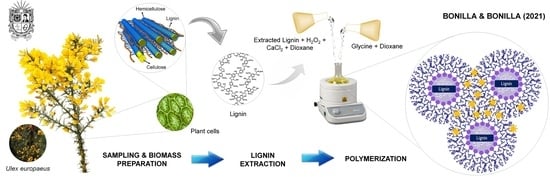
Synthesis and Characterization of a Novel Lignin-Based Biopolymer from Ulex europaeus: A Preliminary Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Lignin Extraction
2.3. Synthesis of the Biopolymer
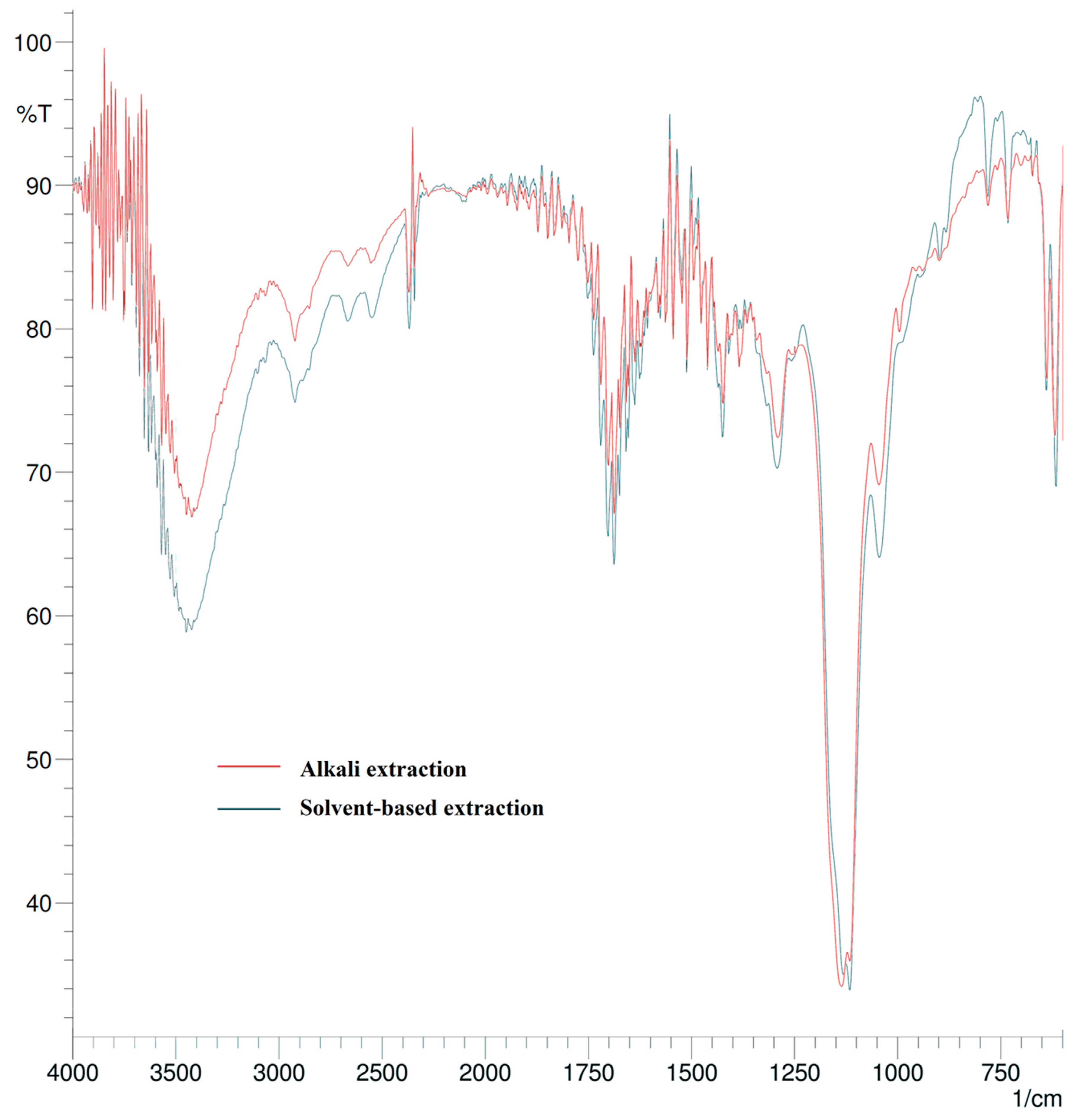
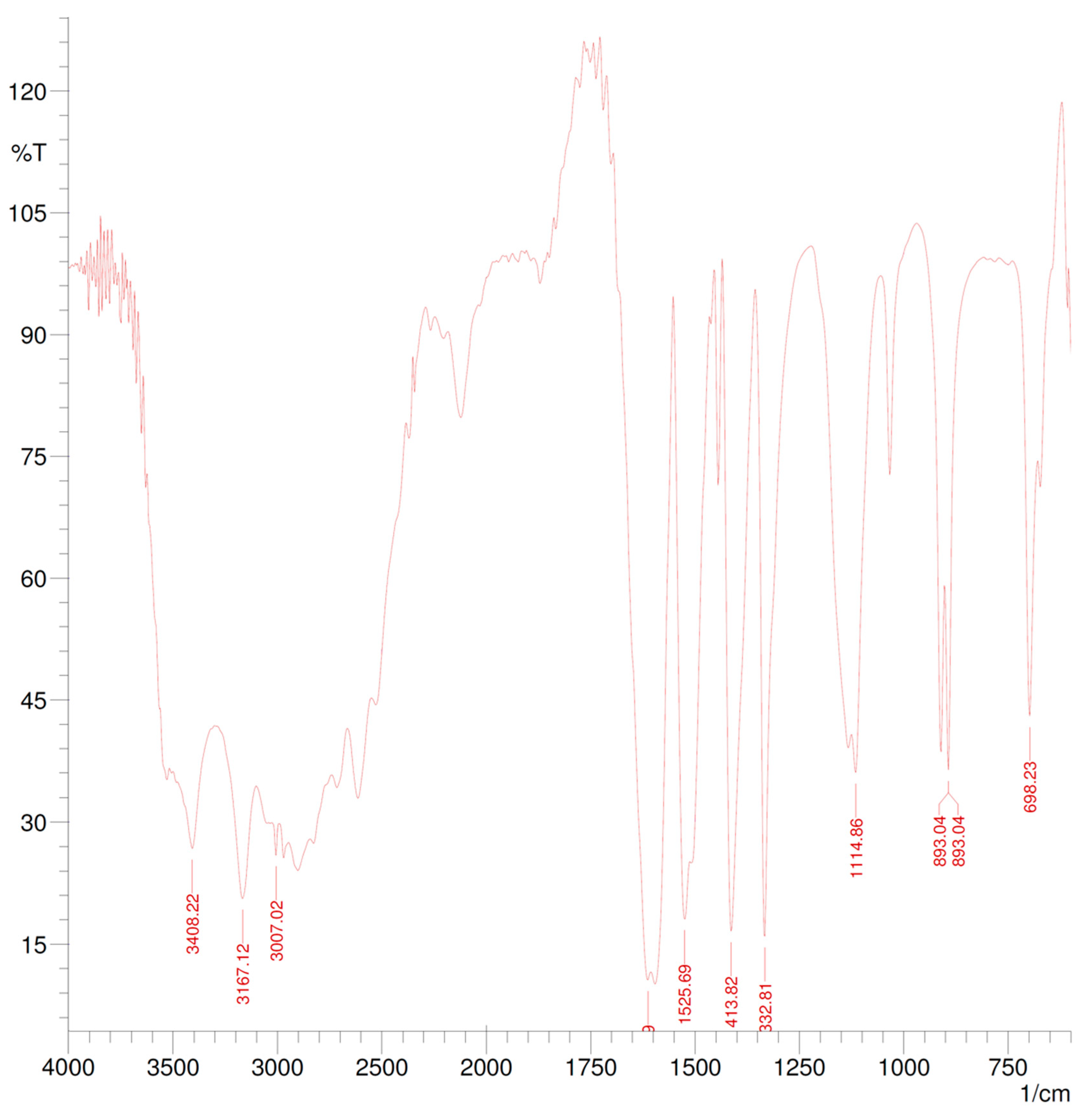
2.4. Spectroscopic Characterization
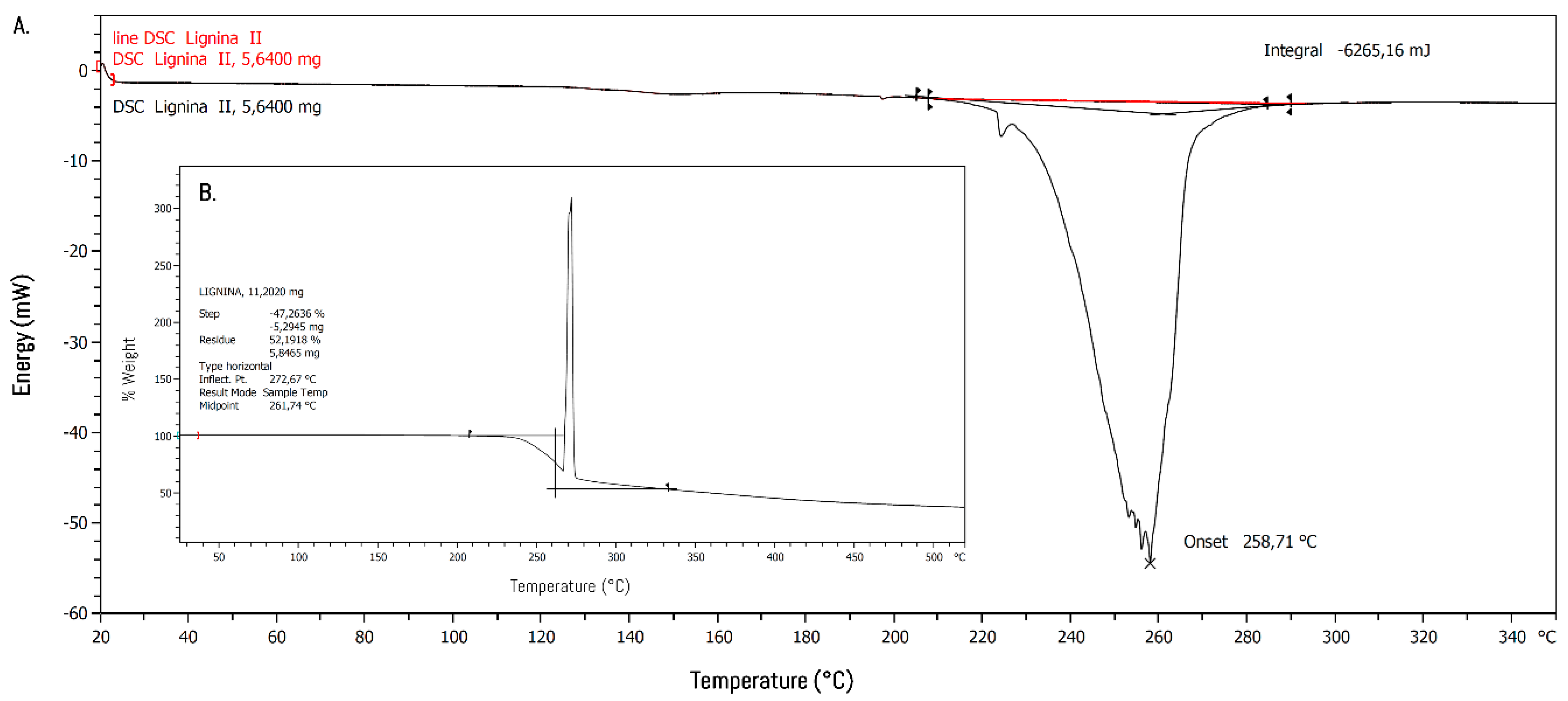
2.5. Thermal Properties
2.6. Biodegradation
3. Results
3.1. Lignin Extraction
3.2. Polymerization and Characterization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Wavenumber (cm−1) | Correspondence |
|---|---|
| 3529.73 | O-H stretch for alcohols |
