A Comparative Study of the Electro-Assisted Grafting of Mono- and Bi-Phosphonic Acids on Nitinol
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals
2.2. Nitinol Substrates Preparation
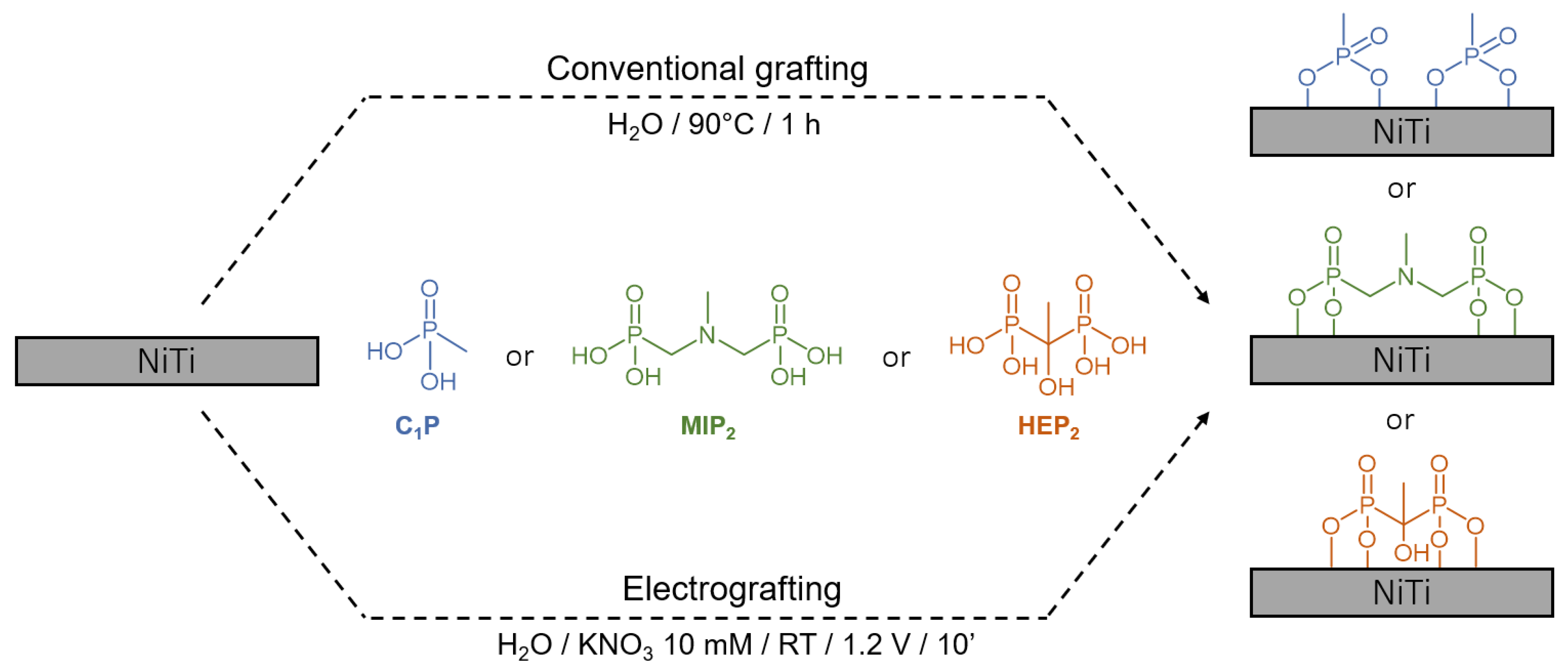
2.3. Conventional Grafting (CG) of the Organophosphonic Acid Derivatives on Nitinol
2.4. Electro-Assisted Grafting (EG) of the Organophosphonic Acid Derivatives on Nitinol
2.5. Substrate Characterization
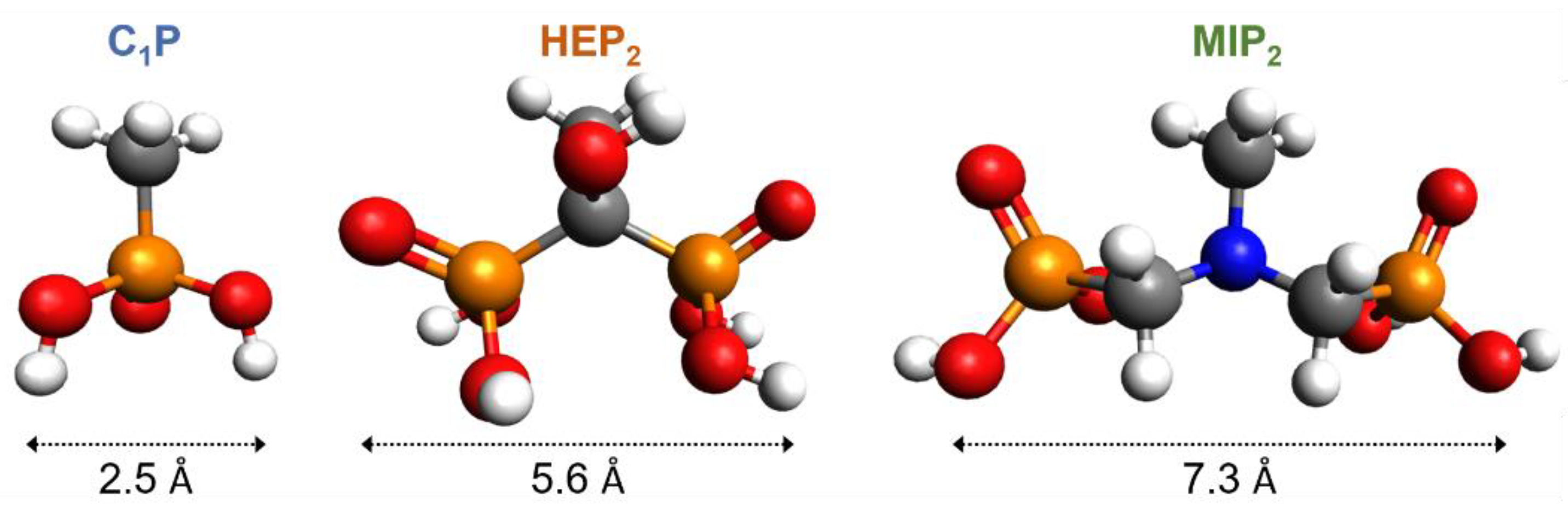
2.6. Molecular Modeling
3. Results and Discussion
Grafting of Organophosphonic Derivatives on HT Nitinol
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Duerig, T.; Pelton, A.; Stöckel, D. An overview of nitinol medical applications. Mater. Sci. Eng. A 1999, 273–275, 149–160. [Google Scholar] [CrossRef]
- Barras, C.D.J.; Myers, K.A. Nitinol—Its Use in Vascular Surgery and Other Applications. Eur. J. Vasc. Endovasc. Surg. 2000, 19, 564–569. [Google Scholar] [CrossRef]
- Shabalovskaya, S.; Anderegg, J.; Van Humbeeck, J. Critical overview of Nitinol surfaces and their modifications for medical applications. Acta Biomater. 2008, 4, 447–467. [Google Scholar] [CrossRef] [PubMed]
- Shayan, M.; Chun, Y. An overview of thin film nitinol endovascular devices. Acta Biomater. 2015, 21, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Elahinia, M.H.; Hashemi, M.; Tabesh, M.; Bhaduri, S.B. Manufacturing and processing of NiTi implants: A review. Prog. Mater. Sci. 2012, 57, 911–946. [Google Scholar] [CrossRef]
- Centre d’Animation Régional en Matériaux Avancés (C.A.R.M.A.). Alliages à Mémoire de Forme; Techniques de l’Ingénieur: Saint-Denis, France, 2001. [Google Scholar]
