee, J.-S. Recent Developments and Key Barriers to Advanced Biofuels: A Short Review. Bioresour. Technol. 2018, 257, 320–333. [Google Scholar] [CrossRef]
- Raj, T.; Chandrasekhar, K.; Kumar, A.N.; Banu, J.R.; Yoon, J.-J.; Bhatia, S.K.; Yang, Y.-H.; Varjani, S.; Kim, S.-H. Recent Advances in Commercial Biorefineries for Lignocellulosic Ethanol Production: Current Status, Challenges and Future Perspectives. Bioresour. Technol. 2022, 344, 126292. [Google Scholar] [CrossRef]
- Vieira, S.; Barros, M.V.; Sydney, A.C.N.; Piekarski, C.M.; de Francisco, A.C.; Vandenberghe, L.P.d.S.; Sydney, E.B. Sustainability of Sugarcane Lignocellulosic Biomass Pretreatment for the Production of Bioethanol. Bioresour. Technol. 2020, 299, 122635. [Google Scholar] [CrossRef]
- Prajapati, B.P.; Jana, U.K.; Suryawanshi, R.K.; Kango, N. Sugarcane Bagasse Saccharification Using Aspergillus tubingensis Enzymatic Cocktail for 2G Bio-Ethanol Production. Renew. Energy 2020, 152, 653–663. [Google Scholar] [CrossRef]
- Vanmarcke, G.; Demeke, M.M.; Foulquié-Moreno, M.R.; Thevelein, J.M. Identification of the Major Fermentation Inhibitors of Recombinant 2G Yeasts in Diverse Lignocellulose Hydrolysates. Biotechnol. Biofuels 2021, 14, 92. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Wang, Z.; Cui, J.; Ferreira, L.F.R.; Bharagava, R.N.; Iqbal, H.M.N. Environmental Impact of Lignocellulosic Wastes and Their Effective Exploitation as Smart Carriers—A Drive towards Greener and Eco-Friendlier Biocatalytic Systems. Sci. Total Environ. 2020, 722, 137903. [Google Scholar] [CrossRef] [PubMed]
- Sjahro, N.; Yunus, R.; Abdullah, L.C.; Rashid, S.A.; Asis, A.J.; Akhlisah, Z.N. Recent Advances in the Application of Cellulose Derivatives for Removal of Contaminants from Aquatic Environments. Cellulose 2021, 28, 7521–7557. [Google Scholar] [CrossRef]
- Sayyed, A.J.; Pinjari, D.V.; Sonawane, S.H.; Bhanvase, B.A.; Sheikh, J.; Sillanpää, M. Cellulose-Based Nanomaterials for Water and Wastewater Treatments: A Review. J. Environ. Chem. Eng. 2021, 9, 106626. [Google Scholar] [CrossRef]
- Tanpichai, S.; Boonmahitthisud, A.; Soykeabkaew, N.; Ongthip, L. Review of the Recent Developments in All-Cellulose Nanocomposites: Properties and Applications. Carbohydr. Polym. 2022, 286, 119192. [Google Scholar] [CrossRef]
- Zhou, Y.; Lu, J.; Zhou, Y.; Liu, Y. Recent Advances for Dyes Removal Using Novel Adsorbents: A Review. Environ. Pollut. 2019, 252, 352–365. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, A.; Ullah, M.W.; Ali, J.; Aziz, K.; Javed, M.A.; Shi, Z.; Manan, S.; Ul-Islam, M.; Nazar, M.; Yang, G. The Versatility of Nanocellulose, Modification Strategies, and Its Current Progress in Wastewater Treatment and Environmental Remediation. Sci. Total Environ. 2023, 858, 159937. [Google Scholar] [CrossRef] [PubMed]
- Ramos, S.N.d.C.; Xavier, A.L.P.; Teodoro, F.S.; Elias, M.M.C.; Gonçalves, F.J.; Gil, L.F.; de Freitas, R.P.; Gurgel, L.V.A. Modeling Mono- and Multi-Component Adsorption of Cobalt(II), Copper(II), and Nickel(II) Metal Ions from Aqueous Solution onto a New Carboxylated Sugarcane Bagasse. Part I: Batch Adsorption Study. Ind. Crops Prod. 2015, 74, 357–371. [Google Scholar] [CrossRef]
- Chen, Z.; Pan, K. Enhanced Removal of Cr(VI) via in-Situ Synergistic Reduction and Fixation by Polypyrrole/Sugarcane Bagasse Composites. Chemosphere 2021, 272, 129606. [Google Scholar] [CrossRef]
- Sankararamakrishnan, N.; Shankhwar, A.; Chauhan, D. Mechanistic Insights on Immobilization and Decontamination of Hexavalent Chromium onto Nano MgS/FeS Doped Cellulose Nanofibres. Chemosphere 2019, 228, 390–397. [Google Scholar] [CrossRef]
- Guleria, A.; Kumari, G.; Lima, E.C. Cellulose-g-Poly-(Acrylamide-Co-Acrylic Acid) Polymeric Bioadsorbent for the Removal of Toxic Inorganic Pollutants from Wastewaters. Carbohydr. Polym. 2020, 228, 115396. [Google Scholar] [CrossRef]
- Montero, J.I.Z.; Monteiro, A.S.C.; Gontijo, E.S.J.; Bueno, C.C.; de Moraes, M.A.; Rosa, A.H. High Efficiency Removal of As(III) from Waters Using a New and Friendly Adsorbent Based on Sugarcane Bagasse and Corncob Husk Fe-Coated Biochars. Ecotoxicol. Environ. Saf. 2018, 162, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Zhang, M.; Niu, B.; Zhang, W.; Wang, X.; Wang, J.; Wu, D.; Wang, L.; Jiang, K. Rotten Sugarcane Bagasse Derived Biochars with Rich Mineral Residues for Effective Pb (II) Removal in Wastewater and the Tech-Economic Analysis. J. Taiwan Inst. Chem. Eng. 2022, 132, 104231. [Google Scholar] [CrossRef]
- Jiang, C.; Zhou, S.; Li, C.; Yue, F.; Zheng, L. Properties and Mechanism of Cr(VI) Removal by a ZnCl2-Modified Sugarcane Bagasse Biochar–Supported Nanoscale Iron Sulfide Composite. Environ. Sci. Pollut. Res. 2022, 30, 26889–26900. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Shao, J.; Li, Z.; Yang, H.; Zhang, S.; Chen, H. Nano Nickel Embedded in N-Doped CNTs-Supported Porous Biochar for Adsorption-Reduction of Hexavalent Chromium. J. Hazard. Mater. 2021, 416, 125693. [Google Scholar] [CrossRef] [PubMed]
