Octyl Gallate as an Intervention Catalyst to Augment Antifungal Efficacy of Caspofungin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Antifungal Bioassay: Saccharomyces cerevisiae
2.3. Antifungal Bioassay: Filamentous Fungi
2.4. Statistical Analysis
3. Results and Discussion
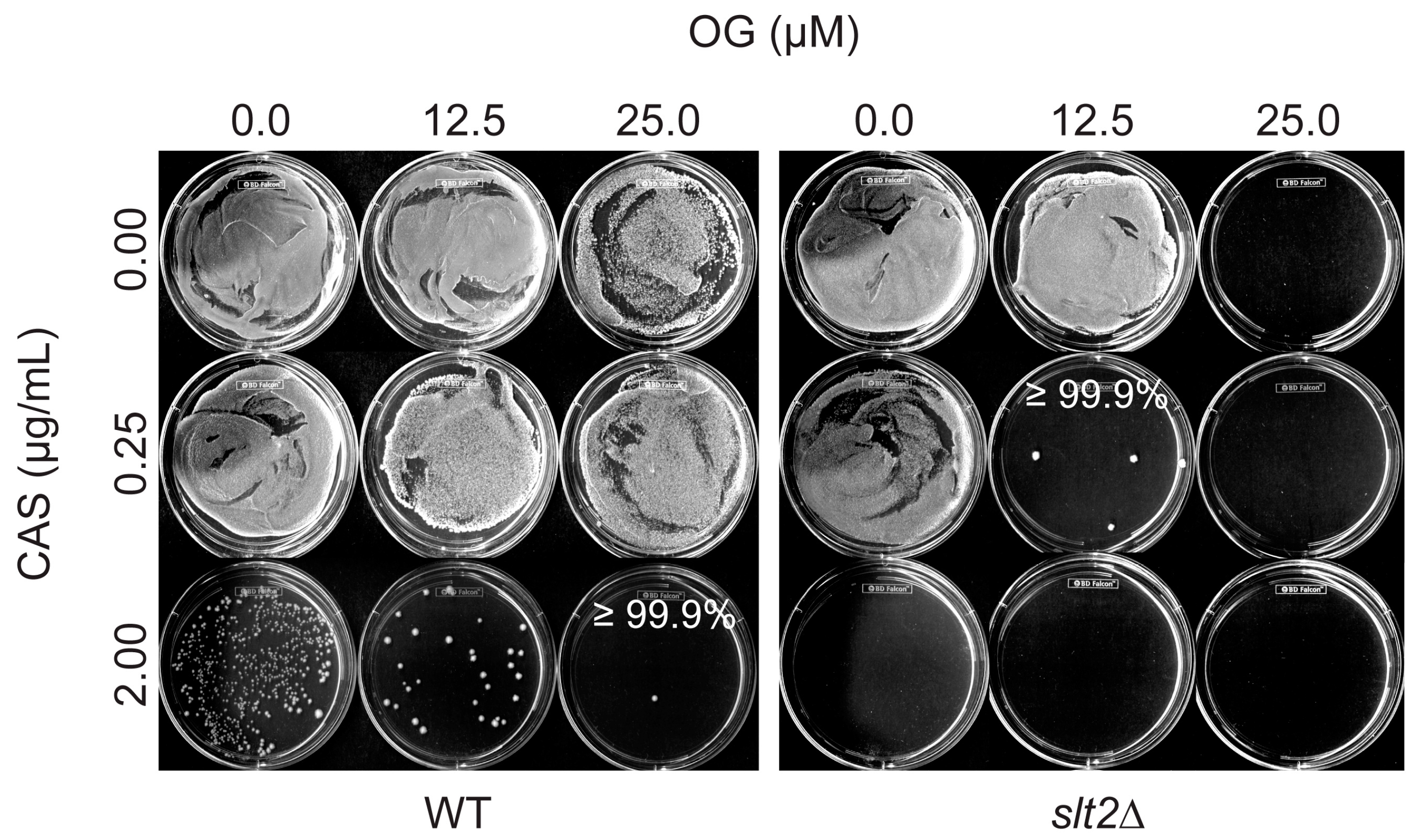
3.1. Octyl Gallate Perturbs the Fungal Cell Wall Integrity System: S. cerevisiae Bioassay
3.2. Octyl Gallate Enhances the Efficacy of Caspofungin: Filamentous Fungi Bioassay
3.3. Octyl Gallate Debilitates Antioxidant Mutants during Chemosensitization
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- McCarthy, M.W.; Kontoyiannis, D.P.; Cornely, O.A.; Perfect, J.R.; Walsh, T.J. Novel agents and drug targets to meet the challenges of resistant fungi. J. Infect. Dis. 2017, 216, S474–S483. [Google Scholar] [CrossRef] [PubMed]
- Beauvais, A.; Latgé, J.P. Membrane and cell wall targets in Aspergillus fumigatus. Drug Resist. Updates 2001, 4, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, B.B.; Mylonakis, E. Our paths might cross: The role of the fungal cell wall integrity pathway in stress response and cross talk with other stress response pathways. Eukaryot. Cell 2009, 8, 1616–1625. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, T.; Mizutani, O.; Furukawa, K.; Sato, N.; Yoshimi, A.; Yamagata, Y.; Nakajima, T.; Abe, K. MpkA-Dependent and -independent cell wall integrity signaling in Aspergillus nidulans. Eukaryot. Cell 2007, 6, 1497–1510. [Google Scholar] [CrossRef] [PubMed]
- Perlin, D.S. Mechanisms of echinocandin antifungal drug resistance. Ann. N. Y. Acad. Sci. 2015, 1354, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Walker, L.A.; Lee, K.K.; Munro, C.A.; Gow, N.A. Caspofungin treatment of Aspergillus fumigatus results in ChsG-dependent upregulation of chitin synthesis and the formation of chitin-rich micro-colonies. Antimicrob. Agents Chemother. 2015, 59, 5932–5941. [Google Scholar] [CrossRef] [PubMed]
