Tuning the Catalytic Activity of a Quantum Nutcracker for Hydrogen Dissociation
Abstract
:1. Introduction
2. Methods
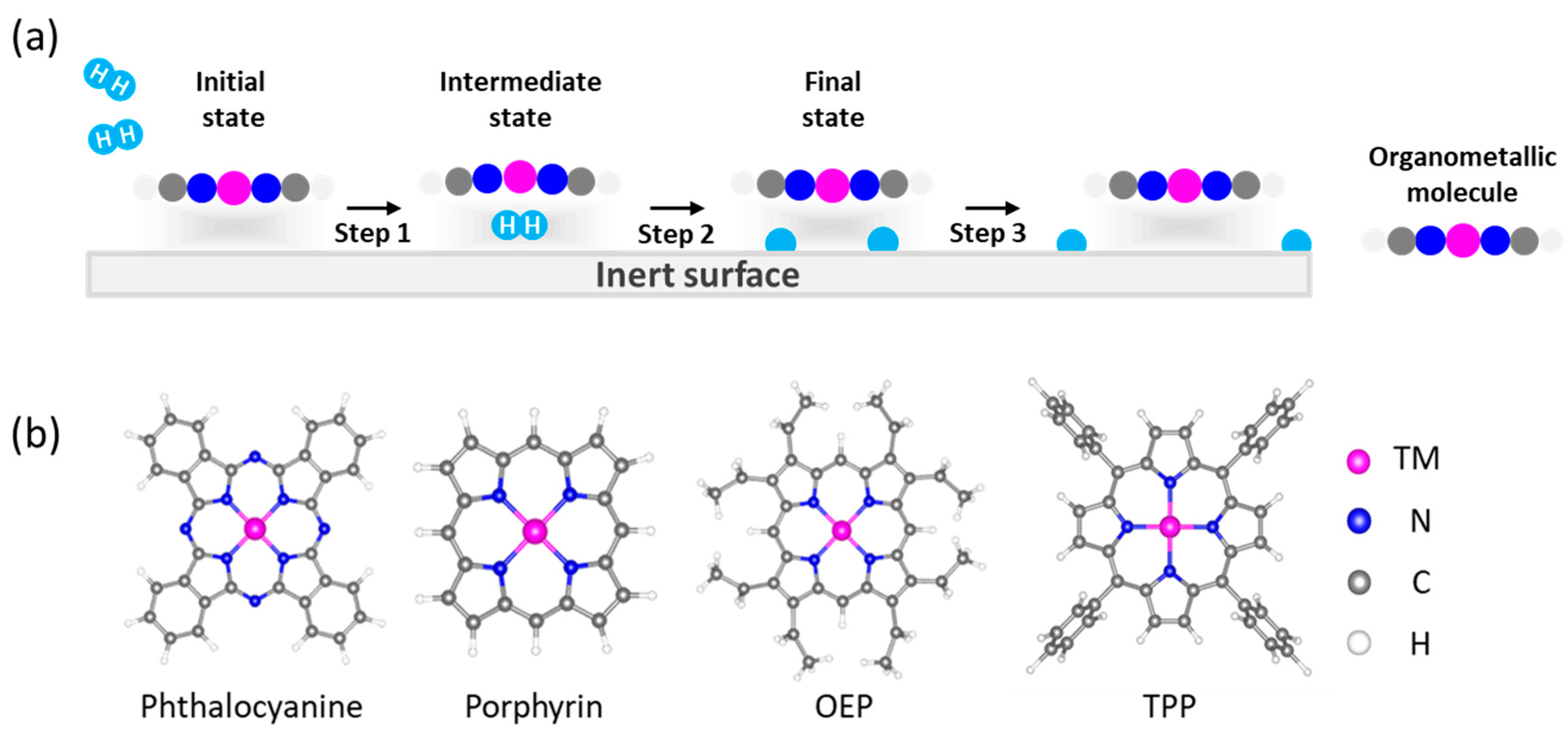
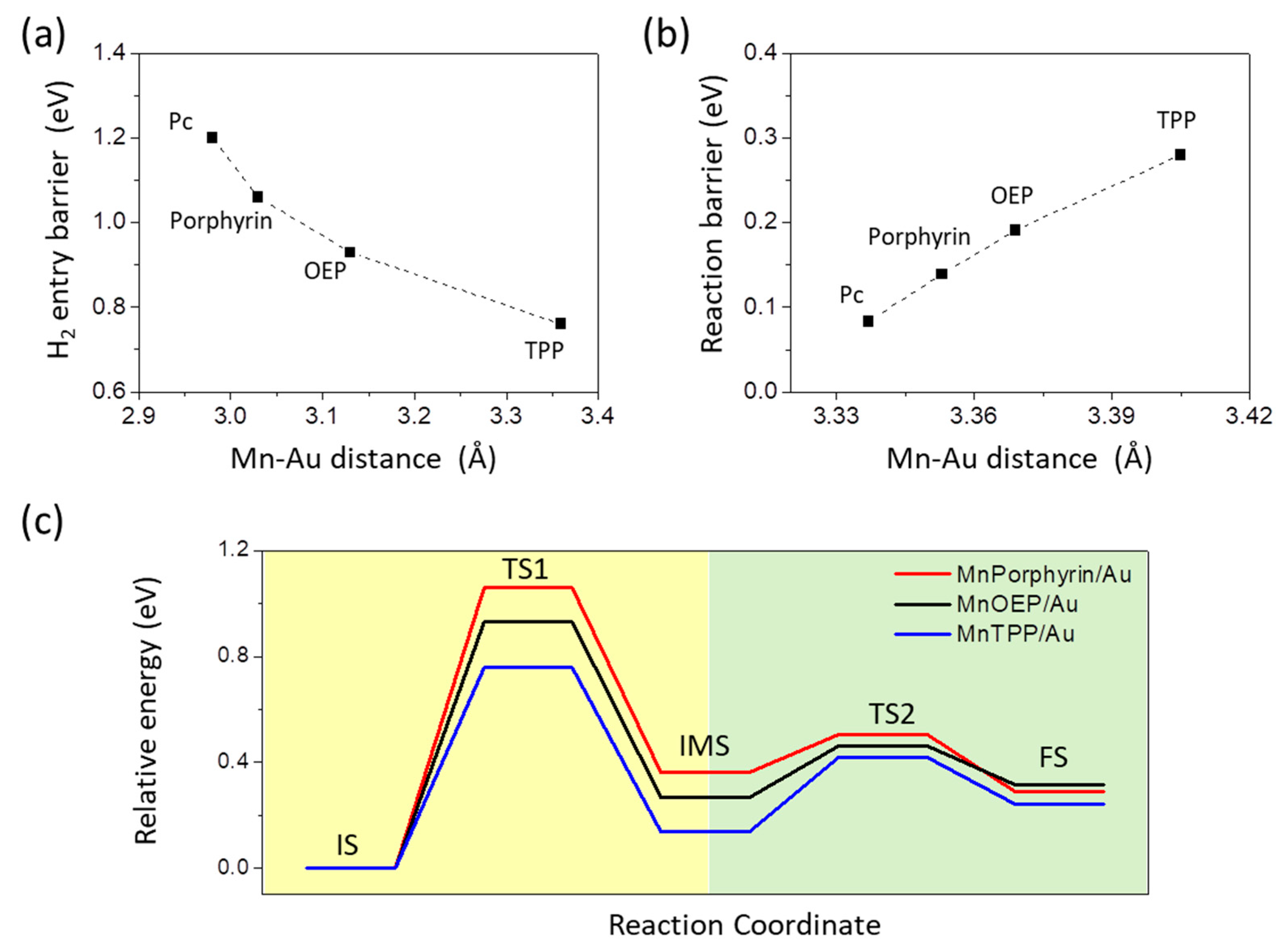
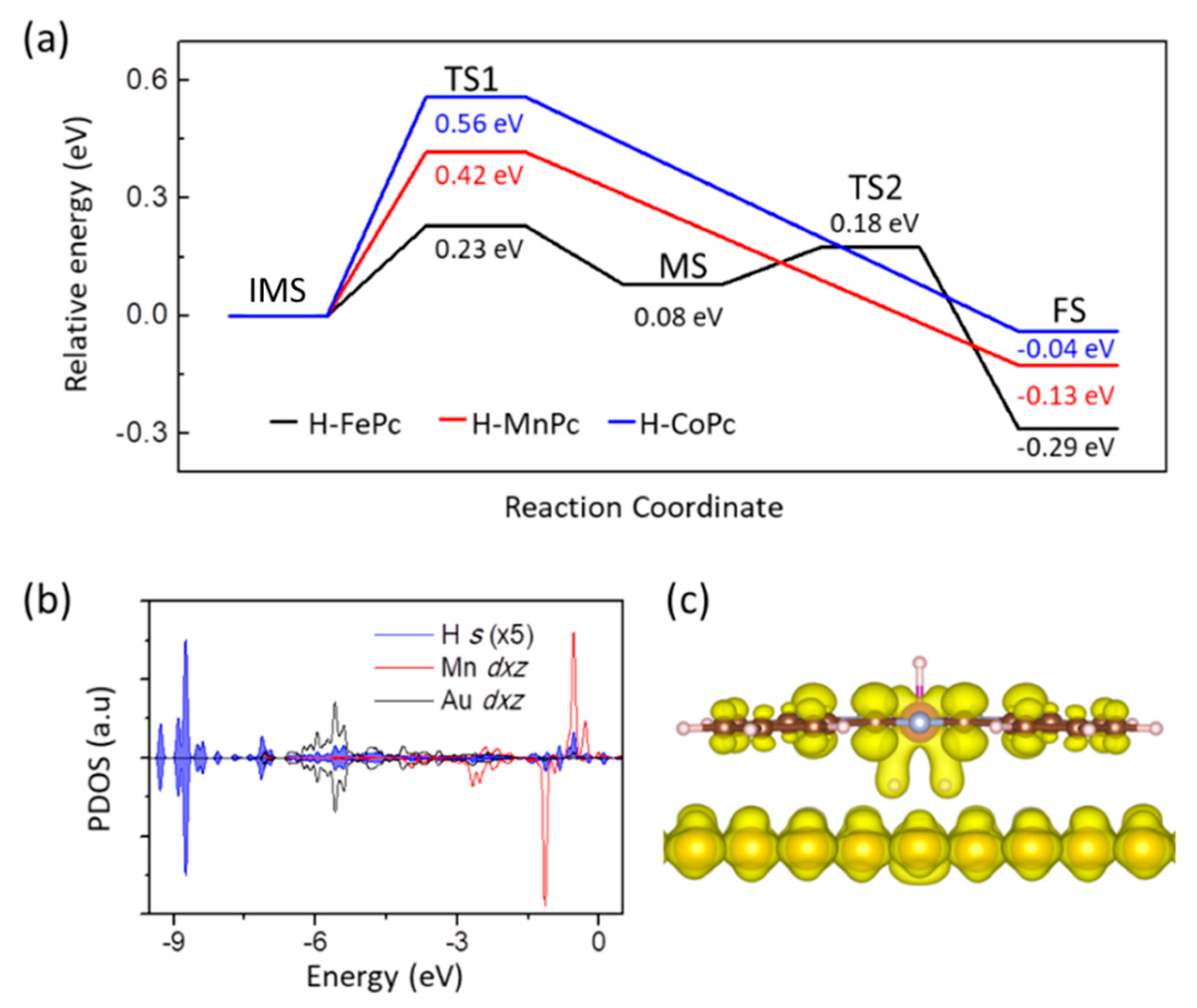
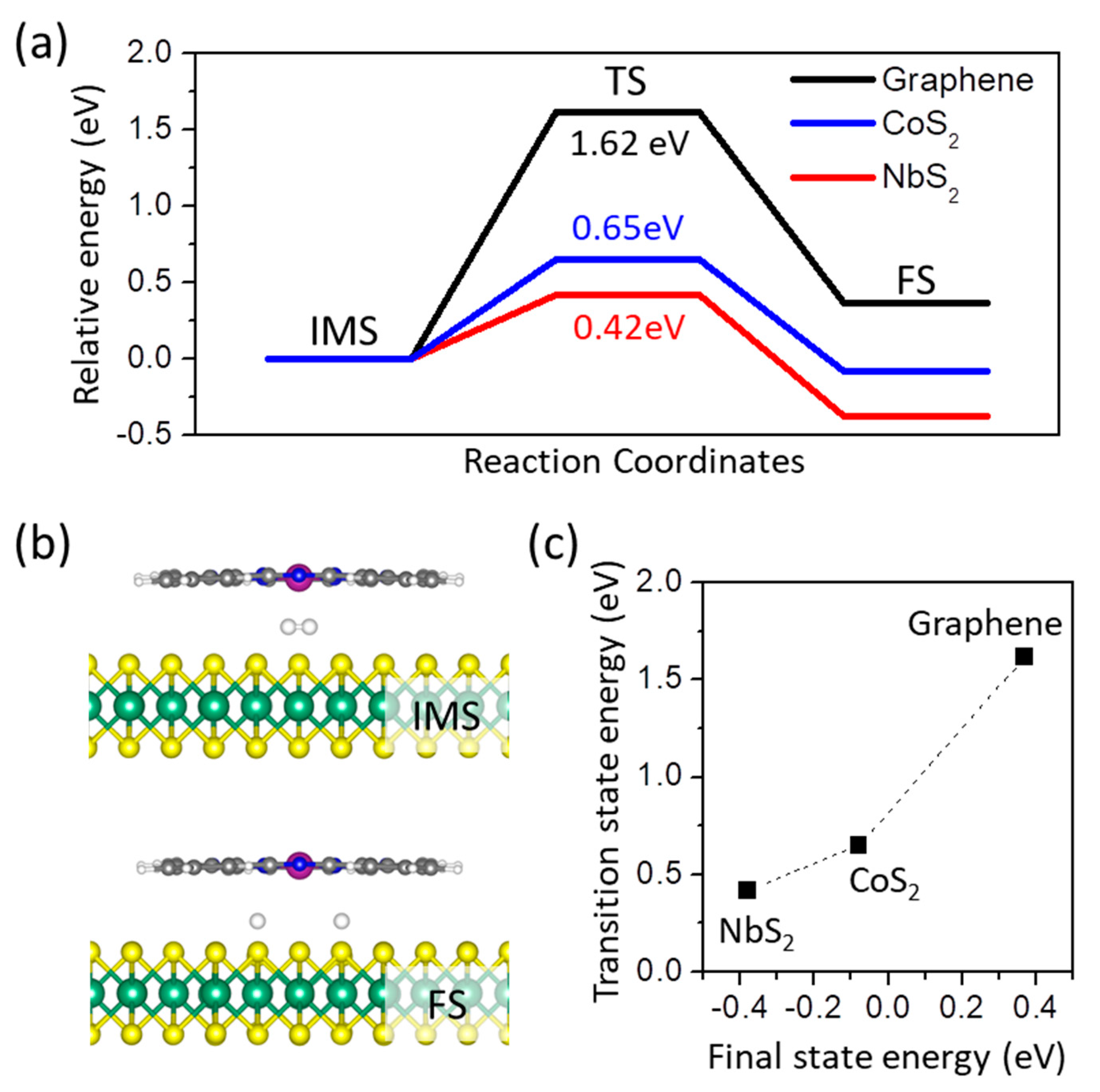
3. Results and Disscusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Darling, G.R.; Holloway, S. The dissociation of diatomic molecules at surfaces. Rep. Prog. Phys. 1995, 58, 1595. [Google Scholar] [CrossRef]
- Yang, X.-F.; Wang, A.; Qiao, B.; Li, J.; Liu, J.; Zhang, T. Single-atom catalysts: A new frontier in heterogeneous catalysis. Acc. Chem. Res. 2013, 46, 1740–1748. [Google Scholar] [CrossRef] [PubMed]
- Rashkeev, S.N.; Lupini, A.R.; Overbury, S.H.; Pennycook, S.J.; Pantelides, S.T. Role of the nanoscale in catalytic CO oxidation by supported Au and Pt nanostructures. Phys. Rev. B 2007, 76, 035438. [Google Scholar] [CrossRef]
- Fujitani, T.; Nakamura, I.; Akita, T.; Okumura, M.; Haruta, M. Hydrogen dissociation by gold clusters. Angew. Chem. Int. Ed. 2009, 48, 9515–9518. [Google Scholar] [CrossRef] [PubMed]
