Hydroxyapatite-Based Adsorbent Materials from Aquaculture Waste for Remediation of Metal-Contaminated Waters: Investigation of Cadmium Removal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Adsorbents
2.2.1. Shells Calcination
2.2.2. Hydroxyapatite Synthesis
2.3. Characterisation
2.3.1. Scanning Electron Microscope and Transmission Electron Microscopy Measurements
2.3.2. Particle Size Distribution Measurements
2.3.3. Chemical Characterisation
2.3.4. X-Ray Powder Diffraction (XRPD)
2.3.5. Thermal Analysis
2.4. Batch Adsorption Experiments
3. Results and Discussion
3.1. Materials Characterisation
3.1.1. SEM/EDS and TEM Imaging
3.1.2. Particle Size Distribution
3.2. Batch Adsorption Results
3.2.1. Effect of Calcination in Adsorption Experiments
3.2.2. Kinetics Experiments
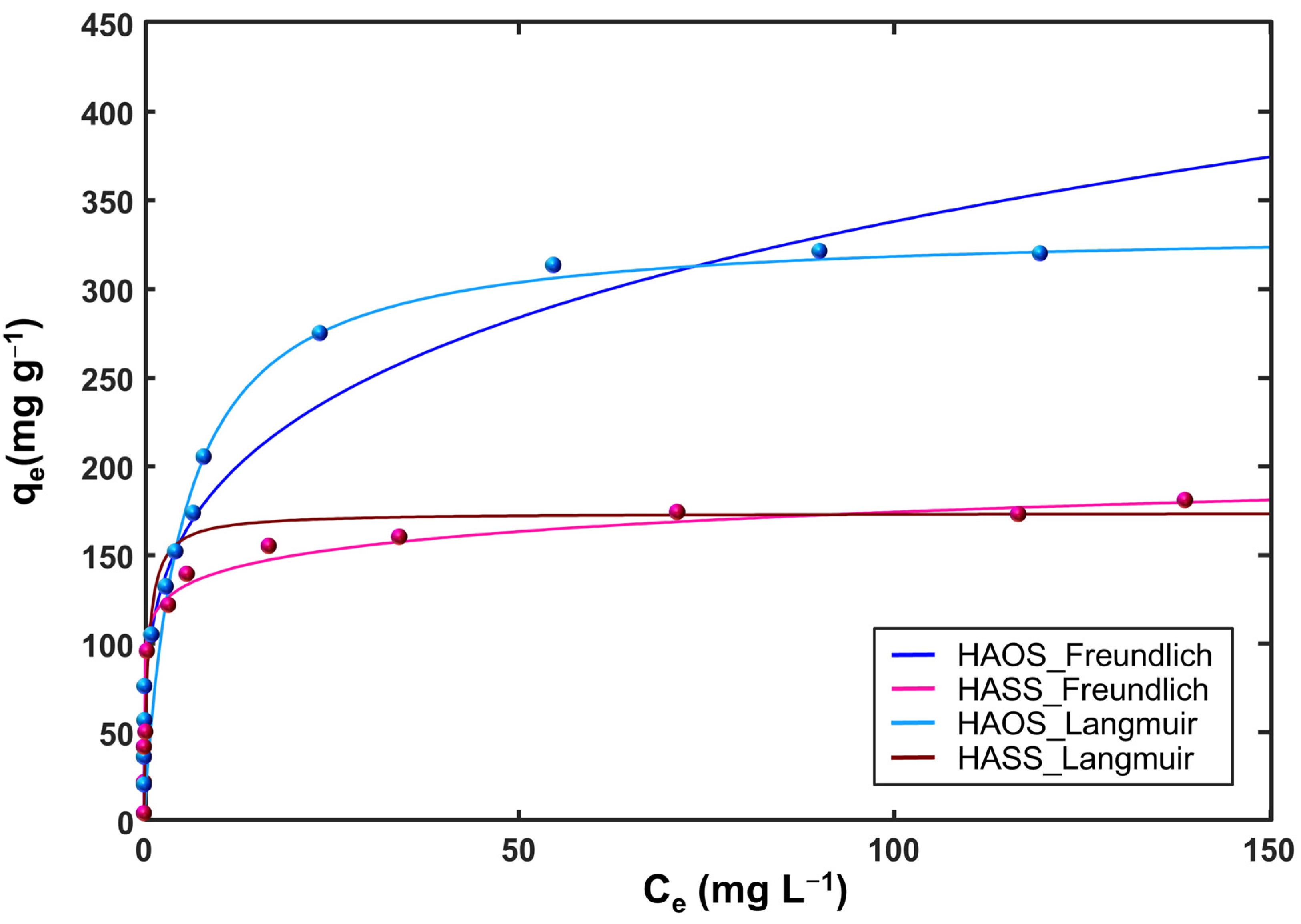
3.2.3. Adsorption Isotherms
| Adsorbents | qs (mg g−1) | References |
|---|---|---|
| Bamboo charcoal | 12.08 | [52] |
| Oyster shells (OS) | 15.03 | [45] |
| Cashew nutshell | 21.11 | [53] |
| Sugar cane | 48.31 | [54] |
| Scallop shells (SS) | 55 ± 17.4 | [10] |
| Orange peel | 56.5 ± 0.50 | [55] |
| Mussel shells HA | 62.5 | [25] |
| Clam shells HA | 62.5 | [49] |
| Small surface area HA | 71.94 | [22] |
| Pine bark biochar (600 °C) | 85.5 | [56] |
| Alumina-HA Spheres | 89.37 | [21] |
| Canna indica biochar (600 °C) | 140.01 ± 14.42 | [57] |
| HASS | 173.7 ± 16.95 | Present study |
| Large surface area HA | 207.97 | [22] |
| nano-HA | 243.90 | [18] |
| HAOS | 334.3 ± 46.20 | Present study |
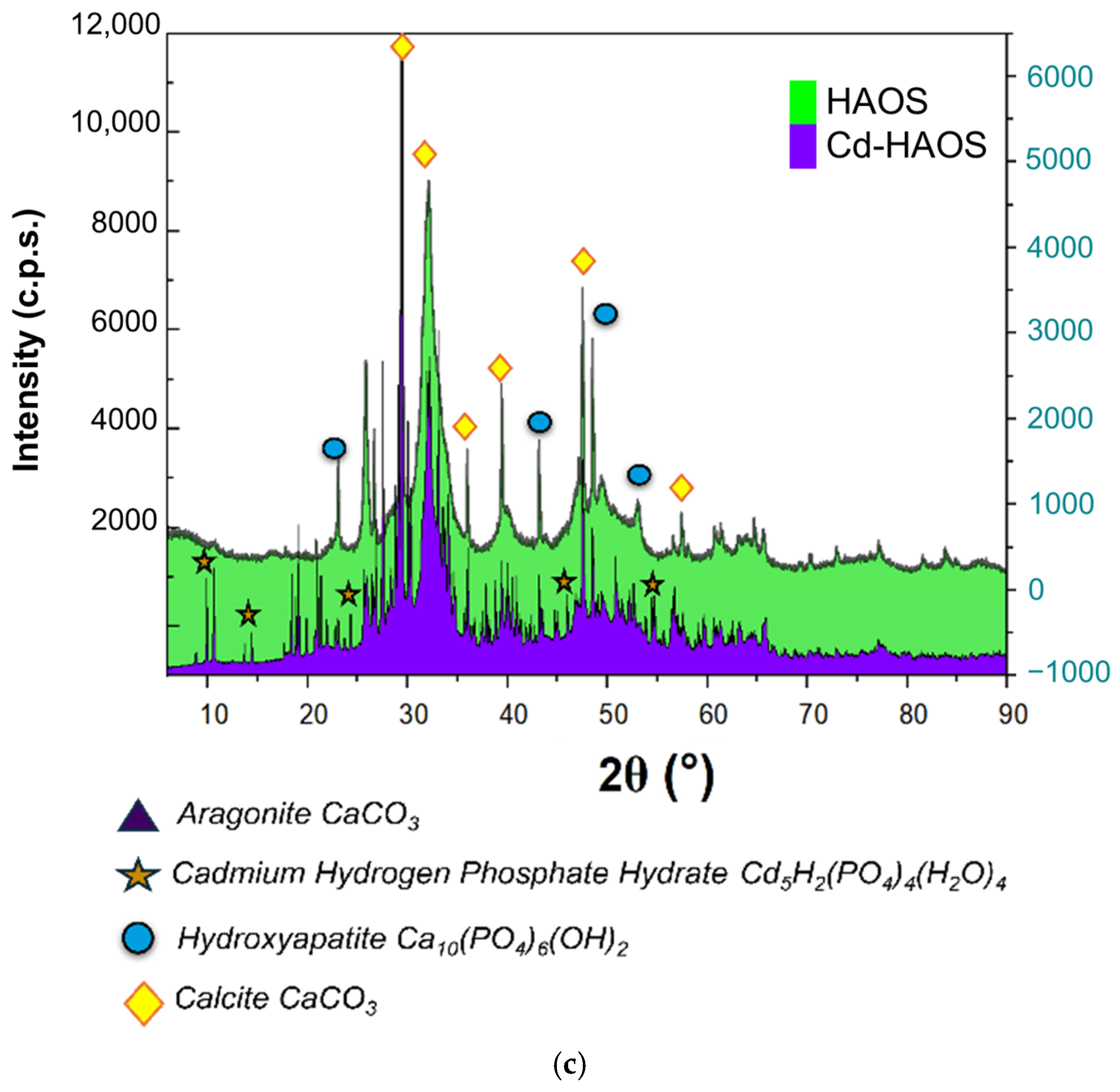
3.3. Structural and Thermal Characterisation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, V.; Parihar, R.D.; Sharma, A.; Bakshi, P.; Singh Sidhu, G.P.; Bali, A.S.; Karaouzas, I.; Bhardwaj, R.; Thukral, A.K.; Gyasi-Agyei, Y.; et al. Global Evaluation of Heavy Metal Content in Surface Water Bodies: A Meta-Analysis Using Heavy Metal Pollution Indices and Multivariate Statistical Analyses. Chemosphere 2019, 236, 124364. [Google Scholar] [CrossRef] [PubMed]
- Suhani, I.; Sahab, S.; Srivastava, V.; Singh, R.P. Impact of Cadmium Pollution on Food Safety and Human Health. Curr. Opin. Toxicol. 2021, 27, 1–7. [Google Scholar] [CrossRef]
- Sinicropi, M.S.; Amantea, D.; Caruso, A.; Saturnino, C. Chemical and Biological Properties of Toxic Metals and Use of Chelating Agents for the Pharmacological Treatment of Metal Poisoning. Arch. Toxicol. 2010, 84, 501–520. [Google Scholar] [CrossRef] [PubMed]
- Waalkes, M.P. Cadmium Carcinogenesis. Mutat. Res.-Fundam. Mol. Mech. Mutagen. 2003, 533, 107–120. [Google Scholar] [CrossRef]