- Cowen, L.E.; Steinbach, W.J. Stress, drugs, and evolution: The role of cellular signaling in fungal drug resistance. Eukaryot. Cell 2008, 7, 747–764. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.C.; Chan, K.L.; Kim, J.H. Chemosensitization as a means to augment commercial antifungal agents. Front. Microbiol. 2012, 3, 79. [Google Scholar] [CrossRef] [PubMed]
- Buckley, H.L.; Hart-Cooper, W.M.; Kim, J.H.; Faulkner, D.M.; Cheng, L.W.; Chan, K.L.; Vulpe, C.D.; Orts, W.J.; Amrose, S.E.; Mulvihill, M.J. Design and testing of safer, more effective preservatives for consumer products. ACS Sustain. Chem. Eng. 2017, 5, 4320–4331. [Google Scholar] [CrossRef]
- Xue, T.; Nguyen, C.K.; Romans, A.; May, G.S. A mitogen-activated protein kinase that senses nitrogen regulates conidial germination and growth in Aspergillus fumigatus. Eukaryot. Cell 2004, 3, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Reyes, G.; Romans, A.; Nguyen, C.K.; May, G.S. Novel mitogen-activated protein kinase MpkC of Aspergillus fumigatus is required for utilization of polyalcohol sugars. Eukaryot. Cell 2006, 5, 1934–1940. [Google Scholar] [CrossRef] [PubMed]
- Li, H.X.; Xiao, C.L. Characterization of fludioxonil-resistant and pyrimethanil-resistant phenotypes of Penicillium expansum from apple. Phytopathology 2008, 98, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Arendrup, M.C.; Cuenca-Estrella, M.; Lass-Flörl, C.; Hope, W. EUCAST technical note on the EUCAST definitive document EDef 7.2: Method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for yeasts EDef 7.2 (EUCAST-AFST). Clin. Microbiol. Infect. 2012, 18, E246–E247. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute (CLSI). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi: Approved Standard, 2nd ed.; CLSI Document M38-A2; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008; Volume 28. [Google Scholar]
- Odds, F.C. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 2003, 52, 1. [Google Scholar] [CrossRef] [PubMed]
- Isenberg, H.D. Clinical Microbiology Procedures Handbook, 1st ed.; American Society for Microbiology: Washington, DC, USA, 1992. [Google Scholar]
- Kirkman, T.W. Statistics to Use. Available online: http://www.physics.csbsju.edu/stats/ (accessed on 23 April 2018).
