A Hypothesis on How the Azolla Symbiosis Mitigates Nitrous Oxide Based on In Silico Analyses
Abstract
:1. Introduction—Nitrous Oxide, Climate Change and Azolla
2. Results and Discussion
2.1. Rationale for an In Silico Study of a Candidate Protein from Trichormus azollae
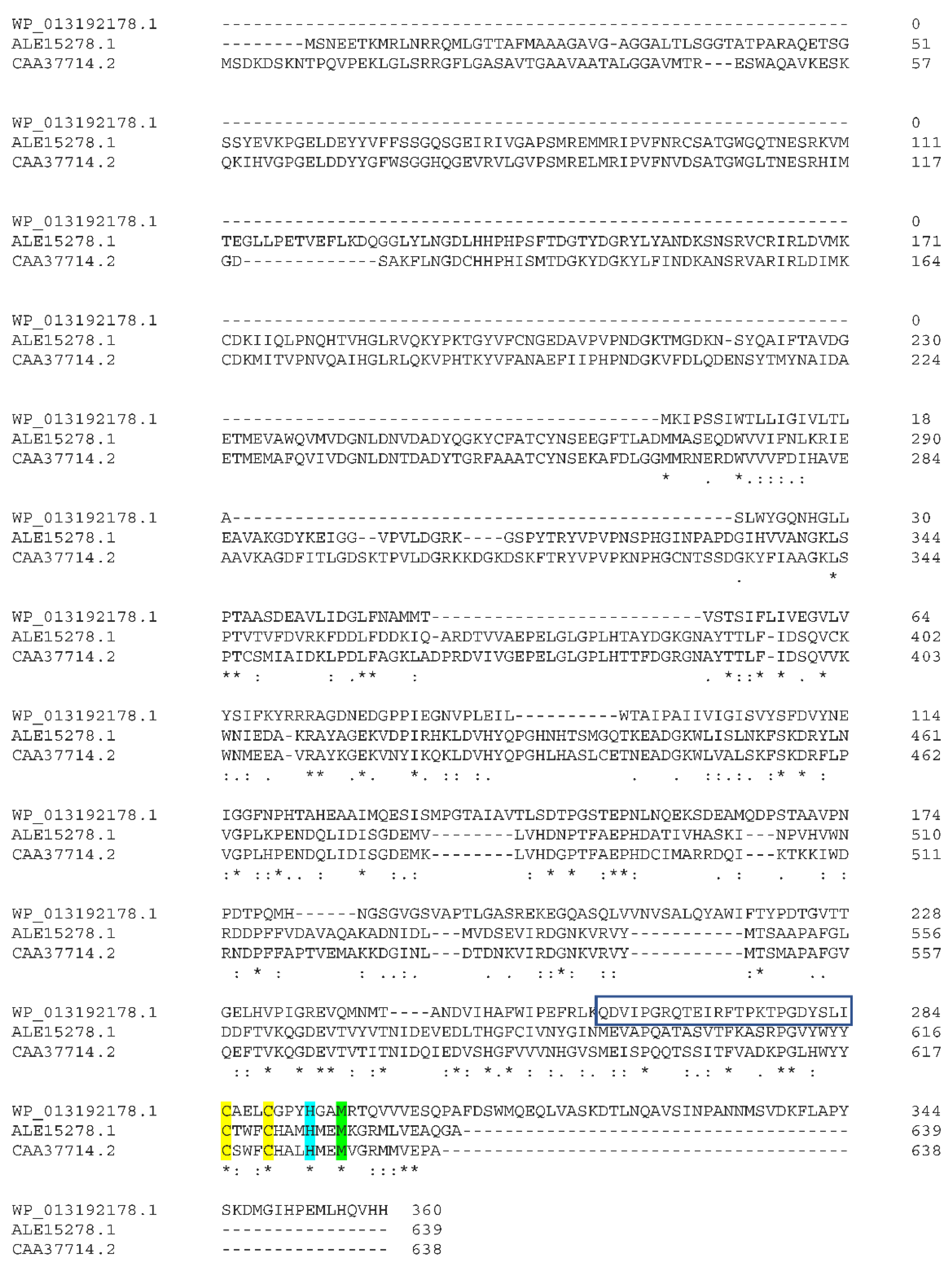
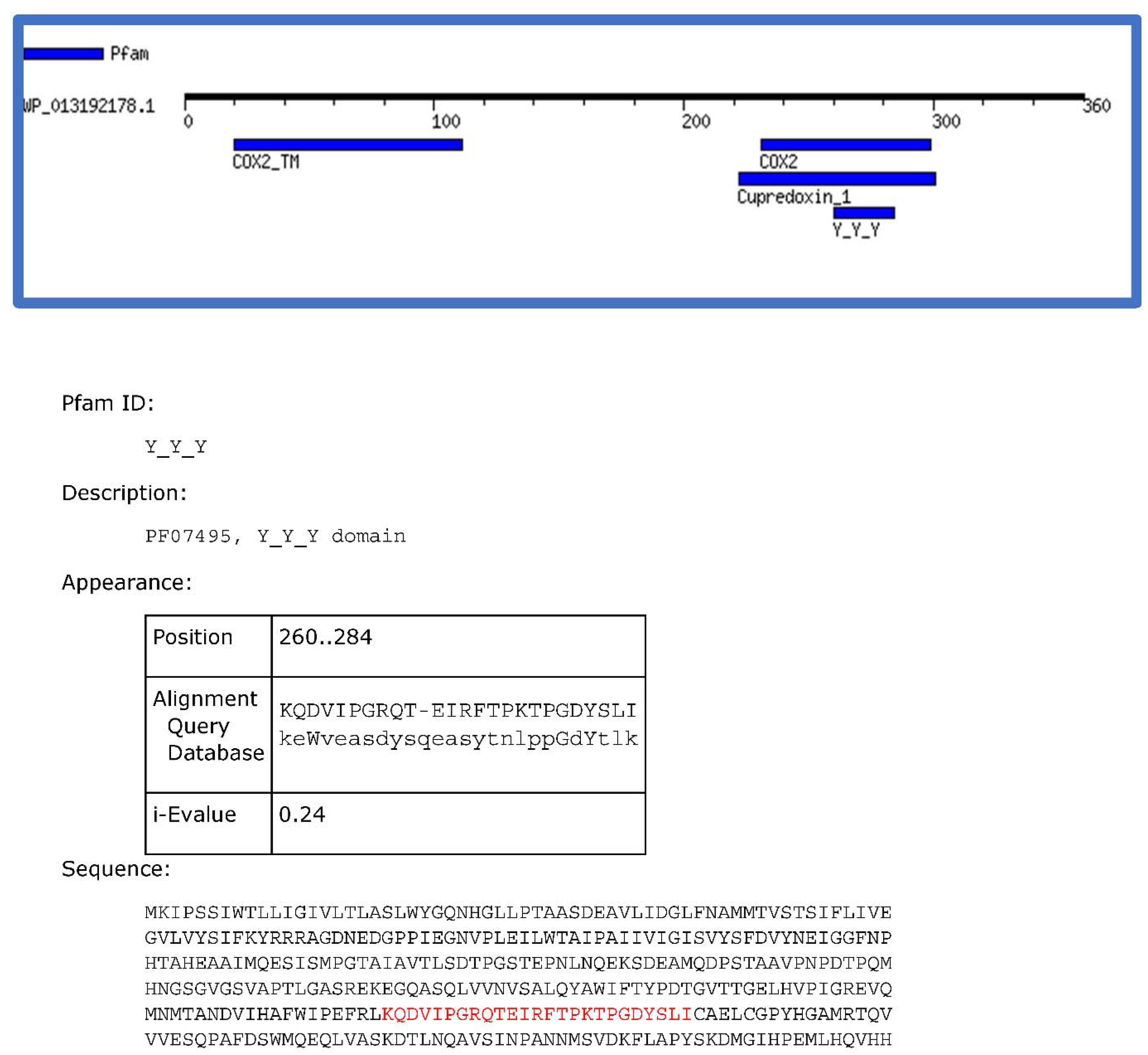
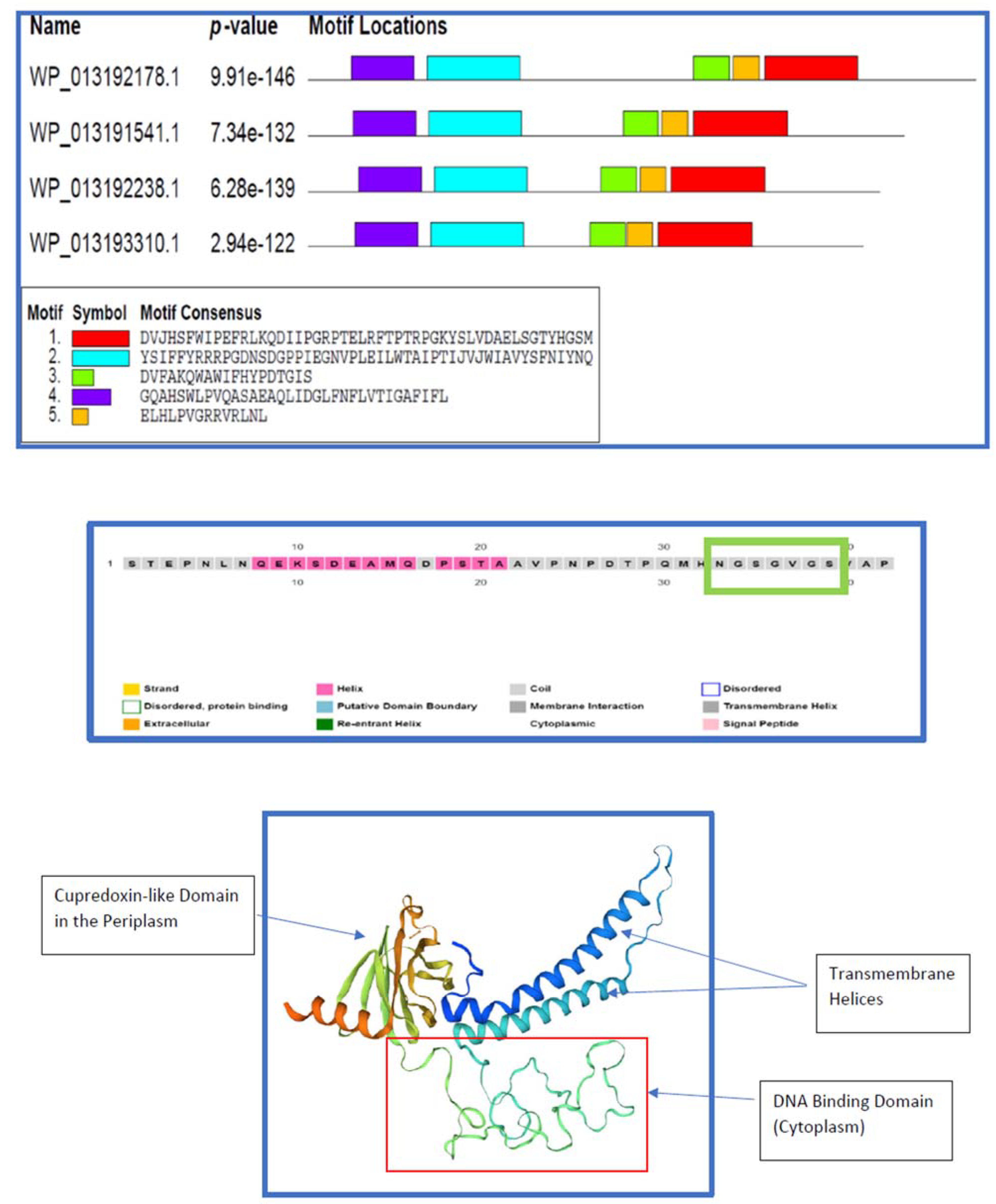
2.2. The Sensory Periplasmic Y_Y_Y Domain
2.3. The Phylogeny of the Cytochrome Oxidase Subunit II Enzymes
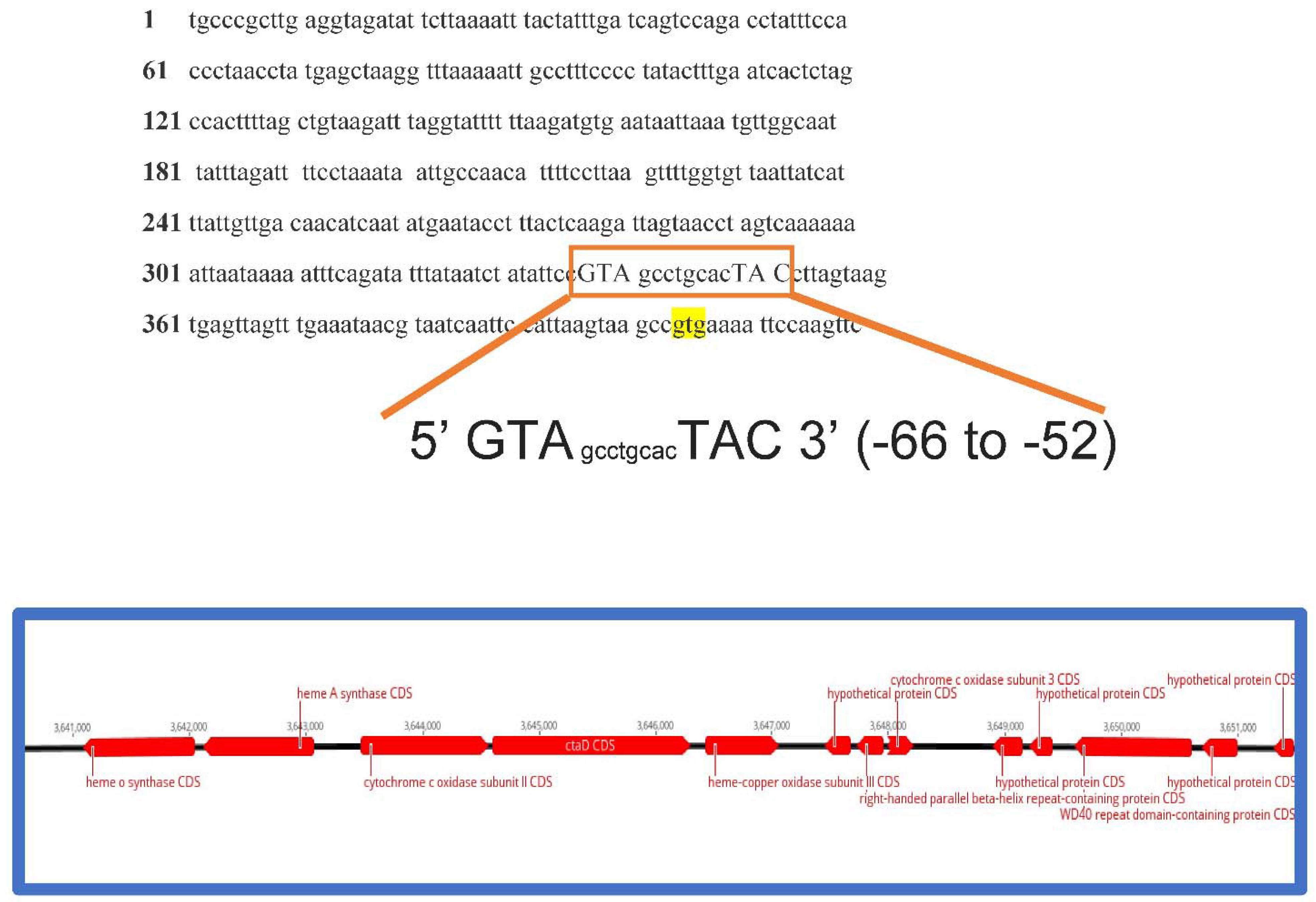
2.4. Gene Synteny Analyses
2.5. Promoter for the NtcA Transcription Factor
2.6. Terminal Histidines
2.7. Translocation of the Periplasmic Domains
