Genome Doubling of Northern Spicebush, Lindera benzoin L.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
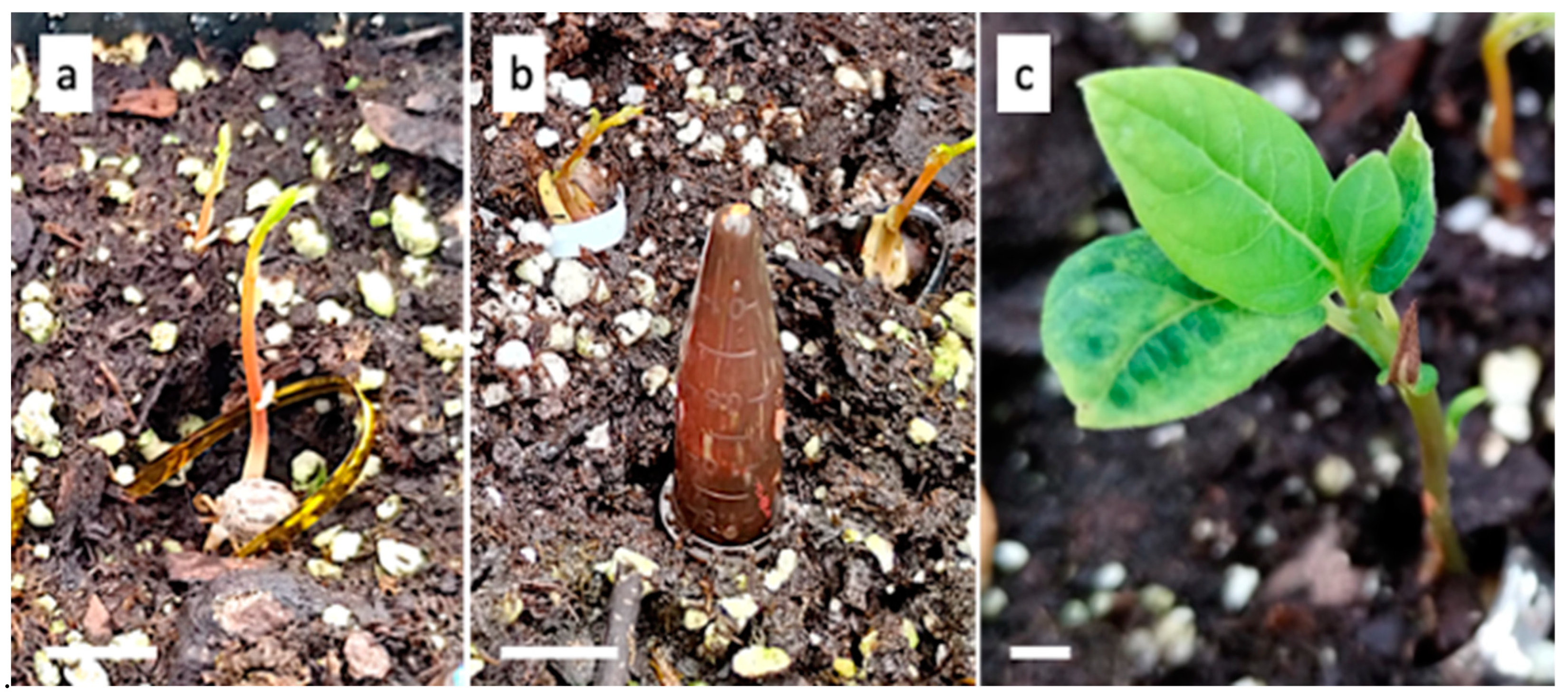
2.2. Seedling Solid Agar Treatment
2.3. Flow Cytometry
2.4. Stomatal Density Size and Measurement and Visual Morphology
3. Results
3.1. Seedling Solid Agar Treatment
3.2. Stomata Size, Density, and Leaf Morphology
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wei, G.; Zhang, J.; Lei, J.; Ma, C.; Tong, Y.; Jiang, H. Chemical constituents from Lindera nacusua (D. Don) Merr. Biochem. Syst. Ecol. 2016, 66, 94–97. [Google Scholar] [CrossRef]
- Small, E. North American Cornucopia: Top 100 Indigenous Food Plants; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Core, E.L. Carolina Flora Manual of the Vascular Flora of the Carolinas. In Bioscience; Radford, A.E., Ahles, H.E., Bell, C.R., Eds.; The University of North Carolina Press: Chapel Hill, NK, USA, 1970; Volume 20, p. 1217. [Google Scholar]
- Hoss, G. Propagation Protocol for Spicebush (Lindera benzoin). Nativ. Plants J. 2006, 7, 134–136. [Google Scholar] [CrossRef]
- USDA-NRCS Spicebush Lindera benzoin (L.) Blume. Available online: https://plants.usda.gov/DocumentLibrary/plantguide/pdf/pg_libe3.pdf (accessed on 20 December 2023).