- Vareda, J.P.; Valente, A.J.M.; Durães, L. Assessment of Heavy Metal Pollution from Anthropogenic Activities and Remediation Strategies: A Review. J. Environ. Manag. 2019, 246, 101–118. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of Heavy Metal Ions from Wastewaters: A Review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Chan, G.Y.S.; Lo, W.H.; Babel, S. Physico-Chemical Treatment Techniques for Wastewater Laden with Heavy Metals. Chem. Eng. J. 2006, 118, 83–98. [Google Scholar] [CrossRef]
- Al-Saydeh, S.A.; El-Naas, M.H.; Zaidi, S.J. Copper Removal from Industrial Wastewater: A Comprehensive Review. J. Ind. Eng. Chem. 2017, 56, 35–44. [Google Scholar] [CrossRef]
- Burakov, A.E.; Galunin, E.V.; Burakova, I.V.; Kucherova, A.E.; Agarwal, S.; Tkachev, A.G.; Gupta, V.K. Adsorption of Heavy Metals on Conventional and Nanostructured Materials for Wastewater Treatment Purposes: A Review. Ecotoxicol. Environ. Saf. 2018, 148, 702–712. [Google Scholar] [CrossRef]
- Chenet, T.; Schwarz, G.; Neff, C.; Hattendorf, B.; Günther, D.; Martucci, A.; Cescon, M.; Baldi, A.; Pasti, L. Scallop Shells as Biosorbents for Water Remediation from Heavy Metals: Contributions and Mechanism of Shell Components in the Adsorption of Cadmium from Aqueous Matrix. Heliyon 2024, 10, e29296. [Google Scholar] [CrossRef]
- Morris, J.P.; Backeljau, T.; Chapelle, G. Shells from Aquaculture: A Valuable Biomaterial, Not a Nuisance Waste Product. Rev. Aquac. 2019, 11, 42–57. [Google Scholar] [CrossRef]
- Annane, K.; Lemlikchi, W.; Tingry, S. Efficiency of Eggshell as a Low-Cost Adsorbent for Removal of Cadmium: Kinetic and Isotherm Studies. Biomass Convers. Biorefin. 2021, 13, 6163–6174. [Google Scholar] [CrossRef]
- Nakajima, S.; Araki, S.; Sasamoto, R.; Kanda, Y.; Yamanaka, S. Key Particle Properties of Shells for Cadmium Chemisorption. Chemosphere 2022, 287, 132257. [Google Scholar] [CrossRef] [PubMed]
- Witek-Krowiak, A.; Szafran, R.G.; Modelski, S. Biosorption of Heavy Metals from Aqueous Solutions onto Peanut Shell as a Low-Cost Biosorbent. Desalination 2011, 265, 126–134. [Google Scholar] [CrossRef]
- Villar da Gama, B.M.; Elisandra do Nascimento, G.; Silva Sales, D.C.; Rodríguez-Díaz, J.M.; Bezerra de Menezes Barbosa, C.M.; Menezes Bezerra Duarte, M.M. Mono and Binary Component Adsorption of Phenol and Cadmium Using Adsorbent Derived from Peanut Shells. J. Clean. Prod. 2018, 201, 219–228. [Google Scholar] [CrossRef]
- Sattar, M.S.; Shakoor, M.B.; Ali, S.; Rizwan, M.; Niazi, N.K.; Jilani, A. Comparative Efficiency of Peanut Shell and Peanut Shell Biochar for Removal of Arsenic from Water. Environ. Sci. Pollut. Res. 2019, 26, 18624–18635. [Google Scholar] [CrossRef]