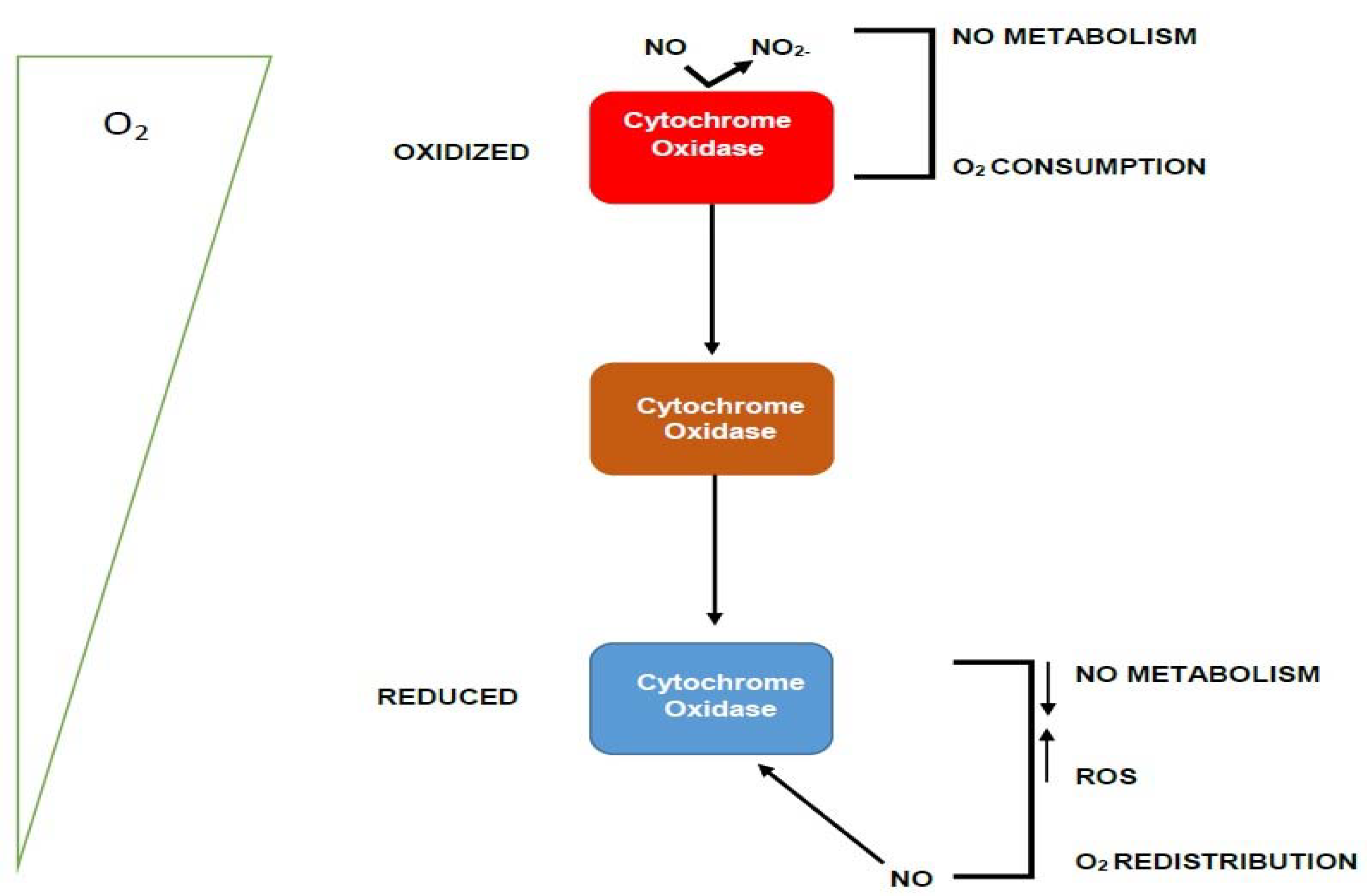
2.8. Proposed Functional Mechanism of the Candidate Protein WP_013192178.1
- The NtcA transcription factor binds to the promoter of the gene to produce mRNA that will be translated into the corresponding WP_013192178.1 protein
- The periplasmic domain of the WP_013192178.1 protein is exported into the periplasmic space.
- The nitric oxide sensing function will negotiate periplasmic NO as the trigger using the Y_Y_Y domain to detect toxic NO in the periphery of the heterocyst membrane. Note, we also envisage direct binding of N2O to the Y_Y_Y domain, since they are both small molecules and known to bind to the same proteins
