Surface-Modified Chitosan: An Adsorption Study of a “Tweezer-Like” Biopolymer with Fluorescein
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Tweezers-Like Adsorbent (CS-Ac-An)
2.3. Characterization
2.3.1. Thermogravimetric Analysis (TGA)
2.3.2. Fourier Transform Infrared (FT-IR) Spectroscopy
2.3.3. 1H- and 13C- Nuclear Magnetic Resonance (NMR) Spectroscopy
2.3.4. Degree of Substitution (DS)
2.3.5. Swelling Properties of the Tweezer-like Adsorbent at Equilibrium
2.3.6. Scanning Electron Microscopy (SEM)
2.3.7. Adsorption Studies
Equilibrium Dye Adsorption
Adsorption Kinetics
Adsorption Isotherms and Data Modelling
Adsorbent Regeneration Studies
3. Results and Discussion
3.1. Characterization of the Tweezers-Like Adsorbent (CS-Ac-An)
3.1.1. TGA
3.1.2. FT-IR Spectroscopy
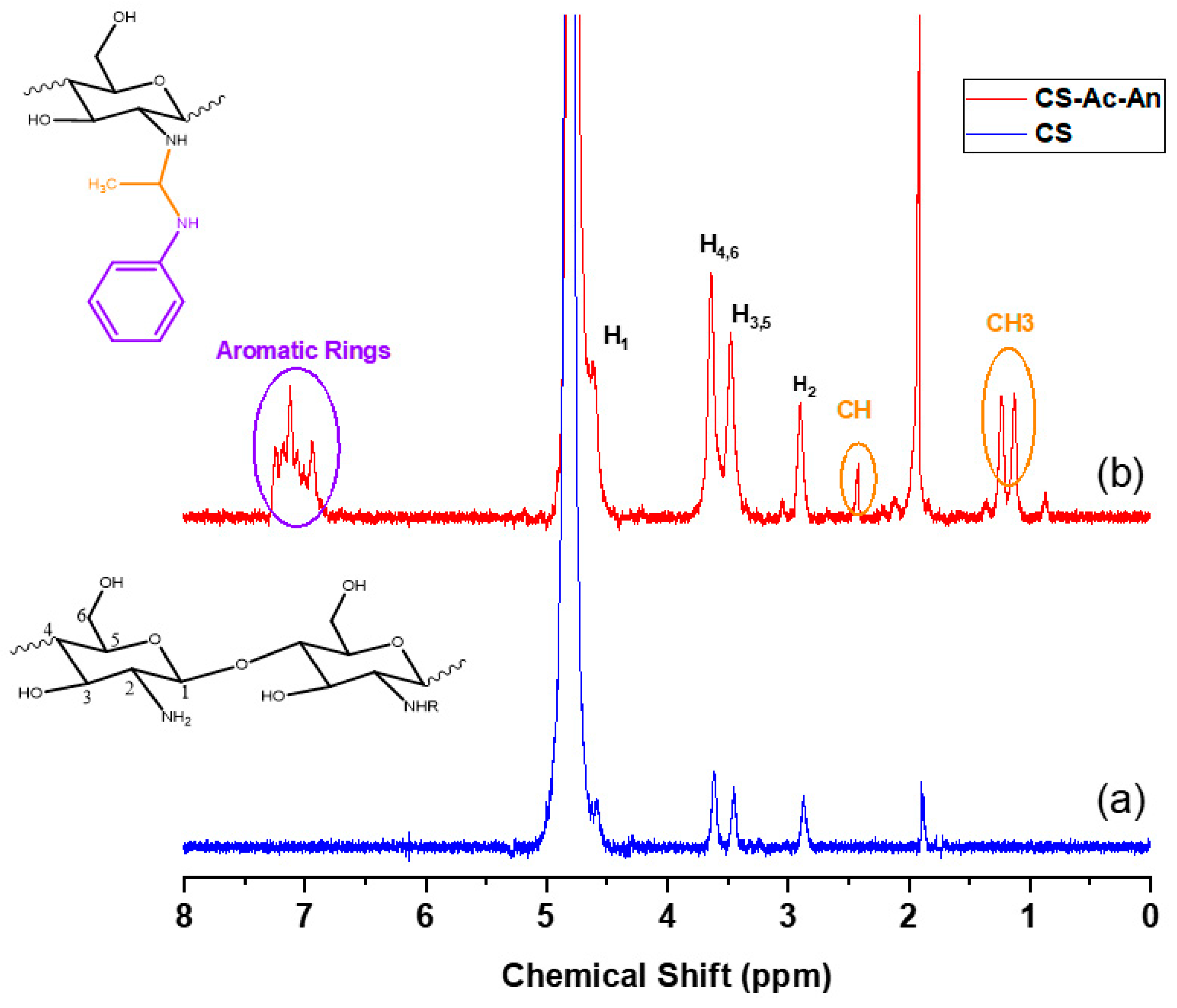
3.1.3. 1H-NMR and 13C-NMR Spectroscopy
3.1.4. Equilibrium Swelling Properties of the Chitosan Adsorbent
3.1.5. SEM
3.1.6. Surface Area (SA)
3.2. Adsorption Studies
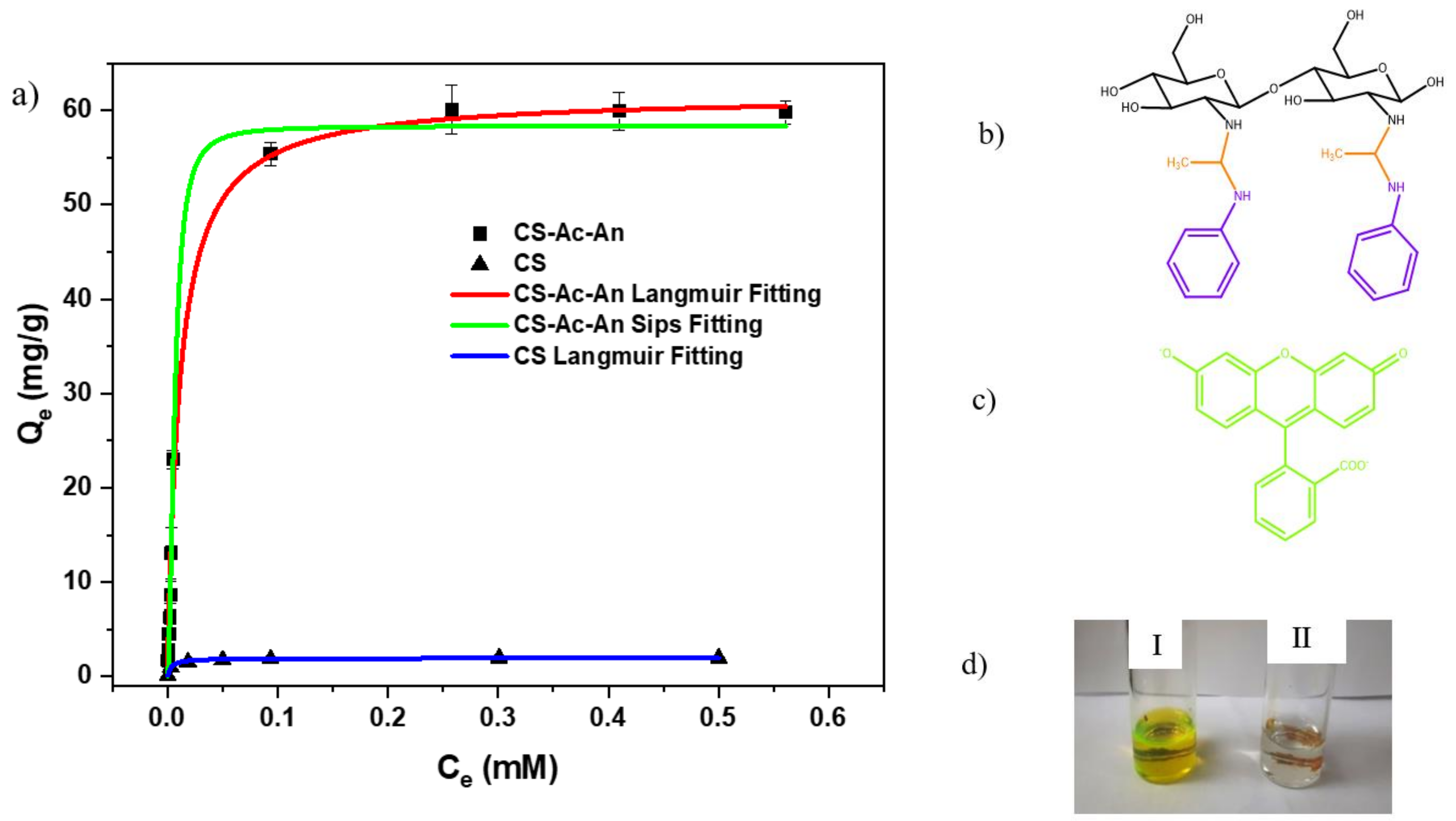
3.2.1. Equilibrium Adsorption of FL
3.2.2. Equilibrium Adsorption of FL Using Surfactant Modified CS-Ac-An
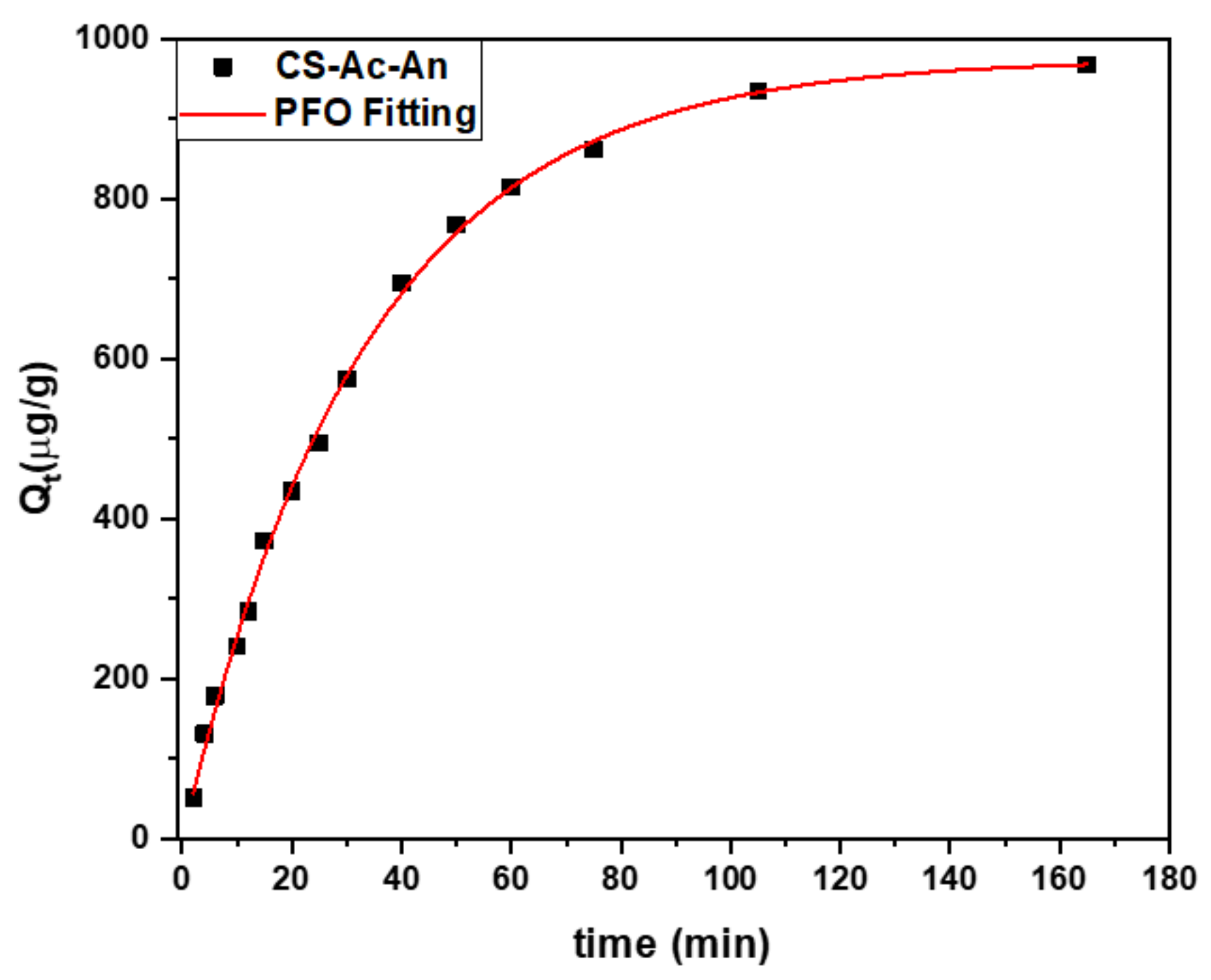
3.2.3. Kinetic Studies of FL Using CS-Ac-An
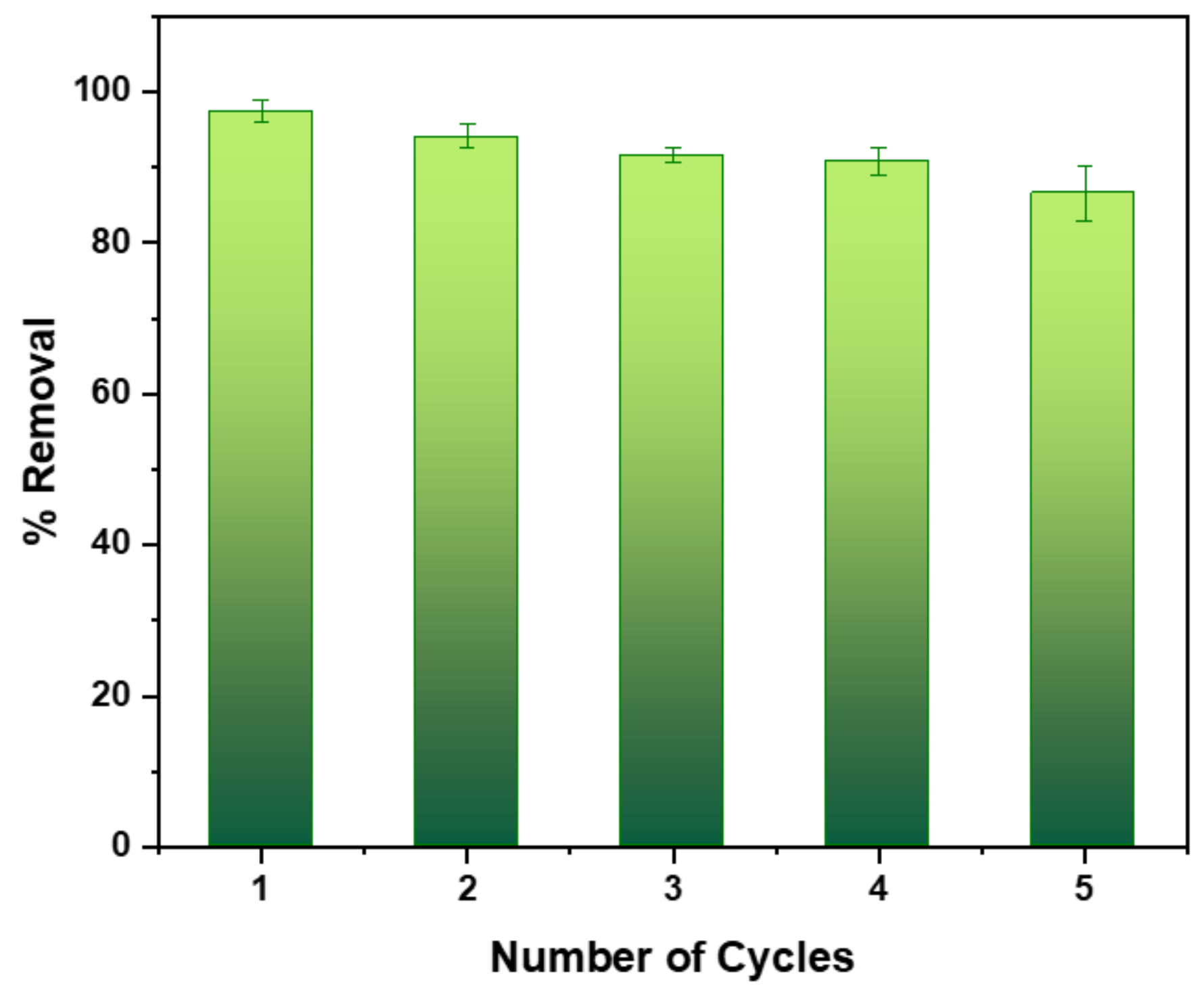
3.2.4. Regeneration Studies
3.2.5. Adsorption Mechanism, 1D/2D NOESY Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Edokpayi, J.; Odiyo, J.; Popoola, O.; Msagati, T. Assessment of Trace Metals Contamination of Surface Water and Sediment: A Case Study of Mvudi River, South Africa. Sustainability 2016, 8, 135. [Google Scholar] [CrossRef]
- Chen, Q.; Luo, Z.; Hills, C.; Xue, G.; Tyrer, M. Precipitation of Heavy Metals from Wastewater Using Simulated Flue Gas: Sequent Additions of Fly Ash, Lime and Carbon dioxide. Water Res. 2009, 43, 2605–2614. [Google Scholar] [CrossRef] [PubMed]
- Hubicki, Z.; Kolodynska, D. Selective Removal of Heavy Metal Ions from Waters and Waste Waters Using Ion Exchange Methods. Ion Exch. Technol. 2012, 7, 193–240. [Google Scholar]
- Bessbousse, H.; Rhlalou, T.; Verchère, J.F.; Lebrun, L. Removal of Heavy Metal Ions from Aqueous Solutions by Filtration with a Novel Complexing Membrane Containing Poly(ethyleneimine) in a Poly(vinyl alcohol) Matrix. J. Membr. Sci. 2008, 307, 249–259. [Google Scholar] [CrossRef]