- Sousa, F.W.; Oliveira, A.G.; Ribeiro, J.P.; Rosa, M.F.; Keukeleire, D.; Nascimento, R.F. Green Coconut Shells Applied as Adsorbent for Removal of Toxic Metal Ions Using Fixed-Bed Column Technology. J. Environ. Manag. 2010, 91, 1634–1640. [Google Scholar] [CrossRef]
- Salah, T.A.; Mohammad, A.M.; Hassan, M.A.; El-Anadouli, B.E. Development of Nano-Hydroxyapatite/Chitosan Composite for Cadmium Ions Removal in Wastewater Treatment. J. Taiwan Inst. Chem. Eng. 2014, 45, 1571–1577. [Google Scholar] [CrossRef]
- Ferri, M.; Campisi, S.; Gervasini, A. Nickel and Cobalt Adsorption on Hydroxyapatite: A Study for the de-Metalation of Electronic Industrial Wastewaters. Adsorption 2019, 25, 649–660. [Google Scholar] [CrossRef]
- Kavasi, R.M.; Coelho, C.C.; Platania, V.; Quadros, P.A.; Chatzinikolaidou, M. In Vitro Biocompatibility Assessment of Nano-hydroxyapatite. Nanomaterials 2021, 11, 1152. [Google Scholar] [CrossRef]
- Silva-Holguín, P.N.; Medellín-Castillo, N.A.; Zaragoza-Contreras, E.A.; Reyes-López, S.Y. Alumina-Hydroxyapatite Millimetric Spheres for Cadmium(II) Removal in Aqueous Medium. ACS Omega 2023, 8, 44675–44688. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Guo, X.; Ye, X. Screening Hydroxyapatite for Cadmium and Lead Immobilization in Aqueous Solution and Contaminated Soil: The Role of Surface Area. J. Environ. Sci. 2017, 52, 141–150. [Google Scholar] [CrossRef]
- Corami, A.; Mignardi, S.; Ferrini, V. Cadmium Removal from Single- and Multi-Metal (Cd + Pb + Zn + Cu) Solutions by Sorption on Hydroxyapatite. J. Colloid. Interface Sci. 2008, 317, 402–408. [Google Scholar] [CrossRef]
- Gibert, O.; Valderrama, C.; Martínez, M.M.; Darbra, R.M.; Moncunill, J.O.; Martí, V. Hydroxyapatite Coatings on Calcite Powder for the Removal of Heavy Metals from Contaminated Water. Water 2021, 13, 1493. [Google Scholar] [CrossRef]
- Meski, S.; Tazibt, N.; Khireddine, H.; Ziani, S.; Biba, W.; Yala, S.; Sidane, D.; Boudjouan, F.; Moussaoui, N. Synthesis of Hydroxyapatite from Mussel Shells for Effective Adsorption of Aqueous Cd(II). Water Sci. Technol. 2019, 80, 1226–1237. [Google Scholar] [CrossRef]
- Wu, S.C.; Hsu, H.C.; Hsu, S.K.; Tseng, C.P.; Ho, W.F. Effects of Calcination on Synthesis of Hydroxyapatite Derived from Oyster Shell Powders. J. Aust. Ceram. Soc. 2019, 55, 1051–1058. [Google Scholar] [CrossRef]
- Mohd Pu’ad, N.A.S.; Alipal, J.; Abdullah, H.Z.; Idris, M.I.; Lee, T.C. Synthesis of Eggshell Derived Hydroxyapatite via Chemical Precipitation and Calcination Method. Mater. Today Proc. 2019, 42, 172–177. [Google Scholar] [CrossRef]
- Alif, M.F.; Aprillia, W.; Arief, S. A Hydrothermal Synthesis of Natural Hydroxyapatite Obtained from Corbicula Moltkiana Freshwater Clams Shell Biowaste. Mater. Lett. 2018, 230, 40–43. [Google Scholar] [CrossRef]