- Mantovani, D. Shape memory alloys: Properties and biomedical applications. JOM 2000, 52, 36–44. [Google Scholar] [CrossRef]
- Mohd Jani, J.; Leary, M.; Subic, A.; Gibson, M.A. A review of shape memory alloy research, applications and opportunities. Mater. Des. 2014, 56, 1078–1113. [Google Scholar] [CrossRef]
- Duerig, T.W.; Melton, K.N.; Stöckel, D. Engineering Aspects of Shape Memory Alloys; Butterworth–Heinemann: London, UK, 1990. [Google Scholar]
- Airoldi, G.; Riva, G.; Vanelli, M. Superelasticity and Shape Memory Effect in NiTi Orthodontic Wires. J. Phys. IV 1995, 5, 1205–1210. [Google Scholar] [CrossRef]
- Laster, Z.; MacBean, A.D.; Ayliffe, P.R.; Newlands, L.C. Fixation of a frontozygomatic fracture with a shape-memory staple. Br. J. Oral Maxillofac. Surg. 2001, 39, 324–325. [Google Scholar] [CrossRef] [PubMed]
- Luebke, N.; Brantley, W.; Alapati, S.; Mitchell, J.; Lausten, L.; Daehn, G. Bending Fatigue Study of Nickel-Titanium Gates Glidden Drills. J. Endod. 2005, 31, 523–525. [Google Scholar] [CrossRef]
- Nematollahi, M.; Baghbaderani, K.S.; Amerinatanzi, A. Application of NiTi in Assistive and Rehabilitation Devices: A Review. Bioengineering 2019, 6, 37. [Google Scholar] [CrossRef]
- Eliaz, N. Corrosion of Metallic Biomaterials: A Review. Materials 2019, 12, 407. [Google Scholar] [CrossRef]
- Shabalovskaya, S.A.; Rondelli, G.C.; Undisz, A.L.; Anderegg, J.W.; Burleigh, T.D.; Rettenmayr, M.E. The electrochemical characteristics of native Nitinol surfaces. Biomaterials 2009, 30, 3662–3671. [Google Scholar] [CrossRef]