- Ulewicz, M.; Walkowiak, W.; Gega, J.; Pospiech, B. Zinc(II) Selective Removal from other Transition Metal Ions by Solvent Extraction and Transport through Polymer Inclusion Memebranes with D2EHPA. Ars Separatoria Acta 2003, 2, 47–55. [Google Scholar]
- Ozaki, H.; Sharma, K.; Saktaywin, W. Performance of an Ultra-low-pressure Reverse Osmosis Membrane (ULPROM) for Separating Heavy Metal: Effects of Interference Parameters. Desalination 2002, 144, 287–294. [Google Scholar] [CrossRef]
- Agbovi, H.K.; Wilson, L.D. Design of Amphoteric Chitosan Flocculants for Phosphate and Turbidity Removal in Wastewater. Carbohydr. Polym. 2018, 189, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Agbovi, H.K.; Wilson, L.D.; Tabil, L.G. Biopolymer Flocculants and Oat Hull Biomass to Aid the Removal of Orthophosphate in Wastewater Treatment. Ind. Eng. Chem. Res. 2017, 56, 37–46. [Google Scholar] [CrossRef]
- Faust, S.D.; Osman, A.M. Adsorption Processes for Water Treatment and Purification; Bonilla-Petriciolet, A., Mendoza-Castillo, D.I., Reynel-Ávila, H.E., Eds.; Springer: Cham, Switzerland, 2017; pp. 8–14. [Google Scholar]
- Babel, S.; Kurniawan, T.A. Low-cost Adsorbents for Heavy Metals Uptake from Contaminated Water: A Review. J. Hazard. Mater. 2003, 97, 219–243. [Google Scholar] [CrossRef]
- Bailey, S.E.; Olin, T.J.; Bricka, R.M.; Adrian, D.D. A Review of Potentially Low-cost Sorbents for Heavy Metals. Water Res. 1999, 33, 2469–2479. [Google Scholar] [CrossRef]
- Demirbas, A. Heavy Metal Adsorption onto Agro-based Waste Materials: A Review. J. Hazard. Mater. 2008, 157, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zeng, Y.; Cheng, Z. Removal of Heavy Metal Ions Using Chitosan and Modified Chitosan: A Review. J. Mol. Liq. 2016, 214, 175–191. [Google Scholar] [CrossRef]
- Lyons, R.G. Identification and Separation of Water Tracing Dyes Using pH Response Characteristics. J. Hydrol. 1993, 152, 13–29. [Google Scholar] [CrossRef]
- Arsnow, G.F.; Vancil, M.A.; Schreiber, R.P.; Ramacciotti, C.N. Dye Tracer Study-Tried and True Method Yields Surprising Results. In Proceedings of the Annual International Conference on Soils, Sediments, Water and Energy, Amherst, MA, USA, 18–21 October 2010; pp. 337–350. [Google Scholar]
- Yan, F.; Fan, K.; Bai, Z.; Zhang, R.; Zu, F.; Xu, J.; Li, X. Fluorescein Applications as Fluorescent Probes for the Detection of Analytes. TrAC Trends Anal. Chem. 2017, 97, 15–35. [Google Scholar] [CrossRef]