- Chen, J.; Wen, Z.; Zhong, S.; Wang, Z.; Wu, J.; Zhang, Q. Synthesis of Hydroxyapatite Nanorods from Abalone Shells via Hydrothermal Solid-State Conversion. Mater. Des. 2015, 87, 445–449. [Google Scholar] [CrossRef]
- Wang, H.; Yan, K.; Chen, J. Preparation of Hydroxyapatite Microspheres by Hydrothermal Self-Assembly of Marine Shell for Effective Adsorption of Congo Red. Mater. Lett. 2021, 304, 130573. [Google Scholar] [CrossRef]
- Zhang, H.-B.; Zhou, K.-C.; Li, Z.-Y.; Huang, S.-P. Plate-like Hydroxyapatite Nanoparticles Synthesized by the Hydrothermal Method. J. Phys. Chem. Solids 2009, 70, 243–248. [Google Scholar] [CrossRef]
- Summa, D.; Lanzoni, M.; Castaldelli, G.; Fano, E.A.; Tamburini, E. Trends and Opportunities of Bivalve Shells’ Waste Valorization in a Prospect of Circular Blue Bioeconomy. Resources 2022, 11, 48. [Google Scholar] [CrossRef]
- Kaplan, D.L. Mollusc Shell Structures: Novel Design Strategies for Synthetic Materials. Curr. Opin. Solid State Mater. Sci. 1998, 3, 232–236. [Google Scholar] [CrossRef]
- Stanmore, B.R.; Gilot, P. Review-Calcination and Carbonation of Limestone during Thermal Cycling for CO2 Sequestration. Fuel Process. Technol. 2005, 86, 1707–1743. [Google Scholar] [CrossRef]
- Ahmed, J.; Al-Attar, H.; Arfat, Y.A. Effect of Particle Size on Compositional, Functional, Pasting and Rheological Properties of Commercial Water Chestnut Flour. Food Hydrocoll. 2016, 52, 888–895. [Google Scholar] [CrossRef]
- Chenet, T.; Sarti, E.; Costa, V.; Cavazzini, A.; Rodeghero, E.; Beltrami, G.; Felletti, S.; Pasti, L.; Martucci, A. Influence of Caffeic Acid on the Adsorption of Toluene onto an Organophilic Zeolite. J. Environ. Chem. Eng. 2020, 8, 104229. [Google Scholar] [CrossRef]
- Zeng, C.; Ramos-Ruiz, A.; Field, J.A.; Sierra-Alvarez, R. Cadmium Telluride (CdTe) and Cadmium Selenide (CdSe) Leaching Behavior and Surface Chemistry in Response to PH and O2. J. Environ. Manag. 2015, 154, 78–85. [Google Scholar] [CrossRef]
- Smičiklas, I.D.; Milonjicámilonjicá, S.K.; Pfendt, P.; Raičevicá, S.R. The Point of Zero Charge and Sorption of Cadmium (II) and Strontium (II) Ions on Synthetic Hydroxyapatite. Sep. Purif. Technol. 2000, 18, 185–194. [Google Scholar] [CrossRef]
- Grassi, S.; Amadori, M.; Pennisi, M.; Cortecci, G. Identifying Sources of B and As Contamination in Surface Water and Groundwater Downstream of the Larderello Geothermal-Industrial Area (Tuscany-Central Italy). J. Hydrol. 2014, 509, 66–82. [Google Scholar] [CrossRef]