- The binding of a Y_Y_Y domain to nitric oxide has been reported at least once before to disentangle the sensor protein from the membrane localization, and this dissociation from a fixed membrane position, has been linked to subsequent binding to DNA to effectuate downstream functions [17]. We propose a similar mechanism here.
- The mid-section of the candidate protein (WP_013192178.1), comprising a loosely random-coiled section as shown in the homology model (Figure 11), is likely to be functional as a DNA binding surface. DNA binding could be involved in transcription of nitric/nitrous oxide quenching/transforming ORFs or other regulatory functions. The evolutionarily “elderly” global fold common to nitrous oxide reductases and cytochrome oxidases (subunit II) may be more adaptable due to its long vigil in time and may even accommodate novel – adaptive - catalytic properties [30]. Long coiled segments are characteristic of DNA and RNA binding proteins, including DNA and RNA chaperones [31] and even as oligomerization interfaces.
2.9. DNA Binding and/or Oligomerization
2.10. Phosphorylation of Serine Residues
2.11. Interpreting a Hydropathy Profile of the WP_013192178.1 Protein
3. Methods
3.1. Phylogenetic Reconstructions
3.2. Multiple Sequence Alignments
3.3. Determination of Protein Families/Superfamilies
3.4. Protein Motif Analyses
3.5. Gene Synteny Analyses
3.6. Building of Homology Models
3.7. Phosphorylation Site Prediction
3.8. Prediction of DNA Binding Residues
3.9. Hydropathy Profile
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mvondo, D.N.; Navarro-González, R.; McKay, C.; Coll, P.; Raulin, F. Production of nitrogen oxides by lightning and coronae discharges in simulated early earth, venus and mars environments. Adv. Space Res. 2001, 27, 217–223. [Google Scholar] [CrossRef]
- BBC. 2021. Available online: https://www.bbc.com/future/article/20210603-nitrous-oxide-the-worlds-forgotten-greenhouse-gas (accessed on 28 January 2022).