- Wenderoth, B.; Gonzalez, M.F.; Dambach, S.; Machetanz, L.; Nitzschke, U. Glycol-free Aqueous Antifreeze Compositions Comprising Dicarboxylic Acid Salts. U.S. Patent 7,704,406, 27 April 2010. [Google Scholar]
- Clark, M. Christie Fluorescent dyes. In Handbook of Textile and Industrial Dyeing Principles, Processes and Types of Dyes; Woodhead Publishing: Cornwall, UK, 2011; pp. 562–587. [Google Scholar]
- Campardelli, R.; Trucillo, P.; Reverchon, E. A Supercritical Fluid-Based Process for the Production of Fluorescein-Loaded Liposomes. Ind. Eng. Chem. Res. 2016, 55, 5359–5365. [Google Scholar] [CrossRef]
- Kaido, M.; Matsumoto, Y.; Shigeno, Y.; Ishida, R.; Dogru, M. Corneal Fluorescein Staining Correlates with Visual Function in Dry Eye Patients. Investig. Ophthalmol. Vis. Sci. 2011, 52, 9516–9522. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Yiu, S.C. Dry Eye Disease: A Review of Diagnostic Approaches and Treatments. Saudi J. Ophthalmol. 2014, 28, 173–181. [Google Scholar] [CrossRef]
- Stockton, K.A.; Moffitt, C.M.; Farmer, C.N. Acute Toxicity of Sodium Fluorescein to Ashy Pebblesnails Fluminicola fuscus. Northwet Sci. 2012, 86, 190–197. [Google Scholar] [CrossRef]
- Pirillo, S.; Cornaglia, L. Removal of Fluorescein Using Different Iron Oxides as Adsorbents: Effect of pH. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2008, 71, 636–643. [Google Scholar] [CrossRef]
- Pirillo, S. Adsorption of Alizarin, Eriochrome Blue Black R and Fluorescein Using Different Iron Oxides as Adsorbents. Ind. Eng. Chem. Res. 2007, 46, 8255–8263. [Google Scholar] [CrossRef]
- Sugita, H.; Komai, T.; Yanagisawa, N.; Matsunaga, I. Study on Adsorption Behavior of Fluorescein on Soils. J. MMIJ 2008, 124, 513–518. [Google Scholar] [CrossRef]
- Tella, A.C.; Owalude, S.O.; Ojekanmi, A. Synthesis of Copper–Isonicotinate Metal–Organic Frameworks Simply by Mixing Solid Reactants and Investigation of Their Adsorptive Properties for the Removal of the Fluorescein dye. New J. Chem. 2014, 38, 4494–4500. [Google Scholar] [CrossRef]
- Shi, B.; Guan, H.; Shangguan, L.; Wang, H.; Xia, D.; Kong, X.; Huang, F. A Pillar arene-based 3D Network Polymer for Rapid Removal of Organic Micropollutants from Water. J. Mater. Chem. A 2017, 5, 24217–24222. [Google Scholar] [CrossRef]
- Abdus-Salam, N.; Buhari, M. Adsorption of Alizarin and Fluorescein Dyes on Adsorbent prepared from Mango Seed. Pac. J. Sci. Technol. 2014, 15, 232–244. [Google Scholar]