- Shabalovskaya, S.A.; Tian, H.; Anderegg, J.W.; Schryvers, D.U.; Carroll, W.U.; Van Humbeeck, J. The influence of surface oxides on the distribution and release of nickel from Nitinol wires. Biomaterials 2009, 30, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Figueira, N.; Silva, T.M.; Carmezim, M.J.; Fernandes, J.C.S. Corrosion behaviour of NiTi alloy. Electrochim. Acta 2009, 54, 921–926. [Google Scholar] [CrossRef]
- Milošev, I.; Kapun, B. The corrosion resistance of Nitinol alloy in simulated physiological solutions Part 1: The effect of surface preparation. Mater. Sci. Eng. C 2012, 32, 1087–1096. [Google Scholar] [CrossRef]
- Yang, J.; Wang, J.; Tong, S. Surface properties of bio-implant Nitinol modified by ECR cold plasma. Mater. Sci. Technol. 2004, 20, 1427–1431. [Google Scholar] [CrossRef]
- Shen, Y.; Wang, G.; Chen, L.; Li, H.; Yu, P.; Bai, M.; Zhang, Q.; Lee, J.; Yu, Q. Investigation of surface endothelialization on biomedical nitinol (NiTi) alloy: Effects of surface micropatterning combined with plasma nanocoatings. Acta Biomater. 2009, 5, 3593–3604. [Google Scholar] [CrossRef]
- Siu, H.T.; Man, H.C. Fabrication of bioactive titania coating on nitinol by plasma electrolytic oxidation. Appl. Surf. Sci. 2013, 274, 181–187. [Google Scholar] [CrossRef]
- Lahann, J.; Klee, D.; Thelen, H.; Bienert, H.; Vorwerk, D.; Höcker, H. Improvement of haemocompatibility of metallic stents by polymer coating. J. Mater. Sci. Mater. Med. 1999, 10, 443–448. [Google Scholar] [CrossRef]
- Catledge, S.A.; Thomas, V.; Vohra, Y.K. Effect of Surface Oxides and Intermetallics on Nanostructured Diamond Coating of Nitinol. Curr. Nanosci. 2006, 2, 9–12. [Google Scholar] [CrossRef]