- Kamran, U.; Bhatti, H.N.; Noreen, S.; Tahir, M.A.; Park, S.-J. Chemically Modified Sugarcane Bagasse-Based Biocomposites for Efficient Removal of Acid Red 1 Dye: Kinetics, Isotherms, Thermodynamics, and Desorption Studies. Chemosphere 2022, 291, 132796. [Google Scholar] [CrossRef]
- Noreen, S.; Bhatti, H.N.; Iqbal, M.; Hussain, F.; Sarim, F.M. Chitosan, Starch, Polyaniline and Polypyrrole Biocomposite with Sugarcane Bagasse for the Efficient Removal of Acid Black Dye. Int. J. Biol. Macromol. 2020, 147, 439–452. [Google Scholar] [CrossRef] [PubMed]
- Mpatani, F.M.; Aryee, A.A.; Kani, A.N.; Guo, Q.; Dovi, E.; Qu, L.; Li, Z.; Han, R. Uptake of Micropollutant-Bisphenol A, Methylene Blue and Neutral Red onto a Novel Bagasse-β-Cyclodextrin Polymer by Adsorption Process. Chemosphere 2020, 259, 127439. [Google Scholar] [CrossRef]
- Syafrudin, M.; Kristanti, R.A.; Yuniarto, A.; Hadibarata, T.; Rhee, J.; Al-onazi, W.A.; Algarni, T.S.; Almarri, A.H.; Al-Mohaimeed, A.M. Pesticides in Drinking Water—A Review. Int. J. Environ. Res. Public Health 2021, 18, 468. [Google Scholar] [CrossRef]
- Dey, S.; Bano, F.; Malik, A. Pharmaceuticals and Personal Care Product (PPCP) Contamination—A Global Discharge Inventory. In Pharmaceuticals and Personal Care Products: Waste Management and Treatment Technology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–26. [Google Scholar]
- Charuaud, L.; Jarde, E.; Jaffrezic, A.; Thomas, M.-F.; Le Bot, B. Veterinary Pharmaceutical Residues from Natural Water to Tap Water: Sales, Occurrence and Fate. J. Hazard. Mater. 2019, 361, 169–186. [Google Scholar] [CrossRef]
- Ahamad, T.; Naushad, M.; Ruksana; Alhabarah, A.N.; Alshehri, S.M. N/S Doped Highly Porous Magnetic Carbon Aerogel Derived from Sugarcane Bagasse Cellulose for the Removal of Bisphenol-A. Int. J. Biol. Macromol. 2019, 132, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Meneses, I.P.; Novaes, S.D.; Dezotti, R.S.; Oliveira, P.V.; Petri, D.F.S. CTAB-Modified Carboxymethyl Cellulose/Bagasse Cryogels for the Efficient Removal of Bisphenol A, Methylene Blue and Cr(VI) Ions: Batch and Column Adsorption Studies. J. Hazard. Mater. 2022, 421, 126804. [Google Scholar] [CrossRef] [PubMed]
- Freitas, J.V.; Farinas, C.S. Sugarcane Bagasse Fly Ash as a No-Cost Adsorbent for Removal of Phenolic Inhibitors and Improvement of Biomass Saccharification. ACS Sustain. Chem. Eng. 2017, 5, 11727–11736. [Google Scholar] [CrossRef]
- Han, J.; Zhang, L.; Zhao, B.; Qin, L.; Wang, Y.; Xing, F. The N-Doped Activated Carbon Derived from Sugarcane Bagasse for CO2 Adsorption. Ind. Crops Prod. 2019, 128, 290–297. [Google Scholar] [CrossRef]
- Komal; Gupta, K.; Kumar, V.; Tikoo, K.B.; Kaushik, A.; Singhal, S. Encrustation of Cadmium Sulfide Nanoparticles into the Matrix of Biomass Derived Silanized Cellulose Nanofibers for Adsorptive Detoxification of Pesticide and Textile Waste. Chem. Eng. J. 2020, 385, 123700. [Google Scholar] [CrossRef]
- Prasannamedha, G.; Kumar, P.S.; Mehala, R.; Sharumitha, T.J.; Surendhar, D. Enhanced Adsorptive Removal of Sulfamethoxazole from Water Using Biochar Derived from Hydrothermal Carbonization of Sugarcane Bagasse. J. Hazard. Mater. 2021, 407, 124825. [Google Scholar] [CrossRef] [PubMed]
- Abo El Naga, A.O.; El Saied, M.; Shaban, S.A.; El Kady, F.Y. Fast Removal of Diclofenac Sodium from Aqueous Solution Using Sugar Cane Bagasse-Derived Activated Carbon. J. Mol. Liq. 2019, 285, 9–19. [Google Scholar] [CrossRef]
- Hassan, M.; Du, J.; Liu, Y.; Naidu, R.; Zhang, J.; Ahsan, M.A.; Qi, F. Magnetic Biochar for Removal of Perfluorooctane Sulphonate (PFOS): Interfacial Interaction and Adsorption Mechanism. Environ. Technol. Innov. 2022, 28, 102593. [Google Scholar] [CrossRef]
- Qu, Y.; Xu, L.; Chen, Y.; Sun, S.; Wang, Y.; Guo, L. Efficient Toluene Adsorption/Desorption on Biochar Derived from in Situ Acid-Treated Sugarcane Bagasse. Environ. Sci. Pollut. Res. 2021, 28, 62616–62627. [Google Scholar] [CrossRef]
- Bezerra, W.F.d.P.; Dognani, G.; Alencar, L.N.d.; Parizi, M.P.S.; Boina, R.F.; Cabrera, F.C.; Job, A.E. Chemical Treatment of Sugarcane Bagasse and Its Influence on Glyphosate Adsorption. Matéria 2022, 27, e13142. [Google Scholar] [CrossRef]
- Ganesan, K.; Dennstedt, A.; Barowski, A.; Ratke, L. Design of Aerogels, Cryogels and Xerogels of Cellulose with Hierarchical Porous Structures. Mater. Des. 2016, 92, 345–355. [Google Scholar] [CrossRef]
- Groult, S.; Buwalda, S.; Budtova, T. Pectin Hydrogels, Aerogels, Cryogels and Xerogels: Influence of Drying on Structural and Release Properties. Eur. Polym. J. 2021, 149, 110386. [Google Scholar] [CrossRef]