- Kim, J.H.; Campbell, B.C.; Yu, J.; Mahoney, N.; Chan, K.L.; Molyneux, R.J.; Bhatnagar, D.; Cleveland, T.E. Examination of fungal stress response genes using Saccharomyces cerevisiae as a model system: Targeting genes affecting aflatoxin biosynthesis by Aspergillus flavus Link. Appl. Microbiol. Biotechnol. 2005, 67, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.Y.; St.Onge, R.P.; Proctor, M.J.; Wallace, I.M.; Nile, A.H.; Spagnuolo, P.A.; Jitkova, Y.; Gronda, M.; Wu, Y.; Kim, M.K.; et al. Mapping the cellular response to small molecules using chemogenomic fitness signatures. Science 2014, 344, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Norris, M.; Lovell, S.; Delneri, D. Characterization and prediction of haploinsufficiency using systems-level gene properties in yeast. G3 2013, 3, 1965–1977. [Google Scholar] [CrossRef] [PubMed]
- Parsons, A.B.; Brost, R.L.; Ding, H.; Li, Z.; Zhang, C.; Sheikh, B.; Brown, G.W.; Kane, P.M.; Hughes, T.R.; Boone, C. Integration of chemical-genetic and genetic interaction data links bioactive compounds to cellular target pathways. Nat. Biotechnol. 2003, 22, 62. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration (FDA). Everything Added to Food in the United States. 2011. Available online: http://www.fda.gov/Food/IngredientsPackagingLabeling/FoodAdditivesIngredients/ucm115326.htm (accessed on 23 April 2018).
- Rúa, J.; Fernandez-Álvarez, L.; de Castro, C.; del Valle, P.; de Arriaga, D.; Garcia-Armesto, M.R. Antibacterial activity against foodborne Staphylococcus aureus and antioxidant capacity of various pure phenolic compounds. Foodborne Pathog. Dis. 2011, 8, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Fernández, J.; García-Armesto, M.R.; Álvarez-Alonso, R.; del Valle, P.; de Arriaga, D.; Rúa, J. Antimicrobial activity of binary combinations of natural and synthetic phenolic antioxidants against Enterococcus faecalis. J. Dairy Sci. 2013, 96, 4912–4920. [Google Scholar] [CrossRef] [PubMed]
- Reinoso-Martín, C.; Schüller, C.; Schuetzer-Muehlbauer, M.; Kuchler, K. The yeast protein kinase C cell integrity pathway mediates tolerance to the antifungal drug caspofungin through activation of Slt2p mitogen-activated protein kinase signaling. Eukaryot. Cell 2003, 2, 1200–1210. [Google Scholar] [CrossRef] [PubMed]
- Parra, E.; Jiménez-Barbero, J.; Bernabe, M.; Leal, J.A.; Prieto, A.; Gómez-Miranda, B. Structural investigation of two cell-wall polysaccharides of Penicillium expansum strains. Carbohydr. Res. 1994, 257, 239–248. [Google Scholar] [CrossRef]
- Gow, N.A.R.; Latge, J.-P.; Munro, C.A. The fungal cell wall: Structure, biosynthesis, and function. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- Joint FAO/WHO Expert Committee on Food Additives. Evaluation of the toxicity of a number of antimicrobials and antioxidants. World Health Organ. Tech. Rep. Ser. 1962, 228, 61–65. [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources Added to Food). Scientific Opinion on the re-evaluation of octyl gallate (E 311) as a food additive. EFSA J. 2015, 13, 4248. [Google Scholar] [CrossRef]
- Fujita, K.; Kubo, I. Antifungal activity of octyl gallate. Int. J. Food Microbiol. 2002, 79, 193–201. [Google Scholar] [CrossRef]