- Costantino, U.; Coletti, N.; Nocchetti, M.; Aloisi, G.G.; Elisei, F.; Latterini, L. Surface Uptake and Intercalation of Fluorescein Anions into Zn-Al-Hydrotalcite. Photophysical Characterization of Materials Obtained. Langmuir 2000, 16, 10351–10358. [Google Scholar] [CrossRef]
- Cvetkovic, A.; Zomerdijk, M.; Straathof, A.; Krishna, R.; Van Der Wielen, L. Adsorption of Fluorescein by Protein Crystals. Biotechnol. Bioeng. 2004, 87, 658–668. [Google Scholar] [CrossRef]
- Silva, P.M.O.; Francisco, J.E.; Cajé, J.C.M.; Cassella, R.J.; Pacheco, W.F.; Francisco, J.E.; Cajé, J.C.M.; Cassella, R.J. A Batch and Fixed Bed Column Study for Fluorescein Removal Using Chitosan Modified by Epichlorohydrin. J. Environ. Sci. Health Part A 2017, 53, 55–64. [Google Scholar] [CrossRef]
- Crini, G. Recent Developments in Polysaccharide-based Materials Used as Adsorbents in Wastewater Treatment. Prog. Polym. Sci. 2005, 30, 38–70. [Google Scholar] [CrossRef]
- Mahaninia, M.H.; Wilson, L.D. Cross-linked Chitosan Beads for Phosphate Removal from Aqueous Solution. J. Appl. Polym. Sci. 2016, 133, 1–10. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A.; Tubertini, O. Chitin and Chitosan as Chromatographic Supports and Adsorbents for Collection of Metal Ions from Organic and Aqueous Solutions and Sea-Water. Talanta 1969, 16, 1571–1577. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Bikiaris, D.N. Recent Modifications of Chitosan for Adsorption Applications: A Critical and Systematic Review. Mar. Drugs 2015, 13, 312–337. [Google Scholar] [CrossRef] [PubMed]
- Piron, E.; Accominotti, M.; Domard, A. Interaction Between Chitosan and Uranyl Ions. Role of Physical and Physicochemical Parameters on the Kinetics of Sorption. Langmuir 1997, 13, 1653–1658. [Google Scholar] [CrossRef]
- Pestov, A.; Bratskaya, S. Chitosan and Its Derivatives as Highly Efficient Polymer Ligands. Molecules 2016, 21, 330. [Google Scholar] [CrossRef] [PubMed]
- Ugraskan, V.; Torman, A.; Yoruc, A.B.H. Chitosan-Based Adsorbents for Wastewater Treatment; Naser, A., Ed.; Materials Research Forum LLC: Millersville, PA, USA, 2018; pp. 2–17. [Google Scholar]
- Chen, C.W.; Whitlock, H.W. Molecular Tweezers: A Simple Model of Bifunctional Intercalation. J. Am. Chem. Soc. 1978, 100, 4921–4922. [Google Scholar] [CrossRef]