- Pan, Y.; Shi, X.; Cai, P.; Guo, T.; Tong, Z.; Xiao, H. Dye Removal from Single and Binary Systems Using Gel-like Bioadsorbent Based on Functional-Modified Cellulose. Cellulose 2018, 25, 2559–2575. [Google Scholar] [CrossRef]
- Adio, S.O.; Ganiyu, S.A.; Usman, M.; Abdulazeez, I.; Alhooshani, K. Facile and Efficient Nitrogen Modified Porous Carbon Derived from Sugarcane Bagasse for CO2 Capture: Experimental and DFT Investigation of Nitrogen Atoms on Carbon Frameworks. Chem. Eng. J. 2020, 382, 122964. [Google Scholar] [CrossRef]
- Alokika; Anu; Kumar, A.; Kumar, V.; Singh, B. Cellulosic and Hemicellulosic Fractions of Sugarcane Bagasse: Potential, Challenges and Future Perspective. Int. J. Biol. Macromol. 2021, 169, 564–582. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.; Heo, S.; Foo, M.; Chew, I.M.; Yoo, C. An Insight into Nanocellulose as Soft Condensed Matter: Challenge and Future Prospective toward Environmental Sustainability. Sci. Total Environ. 2019, 650, 1309–1326. [Google Scholar] [CrossRef] [PubMed]
- Syeda, H.I.; Yap, P.-S. A Review on Three-Dimensional Cellulose-Based Aerogels for the Removal of Heavy Metals from Water. Sci. Total Environ. 2022, 807, 150606. [Google Scholar] [CrossRef]
- Dotto, G.L.; McKay, G. Current Scenario and Challenges in Adsorption for Water Treatment. J. Environ. Chem. Eng. 2020, 8, 103988. [Google Scholar] [CrossRef]
- Nasir, A.M.; Goh, P.S.; Abdullah, M.S.; Ng, B.C.; Ismail, A.F. Adsorptive Nanocomposite Membranes for Heavy Metal Remediation: Recent Progresses and Challenges. Chemosphere 2019, 232, 96–112. [Google Scholar] [CrossRef]
- Hamilton, I.; Rapf, O.; Kockat, D.J.; Zuhaib, D.S.; Abergel, T.; Oppermann, M.; Otto, M.; Loran, S.; Fagotto, I.; Steurer, N.; et al. Global Status Report for Buildings and Construction; United Nations Environmental Programme: Nairobi, Kenya, 2020. [Google Scholar]
- Molin Filho, R.G.D.; Longhi, D.A.; de Souza, R.C.T.; Ferrer, M.M.; Vanderlei, R.D.; Paraíso, P.R.; Jorge, L.M.d.M. Self-Compacting Mortar with Sugarcane Bagasse Ash: Development of a Sustainable Alternative for Brazilian Civil Construction. Environ. Dev. Sustain. 2019, 21, 2125–2143. [Google Scholar] [CrossRef]
- Nguyen, H.; Jamali Moghadam, M.; Moayedi, H. Agricultural Wastes Preparation, Management, and Applications in Civil Engineering: A Review. J. Mater. Cycles Waste Manag. 2019, 21, 1039–1051. [Google Scholar] [CrossRef]
- Juenger, M.C.G.; Snellings, R.; Bernal, S.A. Supplementary Cementitious Materials: New Sources, Characterization, and Performance Insights. Cem. Concr. Res. 2019, 122, 257–273. [Google Scholar] [CrossRef]
- Ribeiro, B.; Yamashiki, Y.; Yamamoto, T. A Study on Mechanical Properties of Mortar with Sugarcane Bagasse Fiber and Bagasse Ash. J. Mater. Cycles Waste Manag. 2020, 22, 1844–1851. [Google Scholar] [CrossRef]
- Torres, S.M.; de Lima, V.E.; de Azevedo Basto, P.; de Araújo Júnior, N.T.; de Melo Neto, A.A. Assessing the Pozzolanic Activity of Sugarcane Bagasse Ash Using X-Ray Diffraction. Constr. Build. Mater. 2020, 264, 120684. [Google Scholar] [CrossRef]
- Cláudia dos Santos, A.; Cardoso, F.G.; da Silva, R.J.; de Fátima Gorgulho, H.; Panzera, T.H. Modification of Short Sugarcane Bagasse Fibres for Application in Cementitious Composites: A Statistical Approach to Mechanical and Physical Properties. Constr. Build. Mater. 2022, 353, 129072. [Google Scholar] [CrossRef]
- Hernández-Olivares, F.; Medina-Alvarado, R.E.; Burneo-Valdivieso, X.E.; Zúñiga-Suárez, A.R. Short Sugarcane Bagasse Fibers Cementitious Composites for Building Construction. Constr. Build. Mater. 2020, 247, 118451. [Google Scholar] [CrossRef]
- Ramlee, N.A.; Naveen, J.; Jawaid, M. Potential of Oil Palm Empty Fruit Bunch (OPEFB) and Sugarcane Bagasse Fibers for Thermal Insulation Application—A Review. Constr. Build. Mater. 2021, 271, 121519. [Google Scholar] [CrossRef]
- Mehrzad, S.; Taban, E.; Soltani, P.; Samaei, S.E.; Khavanin, A. Sugarcane Bagasse Waste Fibers as Novel Thermal Insulation and Sound-Absorbing Materials for Application in Sustainable Buildings. Build. Environ. 2022, 211, 108753. [Google Scholar] [CrossRef]
- Quedou, P.G.; Wirquin, E.; Bokhoree, C. Sustainable Concrete: Potency of Sugarcane Bagasse Ash as a Cementitious Material in the Construction Industry. Case Stud. Constr. Mater. 2021, 14, e00545. [Google Scholar] [CrossRef]
- Li, Q.; Ma, C.-L.; Zhang, P.-Q.; Li, Y.-Y.; Zhu, X.; He, Y.-C. Effective Conversion of Sugarcane Bagasse to Furfural by Coconut Shell Activated Carbon-Based Solid Acid for Enhancing Whole-Cell Biosynthesis of Furfurylamine. Ind. Crops Prod. 2021, 160, 113169. [Google Scholar] [CrossRef]