| 3408.22 | N-H stretch for amides |
| 3026.31 | =CH2 asymmetrical stretch for alkenes |
| 3007.02 | =CH2 symmetrical stretch for alkenes |
| 2827.64 | –OCH3 stretch for ethers |
| 2000–1660 | Combination bands of benzene |
| 1870.95 | Out-of-plane =CH2 vibrations for alkenes |
| 1614.42 | C=O stretch for amides |
| 1612.49 | C=N stretch for imines |
| 1525.69 | N-H bend for amides |
| 1444.68 | CH2-C=C stretch for alkenes |
| 1413.82 | O-H bend for alcohols |
| 1332.81 | C-N stretch for amines |
| 1116.78 | C-O stretch for ethers |
| 1114.86 | C-O stretch for alcohols |
| 1033.84 | =C-O-C stretch for ethers |
| 910.40 | In-plane =CH2 bend for alkenes |
| 893.04 | Out-of-plane =CH2 stretch for alkenes |
| 698.23 | Out-of-plane N-H bend for amines |
| 673.16 | Out-of-plane Ar-H bend for benzene derivates |
References
- Schöb, C.; Atlan, A.; Limbada, F.; Christina, M. Climatic niche shift of an invasive shrub (Ulex europaeus): A global scale comparison in native and introduced regions. J. Plant Ecol. 2020, 13, 42–50. [Google Scholar] [CrossRef]
- Contu, S.R.; Rivers, M.C. Ulex europaeus. The IUCN Red List of Threatened Species 2017: e.T19891755A86138815. Available online: https://dx.doi.org/10.2305/IUCN.UK.2017-3.RLTS.T19891755A86138815.en (accessed on 8 October 2020).