- Sierra-Campos, E.; Valdez-Solana, M.A.; Matuz-Mares, D.; Velazquez, I.; Pardo, J.P. Induction of morphological changes in Ustilago maydis cells by octyl gallate. Microbiology 2009, 155, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Guillen, F.; Evans, C.S. Anisaldehyde and veratraldehyde acting as redox cycling agents for H2O2 production by Pleurotus eryngii. Appl. Environ. Microbiol. 1994, 60, 2811–2817. [Google Scholar] [PubMed]
- Jacob, C. A scent of therapy: Pharmacological implications of natural products containing redox-active sulfur atoms. Nat. Prod. Rep. 2006, 23, 851–863. [Google Scholar] [CrossRef] [PubMed]
- García-Rodriguez, L.J.; Durán, A.; Roncero, C. Calcofluor antifungal action depends on chitin and a functional high-osmolarity glycerol response (HOG) pathway: Evidence for a physiological role of the Saccharomyces cerevisiae HOG pathway under noninducing conditions. J. Bacteriol. 2000, 182, 2428–2437. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Ram, A.F.; Sheraton, J.; Klis, F.M.; Bussey, H. Regulation of cell wall beta-glucan assembly: PTC1 negatively affects PBS2 action in a pathway that includes modulation of EXG1 transcription. Mol. Gen. Genet. 1995, 248, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.H.; Silverman, S.J.; Gaughran, J.P.; Kirsch, D.R. Multiple copies of PBS2, MHP1 or LRE1 produce glucanase resistance and other cell wall effects in Saccharomyces cerevisiae. Yeast 1997, 13, 199–213. [Google Scholar] [CrossRef]
- Alonso-Monge, R.; Navarro-García, F.; Molero, G.; Diez-Orejas, R.; Gustin, M.; Pla, J.; Sánchez, M.; Nombela, C. Role of the mitogen-activated protein kinase Hog1p in morphogenesis and virulence of Candida albicans. J. Bacteriol. 1999, 181, 3058–3068. [Google Scholar] [PubMed]
- Rodríguez-Peña, J.M.; García, R.; Nombela, C.; Arroyo, J. The high-osmolarity glycerol (HOG) and cell wall integrity (CWI) signalling pathways interplay: A yeast dialogue between MAPK routes. Yeast 2010, 27, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Ogita, A.; Fujita, K.; Taniguchi, M.; Tanaka, T. Enhancement of the fungicidal activity of amphotericin B by allicin, an allyl-sulfur compound from garlic, against the yeast Saccharomyces cerevisiae as a model system. Planta Med. 2006, 72, 1247–1250. [Google Scholar] [CrossRef] [PubMed]


| Aspergillus Strains | Strain Characteristics | Source |
| A. fumigatus AF293 | Human pathogen (aspergillosis), parental strain, reference clinical strain used for genome sequencing | [10] |
| A. fumigatus sakA∆ | Mitogen-activated protein kinase (MAPK) gene deletion mutant derived from AF293 | [10] |
| A. fumigatus mpkC∆ | MAPK gene deletion mutant derived from AF293 | [11] |
| A. flavus 3357 | Toxigenic (aflatoxin-producing), human pathogen (aspergillosis), reference strain for genome sequencing | NRRL 1 |
| A. flavus 4212 | Toxigenic (aflatoxin-producing), human pathogen (aspergillosis) | NRRL |
| A. parasiticus 2999 | Toxigenic (aflatoxin-producing) | NRRL |
| A. parasiticus 5862 | Toxigenic (aflatoxin-producing) | NRRL |
| Penicillium Strains | Strain Characteristics | Source |
| P. expansum W1 | Toxigenic (patulin-producing; parental strain) | [12] |