- Pérez, L.M.; Gracia-Villa, L.; Puértolas, J.A.; Arruebo, M.; Irusta, S.; Santamaría, J. Effect of Nitinol surface treatments on its physico-chemical properties. J. Biomed. Mater. Res. B. Appl. Biomater. 2009, 91, 337–347. [Google Scholar] [CrossRef]
- Devillers, S.; Barthélémy, B.; Delhalle, J.; Mekhalif, Z. Induction Heating Vs Conventional Heating for the Hydrothermal Treatment of Nitinol and Its Subsequent 2-(Methacryloyloxy)ethyl 2-(trimethylammonio)ethyl Phosphate Coating by Surface-Initiated Atom Transfer Radical Polymerization. ACS Appl. Mater. Interfaces 2011, 3, 4059–4066. [Google Scholar] [CrossRef] [PubMed]
- Quiñones, R.; Gawalt, E.S. Study of the formation of self-assembled monolayers on nitinol. Langmuir 2007, 23, 10123–10130. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, Z.; Katic, J.; Metikos-Hukovic, M.; Dadafarin, H.; Omanovic, S. Modification of a Nitinol Surface by Phosphonate Self-Assembled Monolayers. J. Electrochem. Soc. 2011, 158, F159–F165. [Google Scholar] [CrossRef]
- Maho, A.; Kanoufi, F.; Combellas, C.; Delhalle, J.; Mekhalif, Z. Electrochemical Investigation of Nitinol/Tantalum Hybrid Surfaces Modified by Alkylphosphonic Self-Assembled Monolayers. Electrochim. Acta 2014, 116, 78–88. [Google Scholar] [CrossRef]
- Marcinko, S.; Fadeev, A.Y. Hydrolytic Stability of Organic Monolayers Supported on TiO2 and ZrO2. Langmuir 2004, 20, 2270–2273. [Google Scholar] [CrossRef] [PubMed]
- Bhure, R.; Mahapatro, A.; Bonner, C.; Abdel-Fattah, T.M. In vitro stability study of organophosphonic self assembled monolayers (SAMs) on cobalt chromium (Co–Cr) alloy. Mater. Sci. Eng. C 2013, 33, 2050–2058. [Google Scholar] [CrossRef] [PubMed]