- La Vigna, F.; Ciadamidaro, S.; Mazza, R.; Mancini, L. Water Quality and Relationship between Superficial and Ground Water in Rome (Aniene River Basin, Central Italy). Environ. Earth Sci. 2010, 60, 1267–1279. [Google Scholar] [CrossRef]
- Ioele, G.; De Luca, M.; Grande, F.; Durante, G.; Trozzo, R.; Crupi, C.; Ragno, G. Assessment of Surface Water Quality Using Multivariate Analysis: Case Study of the Crati River, Italy. Water 2020, 12, 2214. [Google Scholar] [CrossRef]
- Chenet, T.; Martucci, A.; Cescon, M.; Vergine, G.; Beltrami, G.; Gigli, L.; Ardit, M.; Migliori, M.; Catizzone, E.; Giordano, G.; et al. Supramolecular Assembly of L-Lysine on ZSM-5 Zeolites with Different Si/Al Ratio. Microporous Mesoporous Mater. 2021, 323, 111183. [Google Scholar] [CrossRef]
- Ding, J.; Zhao, L.; Chang, Y.; Zhao, W.; Du, Z.; Hao, Z. Transcriptome Sequencing and Characterization of Japanese Scallop Patinopecten Yessoensis from Different Shell Color Lines. PLoS ONE 2015, 10, 0116406. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, X.; Oh, M.; Park, J. Oyster Shell as a Low-Cost Adsorbent for Removing Heavy Metal Ions from Wastewater. Pol. J. Environ. Stud. 2019, 28, 2949–2959. [Google Scholar] [CrossRef]
- Zhao, G.; Wang, X.; Chen, Q.; Cheng, J. Efficacy of Phosphorus Loaded Oyster Shell in Heavy Metal Removal: A Combined Study on Adsorption Behavior, Structure Characterization and Comparative Mechanism. Environ. Technol. Innov. 2024, 33, 103484. [Google Scholar] [CrossRef]
- Beni, A.A.; Esmaeili, A. Biosorption, an Efficient Method for Removing Heavy Metals from Industrial Effluents: A Review. Environ. Technol. Innov. 2020, 17, 100503. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the Modeling of Adsorption Isotherm Systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Araújo, C.S.T.; Almeida, I.L.S.; Rezende, H.C.; Marcionilio, S.M.L.O.; Léon, J.J.L.; de Matos, T.N. Elucidation of Mechanism Involved in Adsorption of Pb(II) onto Lobeira Fruit (Solanum Lycocarpum) Using Langmuir, Freundlich and Temkin Isotherms. Microchem. J. 2018, 137, 348–354. [Google Scholar] [CrossRef]
- Núñez, D.; Serrano, J.A.; Mancisidor, A.; Elgueta, E.; Varaprasad, K.; Oyarzún, P.; Cáceres, R.; Ide, W.; Rivas, B.L. Heavy Metal Removal from Aqueous Systems Using Hydroxyapatite Nanocrystals Derived from Clam Shells. RSC Adv. 2019, 9, 22883–22890. [Google Scholar] [CrossRef]
- Marchat, D.; Bernache-Assollant, D.; Champion, E. Cadmium Fixation by Synthetic Hydroxyapatite in Aqueous Solution-Thermal Behaviour. J. Hazard. Mater. 2007, 139, 453–460. [Google Scholar] [CrossRef]