- Zimmerman, S.C. Rigid Molecular Tweezers as Hosts for the Complexation of Neutral Guests. Top. Curr. Chem. 1993, 165, 71–102. [Google Scholar]
- Leblond, J.; Petitjean, A. Molecular tweezers: Concepts and applications. ChemPhysChem 2011, 12, 1043–1051. [Google Scholar] [CrossRef] [PubMed]
- Klärner, F.G.; Kahlert, B. Molecular Tweezers and Clips as Synthetic Receptors. Molecular Recognition and Dynamics in Receptor-Substrate Complexes. Acc. Chem. Res. 2003, 36, 919–932. [Google Scholar] [CrossRef]
- Hardouin–Lerouge, M.; Hudhomme, P.; Sallé, M. Molecular Clips and Tweezers Hosting Neutral Guests. Chem. Soc. Rev. 2011, 40, 30–43. [Google Scholar] [CrossRef]
- Brouwer, E.B.; Enright, G.D.; Ratcliffe, C.I.; Facey, G.A.; Ripmeester, J.A. Weak Intermolecular Interactions and Molecular Recognition: Structure and Dynamics of the Benzene and Pyridine p-tert-butylcalix arene Inclusions. J. Phys. Chem. B 1999, 103, 10604–10616. [Google Scholar] [CrossRef]
- Bismarck, A.; Chan-park, M.B. Modified Chitosan Emulsifiers: Small Compositional Changes Produce Vastly Different High Internal Phase Emulsion Types. J. Mater. Chem. B 2015, 3, 4118–4122. [Google Scholar]
- Mahanta, A.K.; Mittal, V.; Singh, N.; Dash, D.; Malik, S.; Kumar, M.; Maiti, P. Polyurethane-Grafted Chitosan as New Biomaterials for Controlled Drug Delivery. Macromolecules 2015, 48, 2654–2666. [Google Scholar] [CrossRef]
- Mohamed, M.H.; Wilson, L.D. Sequestration of Agrochemicals from Aqueous Media Using Cross-linked Chitosan-based Sorbents. Adsorption 2016, 22, 1025–1034. [Google Scholar] [CrossRef]
- Lowell, S.; Shields, J.E. Powder Surface Area and Porosity, 3rd ed.; Springer: Hong Kong, China, 1991. [Google Scholar]
- Inel, O.; Tumsek, F. The Measurement of Surface Areas of Some Silicates by Solution Adsorption. Turk. J. Chem. 2000, 24, 9–19. [Google Scholar]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and Interpretation of Adsorption Isotherms. J. Chem. 2017, 2017, 11. [Google Scholar] [CrossRef]
- Sips, R. On the Structure of a Catalyst Surface. J. Chem. Phys. 1948, 16, 490–495. [Google Scholar] [CrossRef]
- Dolatkhah, A.; Wilson, L.D. Magnetite/Polymer Brush Nanocomposites with Switchable Uptake Behavior Toward Methylene Blue. ACS Appl. Mater. Interfaces 2016, 8, 5595–5607. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.S.; Mckay, G. Sorption of Dye from Aqueous Solution by Peat. Chem. Eng. J. 1998, 70, 115–124. [Google Scholar] [CrossRef]