- Broadfield, N.; McHenry, M.T. A World of Gorse: Persistence of Ulex europaeus in Managed Landscapes. Plants 2019, 8, 523. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Bonilla, F.D.P. Estado de Conocimiento de Especies Invasoras. Propuesta de Lineamientos Para el Control de Los Impactos; Instituto de Investigacion de Recursos Biológicos Alexander Von Humboldt: Bogotá, Colombia, 2006. [Google Scholar]
- Díaz-Espinosa, A.; Díaz-Triana, J.; Vargas, O. Catálogo de Plantas Invasoras de los Humedales de Bogotá; Universidad Nacional de Colombia: Bogotá, Colombia, 2012. [Google Scholar]
- Aguilar, M. Restauración Ecológica de Áreas afectadas por Ulex Europaeus l. Serranía El Zuque, Reserva Forestal Bosque Oriental de Bogotá, localidad 4 San Cristóbal, Bogotá DC, Colombia. Master’s Thesis, Universidad de Alcalá, Alcalá de Henares, Spain, 2010. [Google Scholar]
- Udo, N.; Tarayre, M.; Atlan, A. Evolution of germination strategy in the invasive species Ulex europaeus. J. Plant Ecol. 2016. [Google Scholar] [CrossRef]
- Zabaleta, A.; Vargas, O. Expresión in situ del banco de semillas germinable de Ulex europaeus y su relación con la estructura de los matorrales. In Estrategias Para la Restauración Ecológica del Bosque alto Andino. El Caso de la Reserva Forestal Municipal de Cogua, Cundinamarca; Vargas, O., Ambiente, S.D.d., Eds.; Universidad Nacional de Colombia: Bogotá, Colombia, 2009; Volume 372. [Google Scholar]
- Gómez-Ruiz, P.A.; Lindig-Cisneros, R.; Vargas-Ríos, O. Facilitation among plants: A strategy for the ecological restoration of the high-andean forest (Bogotá, D.C.—Colombia). Ecol. Eng. 2013, 57, 267–275. [Google Scholar] [CrossRef]
- Bateman, J.B.; Vitousek, P.M. Soil fertility response to Ulex europaeus invasion and restoration efforts. Biol. Invasions 2018, 20, 2777–2791. [Google Scholar] [CrossRef]
- Edwards, G.R.; Tozer, K.N.; Maxwell, T.M.R.; Marshall, A.J. Control of gorse (Ulex europaeus) in dryland pasture converted from Pinus radiata forest. New Zealand Plant Prot. 2007, 60, 141–145. [Google Scholar] [CrossRef]
- Sánchez Fernández, M.P.; Millán Orduz, D.L.; Manrique Osorio, P.; Rico Torres, D.Z.; Salazar Torres, M.L. Erradicación de retamo espinoso e inicio de restauración ecológica en los cerros orientales de Bogotá (prueba piloto). In Proceedings of the Encuentro Internacional de Educación en Ingeniería, Cartagena, Colombia, 18–21 September 2018. [Google Scholar]