- GadelHak, Y.; El-Azazy, M.; Shibl, M.F.; Mahmoud, R.K. Cost estimation of synthesis and utilization of nano-adsorbents on the laboratory and industrial scales: A detailed review. Sci. Total Environ. 2023, 875, 162629. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.Y.; Wang, H.; Ma, J.W. Adsorption of Cadmium (II) Ions from Aqueous Solution by a New Low-Cost Adsorbent-Bamboo Charcoal. J. Hazard. Mater. 2010, 177, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.S.; Ramalingam, S.; Sathyaselvabala, V.; Kirupha, S.D.; Murugesan, A.; Sivanesan, S. Removal of Cadmium(II) from Aqueous Solution by Agricultural Waste Cashew Nut Shell. Korean J. Chem. Eng. 2012, 29, 756–768. [Google Scholar] [CrossRef]
- Batool, F.; Akbar, J.; Iqbal, S.; Noreen, S.; Bukhari, S.N.A. Study of Isothermal, Kinetic, and Thermodynamic Parameters for Adsorption of Cadmium: An Overview of Linear and Nonlinear Approach and Error Analysis. Bioinorg. Chem. Appl. 2018, 2018, 3463724. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.J.; Chao, H.P. Thermodynamic Parameters of Cadmium Adsorption onto Orange Peel Calculated from Various Methods: A Comparison Study. J. Environ. Chem. Eng. 2016, 4, 2671–2682. [Google Scholar] [CrossRef]
- Park, J.H.; Wang, J.J.; Kim, S.H.; Kang, S.W.; Jeong, C.Y.; Jeon, J.R.; Park, K.H.; Cho, J.S.; Delaune, R.D.; Seo, D.C. Cadmium Adsorption Characteristics of Biochars Derived Using Various Pine Tree Residues and Pyrolysis Temperatures. J. Colloid Interface Sci. 2019, 553, 298–307. [Google Scholar] [CrossRef]
- Cui, X.; Fang, S.; Yao, Y.; Li, T.; Ni, Q.; Yang, X.; He, Z. Potential Mechanisms of Cadmium Removal from Aqueous Solution by Canna Indica Derived Biochar. Sci. Total Environ. 2016, 562, 517–525. [Google Scholar] [CrossRef]
- Rujitanapanich, S.; Kumpapan, P.; Wanjanoi, P. Synthesis of Hydroxyapatite from Oyster Shell via Precipitation. Energy Procedia 2014, 56, 112–117. [Google Scholar] [CrossRef]
- Wang, M.; Wu, S.; Guo, J.; Zhang, X.; Yang, Y.; Chen, F.; Zhu, R. Immobilization of Cadmium by Hydroxyapatite Converted from Microbial Precipitated Calcite. J. Hazard. Mater. 2019, 366, 684–693. [Google Scholar] [CrossRef]
- Evis, Z.; Yilmaz, B.; Usta, M.; Levent Aktug, S. X-Ray Investigation of Sintered Cadmium Doped Hydroxyapatites. Ceram. Int. 2013, 39, 2359–2363. [Google Scholar] [CrossRef]
- Julia, M.; Putnis, C.V.; King, H.E.; Renard, F. Coupled Dissolution-Precipitation and Growth Processes on Calcite, Aragonite, and Carrara Marble Exposed to Cadmium-Rich Aqueous Solutions. Chem. Geol. 2023, 621, 121364. [Google Scholar] [CrossRef]
- Wu, S.C.; Hsu, H.C.; Hsu, S.K.; Tseng, C.P.; Ho, W.F. Preparation and Characterization of Hydroxyapatite Synthesized from Oyster Shell Powders. Adv. Powder Technol. 2017, 28, 1154–1158. [Google Scholar] [CrossRef]