- Mahapatro, A.; Matos Negrón, T.D.; Nguyen, A. Spectroscopic Evaluations of Interfacial Oxidative Stability of Phosphonic Nanocoatings on Magnesium. J. Spectrosc. 2015, 2015, 350630. [Google Scholar] [CrossRef]
- Guerrero, G.; Alauzun, J.G.; Granier, M.; Laurencin, D.; Mutin, P.H. Phosphonate coupling molecules for the control of surface/interface properties and the synthesis of nanomaterials. Dalton Trans. 2013, 42, 12569. [Google Scholar] [CrossRef] [PubMed]
- Mani, G.; Johnson, D.M.; Marton, D.; Dougherty, V.L.; Feldman, M.D.; Patel, D.; Ayon, A.A.; Agrawal, C.M. Stability of Self-Assembled Monolayers on Titanium and Gold. Langmuir 2008, 24, 6774–6784. [Google Scholar] [CrossRef] [PubMed]
- Ishizaki, T.; Okido, M.; Masuda, Y.; Saito, N.; Sakamoto, M. Corrosion resistant performances of alkanoic and phosphonic acids derived self-assembled monolayers on magnesium alloy AZ31 by Vapor-Phase Method. Langmuir 2011, 27, 6009–6017. [Google Scholar] [CrossRef] [PubMed]
- Abohalkuma, T.; Telegdi, J. Corrosion protection of carbon steel by special phosphonic acid nano-layers. Mater. Corros. 2015, 66, 1382–1390. [Google Scholar] [CrossRef]
- Quiñones, R.; Raman, A.; Gawalt, E.S. Functionalization of nickel oxide using alkylphosphonic acid self-assembled monolayers. Thin Solid Films 2008, 516, 8774–8781. [Google Scholar] [CrossRef]
- Devillers, S.; Lemineur, J.F.; Dilimon, V.S.; Barthélémy, B.; Delhalle, J.; Mekhalif, Z. Polyelectrolyte multilayer deposition on nickel modified with self-assembled monolayers of organophosphonic acids for biomaterials: Electrochemical and spectroscopic evaluation. J. Phys. Chem. C 2012, 116, 19252–19261. [Google Scholar] [CrossRef]