- de Sande, V.T.; Sadique, M.; Bras, A.; Pineda, P. Activated Sugarcane Bagasse Ash as Efficient Admixture in Cement-Based Mortars: Mechanical and Durability Improvements. J. Build. Eng. 2022, 59, 105082. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Kroehong, W.; Damrongwiriyanupap, N.; Suriyo, W.; Jaturapitakkul, C. Mechanical Properties, Chloride Resistance and Microstructure of Portland Fly Ash Cement Concrete Containing High Volume Bagasse Ash. J. Build. Eng. 2020, 31, 101415. [Google Scholar] [CrossRef]
- Akarsh, P.K.; Ganesh, G.O.; Marathe, S.; Rai, R. Incorporation of Sugarcane Bagasse Ash to Investigate the Mechanical Behavior of Stone Mastic Asphalt. Constr. Build. Mater. 2022, 353, 129089. [Google Scholar] [CrossRef]
- Perez-Diaz, E.D.; Reyes-Araiza, J.L.; Millán-Malo, B.M.; Londoño-Restrepo, S.M.; Rodríguez-Garcia, M.E. Evaluation of Bamboo Cortex Ash as Supplementary Cementitious Material: Comparative Analysis with Sugarcane Bagasse Ash and Natural Pozzolan. J. Build. Eng. 2023, 66, 105846. [Google Scholar] [CrossRef]
- Minnu, S.N.; Bahurudeen, A.; Athira, G. Comparison of Sugarcane Bagasse Ash with Fly Ash and Slag: An Approach towards Industrial Acceptance of Sugar Industry Waste in Cleaner Production of Cement. J. Clean. Prod. 2021, 285, 124836. [Google Scholar] [CrossRef]
- Amin, M.; Attia, M.M.; Agwa, I.S.; Elsakhawy, Y.; El-hassan, K.A.; Abdelsalam, B.A. Effects of Sugarcane Bagasse Ash and Nano Eggshell Powder on High-Strength Concrete Properties. Case Stud. Constr. Mater. 2022, 17, e01528. [Google Scholar] [CrossRef]
- Chuewangkam, N.; Nachaithong, T.; Chanlek, N.; Thongbai, P.; Pinitsoontorn, S. Mechanical and Dielectric Properties of Fly Ash Geopolymer/Sugarcane Bagasse Ash Composites. Polymers 2022, 14, 1140. [Google Scholar] [CrossRef]
- Yadav, A.L.; Sairam, V.; Srinivasan, K.; Muruganandam, L. Synthesis and Characterization of Geopolymer from Metakaolin and Sugarcane Bagasse Ash. Constr. Build. Mater. 2020, 258, 119231. [Google Scholar] [CrossRef]
- Akbar, A.; Farooq, F.; Shafique, M.; Aslam, F.; Alyousef, R.; Alabduljabbar, H. Sugarcane Bagasse Ash-Based Engineered Geopolymer Mortar Incorporating Propylene Fibers. J. Build. Eng. 2021, 33, 101492. [Google Scholar] [CrossRef]
- Marichelvam, M.K.; Manimaran, P.; Verma, A.; Sanjay, M.R.; Siengchin, S.; Kandakodeeswaran, K.; Geetha, M. A Novel Palm Sheath and Sugarcane Bagasse Fiber Based Hybrid Composites for Automotive Applications: An Experimental Approach. Polym. Compos. 2021, 42, 512–521. [Google Scholar] [CrossRef]
- Ramlee, N.A.; Jawaid, M.; Yamani, S.A.K.; Zainudin, E.S.; Alamery, S. Effect of Surface Treatment on Mechanical, Physical and Morphological Properties of Oil Palm/Bagasse Fiber Reinforced Phenolic Hybrid Composites for Wall Thermal Insulation Application. Constr. Build. Mater. 2021, 276, 122239. [Google Scholar] [CrossRef]
- Guna, V.; Ilangovan, M.; Hu, C.; Venkatesh, K.; Reddy, N. Valorization of Sugarcane Bagasse by Developing Completely Biodegradable Composites for Industrial Applications. Ind. Crops Prod. 2019, 131, 25–31. [Google Scholar] [CrossRef]
- de Paiva, F.F.G.; de Maria, V.P.K.; Torres, G.B.; Dognani, G.; dos Santos, R.J.; Cabrera, F.C.; Job, A.E. Sugarcane Bagasse Fiber as Semi-Reinforcement Filler in Natural Rubber Composite Sandals. J. Mater. Cycles Waste Manag. 2019, 21, 326–335. [Google Scholar] [CrossRef]
- Monteiro, S.N.; Candido, V.S.; Braga, F.O.; Bolzan, L.T.; Weber, R.P.; Drelich, J.W. Sugarcane Bagasse Waste in Composites for Multilayered Armor. Eur. Polym. J. 2016, 78, 173–185. [Google Scholar] [CrossRef]
- Bam, S.A.; Gundu, D.T.; Onu, F.A. The Effect of Chemical Treatments on the Mechanical and Physical Properties of Bagasse Filler Reinforced Low Density Polyethylene Composite. Am. J. Eng. Res. 2019, 8, 95–98. [Google Scholar]
- EL-Zayat, M.M.; Abdel-Hakim, A.; Mohamed, M.A. Effect of Gamma Radiation on the Physico Mechanical Properties of Recycled HDPE/Modified Sugarcane Bagasse Composite. J. Macromol. Sci. Part A 2019, 56, 127–135. [Google Scholar] [CrossRef]
- Sumarji; Sholahuddin, I.; Laksana, D.D.; Syuhri, A.; Aditya, Y. Effect of Steam Pre-Treatment of Bagasse as Fiber Reinforcement SCG Composite. IOP Conf. Ser. Mater. Sci. Eng. 2019, 494, 012042. [Google Scholar] [CrossRef]
- Ramlee, N.A.; Jawaid, M.; Zainudin, E.S.; Yamani, S.A.K. Modification of Oil Palm Empty Fruit Bunch and Sugarcane Bagasse Biomass as Potential Reinforcement for Composites Panel and Thermal Insulation Materials. J. Bionic Eng. 2019, 16, 175–188. [Google Scholar] [CrossRef]
- Abedom, F.; Sakthivel, S.; Asfaw, D.; Melese, B.; Solomon, E.; Kumar, S.S. Development of Natural Fiber Hybrid Composites Using Sugarcane Bagasse and Bamboo Charcoal for Automotive Thermal Insulation Materials. Adv. Mater. Sci. Eng. 2021, 2021, 2508840. [Google Scholar] [CrossRef]
- dos Santos, B.H.; de Souza do Prado, K.; Jacinto, A.A.; da Silva Spinacé, M.A. Influence of Sugarcane Bagasse Fiber Size on Biodegradable Composites of Thermoplastic Starch. J. Renew. Mater. 2018, 6, 176–182. [Google Scholar] [CrossRef]