- Sreelatha, G.; Ageetha, V.; Parmar, J.; Padmaja, P. Equilibrium and Kinetic Studies on Reactive Dye Adsorption Using Palm Shell Powder (An Agrowaste) and Chitosan. J. Chem. Eng. Data 2010, 56, 35–42. [Google Scholar] [CrossRef]
- La, M.; Xia, Z.; Serreqi, A.N.; Berrada, M.; Rodrigues, A.; Wang, D. A validated 1H-NMR Method for the Determination of the Degree of Deacetylation of Chitosan. J. Pharm. Biomed. Anal. 2003, 32, 1149–1158. [Google Scholar]
- Rinaudo, M.; Le Dung, P.; Gey, C.; Milas, M. Substituent Distribution on O,N-carboxymethylchitosans by 1H and 13C-NMR Marguerite. Int. J. Biol. Macromol. 1992, 14, 122–128. [Google Scholar] [CrossRef]
- Wang, H.Y.; Jia, H.R.; Lu, X.; Chen, B.; Zhou, G.; He, N.; Chenb, Z.; Wu, F.G. Imaging Plasma Membranes without Cellular Reagents based on Glycol Cchitosan Derivatives. J. Mater. Chem. B 2015, 3, 6165–6173. [Google Scholar] [CrossRef]
- Dehabadi, L.; Shakouri, M.; Simonson, C.J.; Arjmand, M.; Sundararaj, U.; Wilson, L.D. Vapour and Solution Uptake Properties of Starch and Cellulose Biopolymers. J. Geosci. Environ. Prot. 2018, 6, 101–117. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, J.; Watoo, F.H.; Watoo, M.H.S.; Malik, R.; Trimizi, S.A.; Imran, M.; Ghangro, A.B. Adsorption of Acid Yellow Dye on Flakes of Chitosan Prepared from Fishery Wastes. Arab. J. Chem. 2011, 4, 389–395. [Google Scholar] [CrossRef]
- Panchompoo, J.; Aldous, L.; Baker, M.; Wallace, M.I.; Compton, R.G. One-step Synthesis of Fluorescein Modified Nano-Carbon for Pd(II) Detection via Fluorescence Quenching. Analyst 2012, 137, 2054–2062. [Google Scholar] [CrossRef] [PubMed]
- Ogunmodede1, O.T.; Ojo, A.A.; Adewole, E.; Adebayo, O.L. Adsorptive Removal of Anionic Dye from Aqueous Solutions by Mixture of Kaolin and Bentonite Clay: Characteristics, Isotherm, Kinetic and Thermodynamic Studies. Iran. J. Energy Environ. 2016, 6, 147–153. [Google Scholar]
- Schottel, B.L.; Chifotides, H.T.; Dunbar, K.R.; Gale, P.A.; García-garrido, S.E.; Garric, J.; Schottel, B.L.; Chifotides, H.T.; Dunbar, K.R. Anion-π interactions. Chem. Soc. Rev. 2008, 37, 68–83. [Google Scholar] [CrossRef] [PubMed]
- Mohan, N.; Vijayalakshmi, K.P.; Koga, N.; Suresh, C.H. Comparison of Aromatic NHπ, OHπ, and CHπ Interactions of Alanine Using MP2, CCSD, and DFT Methods. J. Comput. Chem. 2010, 16, 2874–2882. [Google Scholar]