- Metoki, N.; Liu, L.; Beilis, E.; Eliaz, N.; Mandler, D. Preparation and characterization of alkylphosphonic acid self-assembled monolayers on titanium alloy by chemisorption and electrochemical deposition. Langmuir 2014, 30, 6791–6799. [Google Scholar] [CrossRef] [PubMed]
- Vanhooland, A.; Devillers, S.; Issakova, T.; Michaux, C.; Delhalle, J.; Mekhalif, Z. Electroassisted Auto-Assembly of Alkylphosphonic Acids Monolayers on Nitinol. J. Electrochem. Soc. 2016, 163, G173–G177. [Google Scholar] [CrossRef]
- Arrotin, B.; Delhalle, J.; Dubois, P.; Mespouille, L.; Mekhalif, Z. Electroassisted Functionalization of Nitinol Surface, a Powerful Strategy for Polymer Coating through Controlled Radical Surface Initiation. Langmuir 2017, 33, 2977–2985. [Google Scholar] [CrossRef]
- Gupta, R.K.; Singh, R.A. Inhibition of corrosion by poly(N-hexadecylaniline)/docosanol mixed Langmuir–Blodgett films on copper in sea water. Mater. Chem. Phys. 2006, 97, 226–229. [Google Scholar] [CrossRef]
- Li, C.; Li, L.; Wang, C. Study of the inhibitive effect of mixed self-assembled monolayers on copper with SECM. Electrochim. Acta 2014, 115, 531–536. [Google Scholar] [CrossRef]
- Barthélémy, B.; Maheux, S.; Devillers, S.; Kanoufi, F.; Combellas, C.; Delhalle, J.; Mekhalif, Z. Synergistic Effect on Corrosion Resistance of Phynox Substrates Grafted with Surface-Initiated ATRP (Co)polymerization of 2-Methacryloyloxyethyl Phosphorylcholine (MPC) and 2-Hydroxyethyl Methacrylate (HEMA). ACS Appl. Mater. Interfaces 2014, 6, 10060–10071. [Google Scholar] [CrossRef] [PubMed]