- Gunawardana, D. An Exploration of Common Greenhouse Gas Emissions by the Cyanobiont of the Azolla–Nostoc Symbiosis and Clues as to Nod Factors in Cyanobacteria. Plants 2019, 8, 587. [Google Scholar] [CrossRef] [Green Version]
- Saraste, M.; Castresana, J. Cytochrome oxidase evolved by tinkering with denitrification enzymes. FEBS Lett. 1994, 341, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Prosser, J.; Hink, L.; Gubry-Rangin, C.; Nicol, G.W. Nitrous oxide production by ammonia oxidisers: Physiological diversity, niche differentiation and potential mitigation strategies. Glob. Change biol. 2020, 26, 103–118. [Google Scholar] [CrossRef] [PubMed]
- Weathers, P.J.; Niedzielski, J.J. Nitrous oxide production by cyanobacteria. Arch. Microbiol. 1986, 146, 204–206. [Google Scholar] [CrossRef]
- Jones, K.; Haselkorn, R. Newly Identified Cytochrome c Oxidase Operon in the Nitrogen-Fixing Cyanobacterium Anabaena sp. Strain PCC 7120 Specifically Induced in Heterocysts. J. Bacteriol. 2002, 184, 2491–2499. [Google Scholar] [CrossRef] [Green Version]
- Christiansen, J.; Seefeldt, L.; Dean, D.R. Competitive Substrate and Inhibitor Interactions at the Physiologically Relevant Active Site of Nitrogenase. J. Biol. Chem. 2000, 275, 36104–36107. [Google Scholar] [CrossRef] [Green Version]
- Pushpakumara, B.; Gunawardana, D. Preliminary data on the presence of an alternate vanadium nitrogenase in a culturable cyanobiont of Azolla pinnata R. Brown: Implications on Chronic Kidney Disease of an unknown etiology (CKDu). Data Brief 2018, 21, 2590–2597. [Google Scholar] [CrossRef]
- Kimani, S.M.; Bimantara, P.O.; Hattori, S.; Tawaraya, K.; Sudo, S.; Xu, X.; Cheng, W. Co-application of poultry-litter biochar with Azolla has synergistic effects on CH4 and N2O emissions from rice paddy soils. Heliyon 2020, 6, e05042. [Google Scholar] [CrossRef]
- Malyan, S.K.; Bhatia, A.; Tomer, R.; Harit, R.C.; Jain, N.; Bhowmik, A.; Kaushik, R. Mitigation of yield-scaled greenhouse gas emissions from irrigated rice through Azolla, Blue-green algae, and plant growth–promoting bacteria. Environ. Sci. Pollut. Res. 2021, 28, 51425–51439. [Google Scholar] [CrossRef]
- Pereira, A.L.; Vasconcelos, V. Classification and phylogeny of the cyanobiont Anabaena azollae Strasburger: An answered question? Int. J. Syst. Evol. Microbiol. 2014, 64, 1830–1840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ran, L.; Larsson, J.; Vigil-Stenman, T.; Nylander, J.A.A.; Ininbergs, K.; Zheng, W.-W.; Lapidus, A.; Lowry, S.; Haselkorn, R.; Bergman, B. Genome Erosion in a Nitrogen-Fixing Vertically Transmitted Endosymbiotic Multicellular Cyanobacterium. PLoS ONE 2010, 5, e11486. [Google Scholar] [CrossRef]
- Conte, L.L.; Ailey, B.; Hubbard, T.J.; Brenner, S.E.; Murzin, A.G.; Chothia, C. SCOP: A Structural Classification of Proteins database. Nucleic Acids Res. 2000, 28, 257–259. [Google Scholar] [CrossRef] [Green Version]
- Einarsdóttir, O.; Caughey, W.S. Interactions of the anesthetic nitrous oxide with bovine heart cytochrome c oxidase. Effects on protein structure, oxidase activity, and other properties. J. Biol. Chem. 1988, 263, 9199–9205. [Google Scholar] [CrossRef]
- Taylor, C.; Moncada, S. Nitric Oxide, Cytochrome C Oxidase, and the Cellular Response to Hypoxia. Arter. Thromb. Vasc. Biol. 2010, 30, 643–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guttierez, E.; Long, P.F. Abstract. Available online: https://aaas.confex.com/aaas/2012/webprogram/Paper8031.html (accessed on 28 January 2022).