- Antolini, E. Graphene as a new carbon support for low-temperature fuel cell catalysts. Appl. Catal. B Environ. 2012, 123–124, 52–68. [Google Scholar] [CrossRef]
- Asadi, M.; Kim, K.; Liu, C.; Addepalli, A.V.; Abbasi, P.; Yasaei, P.; Phillips, P.; Behranginia, A.; Cerrato, J.M.; Haasch, R.; et al. Nanostructured transition metal dichalcogenide electrocatalysts for CO2 reduction in ionic liquid. Science 2016, 353, 467. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.-L.; Lei, B.; Zhu, Z.L.; Tao, L.; Qi, J.; Bao, D.L.; Wu, X.; Huang, L.; Zhang, Y.Y.; Lin, X.; et al. Spontaneous Formation of 1D Pattern in Monolayer VSe2 with Dispersive Adsorption of Pt Atoms for HER Catalysis. Nano Lett. 2019, 19, 4897–4903. [Google Scholar] [CrossRef]
- Lu, Q.; Yu, Y.; Ma, Q.; Vhen, B.; Zhuang, H. 2D Transition-Metal-Dichalcogenide-Nanosheet-Based Composites for Photocatalytic and Electrocatalytic Hydrogen Evolution Reactions. Adv. Mater. 2016, 28, 1917–1933. [Google Scholar] [CrossRef]