- Shrestha, P.; Lubell, J.D. Suitability of eight northeastern US native shrubs as replacements for invasive plants in a difficult landscape site with white-tailed deer pressure. HortTechnology 2015, 25, 171–176. [Google Scholar] [CrossRef]
- Azhar, M.A.M.; Salleh, W.M.N.H.W. Chemical composition and biological activities of essential oils of the genus Litsea (Lauraceae)—A review. Agric. Conspec. Sci. 2020, 85, 97–103. [Google Scholar]
- Tucker, A.O.; Maciarello, M.J.; Burbage, P.W.; Sturtz, G. Spicebush [Lindera benzoin (L.) Blume var. benzoin, Lauraceae]: A Tea, Spice, and Medicine. Econ. Bot. 1994, 48, 333–336. [Google Scholar] [CrossRef]
- Tantaquidgeon, G. Folk Medicine of the Delaware and Related Algonkian Indians; DIANE Publishing Inc.: Darby, PA, USA, 2007. [Google Scholar]
- Hutton, K. A Comparative Study of the Plants Used for Medicinal Purposes by the Creek and Seminoles Tribes. Master’s Thesis, University of South Florida, Tampa, FL, USA, 2010. [Google Scholar]
- Hamel, P.B.; Chiltoskey, M.U. Cherokee Plants and Their Uses: A 400 Year History; Herald Publishing Company: Sylva, NC, USA, 1975. [Google Scholar]
- Anderson, J.E.; Ma, W.; Smith, D.L.; Chang, C.; McLaughlin, J.L. Biologically active γ-lactones and methylketoalkenes from Lindera benzoin. J. Nat. Prod. 1992, 55, 71–83. [Google Scholar] [CrossRef]
- Haque, M.E.; Azam, S.; Balakrishnan, R.; Akther, M.; Kim, I. Therapeutic potential of Lindera obtusiloba: Focus on antioxidative and pharmacological properties. Plants 2020, 9, 1765. [Google Scholar] [CrossRef]
- Henz Ryen, A.; Göls, T.; Steinmetz, J.; Tahir, A.; Jakobsson, P.; Backlund, A.; Urban, E.; Glasl, S. Bisabolane sesquiterpenes from the leaves of Lindera benzoin reduce prostaglandin E2 formation in A549 cells. Phytochem. Lett. 2020, 38, 6–11. [Google Scholar] [CrossRef]
- Cao, Y.; Xuan, B.; Peng, B.; Li, C.; Chai, X.; Tu, P. The genus Lindera: A source of structurally diverse molecules having pharmacological significance. Phytochem. Rev. 2016, 15, 869–906. [Google Scholar] [CrossRef]
- Smith, R.; Pomper, K.W.; Lowe, J.D.; Botkins, J.; Crabtree, S.B. Genetic Diversity in Kentucky Spicebush Populations using Simple Sequence Repeat Markers. J. Ky. Acad. Sci. 2012, 73, 101–109. [Google Scholar] [CrossRef]
- Poston, T.; Lee, C.N.; Choie, Y.; Kwon, Y. Local minima and back propagation. In Proceedings of the IJCNN-91-Seattle International Joint Conference on Neural Networks, Seattle, WA, USA, 8–12 July 1991; Volume 2, pp. 173–176. [Google Scholar]
- Templeton, L.K.; Neel, M.C.; Groffman, P.M.; Cadenasso, M.L.; Sullivan, J.H. Changes in vegetation structure and composition of urban and rural forest patches in Baltimore from 1998 to 2015. For. Ecol. Manag. 2019, 454, 117665. [Google Scholar] [CrossRef]