- Meyer, E.E.; Rosenberg, K.J.; Israelachvili, J. Recent Progress in Understanding Hydrophobic Interactions. Proc. Natl. Acad. Sci. USA 2006, 103, 15739–15746. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, S.; Ramanathan, V.; Mishra, B.K. Influence of the Substituents on the CHπ Interaction: Benzene—Methane Complex. J. Phys. Chem. A 2013, 117, 6687–6694. [Google Scholar] [CrossRef]
- Hunter, C.A.; Sanders, J.K.M. The Nature of π-π Interactions. J. Am. Chem. Soc. 1990, 112, 5525–5534. [Google Scholar] [CrossRef]
- Meroufel, B.; Benali, O.; Benyahia, M.; Benmoussa, Y.; Zenasni, M.A. Adsorptive Removal of Anionic Dye from Aqueous Solutions by Algerian Kaolin: Characteristics, Isotherm, Kinetic and Thermodynamic Studies. J. Mater. Environ. Sci. 2013, 4, 482–491. [Google Scholar]
- Pal, P.; Pal, A. Surfactant-Modified Chitosan Beads for Cadmium Ion Adsorption. Int. J. Biol. Macromol. 2017, 104, 1548–1555. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Li, S.; Chen, M.; Jiang, R. Removal of Congo Red Dye by Gemini Surfactant C12-4-C12 2Br-Modified Chitosan Hydrogel Beads. J. Dispers. Sci. Technol. 2017, 38, 46–57. [Google Scholar] [CrossRef]
- Chiappisi, L.; Gradzielski, M. Co-assembly in Chitosan–Surfactant Mixtures: Thermodynamics, Structures, Interfacial Properties and Applications. Adv. Colloid Interface Sci. 2015, 220, 92–107. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Lee, D.S.; Lee, M.W.; Woo, S.H. Enhanced Molar Sorption Ratio for Naphthalene Through the Impregnation of Surfactant into Chitosan Hydrogel Beads. Bioresour. Technol. 2010, 101, 4315–4321. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.C.; Hudson, S.M. Binding of Sodium Dodecyl Sulfate to Polyelectrolyte Based on Chitosan. Macromolecules 1993, 26, 4151–4154. [Google Scholar] [CrossRef]
- Dougherty, D.A. The Cation-π Interaction. Chem. Res. 2013, 46, 885–893. [Google Scholar] [CrossRef]
- Zhao, Z.; Shent, T.A.O. The Absorption and Structure of Fluorescein and its Ethyl Derivatives in Various Solutions. Spectrochim. Acta Part A Mol. Spectrosc. 1989, 45, 1113–1116. [Google Scholar] [CrossRef]
- Diehl, H.; Markuszewski, R. Studies on Fluorescein-II. Talanta 1985, 32, 159–165. [Google Scholar] [CrossRef]
- Karoyo, A.; Dehabadi, L.; Wilson, L.D. Renewable Starch Carrieres with Switchable Adsorption Properties. ACS Sustain. Chem. Eng. 2018, 6, 4603–4613. [Google Scholar] [CrossRef]