- Da Silva, P.S.; de Moura Farias, W.; Gomez, M.R.; Torrecilha, J.K.; Rocha, F.R.; Scapin, M.A.; Garcia, R.H.; De Simone, L.R.; De Amaral, V.S.; Vincent, M.; et al. Oyster shell element composition as a proxy for environmental studies. J. S. Am. Earth Sci. 2024, 134, 104749. [Google Scholar] [CrossRef]
- Filiberto, L.H.; Putnis, C.V.; Julia, M. Factors Controlling Reaction Pathways during Fluid–Rock Interactions. Contrib. Mineral. Petrol. 2023, 178, 53. [Google Scholar] [CrossRef]
- Kaddah, F.; Roziere, E.; Ranaivomanana, H.; Amiri, O. Complementary Use of Thermogravimetric Analysis and Oven to Assess the Composition and Bound CO2 Content of Recycled Concrete Aggregates. Dev. Built Environ. 2023, 15, 100184. [Google Scholar] [CrossRef]
- Tõnsuaadu, K.; Peld, M.; Bender, V. Thermal analysis of apatite structure. J. Therm. Anal. Calorim. 2003, 72, 363–371. [Google Scholar] [CrossRef]
- Lafon, J.P.; Champion, E.; Bernache-Assollant, D.; Gibert, R.; Danna, A.M. Thermal decomposition of carbonated calcium phosphate apatites. J. Therm. Anal. Calorim. 2003, 72, 1127–1134. [Google Scholar] [CrossRef]

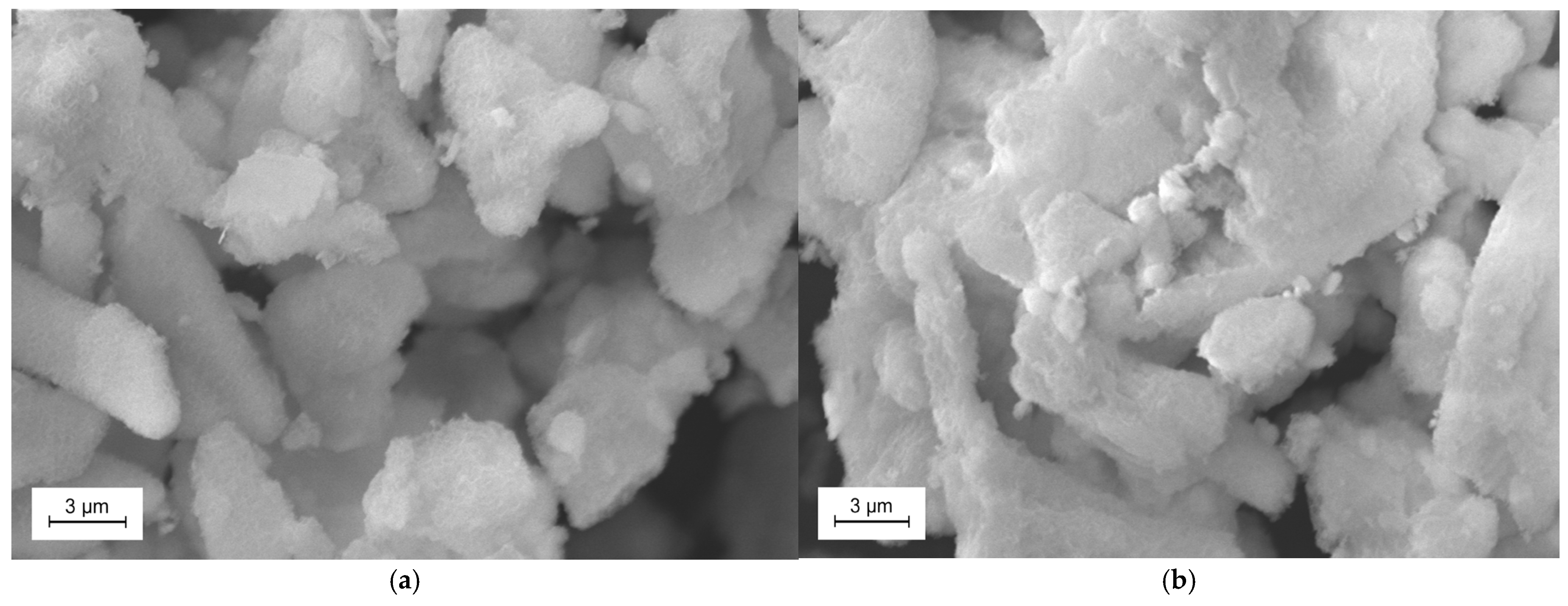
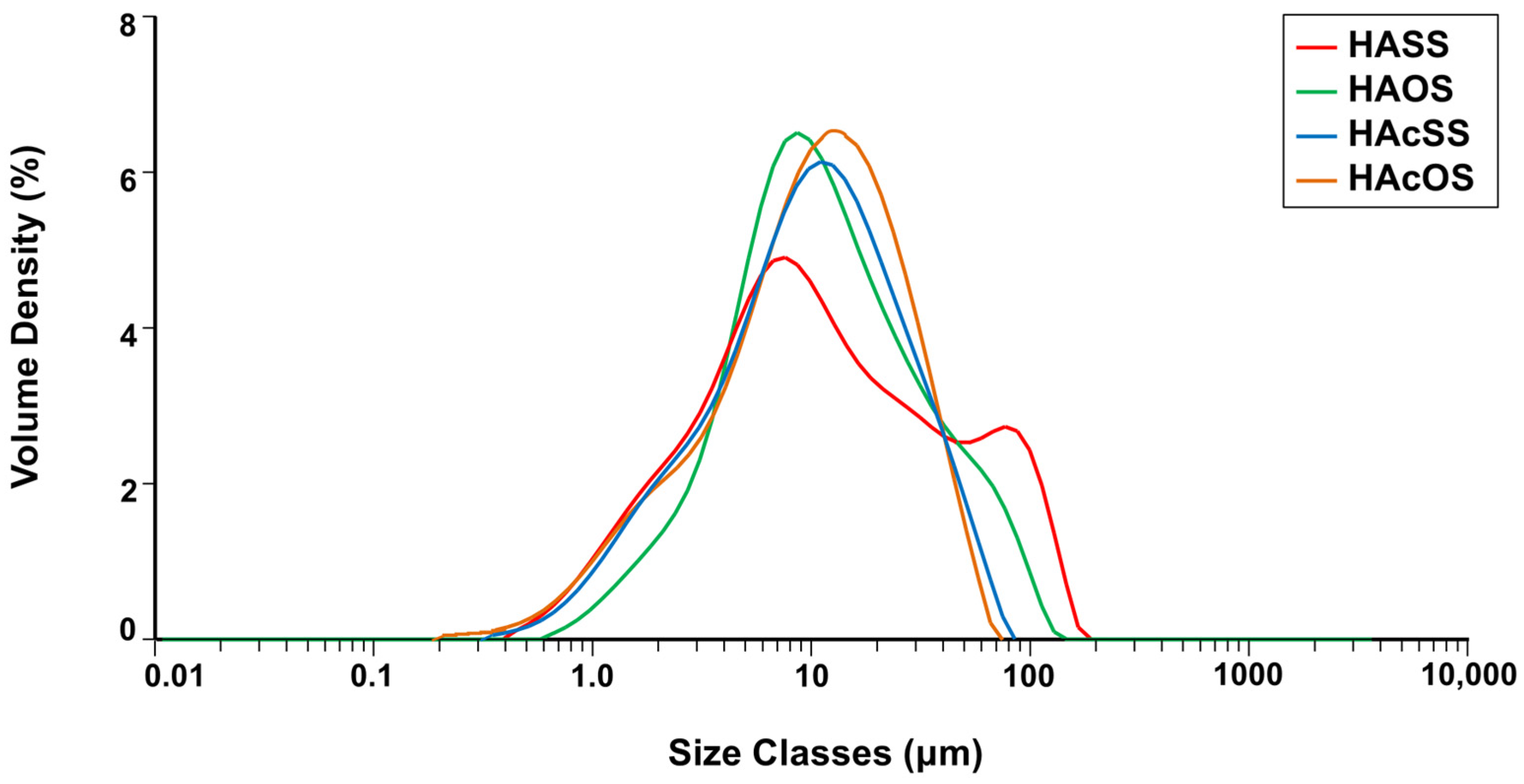
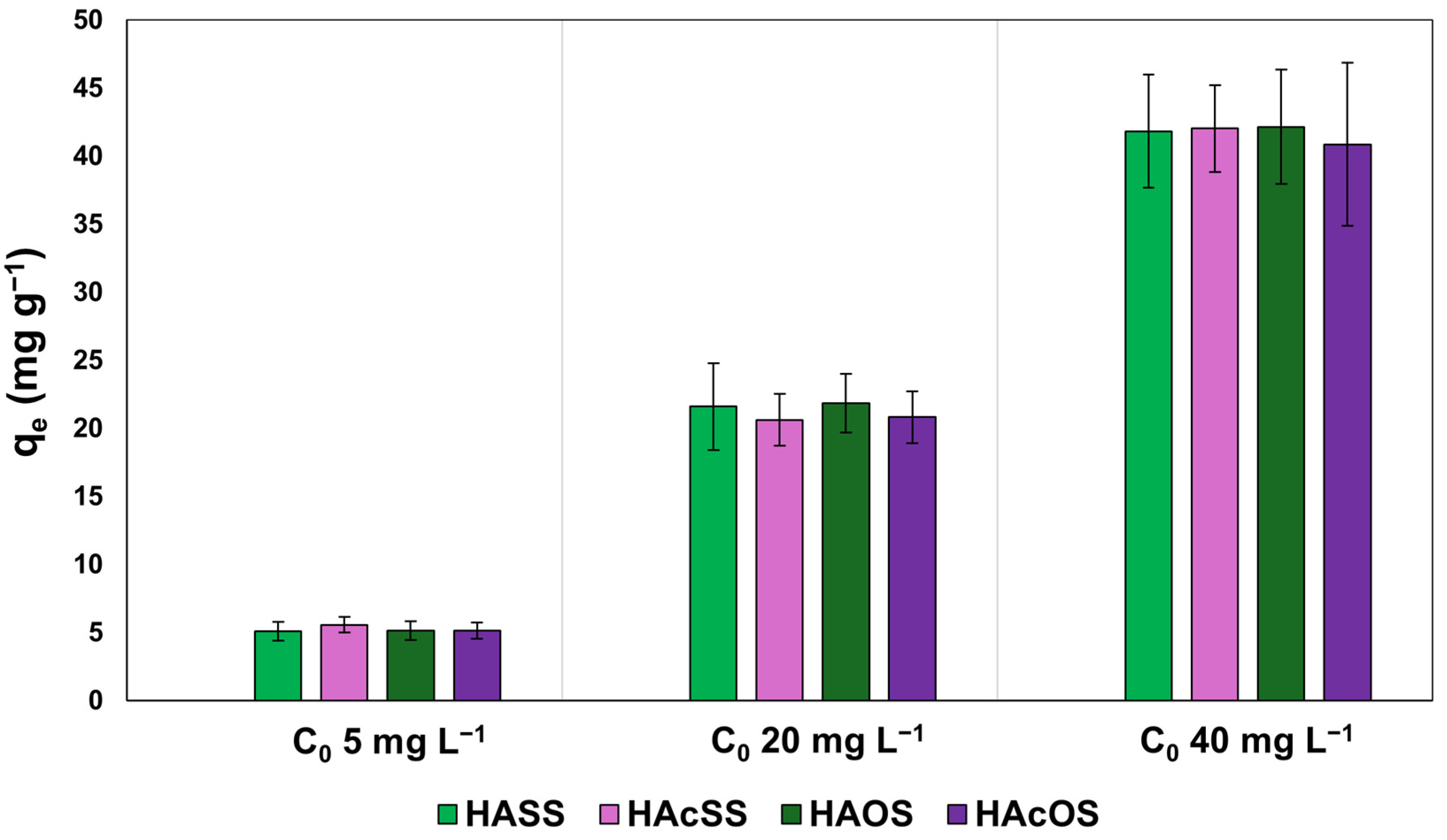
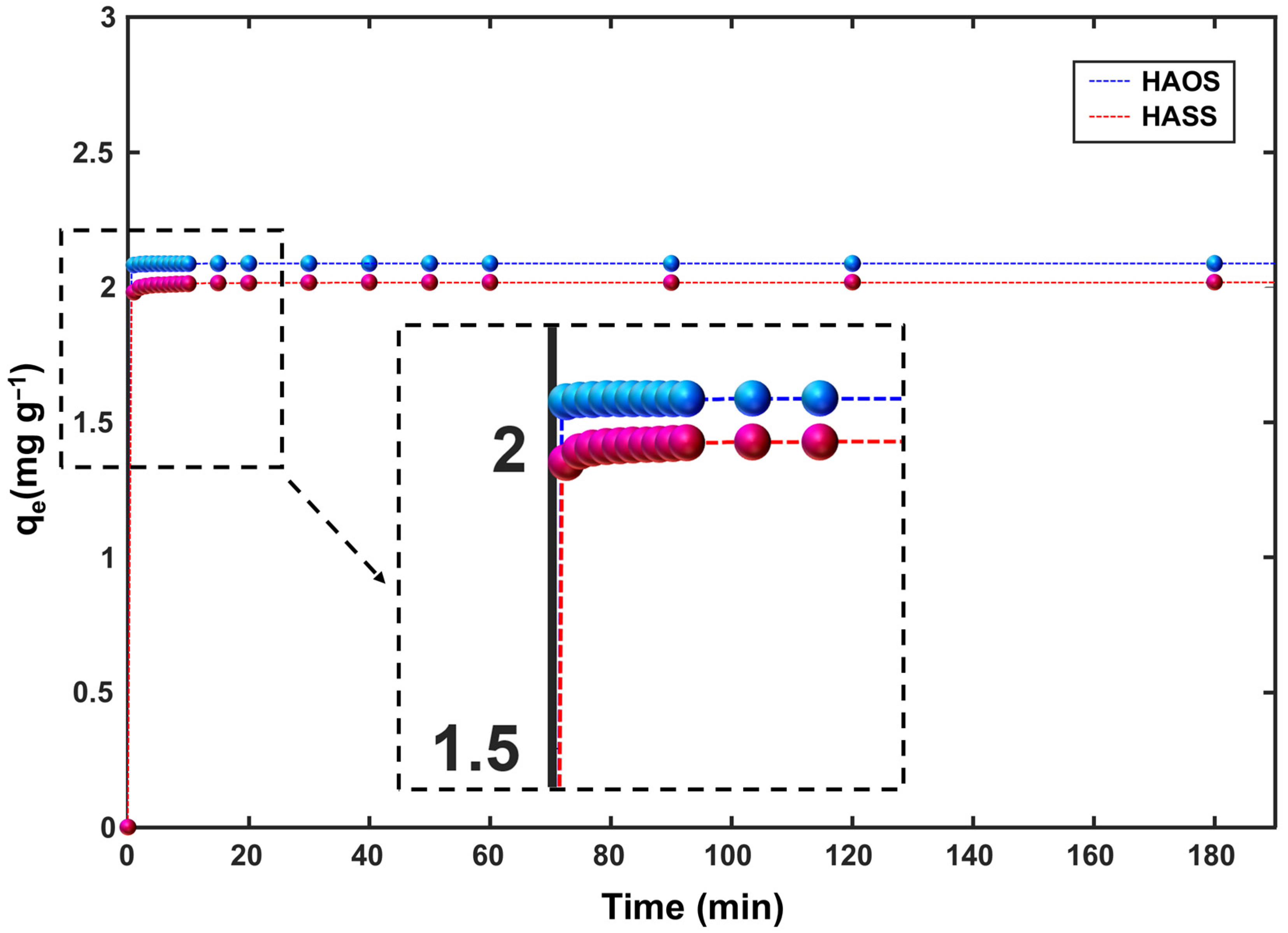



| Parameters | HASS | HAOS | HAcSS | HAcOS |
|---|---|---|---|---|
| D [4;3] (µm) | 22.389 ± 1.009 | 17.291 ± 0.765 | 13.519 ± 0.696 | 13.578 ± 0.779 |
| D [3;4] (µm) | 4.895 ± 0.145 | 6.588 ± 0.301 | 4.695 ± 0.325 | 4.883 ± 0.344 |
| Dv (10) (µm) | 1.913 ± 0.096 | 3.063 ± 0.153 | 2.017 ± 0.110 | 2.023 ± 0.125 |
| Dv (50) (µm) | 9.582 ± 0.379 | 10.113 ± 0.406 | 10.330 ± 0.617 | 9.506 ± 0.467 |
| Dv (90) (µm) | 67.212 ± 3.261 | 43.153 ± 1.878 | 29.910 ± 1.669 | 31.418 ± 1.171 |
| Dv (98) (µm) | 110.072 ± 5.306 | 78.134 ± 3.709 | 45.799 ± 2.156 | 51.669 ± 2.283 |
| Dv (100) (µm) | 162.552 ± 7.128 | 125.641 ± 6.582 | 66.712 ± 3.036 | 75.878 ± 3.294 |
| Volume Below 1 μm (%) | 2.93 | 0.75 | 3.21 | 2.67 |
| Volume Below 10 μm (%) | 51.26 | 49.54 | 48.66 | 52.02 |
| Volume Above 45 μm (%) | 16.80 | 9.30 | 2.22 | 3.64 |
| Materials | k2 (g mg−1 min−1) | qe (mg L−1) | R2 |
|---|---|---|---|
| HASS | 25.8 ± 1.6 | 2.0170 ± 0.0005 | 1 |
| HAOS | 189.9 ± 8.4 | 2.0870 ± 0.0001 | 1 |
| Isotherm | HASS | HAOS |
|---|---|---|
| Langmuir | ||
| qs (mg g−1) | 173.70 ± 16.95 | 334.30 ± 46.20 |
| b (L mg−1) | 2.04 ± 1.58 | 0.31 ± 0.10 |
| R2 | 0.9154 | 0.9443 |
| Freundlich | ||
| kF (mg g−1) (L mg−1)1/n | 112.90 ± 14.87 | 105.90 ± 12.19 |
| n | 10.63 ± 3.72 | 3.97 ± 0.26 |
| R2 | 0.9577 | 0.9219 |
| HAOS | HASS | Cd-HAOS | Cd-HASS | |
|---|---|---|---|---|