- Arrotin, B.; Noël, J.-M.; Delhalle, J.; Mespouille, L.; Mekhalif, Z. Electrografting of mixed organophosphonic monolayers for SI-ATRP of 2-methacryloyloxyethyl phosphorylcholine. J. Coat. Technol. Res. 2019, 16, 1121–1132. [Google Scholar] [CrossRef]
- Chastain, J. (Ed.) Handbook of X-ray Photoelectron Spectroscopy; Perkin Elmer Corporation: Waltham, MA, USA, 1992. [Google Scholar]
- Miomandre, F.; Sadki, S.; Audebert, P.; Méallet-Renaut, R. Électrochimie: Des Concepts aux Applications, 3rd ed.; Dunod: Malakoff, France, 2014. [Google Scholar]
- Lukács, Z. Evaluation of model and dispersion parameters and their effects on the formation of constant-phase elements in equivalent circuits. J. Electroanal. Chem. 1999, 464, 68–75. [Google Scholar] [CrossRef]

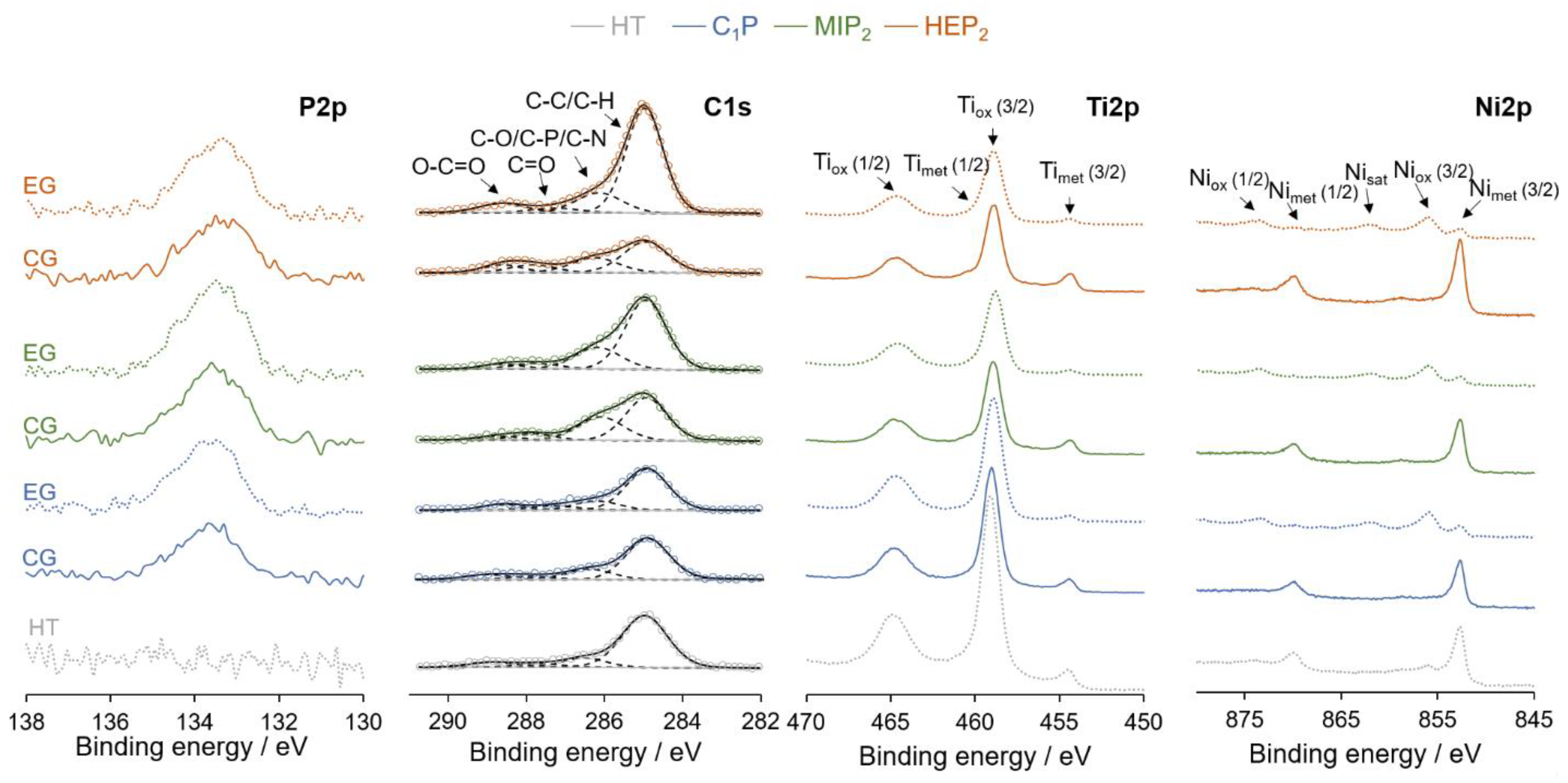
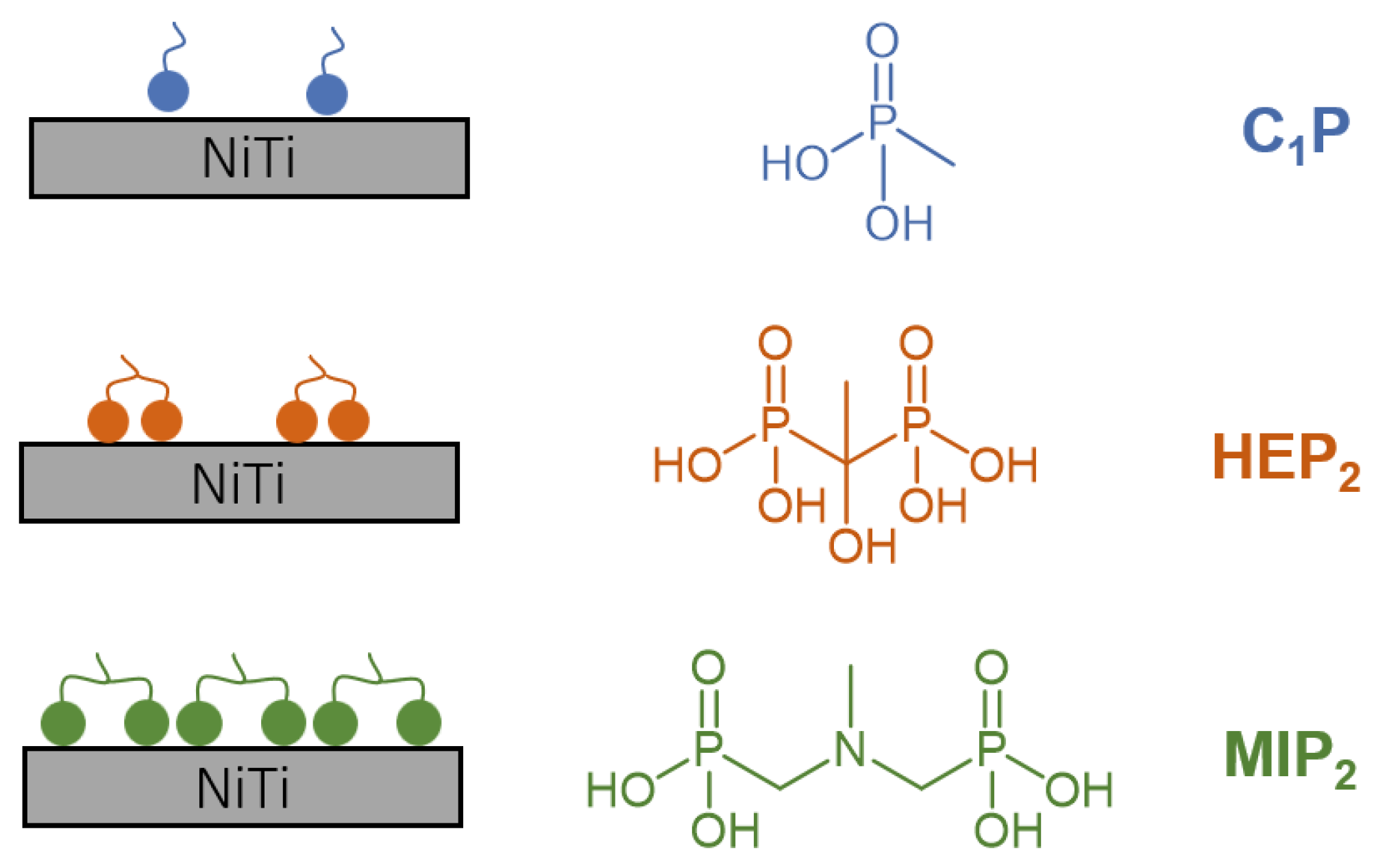

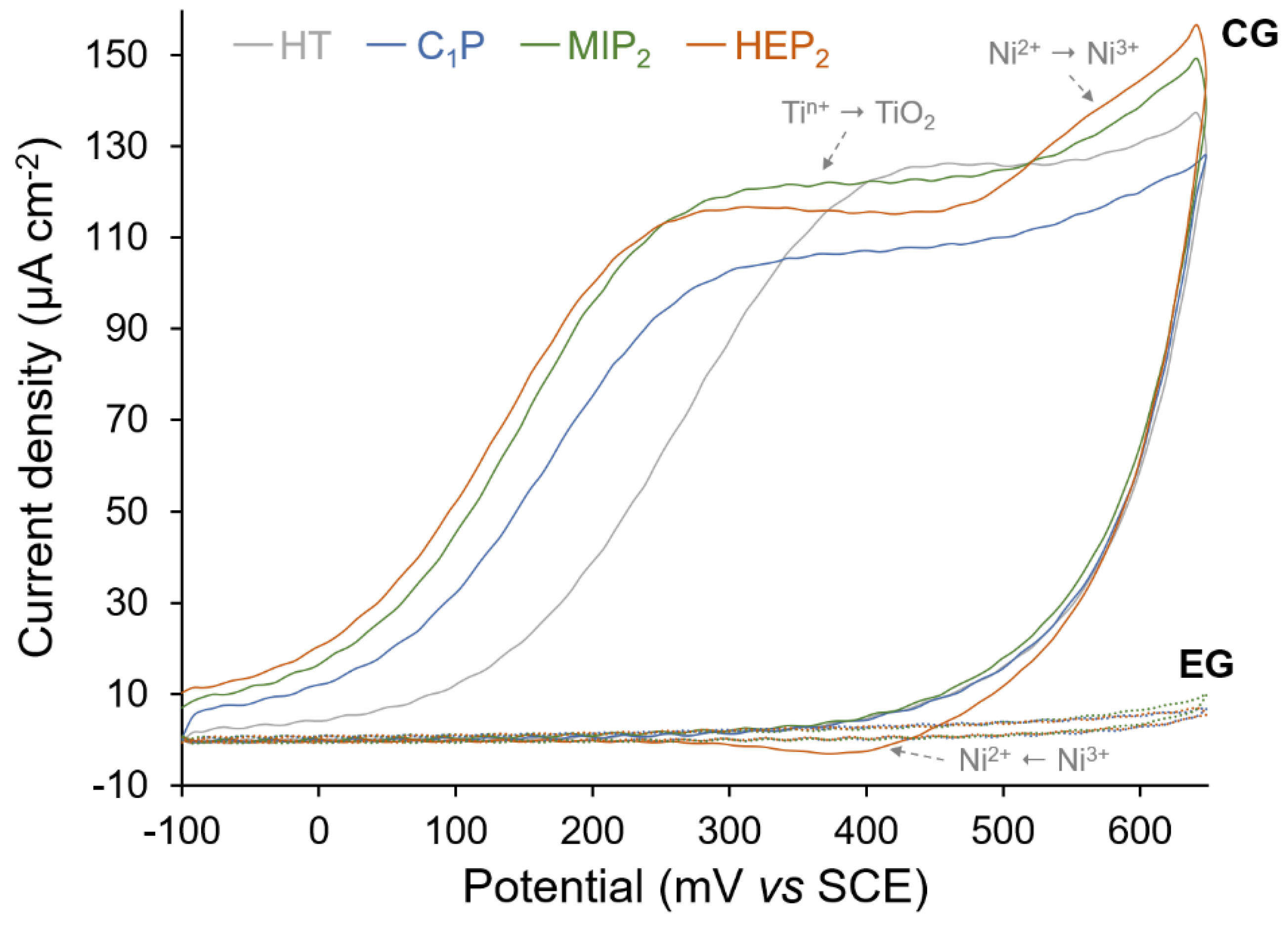
| Sample | P/NiTi | O/NiTi | Ni/NiTi | Ti/NiTi |
|---|---|---|---|---|
| HT | - | 4.27 ± 0.70 | 0.15 ± 0.01 | 0.85 ± 0.01 |
| C1P (CG) | 0.02 ± 0.01 | 2.65 ± 0.56 | 0.18 ± 0.04 | 0.82 ± 0.04 |
| C1P (EG) | 0.05 ± 0.01 | 3.12 ± 0.21 | 0.13 ± 0.02 | 0.87 ± 0.02 |
| MIP2 (CG) | 0.09 ± 0.01 | 2.83 ± 0.23 | 0.21 ± 0.03 | 0.79 ± 0.03 |
| MIP2 (EG) | 0.14 ± 0.02 | 3.92 ± 0.36 | 0.15 ± 0.02 | 0.85 ± 0.02 |
| HEP2 (CG) | 0.08 ± 0.01 | 2.80 ± 0.23 | 0.30 ± 0.03 | 0.70 ± 0.03 |
| HEP2 (EG) | 0.12 ± 0.02 | 3.51 ± 0.90 | 0.15 ± 0.02 | 0.85 ± 0.02 |
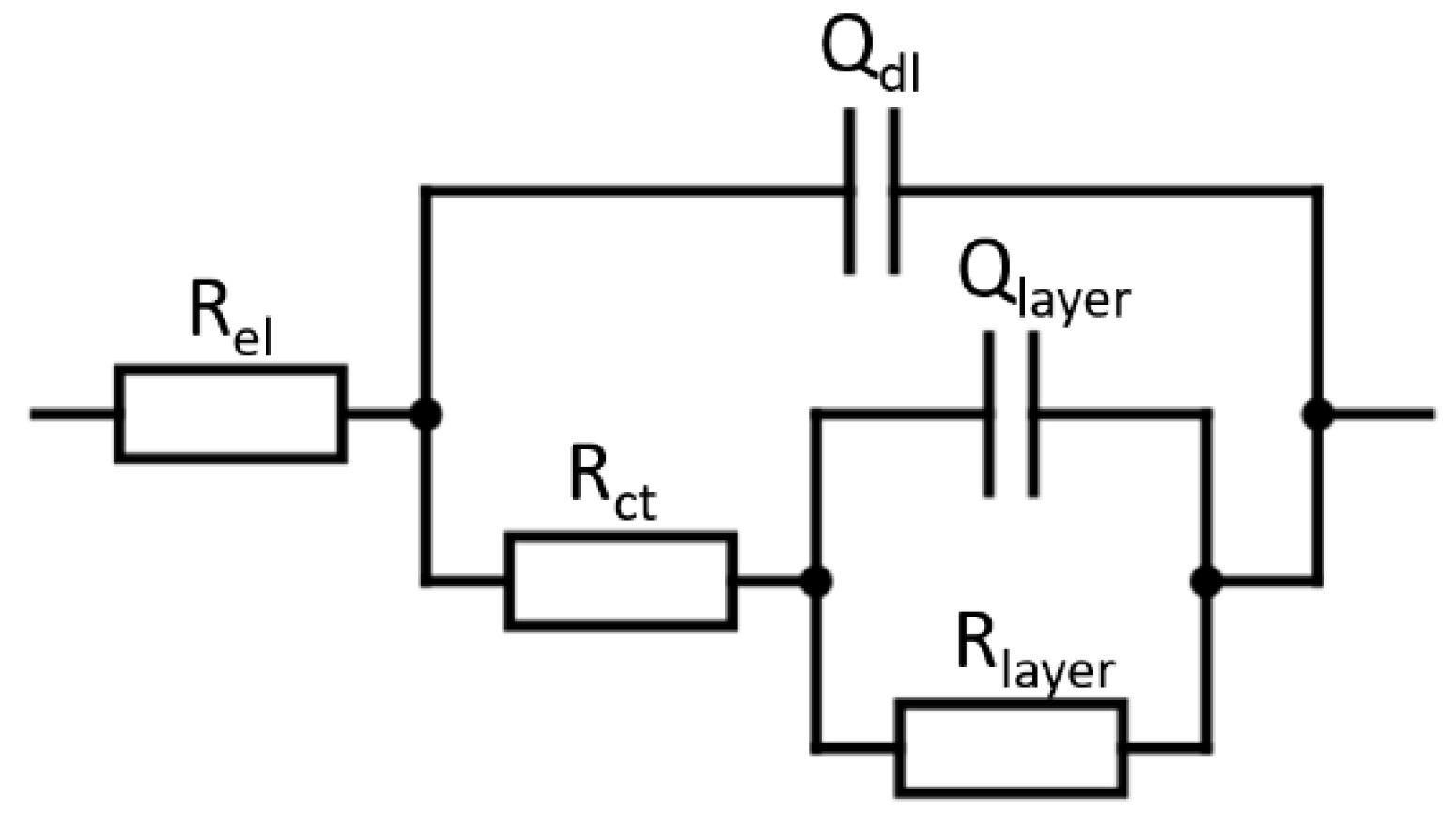
| Sample | Ecorr (mV vs. SCE) | jcorr (nA cm−2) | IE (%) | BF (%) | Rlayer (MΩ cm-2) |
|---|---|---|---|---|---|
| HT | −284 ± 88 | 103.6 ± 22.2 | - | - | 2.900 ± 0.231 |