- Amaya-Villarreal, Á.M.; Renjifo, L.M.J.O.C. Efecto del retamo espinoso (Ulex europaeus) sobre las aves de borde en un bosque altoandino. Ornitol. Colomb. 2010, 10, 11–25. [Google Scholar]
- Gong, X.; Chen, Y.; Wang, T.; Jiang, X.; Hu, X.; Feng, J. Double-edged effects of climate change on plant invasions: Ecological niche modeling global distributions of two invasive alien plants. Sci. Total Environ. 2020, 740. [Google Scholar] [CrossRef] [PubMed]
- Mgidi, T.N.; Le Maitre, D.C.; Schonegevel, L.; Nel, J.L.; Rouget, M.; Richardson, D.M. Alien plant invasions—incorporating emerging invaders in regional prioritization: A pragmatic approach for Southern Africa. J. Environ. Manag. 2007, 84, 173–187. [Google Scholar] [CrossRef]
- Altamirano, A.; Cely, J.P.; Etter, A.; Miranda, A.; Fuentes-Ramirez, A.; Acevedo, P.; Salas, C.; Vargas, R. The invasive species Ulex europaeus (Fabaceae) shows high dynamism in a fragmented landscape of south-central Chile. Environ. Monit. Assess. 2016, 188. [Google Scholar] [CrossRef]
- McLean, J.; Thomson, J.B. Some constituents of Ulex europaeus L. Phytochemistry 1963, 2, 179–181. [Google Scholar] [CrossRef]
- Osorio-Castiblanco, D.F.; Peyre, G.; Saldarriaga, J.F. Physicochemical Analysis and Essential Oils Extraction of the Gorse (Ulex europaeus) and French Broom (Genista monspessulana), Two Highly Invasive Species in the Colombian Andes. Sustainability 2019, 12, 57. [Google Scholar] [CrossRef]
- Núñez-Moreno, A.; Barbieri, G.; Gordillo, G. Analysis of the Feasibility of Generating Solid Biofuel from Ulex Europaeus Plants. Rev. Fac. Ing. 2019, 29. [Google Scholar] [CrossRef]
- Celis, R.; Torres, M.; Valenzuela, P.; Rios, R.; Gacitúa, W.; Pesenti, H. Characterizing Cellulosic Fibers from Ulex europaeus. BioResources 2014, 9, 13. [Google Scholar] [CrossRef]
- Pesenti, H.; Torres, M.; Oliveira, P.; Gacitua, W.; Leoni, M. Exploring Ulex europaeus to Produce Nontoxic Binderless Fibreboard. BioResources 2017, 12, 13. [Google Scholar] [CrossRef][Green Version]