- Touchell, D.H.; Palmer, I.E.; Ranney, T.G. In vitro Ploidy Manipulation for Crop Improvement. Front. Plant Sci. 2020, 11, 722. [Google Scholar] [CrossRef] [PubMed]
- Singh, F.; Khoshoo, T.N. Chromosomal polymorphism within the Hibiscus rosa-sinensis complex. Caryologia 1970, 23, 19–27. [Google Scholar] [CrossRef]
- Chen, H.; Contreras, R.N. Near-hexaploid and near-tetraploid aneuploid progenies derived from backcrossing tetraploid parents Hibiscus syriacus × (H. syriacus × H. paramutabilis). Genes 2022, 13, 1022. [Google Scholar] [CrossRef]
- Pan, I.; Lu, Y.; Wen, P.; Chen, Y. Using colchicine to create poinsettia (Euphorbia pulcherrima × Euphorbia cornastra) mutants with various morphological traits. HortScience 2019, 54, 1667–1672. [Google Scholar] [CrossRef]
- Ye, Y.M.; Tong, J.; Shi, X.P.; Yuan, W.; Li, G.R. Morphological and cytological studies of diploid and colchicine-induced tetraploid lines of crape myrtle (Lagerstroemia indica L.). Sci. Hortic. 2010, 124, 95–101. [Google Scholar] [CrossRef]
- Defiani, M.R.; Astarini, I.A.; Kriswiyanti, E. Oryzalin and gamma radiation induced polyploidization in garden balsam plants (Impatiens balsamina L.) in vitro. Curr. Agric. Res. J. 2017, 5, 1–5. [Google Scholar] [CrossRef]
- Contreras, R.N.; Ruter, J.M.; Hanna, W.W. An oryzalin-induced autoallooctoploid of Hibiscus acetosella ‘Panama Red’. J. Am. Soc. Hort. Sci. 2009, 134, 553–559. [Google Scholar] [CrossRef]
- Fetouh, M.I.; Deng, Z.; Wilson, S.B.; Adams, C.R.; Knox, G.W. Induction and characterization of tetraploids in chinese privet (Ligustrum sinense Lour.). Sci. Hortic. 2020, 271, 109482. [Google Scholar] [CrossRef]
- Mo, L.; Chen, J.; Lou, X.; Xu, Q.; Dong, R.; Tong, Z.; Huang, H.; Lin, E. Colchicine-induced polyploidy in Rhododendron fortunei Lindl. Plants 2020, 9, 424. [Google Scholar] [CrossRef]
- Tong, J.; Ye, Y.; Feng, B.; Yuan, W. Colchicines induced polyploid plants and their identification in three species of Lagerstroemia indica. Acta Hortic. Sin. 2009, 36, 127–132. [Google Scholar]
- Ning, G.; Shi, X.; Hu, H.; Yan, Y.; Bao, M. Development of a range of polyploid lines in Petunia hybrida and the relationship of ploidy with the single-/double-flower trait. HortScience 2009, 44, 250–255. [Google Scholar] [CrossRef]
- Luo, J.; Ren, W.; Cai, G.; Huang, L.; Shen, X.; Li, N.; Nie, C.; Li, Y.; Wang, N. The chromosome-scale genome sequence of Triadica sebifera provides insight into fatty acids and anthocyanin biosynthesis. Commun. Biol. 2022, 5, 786. [Google Scholar] [CrossRef] [PubMed]
- Takamura, T.; Sugimura, T.; Tanaka, M.; Kage, T. Breeding of the tetraploid yellow-flowered cyclamen with “eye”. In Proceedings of the III International Symposium on New Floricultural Crops, Perth, Australia, 1–4 October 1996; pp. 119–126. [Google Scholar]