| C1P (CG) | −392 ± 20 | 8.5 ± 2.6 | 91.8 ± 2.5 | 12.5 ± 3.2 | 3.904 ± 0.514 |
| C1P (EG) | −189 ± 10 | 5.4 ± 1.6 | 94.8 ± 1.5 | 97.2 ± 0.5 | 10.562 ± 0.237 |
| MIP2 (CG) | −463 ± 29 | 16.6 ± 2.6 | 83.9 ± 2.5 | 2.4 ± 0.4 | 3.149 ± 0.371 |
| MIP2 (EG) | −371 ± 56 | 6.1 ± 2.4 | 94.1 ± 2.5 | 95.9 ± 2.9 | 9.247 ± 0.216 |
| HEP2 (CG) | −354 ± 11 | 15.5 ± 2.6 | 85.1 ± 2.5 | 4.9 ± 1.1 | 3.576 ± 0.420 |
| HEP2 (EG) | −182 ± 3 | 15.3 ± 0.9 | 94.9 ± 0.9 | 97.5 ± 0.5 | 10.934 ± 0.206 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arrotin, B.; Libioulle, C.; Issakova, T.; Mespouille, L.; Dubois, P.; Delhalle, J.; Mekhalif, Z. A Comparative Study of the Electro-Assisted Grafting of Mono- and Bi-Phosphonic Acids on Nitinol. Surfaces 2019, 2, 520-530. https://doi.org/10.3390/surfaces2040038
Arrotin B, Libioulle C, Issakova T, Mespouille L, Dubois P, Delhalle J, Mekhalif Z. A Comparative Study of the Electro-Assisted Grafting of Mono- and Bi-Phosphonic Acids on Nitinol. Surfaces. 2019; 2(4):520-530. https://doi.org/10.3390/surfaces2040038
Chicago/Turabian StyleArrotin, Bastien, Corentin Libioulle, Tatiana Issakova, Laetitia Mespouille, Philippe Dubois, Joseph Delhalle, and Zineb Mekhalif. 2019. "A Comparative Study of the Electro-Assisted Grafting of Mono- and Bi-Phosphonic Acids on Nitinol" Surfaces 2, no. 4: 520-530. https://doi.org/10.3390/surfaces2040038
APA StyleArrotin, B., Libioulle, C., Issakova, T., Mespouille, L., Dubois, P., Delhalle, J., & Mekhalif, Z. (2019). A Comparative Study of the Electro-Assisted Grafting of Mono- and Bi-Phosphonic Acids on Nitinol. Surfaces, 2(4), 520-530. https://doi.org/10.3390/surfaces2040038