- Fischer, W.N. Manual Do PFI—Testing and Research Institute for Footwear Production; PFI: Pirmasens, Germany, 1987. [Google Scholar]
- Nakanishi, E.Y.; Cabral, M.R.; Gonçalves, P.d.S.; dos Santos, V.; Savastano Junior, H. Formaldehyde-Free Particleboards Using Natural Latex as the Polymeric Binder. J. Clean. Prod. 2018, 195, 1259–1269. [Google Scholar] [CrossRef]
- Liu, H.; He, H.; Peng, X.; Huang, B.; Li, J. Three-dimensional Printing of Poly(Lactic Acid) Bio-based Composites with Sugarcane Bagasse Fiber: Effect of Printing Orientation on Tensile Performance. Polym. Adv. Technol. 2019, 30, 910–922. [Google Scholar] [CrossRef]
- de Soares, M.M.N.S.; Garcia, D.C.S.; Figueiredo, R.B.; Aguilar, M.T.P.; Cetlin, P.R. Comparing the Pozzolanic Behavior of Sugar Cane Bagasse Ash to Amorphous and Crystalline SiO2. Cem. Concr. Compos. 2016, 71, 20–25. [Google Scholar] [CrossRef]
- Kanking, S.; Niltui, P.; Wimolmala, E.; Sombatsompop, N. Use of Bagasse Fiber Ash as Secondary Filler in Silica or Carbon Black Filled Natural Rubber Compound. Mater. Des. 2012, 41, 74–82. [Google Scholar] [CrossRef]
- dos Santos, R.J.; Agostini, D.L.d.S.; Cabrera, F.C.; dos Reis, E.A.P.; Ruiz, M.R.; Budemberg, E.R.; Teixeira, S.R.; Job, A.E. Sugarcane Bagasse Ash: New Filler to Natural Rubber Composite. Polímeros 2014, 24, 646–653. [Google Scholar] [CrossRef]
- Chandrika, V.S.; Anamika, A.; Jeeva, C.; Perumal, B.; Kumar, S.S.; Roseline, J.F.; Raghavan, I.K. Natural Fiber Incorporated Polymer Matrix Composites for Electronic Circuit Board Applications. Adv. Mater. Sci. Eng. 2022, 2022, 3035169. [Google Scholar] [CrossRef]
- Balachandran, G.B.; David, P.W.; Alexander, A.B.; Mariappan, R.K.; Balasundar, P.; Parrthipan, B.K.; Saravanakumar, S.S.; Kannan, P.S. Saccharum barberi Grass Bagasse Ash-Based Silicone Rubber Composites for Electrical Insulator Applications. Iran. Polym. J. 2021, 30, 1285–1296. [Google Scholar] [CrossRef]
- Huabcharoen, P.; Wimolmala, E.; Markpin, T.; Sombatsompop, N. Purification and Characterization of Silica from Sugarcane Bagasse Ash as a Reinforcing Filler in Natural Rubber Composites. Bioresources 2017, 12, 1228–1245. [Google Scholar] [CrossRef]
- Boonmee, A.; Jarukumjorn, K. Preparation and Characterization of Silica Nanoparticles from Sugarcane Bagasse Ash for Using as a Filler in Natural Rubber Composites. Polym. Bull. 2020, 77, 3457–3472. [Google Scholar] [CrossRef]
- Sharma, P.; Prakash, J.; Kaushal, R. An Insight into the Green Synthesis of SiO2 Nanostructures as a Novel Adsorbent for Removal of Toxic Water Pollutants. Environ. Res. 2022, 212, 113328. [Google Scholar] [CrossRef]
- Seroka, N.S.; Taziwa, R.T.; Khotseng, L. Extraction and Synthesis of Silicon Nanoparticles (SiNPs) from Sugarcane Bagasse Ash: A Mini-Review. Appl. Sci. 2022, 12, 2310. [Google Scholar] [CrossRef]
- Ifijen, I.H.; Itua, A.B.; Maliki, M.; Ize-Iyamu, C.O.; Omorogbe, S.O.; Aigbodion, A.I.; Ikhuoria, E.U. The Removal of Nickel and Lead Ions from Aqueous Solutions Using Green Synthesized Silica Microparticles. Heliyon 2020, 6, e04907. [Google Scholar] [CrossRef]
- September, L.A.; Kheswa, N.; Seroka, N.S.; Khotseng, L. Green Synthesis of Silica and Silicon from Agricultural Residue Sugarcane Bagasse Ash—A Mini Review. RSC Adv. 2023, 13, 1370–1380. [Google Scholar] [CrossRef]
- Negash, Y.; Tolosa, B.; Bantewesen, T.; Bekele, G. ASTU Antibacterial Activities of the CuO/ZnO Nanocomposite Grown on Silica Extracted from Bagasse Ash. Ethiop. J. Sci. Sustain. Dev. 2020, 8, 2021. [Google Scholar] [CrossRef]
- Hikmah, N.; Agustiningsih, D.; Nuryono, N.; Kunarti, E.S. Preparation of Iron-Doped SiO2/TiO2 Using Silica from Sugarcane Bagasse Ash for Visible Light Degradation of Congo Red. Indones. J. Chem. 2021, 22, 402. [Google Scholar] [CrossRef]
- Hendra Saputera, W.; Egiyawati, C.; Setyani Putrie, A.; Fathoni Amri, A.; Rizkiana, J.; Sasongko, D. Titania Modified Silica from Sugarcane Bagasse Waste for Photocatalytic Wastewater Treatment. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1143, 012073. [Google Scholar] [CrossRef]
- Goswami, P.; Mathur, J. Application of Agro-Waste-Mediated Silica Nanoparticles to Sustainable Agriculture. Bioresour Bioprocess 2022, 9, 9. [Google Scholar] [CrossRef]
- Natarajan, S.; Subramaniyam, S.T.; Kumaravel, V. Fabrication of Hydrophobic Coatings Using Sugarcane Bagasse Waste Ash as Silica Source. Appl. Sci. 2019, 9, 190. [Google Scholar] [CrossRef]
- Saed, B.; Ziaee, M.; Kiasat, A.; Jafari Nasab, M. Evaluation of Iranian Diatomaceous Earth in Combination with Nanosilica from Sugarcane Bagasse Ash Applied on Three Different Storage Surfaces against Two Insect Pests of Stored Products. Int. J. Trop. Insect Sci. 2021, 41, 1747–1752. [Google Scholar] [CrossRef]