- Menke, M.; Berger, B.; Cowen, L. Markov random fields reveal an N-terminal double beta-propeller motif as part of a bacterial hybrid two-component sensor system. Proc. Natl. Acad. Sci. USA 2010, 107, 4069–4074. [Google Scholar] [CrossRef] [Green Version]
- Murphy, M.E.P.; Lindley, P.F.; Adman, E.T. Structural comparison of cupredoxin domains: Domain recycling to construct proteins with novel functions. Protein Sci. 1997, 6, 761–770. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.K.-M.; Chan, N.-L.; Wang, A.H.-J. The many blades of the β-propeller proteins: Conserved but versatile. Trends Biochem. Sci. 2011, 36, 553–561. [Google Scholar] [CrossRef]
- Veltri, D.; Malapi-Wight, M.; Crouch, J.A. SimpleSynteny: A web-based tool for visualization of microsynteny across multiple species. Nucleic Acids Res. 2016, 44, W41–W45. [Google Scholar] [CrossRef]
- Gunawardana, D. An in silico Study of Two Transcription Factors Controlling Diazotrophic Fates of the Azolla Major Cyanobiont Trichormus azollae. Bioinform. Biol. Insights 2020, 14, 1177932220977490. [Google Scholar] [CrossRef]
- Vázquez-Bermúdez, M.F.; Flores, E.; Herrero, A. Analysis of binding sites for the nitrogen-control transcription factor NtcA in the promoters of Synechococcus nitrogen-regulated genes. Biochim. Biophys. Acta-Gene Struct. Expr. 2002, 1578, 95–98. [Google Scholar] [CrossRef]
- Milligan, A.J.; Berman-Frank, I.; Gerchman, Y.; Dismukes, G.C.; Falkowski, P.G. Light-dependent oxygen consumption in nitrogen-fixing cyanobacteria plays a key role in nitrogenase protection1. J. Phycol. 2007, 43, 845–852. [Google Scholar] [CrossRef]
- Torrado, A.; Ramírez-Moncayo, C.; Navarro, J.A.; Mariscal, V.; Molina-Heredia, F.P. Cytochrome c6 is the main respiratory and photosynthetic soluble electron donor in heterocysts of the cyanobacterium Anabaena sp. PCC 7120. Biochim. Biophys. Acta 2018, 1860, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Schilling, K.M.; Tao, L.; Wu, B.; Kiblen, J.T.; Ubilla-Rodriguez, N.C.; Pushie, M.J.; Britt, R.D.; Roseman, G.P.; Harris, D.A.; Millhauser, G.L. Both N-Terminal and C-Terminal Histidine Residues of the Prion Protein Are Essential for Copper Coordination and Neuroprotective Self-Regulation. J. Mol. Biol. 2020, 432, 4408–4425. [Google Scholar] [CrossRef]
- Hoeren, F.U.; Berks, B.C.; Ferguson, S.J.; McCarthy, J.E.G. Sequence and expression of the gene encoding the respiratory nitrous-oxide reductase from Paracoccus denitrificans. New and conserved structural and regulatory motifs. Eur. J. Biochem. 1993, 218, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Cutruzzolà, F. Bacterial nitric oxide synthesis. Biochim. Biophys. Acta Bioenerg. 1999, 1411, 231–249. [Google Scholar] [CrossRef] [Green Version]
- Timilsina, A.; Zhang, C.; Pandey, B.; Bizimana, F.; Dong, W.; Hu, C. Potential Pathway of Nitrous Oxide Formation in Plants. Front. Plant Sci. 2020, 11, 1177. [Google Scholar] [CrossRef]
- Rani, S.; Dasgupta, B.; Bhati, G.K.; Tomar, K.; Rakshit, S.; Maiti, S. Superior Proton-Transfer Catalytic Promiscuity of Cytochrome c in Self-Organized Media. ChemBioChem 2020, 22, 1285–1291. [Google Scholar] [CrossRef]
- Tompa, P.; Csermely, P. The role of structural disorder in the function of RNA and protein chaperones. FASEB J. 2004, 18, 1169–1175. [Google Scholar] [CrossRef]
- Yan, J.; Kurgan, L. DRNApred, fast sequence-based method that accurately predicts and discriminates DNA- and RNA-binding residues. Nucleic Acids Res. 2017, 45, e84. [Google Scholar] [CrossRef] [Green Version]
- Dongre, M.; Singh, N.S.; Dureja, C.; Peddada, N.; Solanki, A.K.; Ashish, D.; Raychaudhuri, S. Evidence on How a Conserved Glycine in the Hinge Region of HapR Regulates Its DNA Binding Ability. J. Biol. Chem. 2011, 286, 15043–15049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy Chichili, V.P.; Kumar, V.; Sivaraman, J. Linkers in the structural biology of protein-protein interactions. Protein Sci. 2013, 22, 153–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, J.A.; Wong, W.-C.; Eisenhaber, B.; Warwicker, J.; Eisenhaber, F. Charged residues next to transmembrane regions revisited: “Positive-inside rule” is complemented by the “negative inside depletion/outside enrichment rule”. BMC Biol. 2017, 15, 66. [Google Scholar] [CrossRef] [Green Version]