- Brits, G.J. Polyploid breeding of wild South African plectranthus (spurflowers) as new flowering pot plants, XXVII International, Horticultural Congress-IHC2006: International Symposium on Endogenous and Exogenous Plant. Bioregulators 2006, 774, 437–442. [Google Scholar]
- Guerra, D.; Wittmann, M.T.S.; Schwarz, S.F.; Souza, P.V.D.d.; Gonzatto, M.P.; Weiler, R.L. Comparison between diploid and tetraploid citrus rootstocks: Morphological characterization and growth evaluation. Bragantia 2014, 73, 1–7. [Google Scholar] [CrossRef]
- Kataoka, I.; Mizugami, T.; Kim, J.G.; Beppu, K.; Fukuda, T.; Sugahara, S.; Tanaka, K.; Satoh, H.; Tozawa, K. Ploidy variation of hardy kiwifruit (Actinidia arguta) resources and geographic distribution in Japan. Sci. Hortic. 2010, 124, 409–414. [Google Scholar] [CrossRef]
- Rosati, A.; Caporali, S.; Hammami, S.B.; Moreno-Alías, I.; Rapoport, H. Fruit growth and sink strength in olive (Olea europaea) are related to cell number, not to tissue size. Funct. Plant Biol. 2020, 47, 1098–1104. [Google Scholar] [CrossRef] [PubMed]
- Crawford, S.; Rojas, B.M.; Crawford, E.; Otten, M.; Schoenenberger, T.A.; Garfinkel, A.R.; Chen, H. Characteristics of the diploid, triploid, and tetraploid versions of a cannabigerol-fominant F1 Hybrid Industrial Hemp Cultivar, Cannabis sativa ‘Stem Cell CBG’. Genes 2021, 12, 923. [Google Scholar] [CrossRef]
- Chen, E.G.; Tsai, K.; Chung, H.; Chen, J. Chromosome doubling-enhanced biomass and dihydrotanshinone I production in Salvia miltiorrhiza, a traditional Chinese medicinal plant. Moleculars 2018, 23, 3106. [Google Scholar] [CrossRef]
- Barandalla, L.; Ritter, E.; Ruiz De Galarreta, J.I. Oryzalin treatment of potato diploids yields tetraploid and chimeric plants from which euploids could be derived by callus induction. Potato Res. 2006, 49, 143–154. [Google Scholar] [CrossRef]
- Gallone, A.; Hunter, A.; Douglas, G.C. Polyploid induction in vitro using colchicine and oryzalin on Hebe ‘Oratia Beauty’: Production and characterization of the vegetative traits. Sci. Hortic. 2014, 179, 59–66. [Google Scholar] [CrossRef]
- Contreras, R.N.; Ruter, J.M.; Schwartz, B.M. Oryzalin-induced tetraploidy in Cryptomeria japonica (Cupressaceae). HortScience 2010, 45, 316–319. [Google Scholar] [CrossRef]
- Contreras, R.N.; Meneghelli, L. In vitro chromosome doubling of Prunus laurocerasus ‘Otto Luyken’and ‘Schipkaensis’. HortScience 2016, 51, 1463–1466. [Google Scholar] [CrossRef]
- Deans, L.E.; Palmer, I.E.; Touchell, D.H.; Ranney, T.G. In Vitro induction and characterization of polyploid Hydrangea macrophylla and H. serrata. HortScience 2021, 56, 709–715. [Google Scholar] [CrossRef]
- Melsen, K.; van de Wouw, M.; Contreras, R. Mutation breeding in ornamentals. HortScience 2021, 56, 1154–1165. [Google Scholar] [CrossRef]