- Mulyati, S.; Muchtar, S.; Yusuf, M.; Arahman, N.; Sofyana, S.; Rosnelly, C.M.; Fathanah, U.; Takagi, R.; Matsuyama, H.; Shamsuddin, N.; et al. Production of High Flux Poly(Ether Sulfone) Membrane Using Silica Additive Extracted from Natural Resource. Membranes 2020, 10, 17. [Google Scholar] [CrossRef]
- Singh, R.V.; Sharma, P.; Sambyal, K. Application of Sugarcane Bagasse in Chemicals and Food Packaging Industry: Potential and Challenges. Circ. Econ. Sustain. 2022, 2, 1479–1500. [Google Scholar] [CrossRef]
- Kurian, M.; Paul, A. Recent Trends in the Use of Green Sources for Carbon Dot Synthesis–A Short Review. Carbon Trends 2021, 3, 100032. [Google Scholar] [CrossRef]
- Kasinathan, K.; Samayanan, S.; Marimuthu, K.; Yim, J.-H. Green Synthesis of Multicolour Fluorescence Carbon Quantum Dots from Sugarcane Waste: Investigation of Mercury (II) Ion Sensing, and Bio-Imaging Applications. Appl. Surf. Sci. 2022, 601, 154266. [Google Scholar] [CrossRef]
- Qiu, Y.; Li, D.; Li, Y.; Ma, X.; Li, J. Green Carbon Quantum Dots from Sustainable Lignocellulosic Biomass and Its Application in the Detection of Fe3+. Cellulose 2022, 29, 367–378. [Google Scholar] [CrossRef]
- Alfi, A.A.; Alamrani, N.A.; Azher, O.A.; Snari, R.M.; Abumelha, H.M.; Al-Ahmed, Z.A.; El-Metwaly, N.M. Development of Carbon Dots Sensor Dipstick from Sugarcane Bagasse Agricultural Waste toward All-Cellulose-Derived Tetracycline Sensor. J. Mater. Res. Technol. 2022, 19, 4697–4707. [Google Scholar] [CrossRef]
- Jiang, B.; Zhou, B.; Shen, X.; Yu, Y.; Ji, S.; Wen, C.; Liang, H. Selective Probing of Gaseous Ammonia Using Red-Emitting Carbon Dots Based on an Interfacial Response Mechanism. Chem. Eur. J. 2015, 21, 18993–18999. [Google Scholar] [CrossRef]
- Pandiyan, S.; Arumugam, L.; Srirengan, S.P.; Pitchan, R.; Sevugan, P.; Kannan, K.; Pitchan, G.; Hegde, T.A.; Gandhirajan, V. Biocompatible Carbon Quantum Dots Derived from Sugarcane Industrial Wastes for Effective Nonlinear Optical Behavior and Antimicrobial Activity Applications. ACS Omega 2020, 5, 30363–30372. [Google Scholar] [CrossRef]
- Chung, H.K.; Wongso, V.; Sambudi, N.S. Isnaeni Biowaste-Derived Carbon Dots/Hydroxyapatite Nanocomposite as Drug Delivery Vehicle for Acetaminophen. J. Solgel Sci. Technol. 2020, 93, 214–223. [Google Scholar] [CrossRef]
- Hesam, F.; Tarzi, B.G.; Honarvar, M.; Jahadi, M. Valorization of Sugarcane Bagasse to High Value-Added Xylooligosaccharides and Evaluation of Their Prebiotic Function in a Synbiotic Pomegranate Juice. Biomass Convers. Biorefin. 2023, 13, 787–799. [Google Scholar] [CrossRef]
- Eslami, A.; Borghei, S.M.; Rashidi, A.; Takdastan, A. Preparation of Activated Carbon Dots from Sugarcane Bagasse for Naphthalene Removal from Aqueous Solutions. Sep. Sci. Technol. 2018, 53, 2536–2549. [Google Scholar] [CrossRef]
- Nugraha, M.W.; Zainal Abidin, N.H.; Supandi; Sambudi, N.S. Synthesis of Tungsten Oxide/ Amino-Functionalized Sugarcane Bagasse Derived-Carbon Quantum Dots (WO3/N-CQDs) Composites for Methylene Blue Removal. Chemosphere 2021, 277, 130300. [Google Scholar] [CrossRef] [PubMed]
- So, S.; Cherdthong, A.; Wanapat, M. Improving Sugarcane Bagasse Quality as Ruminant Feed with Lactobacillus, Cellulase, and Molasses. J. Anim. Sci. Technol. 2020, 62, 648–658. [Google Scholar] [CrossRef] [PubMed]
- Lumsangkul, C.; Tapingkae, W.; Sringarm, K.; Jaturasitha, S.; Le Xuan, C.; Wannavijit, S.; Outama, P.; Van Doan, H. Effect of Dietary Sugarcane Bagasse Supplementation on Growth Performance, Immune Response, and Immune and Antioxidant-Related Gene Expressions of Nile Tilapia (Oreochromis niloticus) Cultured under Biofloc System. Animals 2021, 11, 2035. [Google Scholar] [CrossRef] [PubMed]
- Afrazeh, M.; Tadayoni, M.; Abbasi, H.; Sheikhi, A. Extraction of Dietary Fibers from Bagasse and Date Seed, and Evaluation of Their Technological Properties and Antioxidant and Prebiotic Activity. J. Food Meas. Charact. 2021, 15, 1949–1959. [Google Scholar] [CrossRef]
- Gil-López, D.I.L.; Lois-Correa, J.A.; Sánchez-Pardo, M.E.; Domínguez-Crespo, M.A.; Torres-Huerta, A.M.; Rodríguez-Salazar, A.E.; Orta-Guzmán, V.N. Production of Dietary Fibers from Sugarcane Bagasse and Sugarcane Tops Using Microwave-Assisted Alkaline Treatments. Ind. Crops Prod. 2019, 135, 159–169. [Google Scholar] [CrossRef]
- So, S.; Cherdthong, A.; Wanapat, M. Growth Performances, Nutrient Digestibility, Ruminal Fermentation and Energy Partition of Thai Native Steers Fed Exclusive Rice Straw and Fermented Sugarcane Bagasse with Lactobacillus , Cellulase and Molasses. J. Anim. Physiol. Anim. Nutr. 2022, 106, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Molavian, M.; Ghorbani, G.R.; Rafiee, H.; Beauchemin, K.A. Substitution of Wheat Straw with Sugarcane Bagasse in Low-Forage Diets Fed to Mid-Lactation Dairy Cows: Milk Production, Digestibility, and Chewing Behavior. J. Dairy Sci. 2020, 103, 8034–8047. [Google Scholar] [CrossRef] [PubMed]