- Udoetok, I.A.; Wilson, L.D.; Headley, J.V. Self-Assembled and Cross-Linked Animal and Plant-Based Polysaccharides: Chitosan-Cellulose Composites and Their Anion Uptake Properties. ACS Appl. Mater. Interfaces 2016, 8, 33197–33209. [Google Scholar] [CrossRef] [PubMed]
- Danquah, M.K.; Aruei, R.C.; Wilson, L.D. Phenolic Pollutant Uptake Properties of Molecular Templated Polymers Containing β-Cyclodextrin. J. Phys. Chem. B 2018, 122, 4748–4757. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.; Kaware, J. Regeneration and Recovery in Adsorption—A Review. Int. J. Innov. Sci. Eng. Technol. 2014, 1, 61–64. [Google Scholar]
- Poorghorban, M.; Karoyo, A.H.; Grochulski, P.; Verrall, R.E.; Wilson, L.D.; Badea, I. A 1H-NMR Study of Host/Guest Supramolecular Complexes of a Curcumin Analogue with β-Cyclodextrin and a β-Cyclodextrin-Conjugated Gemini Surfactant. Mol. Pharm. 2015, 12, 2993–3006. [Google Scholar] [CrossRef] [PubMed]







| CS | CS-Ac-An | ||||||
|---|---|---|---|---|---|---|---|
| Qm (mg g−1) | KL (L mg−1) | Chi-Square | Adj-R2 | Qm (mg g−1) | KL (L mg−1) | Chi-Square | Adj-R2 |
| 1.96 | 154 | 3.47 | 0.992 | 61.8 | 91.8 | 2.12 | 0.993 |
| Pseudo First Order (PFO) | Pseudo Second Order (PSO) | ||||||
|---|---|---|---|---|---|---|---|
| Qm (µg g−1) | k1 (min−1) | Chi-Square | Adj-R2 | Qm (µg g−1) | k2 (g μg−1 min−1) | Chi-Square | Adj-R2 |
| 975.2 | 0.03 | 71 | 0.998 | 239 | 94.7 | 785 | 0.991 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vafakish, B.; Wilson, L.D. Surface-Modified Chitosan: An Adsorption Study of a “Tweezer-Like” Biopolymer with Fluorescein. Surfaces 2019, 2, 468-484. https://doi.org/10.3390/surfaces2030035
Vafakish B, Wilson LD. Surface-Modified Chitosan: An Adsorption Study of a “Tweezer-Like” Biopolymer with Fluorescein. Surfaces. 2019; 2(3):468-484. https://doi.org/10.3390/surfaces2030035
Chicago/Turabian StyleVafakish, Bahareh, and Lee D. Wilson. 2019. "Surface-Modified Chitosan: An Adsorption Study of a “Tweezer-Like” Biopolymer with Fluorescein" Surfaces 2, no. 3: 468-484. https://doi.org/10.3390/surfaces2030035
APA StyleVafakish, B., & Wilson, L. D. (2019). Surface-Modified Chitosan: An Adsorption Study of a “Tweezer-Like” Biopolymer with Fluorescein. Surfaces, 2(3), 468-484. https://doi.org/10.3390/surfaces2030035