| Calcite (wt%) | 24.5 (4) | 22.8 (2) | 18.4 (4) | 17.0 (4) |
| Aragonite (wt%) | - | 4.2 (2) | - | 4.2 (5) |
| Hydroxyapatite (wt%) | 75.5 (4) | 73.0 (3) | 68.1 (5) | 78.8 (6) |
| Cadmium Hydrogen Phosphate Hydrate (wt%) | - | - | 13.5 (3) | - |
| Crystallite size (nm) | 24.8 | 25.2 | 31.9 | 32.0 |
| Crystallinity (%) | 63.2 | 65.4 | 63.1 | 62.1 |
| Calcite CaCO3 | |||||
| R-3 c H | a = b (Å) | c (Å) | Volume (Å3) | ||
| HAOS | 4.9989 (7) | 17.111 (6) | 370.3 (3) | ||
| Cd-HAOS | 4.9927 (8) | 17.082 (3) | 368.8 (1) | ||
| HAAS | 4.9917 (1) | 17.0846 (4) | 368.6 (1) | ||
| Cd-HAAS | 4.9890 (4) | 17.0746 (2) | 368.0 (1) | ||
| Hydroxyapatite Ca10(PO4)6(OH)2 | |||||
| P 63/m | a = b (Å) | c (Å) | Volume (Å3) | ||
| HAOS | 9.404 (5) | 6.891 (4) | 527.8 (6) | ||
| Cd-HAOS | 9.455 (7) | 6.717 (6) | 520.1 (9) | ||
| HAAS | 9.396 (1) | 6.877 (1) | 525.8 (3) | ||
| Cd-HAAS | 9.400 (3) | 6.805 (4) | 520.7 (4) | ||
| Aragonite CaCO3 | |||||
| P mcn | a (Å) | b (Å) | c (Å) | Volume (Å3) | |
| HAAS | 4.968 (3) | 7.962 (7) | 5.752 (4) | 227.5 (3) | |
| Cd-HAAS | 4.964 (2) | 7.955 (3) | 5.745 (2) | 226.9 (1) | |
| Cadmium Hydrogen Phosphate Hydrate Cd5H2(PO4)4(H2O)4 | |||||
| C 1 2/c 1 | a (Å) | b (Å) | c (Å) | Volume (Å3) | β (°) |
| Cd-HAOS | 17.9553 (1) | 9.4258 (4) | 9.7121 (4) | 1632.9 (4) | 96.57 (1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cescon, M.; Chiefa, F.; Chenet, T.; Mancinelli, M.; Stevanin, C.; Martucci, A.; Pasti, L. Hydroxyapatite-Based Adsorbent Materials from Aquaculture Waste for Remediation of Metal-Contaminated Waters: Investigation of Cadmium Removal. Clean Technol. 2025, 7, 34. https://doi.org/10.3390/cleantechnol7020034
Cescon M, Chiefa F, Chenet T, Mancinelli M, Stevanin C, Martucci A, Pasti L. Hydroxyapatite-Based Adsorbent Materials from Aquaculture Waste for Remediation of Metal-Contaminated Waters: Investigation of Cadmium Removal. Clean Technologies. 2025; 7(2):34. https://doi.org/10.3390/cleantechnol7020034
Chicago/Turabian StyleCescon, Mirco, Francesco Chiefa, Tatiana Chenet, Maura Mancinelli, Claudia Stevanin, Annalisa Martucci, and Luisa Pasti. 2025. "Hydroxyapatite-Based Adsorbent Materials from Aquaculture Waste for Remediation of Metal-Contaminated Waters: Investigation of Cadmium Removal" Clean Technologies 7, no. 2: 34. https://doi.org/10.3390/cleantechnol7020034
APA StyleCescon, M., Chiefa, F., Chenet, T., Mancinelli, M., Stevanin, C., Martucci, A., & Pasti, L. (2025). Hydroxyapatite-Based Adsorbent Materials from Aquaculture Waste for Remediation of Metal-Contaminated Waters: Investigation of Cadmium Removal. Clean Technologies, 7(2), 34. https://doi.org/10.3390/cleantechnol7020034