- Dantas, E.R.S.; Bonhivers, J.-C.; Maciel Filho, R.; Mariano, A.P. Biochemical Conversion of Sugarcane Bagasse into the Alcohol Fuel Mixture of Isopropanol-Butanol-Ethanol (IBE): Is It Economically Competitive with Cellulosic Ethanol? Bioresour. Technol. 2020, 314, 123712. [Google Scholar] [CrossRef] [PubMed]
- Antunes, F.A.F.; Thomé, L.C.; Santos, J.C.; Ingle, A.P.; Costa, C.B.; Dos Anjos, V.; Bell, M.J.V.; Rosa, C.A.; Silva, S.S.D. Multi-Scale Study of the Integrated Use of the Carbohydrate Fractions of Sugarcane Bagasse for Ethanol and Xylitol Production. Renew. Energy 2021, 163, 1343–1355. [Google Scholar] [CrossRef]
- Ahuja, V.; Bhatt, A.K.; Mehta, S.; Sharma, V.; Rathour, R.K. Sheetal Xylitol Production by Pseudomonas Gessardii VXlt-16 from Sugarcane Bagasse Hydrolysate and Cost Analysis. Bioprocess Biosyst. Eng. 2022, 45, 1019–1031. [Google Scholar] [CrossRef]
- Narisetty, V.; Castro, E.; Durgapal, S.; Coulon, F.; Jacob, S.; Kumar, D.; Awasthi, M.K.; Pant, K.K.; Parameswaran, B.; Kumar, V. High Level Xylitol Production by Pichia Fermentans Using Non-Detoxified Xylose-Rich Sugarcane Bagasse and Olive Pits Hydrolysates. Bioresour. Technol. 2021, 342, 126005. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, S.d.S.; Jofre, F.M.; dos Santos, H.A.; Hernández-Pérez, A.F.; Felipe, M.d.G.d.A. Xylitol and Ethanol Co-Production from Sugarcane Bagasse and Straw Hemicellulosic Hydrolysate Supplemented with Molasses. Biomass Convers. Biorefin. 2023, 13, 3143–3152. [Google Scholar] [CrossRef]
- Ntimbani, R.N.; Farzad, S.; Görgens, J.F. Furfural Production from Sugarcane Bagasse along with Co-Production of Ethanol from Furfural Residues. Biomass Convers. Biorefin. 2022, 12, 5257–5267. [Google Scholar] [CrossRef]
- Trung, T.Q.; Thinh, D.B.; Anh, T.N.M.; Nguyet, D.M.; Quan, T.H.; Viet, N.Q.; Tuan, T.T.; Dat, N.M.; Nam, H.M.; Hieu, N.H.; et al. Synthesis of Furfural from Sugarcane Bagasse by Hydrolysis Method Using Magnetic Sulfonated Graphene Oxide Catalyst. Vietnam. J. Chem. 2020, 58, 245–250. [Google Scholar] [CrossRef]
- Silva, T.A.L.; da Silva, A.C.; Pasquini, D. Synthesis and Characterization of Acid-Activated Carbon Prepared from Sugarcane Bagasse for Furfural Production in Aqueous Media. Catalysts 2023, 13, 1372. [Google Scholar] [CrossRef]
- Gupta, H.; Kumar, H.; Kumar, M.; Gehlaut, A.K.; Gaur, A.; Sachan, S.; Park, J.-W. Synthesis of Biodegradable Films Obtained from Rice Husk and Sugarcane Bagasse to Be Used as Food Packaging Material. Environ. Eng. Res. 2019, 25, 506–514. [Google Scholar] [CrossRef]
- Lu, P.; Yang, Y.; Liu, R.; Liu, X.; Ma, J.; Wu, M.; Wang, S. Preparation of Sugarcane Bagasse Nanocellulose Hydrogel as a Colourimetric Freshness Indicator for Intelligent Food Packaging. Carbohydr. Polym. 2020, 249, 116831. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, R.A.; Rodrigues, J.S.; Gonçalves, M.P.; Borsagli, F.G. Ecofriendly Bio-Packing Based on Sugarcane Bagasse Fiber for Potential Application in Agroindustry. Sustain. Chem. Eng. 2022, 3, 66–74. [Google Scholar] [CrossRef]
- Nida, S.; Moses, J.A.; Anandharamakrishnan, C. 3D Printed Food Package Casings from Sugarcane Bagasse: A Waste Valorization Study. Biomass Convers. Biorefin. 2021, 1–11. [Google Scholar] [CrossRef]



| Thermoconversion | Temperature (°C) | Length of Stay | Efficiency (%) | Reference |
|---|---|---|---|---|
| Pyrolysis | 300–500 (slow) | h/days | 30–40 | [98,99] |
| 300–700 (intermediate) | 10–30 s | 25–30 | ||
| 300–750 (fast) | 1–2 s | 12–20 | ||
| Hydrothermal carbonization | 150–200 | min/h | 75–80 | [100,101,102] |
| Gasification | 700–1400 | 10–30 min | 10–13 | [103,104,105] |
| Torrefaction | 200–300 | 30 min–2 h | 55–95 | [106,107] |
| Adsorbent | Treatment/Functionalization | Contaminant Removed | pH | Dosage | Contact Time | Temperature (°C) | Adsorption Capacity | Removal Efficiency (%) | Reference |
|---|---|---|---|---|---|---|---|---|---|
| (g·L−1) | |||||||||
| Carboxylated-functionalized sugarcane bagasse (STA) | Chemical modification with trimellitic anhydride (TA) | Co2+, Cu2+, and Ni2+ | 5,75, 5.75, and 5.50 | 0.2 | 180, 250, and 75 min | 25 | 1.140, 1.197, and 1.563 mmol·g−1 | n.c. | [158] |
| Polypyrrole/sugarcane bagasse composites (Ppy-SB) | Chemical modification with pyrrole monomer | Cr6+ | 2.0 | 1.0 | 16 h | 25 and 45 | 156 and 251 mg·g−1 | n.c. | [159] |
| NanoMgS/FeS doped cellulose nanofibers (FeMgSCNF) | Functionalization with FeSO4·7H2O and Na2S | Cr6+ | 5.0 | 0.5 | 16 h | 25 | 142.8 mg·g−1 | n.c. | [160] |
| Cellulose-g-poly-(acrylamide-co-acrylic acid) | Free radical-induced graft copolymerization of acrylamide and acrylic acid | Cd2+, Cu2+, Pb2+, and Zn2+ | 6.0, 5.5, 5.5, and 6.5 | 1.0 | 90, 70, 120, and 120 min | 25 | 101.73, 61.84, 209.64, and 55.04 mg·g−1 | n.c. | [161] |