- An, X.; Yu, J.C. Graphene-based photocatalytic composites. RSC Adv. 2011, 1, 1426–1434. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Yao, W.; Song, S.; Sun, J.T.; Pan, J.; Ren, X.; Li, C.; Okunishi, E.; Wang, Y.Q.; et al. Monolayer PtSe2, a New Semiconducting Transition-Metal-Dichalcogenide, Epitaxially Grown by Direct Selenization of Pt. Nano Lett. 2015, 15, 4013–4018. [Google Scholar] [CrossRef]
- Machado, B.F.; Serp, P. Graphene-based materials for catalysis. Catal. Sci. Technol. 2012, 2, 54–75. [Google Scholar] [CrossRef]
- Jaramillo, T.F.; Jørgensen, K.P.; Bonde, J.; Nielsen, J.H.; Horch, S.; Chorkendorff, I. Identification of Active Edge Sites for Electrochemical H2 Evolution from MoS2 Nanocatalysts. Science 2007, 317, 100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, D.; Liu, Y.; Wang, Y.; Zhang, H.; Huang, L.; Yu, G. Synthesis of N-Doped Graphene by Chemical Vapor Deposition and Its Electrical Properties. Nano Lett. 2009, 9, 1752–1758. [Google Scholar] [CrossRef]
- Liu, F.; Sun, J.; Zhu, L.; Meng, X.; Qi, C.; Xiao, F.-S. Sulfated graphene as an efficient solid catalyst for acid-catalyzed liquid reactions. J. Mater. Chem. 2012, 22, 5495–5502. [Google Scholar] [CrossRef]