- Nadler, J.D.; Pooler, M.; Olsen, R.T.; Coleman, G.D. In vitro induction of polyploidy in Cercis glabra Pamp. Sci. Hortic. 2012, 148, 126–130. [Google Scholar] [CrossRef]
- Nadler, J.D. In Vitro Induction of Polyploidy in Cercis yunnanensis Hu et Cheng. Master’s Thesis, University of Maryland, College Park, MD, USA, 2009. [Google Scholar]
- Väinölä, A. Polyploidization and early screening of Rhododendron hybrids. Euphytica 2000, 112, 239–244. [Google Scholar] [CrossRef]
- Denaeghel, H.; Van Laere, K.; Leus, L.; Van Huylenbroeck, J.; Van Labeke, M. Induction of Tetraploids in Escallonia spp. In Proceedings of the XXV International EUCARPIA Symposium Section Ornamentals: Crossing Borders 1087, Melle, Belgium, 28 June–2 July 2015; pp. 453–458. [Google Scholar]
- Lam, H.K.; Harbard, J.L.; Koutoulis, A. Tetraploid induction of Acacia crassicarpa using colchicine and oryzalin. J. Trop. For. Sci. 2014, 26, 347–354. [Google Scholar]
- Teng, E.S.; Leonhardt, K.W. In vitro and in vivo polyploidization of Dracaena with oryzalin. In Proceedings of the VI International Symposium on New Floricultural Crops, Funchal, Portugal, 11–15 June 2007; Volume 813, pp. 509–516. [Google Scholar]
- Li, Z.; Ruter, J.M. Development and evaluation of diploid and polyploid Hibiscus moscheutos. HortScience 2017, 52, 676–681. [Google Scholar] [CrossRef]
- Hebert, C.J.; Touchell, D.H.; Ranney, T.G.; LeBude, A.V. In Vitro Shoot Regeneration and Polyploid Induction of Rhododendron ‘Fragrantissimum Improved’. HortScience 2010, 45, 801–804. [Google Scholar] [CrossRef]
- Oates, K.M.; Ranney, T.G.; Touchell, D.H. Influence of induced polyploidy on fertility and morphology of Rudbeckia species and hybrids. HortScience 2012, 47, 1217–1221. [Google Scholar] [CrossRef]
- Lattier, J.D.; Chen, H.; Contreras, R.N. Variation in genome size, ploidy, stomata, and rDNA signals in Althea. J. Am. Soc. Hort. Sci. 2019, 144, 130–140. [Google Scholar] [CrossRef]
- Alexander, L. Production of triploid Hydrangea macrophylla via unreduced gamete breeding. HortScience 2017, 52, 221–224. [Google Scholar] [CrossRef]
- Caine, R.S.; Yin, X.; Sloan, J.; Harrison, E.L.; Mohammed, U.; Fulton, T.; Biswal, A.K.; Dionora, J.; Chater, C.C.; Coe, R.A. Rice with reduced stomatal density conserves water and has improved drought tolerance under future climate conditions. N. Phytol. 2019, 221, 371–384. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Zhou, M.; Shabala, S. How does stomatal density and residual transpiration contribute to osmotic stress tolerance? Plants 2023, 12, 494. [Google Scholar] [CrossRef]
- Ranney, T.G. Polyploidy: From evolution to new plant development. In Combined Proceedings International Plant Propagators’ Society; IPPS: Bellefonte, PA, USA, 2006; Available online: https://ena.ipps.org/uploads/docs/56_85.pdf (accessed on 10 December 2023).