| P. expansum FR2 | Fludioxonil resistant mutant derived from P. expansum W1 | [12] |
| P. expansum W2 | Toxigenic (patulin-producing; parental strain) | [12] |
| P. expansum FR3 | Fludioxonil resistant mutant derived from P. expansum W2 | [12] |
| P. glabrum 766 | Environmental contaminant | NRRL |
| P. chrysogenum 824 | Fleming’s penicillin-producing strain | NRRL |
| P. griseofulvum 2159 | Environmental contaminant | NRRL |
| P. italicum 983 | Environmental contaminant | NRRL |
| Strains | Compounds | MIC Alone | MIC Combined | FICI | MFC Alone | MFC Combined | FFCI |
|---|---|---|---|---|---|---|---|
| A. fumigatus AF293 | CAS OG | 128 0.2 | 32 0.1 | 0.8 | 128 0.4 | 64 0.2 | 1.0 |
| A. fumigatus sakA∆ | CAS OG | 128 0.2 | 8 0.1 | 0.6 | 128 0.2 | 64 0.1 | 1.0 |
| A. fumigatus mpkC∆ | CAS OG | 128 0.2 | 8 0.1 | 0.6 | 128 0.4 | 64 0.1 | 0.8 |
| A. flavus 4212 | CAS OG | 128 0.2 | 64 0.1 | 1.0 | 128 0.4 | 64 0.2 | 1.0 |
| A. flavus 3357 | CAS OG | 128 0.4 | 2 0.2 | 0.5 | 128 1.6 | 128 1.6 | 2.0 |
| A. parasiticus 5862 | CAS OG | 128 0.4 | 64 0.2 | 1.0 | 128 1.6 | 128 1.6 | 2.0 |
| A. parasiticus 2999 | CAS OG | 128 0.4 | 4 0.2 | 0.5 | 128 1.6 | 64 0.2 | 0.6 |
| Mean, Aspergillus 2 | CAS OG | 128.00 0.32 | 33.20 0.16 | 0.8 | 128.00 1.12 | 89.60 0.76 | 1.4 |
| t-test 3 | CAS OG | - - | p < 0.001 p < 0.05 | - | - - | p < 0.05 p < 0.5 | - |
| P. expansum W1 | CAS OG | 128 0.2 | 32 0.05 | 0.5 | 128 1.6 | 324 0.8 | 0.8 |
| P. expansum FR2 | CAS OG | 128 0.2 | 32 0.05 | 0.5 | 128 1.6 | 32 0.8 | 0.8 |
| P. expansum W2 | CAS OG | 128 0.2 | 32 0.05 | 0.5 | 128 1.6 | 32 0.8 | 0.8 |
| P. expansum FR3 | CAS OG | 128 0.2 | 32 0.05 | 0.5 | 128 1.6 | 32 0.8 | 0.8 |
| P. glabrum 766 | CAS OG | 128 0.2 | 16 0.025 | 0.3 | 128 1.6 | 32 0.05 | 0.3 |
| P. italicum 983 | CAS OG | 64 0.2 | 16 0.05 | 0.5 | 64 0.4 | 16 0.2 | 0.8 |
| P. griseofulvum 2159 | CAS OG | 128 0.2 | 8 0.1 | 0.6 | 128 0.8 | 16 0.2 | 0.4 |
| P. chrysogenum 824 | CAS OG | 128 0.2 | 16 0.05 | 0.4 | 128 0.2 | 32 0.05 | 0.5 |
| Mean, Penicillium 2 | CAS OG | 117.33 0.20 | 20.00 0.05 | 0.4 | 117.33 1.03 | 26.67 0.35 | 0.6 |
| t-test 3 | CAS OG | - - | p < 0.001 p < 0.001 | - | - - | p = 0.05 p < 0.05 | - |
| Mean, TOTAL 2 | CAS OG | 122.18 0.25 | 26.00 0.10 | 0.6 | 122.18 1.07 | 55.27 0.54 | 1.0 |
| t-test 3 | CAS OG | - - | p < 0.001 p < 0.001 | - | - - | p < 0.001 p < 0.05 | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.H.; Chan, K.L.; Cheng, L.W. Octyl Gallate as an Intervention Catalyst to Augment Antifungal Efficacy of Caspofungin. J 2018, 1, 19-28. https://doi.org/10.3390/j1010004
Kim JH, Chan KL, Cheng LW. Octyl Gallate as an Intervention Catalyst to Augment Antifungal Efficacy of Caspofungin. J. 2018; 1(1):19-28. https://doi.org/10.3390/j1010004
Chicago/Turabian StyleKim, Jong H., Kathleen L. Chan, and Luisa W. Cheng. 2018. "Octyl Gallate as an Intervention Catalyst to Augment Antifungal Efficacy of Caspofungin" J 1, no. 1: 19-28. https://doi.org/10.3390/j1010004
APA StyleKim, J. H., Chan, K. L., & Cheng, L. W. (2018). Octyl Gallate as an Intervention Catalyst to Augment Antifungal Efficacy of Caspofungin. J, 1(1), 19-28. https://doi.org/10.3390/j1010004