- Sudhir, K.; Glen, S.; Michael, L.; Christina, K.; Koichiro, T. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar]
- Andreeva, A.; Kulesha, E.; Gough, J.; Murzin, A.G. The SCOP database in 2020: Expanded classification of representative family and superfamily domains of known protein structures. Nucleic Acids Res. 2019, 48, D376–D382. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Elkan, C. Fitting a mixture model by expectation maximization to discover motifs in biopolymers. In Proceedings of the Second International Conference on Intelligent Systems for Molecular Biology; AAAI Press: Menlo Park, CA, USA, 1994; pp. 28–36. [Google Scholar]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; De Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef] [Green Version]
- Miller, M.L.; Soufi, B.; Jers, C.; Blom, N.; Macek, B.; Mijakovic, I. NetPhosBac-A predictor for Ser/Thr phosphorylation sites in bacterial proteins. Proteomics 2009, 9, 116–125. [Google Scholar] [CrossRef]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Duvaud, S.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. Protein Identification and Analysis Tools on the ExPASy Server. In The Proteomics Protocols Handbook; Walker, J.M., Ed.; Humana Press: Totowa, NJ, USA, 2005; pp. 571–607. [Google Scholar]
- Xue, L.-G.; Li, S.W.; Xu, S.-J.; An, L.-Z.; Wang, X.-L. Alleviative effects of nitric oxide on the biological damage of spirulina platensis induced by enhanced ultraviolet-B. Acta Microbiol. Sin. 2006, 46, 561–564. [Google Scholar]
- Polacco, J.C.; Mazzafera, P.; Tezotto, T. Opinion–Nickel and urease in plants: Still many knowledge gaps. Plant Sci. 2013, 199-200, 79–90. [Google Scholar] [CrossRef]
- Revitt-Mills, S.A.; Robinson, A. Antibiotic-Induced Mutagenesis: Under the Microscope. Front. Microbiol. 2020, 11, 2611. [Google Scholar] [CrossRef]
- Petsko, G.A.; Kenyon, G.L.; Gerlt, J.A.; Ringe, D.; Kozarich, J.W. On the origin of enzymatic species. Trends Biochem. Sci. 1993, 18, 372–376. [Google Scholar] [CrossRef]
- Gunawardana, D.; Cheng, H.-C.; Gayler, K.R. Identification of functional domains in Arabidopsis thaliana mRNA decapping enzyme (AtDcp2). Nucleic Acids Res. 2007, 36, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.R.; Longley, R.; Hutchins, P.R.; Bauersachs, T. Cellular Innovation of the Cyanobacterial Heterocyst by the Adaptive Loss of Plasticity. Curr. Biol. 2020, 30, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.N. Rapid evolution as an ecological process. Trends Ecol. Evol. 1998, 13, 329–332. [Google Scholar] [CrossRef]
- Hairston, N.G.; Ellner, S.P.; Geber, M.A.; Yoshida, T.; Fox, J.A. Rapid evolution and the convergence of ecological and evolutionary time. Ecol. Lett. 2005, 8, 1114–1127. [Google Scholar] [CrossRef]








| Cyanobiont | Host Plant | Cytochrome Oxidase Subunit II Enzymes |
| Trichormus azollae | Genus Azolla (Monilophyte/Pteridophyte) | WP_013192178.1 WP_013191541.1 WP_013192238.1 WP_013193310.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gunawardana, D.; Herath, V. A Hypothesis on How the Azolla Symbiosis Mitigates Nitrous Oxide Based on In Silico Analyses. J 2022, 5, 166-185. https://doi.org/10.3390/j5010013
Gunawardana D, Herath V. A Hypothesis on How the Azolla Symbiosis Mitigates Nitrous Oxide Based on In Silico Analyses. J. 2022; 5(1):166-185. https://doi.org/10.3390/j5010013
Chicago/Turabian StyleGunawardana, Dilantha, and Venura Herath. 2022. "A Hypothesis on How the Azolla Symbiosis Mitigates Nitrous Oxide Based on In Silico Analyses" J 5, no. 1: 166-185. https://doi.org/10.3390/j5010013
APA StyleGunawardana, D., & Herath, V. (2022). A Hypothesis on How the Azolla Symbiosis Mitigates Nitrous Oxide Based on In Silico Analyses. J, 5(1), 166-185. https://doi.org/10.3390/j5010013