- Zhao, J.; Deng, Q.; Bachmatiuk, A.; Sandeep, G.; Popov, A.; Eckert, J.; Rümmeli, M.H. Free-Standing Single-Atom-Thick Iron Membranes Suspended in Graphene Pores. Science 2014, 343, 1228. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Cheng, H.; Yi, H.; Lin, Y.; Yao, T.; Wang, C.; Li, J.; Wei, S.; Lu, J. Single-Atom Pd1/Graphene Catalyst Achieved by Atomic Layer Deposition: Remarkable Performance in Selective Hydrogenation of 1,3-Butadiene. J. Am. Chem.Soc. 2015, 137, 10484–10487. [Google Scholar] [CrossRef] [PubMed]
- Deng, D.; Novoselov, K.S.; Fu, Q.; Zheng, N.; Tian, Z.; Bao, X. Catalysis with two-dimensional materials and their heterostructures. Nat. Nanotechnol. 2016, 11, 218. [Google Scholar] [CrossRef]
- Suenaga, K.; Kobayashi, H.; Koshino, M. Core-Level Spectroscopy of Point Defects in Single Layer h-BN. Phys. Rev. Lett. 2012, 108, 075501. [Google Scholar] [CrossRef]
- Tao, L.; Guo, W.; Zhang, Y.-Y.; Zhang, Y.-F.; Sun, J.; Du, S.; Pantelides, S.T. Quantum nutcracker for near-room-temperature H2 dissociation. Sci. Bull. 2019, 64, 4–7. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Yang, K.; Jiang, Y.; Song, B.; Xiao, W.; Li, L.; Zhou, H.; Wang, Y.; Du, H.; Ouyang, M.; et al. Reversible Single Spin Control of Individual Magnetic Molecule by Hydrogen Atom Adsorption. Sci. Rep. 2013, 3, 1210. [Google Scholar] [CrossRef]