- Hüttermann, A.; Mai, C.; Kharazipour, A. Modification of lignin for the production of new compounded materials. Appl. Microbiol. Biotechnol. 2001, 55, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, A.; Pereira, H. Compositional Variability of Lignin in Biomass. In Lignin—Trends and Applications; Poletto, M., Ed.; IntechOpen Limited: London, UK, 2018. [Google Scholar] [CrossRef]
- Sharma, S.; Sharma, A.; Mulla, S.I.; Pant, D.; Sharma, T.; Kumar, A. Lignin as Potent Industrial Biopolymer: An Introduction. In Lignin: Biosynthesis and Transformation for Industrial Applications; Sharma, S., Kumar, A., Eds.; Springer: Basel, Switzerland, 2020; pp. 1–15. [Google Scholar]
- Toledano-Zabaleta, A. Lignin Extraction, Purification and Depolymerization Study; Universidad del País Vasco-Euskal Herriko Unibertsitatea: San Sebastián, Spain, 2012. [Google Scholar]
- Gautam, N.; Kaur, I. Soil burial biodegradation studies of starch grafted polyethylene and identification of Rhizobium meliloti therefrom. J. Environ. Chem. Ecotoxicol. 2013, 5, 147–158. [Google Scholar] [CrossRef]
- Ekielski, A.; Mishra, P.K. Lignin for Bioeconomy: The Present and Future Role of Technical Lignin. Int. J. Mol. Sci. 2020, 22, 63. [Google Scholar] [CrossRef]
- Bykov, I. Characterization of Natural and Technical Lignins Using FT-IR Spectroscopy. Master’s Thesis, Luleå University of Technology, Luleå, Sweden, 2008. [Google Scholar]
- El Mansouri, N.-E. Despolimerización de Lignina Para su Aprovechamiento en Adhesivos Para Producir Tableros de Partículas; Universitat Rovira i Virgili: Tarragona, Spain, 2007. [Google Scholar]
- Douglas, C.J. Phenylpropanoid metabolism and lignin biosynthesis: From weeds to trees. Trends Plant Sci. 1996, 1, 171–178. [Google Scholar] [CrossRef]
- Meister, J.J.; Patil, D.R.; Field, L.R.; Nicholson, J.C. Synthesis and characterization of graft copolymers from lignin and 2-propenamide. J. Polym. Sci. Polym. Chem. Ed. 1984, 22, 1963–1980. [Google Scholar] [CrossRef]
- Chen, R.L.; Kokta, B.V.; Daneault, C.; Valade, J.L. Some water-soluble copolymers from lignin. J. Appl. Polym. Sci. 1986, 32, 4815–4826. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, B.; Zheng, G.; He, S.; Gao, J. Graft copolymerization of methyl methacrylate on stone ground wood using the H2O2Fe2 method. J. Appl. Polym. Sci. 1992, 45, 71–77. [Google Scholar] [CrossRef]