| Bio-based nanoheterojunctio of cadmium sulfide nanoparticles (CdS@10%SCNF) | CdS nanoparticles deposited onto the matrix of bacterial cellulose nanofibers by hydrothermal technique | Methylene blue, safranin O, and chlorpyrifos | 3, 3, and 11 | 1.0 | 60, and 120 min | 25 | 26.66, 17.86, and 86.96 mg·g−1 | 96.04, 91.30, and 82.28 * | [176] |
| Iron-biocomposite (FeCl3@NaBH4-SB) and Polypyrrole-biocomposite (Ppy-SB) | Chemically modified sugarcane bagasse with FeCl3·6H2O and NaBH4 (FeCl3@NaBH4-SB), and pyrrole solution (Ppy-SB) | Acid red 1 | 2 | 1.0 | 60 and 75 min | 30 | 192.2 (FeCl3@NaBH4-SB) and 205.1 (Ppy-SB) mg·g−1 | n.c. | [166] |
| Polymeric biocomposite polypyrrole/sugarcane bagasse (Ppy-SB) | Chemical modification with polypyrrole | Acid Black-234 (AB-234) | 3 | 1.0 | 60 min | 30 | 100 mg·g−1 | n.c. | [167] |
| Bagasse-β-cyclodextrin polymer (SB-β-CD) | Chemical modification with β-cyclodextrin and citric acid | Bisphenol A (BPA), methylene blue (MB), and neutral red (NR) | 7.0, 9.0, and 6.0 | 1.0 | 300, 300, and 240 min | 25 | 121, 963, and 685 mg·g−1 | n.c. | [168] |
| Highly porous N/S doped magnetic carbon aerogel (N/S-MCA) | Chemically activated with potassium hydroxide; functionalization with thiourea and hydrothermal process | Bisphenol A (BPA) | 7.0 | 5.0 | 120 min | 25 | 197.6 mg·g−1 | 99.5 | [172] |
| CTAB-modified carboxymethyl cellulose/bagasse cryogel | Crosslinking method and freeze-drying under vacuum | Bisphenol A, methylene blue, and Cr6+ | 7.0, 2.0, and 5.5 | 5 × 10−5 | 110 min | 23 ± 1 | n.c., n.c., and 899 mg·g−1 | 100, 70, and 95 | [173] |
| Sugarcane Bagasse Fly Ash | Pretreatment liquor followed by enzymatic hydrolysis | Vanillin and tannic acid | 5 | 10 | 24 h | 30 | 0.11 and 0.16 g·g−1 | 95 | [174] |
| N-doped activated carbon | Activation via urea-KOH method | CO2 | n.c. | n.c. | n.c. | 25 | 4.8 mmol·g−1 | n.c. | [175] |
| Activated biochar (ABC) | Hydrothermal carbonization (HTC) | Sulfamethoxazole (SMX) | 5.4 | 0.1 | 10–150 min | 25 | 400 mg·g−1 | 99.41 | [177] |
| Porous-activated carbon (SCB-AC) | ZnCl2 activation | diclofenac sodium (DFC) | 2.0 | 0.4 | 15 min | 25 | 315 mg·g−1 | 92.4 | [178] |
| Magnetic biochar (MBC) | Pre-modification of mineral-rich sugarcane bagasse with hematite nanoparticles | Perfluorooctane sulphonate (PFOS) | 5.0 | 1.0 | 24 h | 25 | 120.44 mg·g−1 | n.c. | [179] |
| Sugarcane bagasse biochar (SBAC-7) | In situ sulfuric acid–modified biochar by hydrothermal carbonization process | Toluene | n.c. | n.c. | 80 min | 30 | 771.1 mg·g−1 | n.c. | [180] |
| SBNAOH | Alkaline treatment | Glyphosate | 9.0 | 10.0 | 40 min | 25 | 13.72 mg·g−1 | 86.2 | [181] |
| N/S doped magnetic carbon aerogel (N/S-MCA) | Reaction with thiourea and FeCl3 and hydrothermal carbonization | Bisphenol A (BPA) | 7.0 | 0.1 | 60 min | 70 | 199.8 mg·g−1 | 98–99 | [172] |
| Gel-like bioadsorbert | By crosslinking thiourea-modified sugarcane bagasse cellulose | Methylene blue (MB) and crystal violet (CV) | 9.0 | 0.5 | n.c. | 25 | 632.9 and 574.7 mg·g−1 | n.c. | [184] |
| (TCH) | |||||||||
| Porous activated carbons from sugarcane bagasse | Chemically activate with different agents (air, CO2, H3PO4, and NaOH) | CO2 | n.c. | 1.0 (g/column) | n.c. | 0 | 5.50 mmol·g−1 | n.c. | [21] |
| Nitrogen-modified porous carbon material | Functionalization by melamine | CO2 | n.c. | 0.1 (g/column) | n.c. | 40 | 3.34 mmol·g−1 | n.c. | [185] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hiranobe, C.T.; Gomes, A.S.; Paiva, F.F.G.; Tolosa, G.R.; Paim, L.L.; Dognani, G.; Cardim, G.P.; Cardim, H.P.; dos Santos, R.J.; Cabrera, F.C. Sugarcane Bagasse: Challenges and Opportunities for Waste Recycling. Clean Technol. 2024, 6, 662-699. https://doi.org/10.3390/cleantechnol6020035
Hiranobe CT, Gomes AS, Paiva FFG, Tolosa GR, Paim LL, Dognani G, Cardim GP, Cardim HP, dos Santos RJ, Cabrera FC. Sugarcane Bagasse: Challenges and Opportunities for Waste Recycling. Clean Technologies. 2024; 6(2):662-699. https://doi.org/10.3390/cleantechnol6020035
Chicago/Turabian StyleHiranobe, Carlos T., Andressa S. Gomes, Fábio F. G. Paiva, Gabrieli R. Tolosa, Leonardo L. Paim, Guilherme Dognani, Guilherme P. Cardim, Henrique P. Cardim, Renivaldo J. dos Santos, and Flávio C. Cabrera. 2024. "Sugarcane Bagasse: Challenges and Opportunities for Waste Recycling" Clean Technologies 6, no. 2: 662-699. https://doi.org/10.3390/cleantechnol6020035
APA StyleHiranobe, C. T., Gomes, A. S., Paiva, F. F. G., Tolosa, G. R., Paim, L. L., Dognani, G., Cardim, G. P., Cardim, H. P., dos Santos, R. J., & Cabrera, F. C. (2024). Sugarcane Bagasse: Challenges and Opportunities for Waste Recycling. Clean Technologies, 6(2), 662-699. https://doi.org/10.3390/cleantechnol6020035