- Cecala, J.M.; Wilson Rankin, E.E. Wild bee functional diversity and plant associations in native and conventional plant nurseries. Ecol. Entomol. 2021, 46, 1283–1292. [Google Scholar] [CrossRef]
- Rihn, A.L.; Knuth, M.J.; Peterson, B.J.; Torres, A.P.; Campbell, J.H.; Boyer, C.R.; Palma, M.A.; Khachatryan, H. Investigating drivers of native plant production in the United States green industry. Sustainability 2022, 14, 6774. [Google Scholar] [CrossRef]
- Kauth, P.J.; Pérez, H.E. Industry survey of the native wildflower market in Florida. HortTechnology 2011, 21, 779–788. [Google Scholar] [CrossRef]
- Zadegan, Y.R.; Behe, B.K.; Gough, R. Consumer preferences for native plants in Montana residential landscapes and perceptions for naturalistic designs. J. Environ. Hortic. 2008, 26, 109–114. [Google Scholar] [CrossRef]
- Graham, B. Native Plants Policy, Conservation funding increases pass the Legislature. Audubon North Carol. 2023. Available online: https://nc.audubon.org/news/native-plants-policy-conservation-funding-increases-pass-legislature (accessed on 3 January 2024).
- Beck, T.B. Gardeners perceptions of the aesthetics, manageability, and sustainability of residential landscapes. Appl. Environ. Educ. Commun. 2002, 1, 163–172. [Google Scholar] [CrossRef]
- Norcini, J. Native plants: An overview. Environ. Hort. Dept. Florida Coop. Ext. Serv. Inst. Food Agr. Sci. Univ. Florida, Document ENH1045. 2006. Available online: https://edis.ifas.ufl.edu/publication/EP297 (accessed on 15 January 2024).

| Accession | Duration (H) | Treated Plants # | Survival | Diploid # | Mixoploid # | Tetraploid # |
|---|---|---|---|---|---|---|
| C2023-008 | 24 | 41 | 17 | 6 | 6 | 5 |
| 72 | 40 | 28 | 16 | 8 | 4 | |
| 120 | 40 | 14 | 5 | 6 | 3 | |
| C2023-005 | 24 | 58 | 29 | 19 | 8 | 2 |
| 72 | 56 | 27 | 18 | 7 | 2 | |
| 120 | 53 | 26 | 20 | 6 | 0 | |
| Total | 24 | 99 | 46a * | 25a * | 14a * | 7a * |
| 72 | 96 | 55b | 34a | 15a | 6a | |
| 120 | 93 | 40a | 25a | 12a | 3a |
| n | Length (µm) | SD | Width (µm) | SD | n | Density (per nm2) | SD | |
|---|---|---|---|---|---|---|---|---|
| 2x | 44 | 18.14 | 1.76 | 18.97 | 2.67 | 6 | 300.44 | 28.87 |
| 4x | 42 | 29.13 | 2.50 | 24.19 | 2.22 | 6 | 123.59 | 19.25 |
| p-value * | 7.65 × 10−36 | 1.21 × 10−15 | 7.41 × 10−7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arram, R.F.; Morgan, T.B.; Nix, J.T.; Kao, Y.-L.; Chen, H. Genome Doubling of Northern Spicebush, Lindera benzoin L. J 2024, 7, 116-126. https://doi.org/10.3390/j7020007
Arram RF, Morgan TB, Nix JT, Kao Y-L, Chen H. Genome Doubling of Northern Spicebush, Lindera benzoin L. J. 2024; 7(2):116-126. https://doi.org/10.3390/j7020007
Chicago/Turabian StyleArram, Ramsey F., Thomas B. Morgan, John T. Nix, Yu-Lin Kao, and Hsuan Chen. 2024. "Genome Doubling of Northern Spicebush, Lindera benzoin L." J 7, no. 2: 116-126. https://doi.org/10.3390/j7020007
APA StyleArram, R. F., Morgan, T. B., Nix, J. T., Kao, Y.-L., & Chen, H. (2024). Genome Doubling of Northern Spicebush, Lindera benzoin L. J, 7(2), 116-126. https://doi.org/10.3390/j7020007