- Koshijima, T.; Muraki, E. Radical grafting on lignin. Part, I. Radiation-induced grafting of styrene onto hydrochloric acid lignin. J. Polym. Sci. Part A-1 Polym. Chem. 1968, 6, 1431–1440. [Google Scholar] [CrossRef]
- Phillips, R.B.; Brown, W.; Stannett, V.T. The graft copolymerization of styrene and lignin. II. Kraft softwood lignin. J. Appl. Polym. Sci. 1972, 16, 1–14. [Google Scholar] [CrossRef]
- Nudelman, N. Química Sustentable; Universidad Nacional del Litoral: Santa Fe, Argentina, 2004. [Google Scholar]
- Vroman, I.; Tighzert, L. Biodegradable Polymers. Materials 2009, 2, 307. [Google Scholar] [CrossRef]
- Alvarado Santillán, O.; Sánchez Fernández, E. Obtención de un Polímero Biodegradable a Partir de la Mezcla de Alcohol Polivinílico y Amilosa Extraída del Almidón de Colocasia Esculenta (Vituca) Proveniente del Distrito de Yambrasbamba; Universidad Nacional Toribio Rodríguez de Mendoza de Amazonas: Chachapoyas, Perú, 2015. [Google Scholar]
- Rodríguez Galán, R.A.; Franco García, M.L.; Puiggalí Bellalta, J. Biodegradable Poly(Ester Amide)s: Synthesis and Applications. In Biodegradable Polymers: Processing, Degradation and Applications; Nova Publishers: Hauppauge, NY, USA, 2011; pp. 207–272. [Google Scholar]
- Hans, M.L. Synthesis, Characterization, and Application of Biodegradable Polymeric Prodrug Micelles for Long-Term Drug Delivery; Drexel University: Philadelphia, PA, USA, 2006. [Google Scholar]
- Kawamoto, H. Lignin pyrolysis reactions. J. Wood Sci. 2017, 63, 117–132. [Google Scholar] [CrossRef]
- Caballero, J.A. Estudio Cinético de la Pirólisis de Lignina: Diseño de un Reactor Para el Estudio de las Reacciones Secundarias; Universidad de Alicante: Alicante, Spain, 1995. [Google Scholar]
- Balat, M. Mechanisms of Thermochemical Biomass Conversion Processes. Part 1: Reactions of Pyrolysis. Energy Sources Part A Recovery Util. Environ. Eff. 2008, 30, 620–635. [Google Scholar] [CrossRef]
- Beall, F.C.; Eickner, H.W. Thermal Degradation of Wood Components: A Review of the Literature; US Forest Products Laboratory: Madison, MI, USA, 1970; Volume 130. [Google Scholar]
- Poletto, M. Assessment of the thermal behavior of lignins from softwood and hardwood species. Maderas. Cienc. Y Tecnol. 2017, 19, 63–74. [Google Scholar] [CrossRef]
- Ganewatta, M.S.; Lokupitiya, H.N.; Tang, C. Lignin Biopolymers in the Age of Controlled Polymerization. Polymers 2019, 11, 1176. [Google Scholar] [CrossRef] [PubMed]
- Tuomela, M.; Vikman, M.; Hatakka, A.; Itävaara, M. Biodegradation of lignin in a compost environment: A review. Bioresour. Technol. 2000, 72, 169–183. [Google Scholar] [CrossRef]
- Stucki, G.; Alexander, M. Role of dissolution rate and solubility in biodegradation of aromatic compounds. Appl. Environ. Microbiol. 1987, 53, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Ruggero, F.; Carretti, E.; Gori, R.; Lotti, T.; Lubello, C. Monitoring of degradation of starch-based biopolymer film under different composting conditions, using TGA, FTIR and SEM analysis. Chemosphere 2020, 246. [Google Scholar] [CrossRef]
- Luyt, A.S.; Malik, S.S. Can Biodegradable Plastics Solve Plastic Solid Waste Accumulation? In Plastics to Energy; Al-Salem, S.M., Ed.; Elsevier Inc.: Oxford, UK, 2019; pp. 403–423. [Google Scholar] [CrossRef]
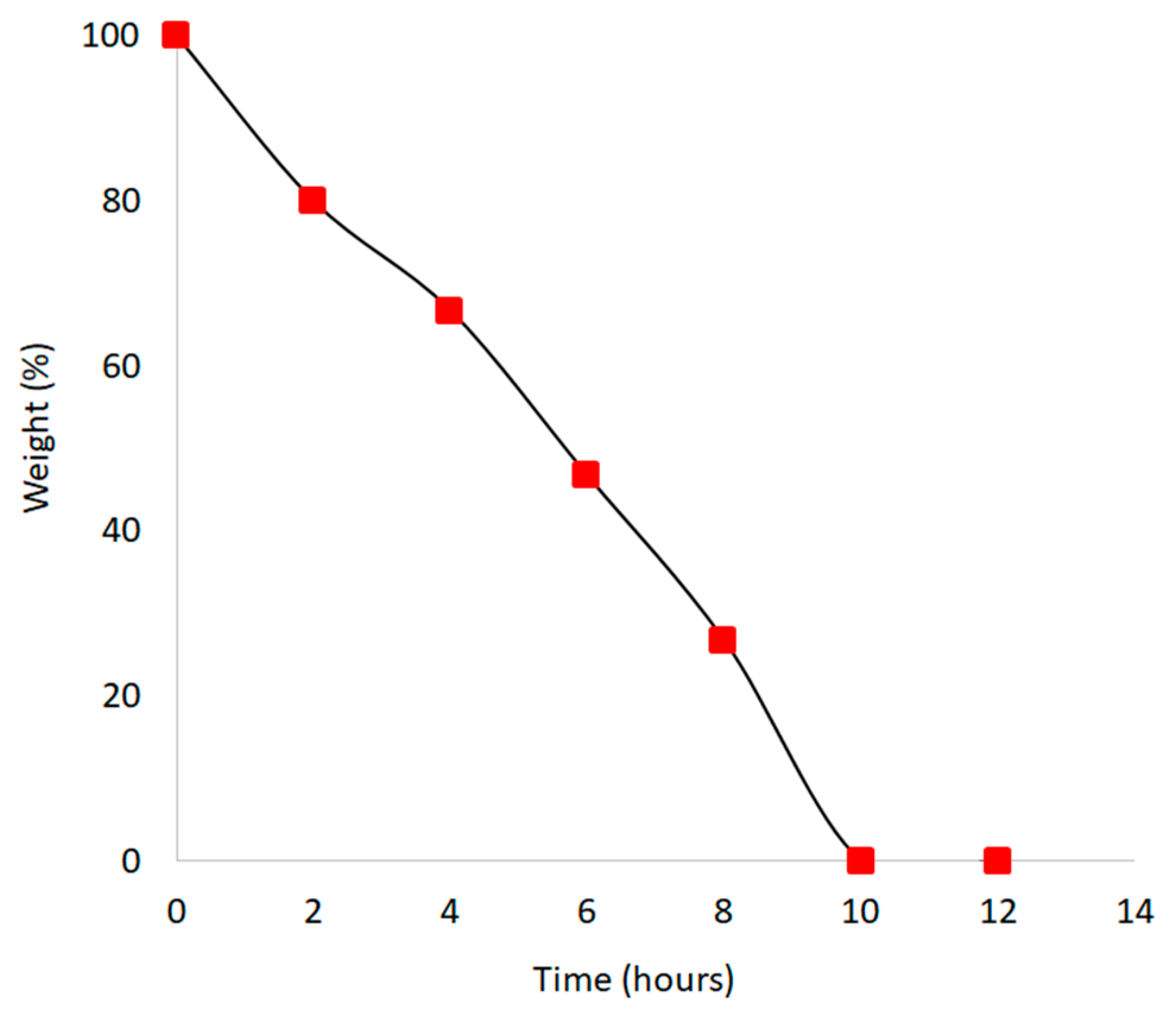
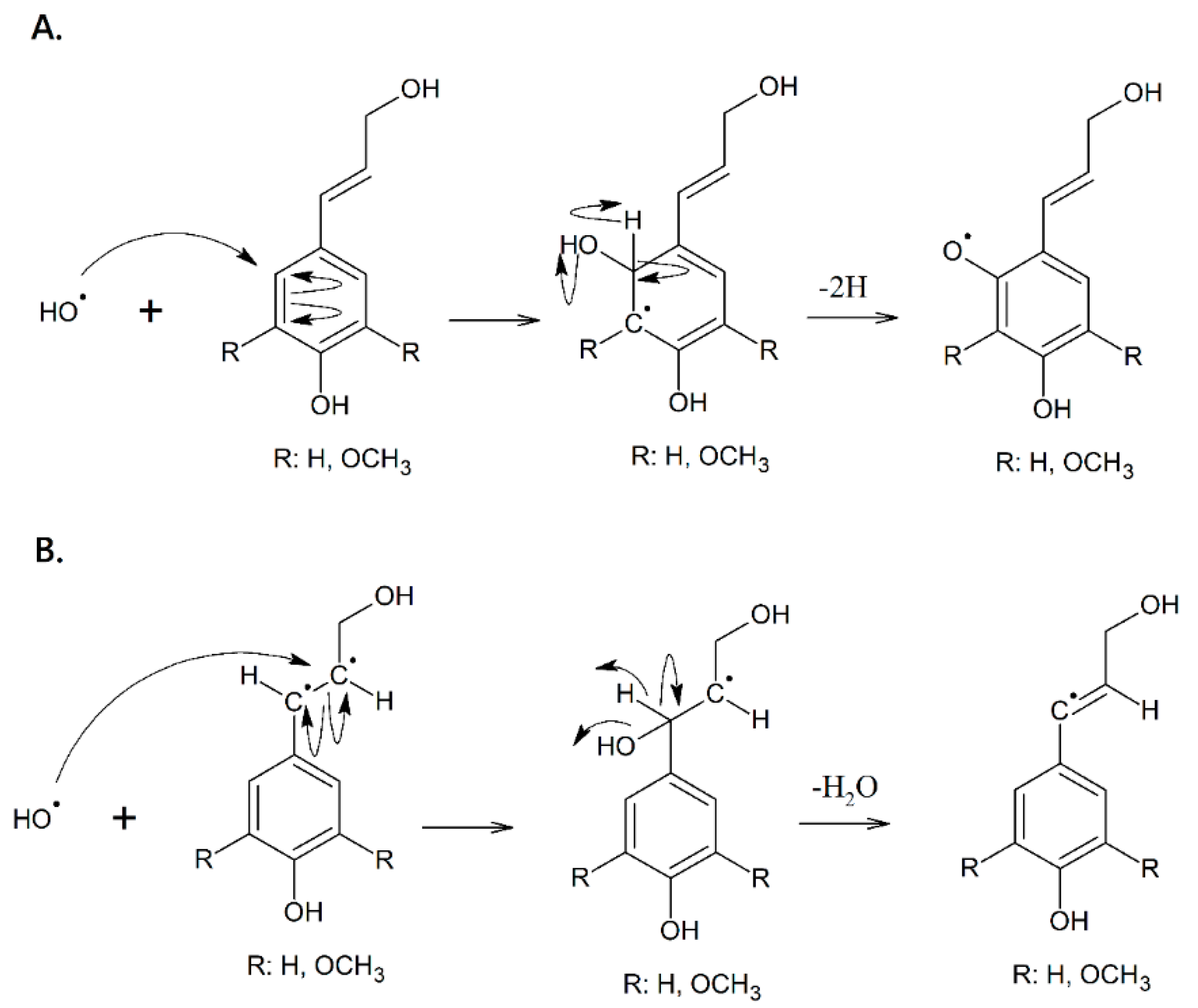


| Extraction Method | Stem (g) | Theoretical Yield * (g) | Actual Yield (g) | Percentage Yield (%) |
|---|---|---|---|---|
| Alkaline | 10.0 | 2.25 | 1.575 | 70 |
| Solvent-based | 10.0 | 2.25 | 1.120 | 50 |
| Solution | Reactants | Amount | Solvent | Product |
|---|---|---|---|---|
| 1 | Lignin * | 0.5 g | 20 mL of 70% 1,4-Dioxane | 1.2 g |
| H2O2 | 5 mL | |||
| CaCl2 | 0.2 g | |||
| 2 | Glycine | 1.5 | 30 mL of 70% 1,4-Dioxane |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonilla, A.F.; Bonilla, D.A. Synthesis and Characterization of a Novel Lignin-Based Biopolymer from Ulex europaeus: A Preliminary Study. J 2021, 4, 101-115. https://doi.org/10.3390/j4020009
Bonilla AF, Bonilla DA. Synthesis and Characterization of a Novel Lignin-Based Biopolymer from Ulex europaeus: A Preliminary Study. J. 2021; 4(2):101-115. https://doi.org/10.3390/j4020009
Chicago/Turabian StyleBonilla, Andrés F., and Diego A. Bonilla. 2021. "Synthesis and Characterization of a Novel Lignin-Based Biopolymer from Ulex europaeus: A Preliminary Study" J 4, no. 2: 101-115. https://doi.org/10.3390/j4020009
APA StyleBonilla, A. F., & Bonilla, D. A. (2021). Synthesis and Characterization of a Novel Lignin-Based Biopolymer from Ulex europaeus: A Preliminary Study. J, 4(2), 101-115. https://doi.org/10.3390/j4020009