- Liu, L.W.; Yang, K.; Xiao, W.; Jiang, Y.H.; Song, B.; Du, S.; Jiang, H.-J. Selective adsorption of metal-phthalocyanine on Au(111) surface with hydrogen atoms. Appl. Phys. Lett. 2013, 103, 023110. [Google Scholar] [CrossRef]
- Bishop, S.R.; Tran, N.L.; Poon, G.C.; Kummel, A.C. Dynamics of analyte binding onto a metallophthalocyanine: NO/FePc. J. Chem. Phys. 2007, 127, 214702. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-Y.; Du, S.-X.; Gao, H.-J. The construction and structure-property manipulation of “small-molecule machines”. Chin. Sci. Bull. 2018, 63, 1255. [Google Scholar]
- Chen, H.; Pope, T.; Wu, Z.Y.; Wang, D.; Tao, L.; Bao, D.L.; Xiao, W.; Zhang, J.L.; Zhang, Y.Y.; Du, S.; et al. Evidence for Ultralow-Energy Vibrations in Large Organic Molecules. Nano Lett. 2017, 17, 4929–4933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin-Ming, C.; Zhnag, Y.-Y.; Hu, H.; Bao, L.-H.; Pan, L.-D.; Tang, W.; Li, G.; Du, S.; Shen, J.; Gao, H.-J. Electric dipolar interaction assisted growth of single crystalline organic thin films. Chin. Phys. B 2010, 19, 067101. [Google Scholar] [CrossRef]
- Nørskov, J.K.; Bligaard, T.; Logadottir, A.; Bahn, S.; Hansen, L.B.; Bollinger, M.; Bengaard, H.; Hammer, B.; Sljivancanin, Z.; Mavrikakis, M.; et al. Universality in heterogeneous catalysis. J. Catal. 2002, 209, 275–278. [Google Scholar]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.; Murray, E.M.; Kong, L.; Lungqvist, B.; Langreth, D.C. Higher-accuracy van der Waals density functional. Phys. Rev. B 2010, 82, 081101. [Google Scholar] [CrossRef] [Green Version]
- Dion, M.; Rydberg, H.; Schröder, E.; Langreth, D.C.; Lungqvist, B.I. Van der waals density functional for general geometries. Phys. Rev. Lett. 2004, 92, 246401. [Google Scholar] [CrossRef] [Green Version]
- Henkelman, G.; Uberuaga, B.P.; Jonsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 2000, 113, 9901–9904. [Google Scholar] [CrossRef] [Green Version]
- Mills, G.; Jonsson, H.; Schenter, G.K. Reversible work transition state theory: Application to dissociative adsorption of hydrogen. Surf. Sci. 1995, 324, 305–337. [Google Scholar] [CrossRef] [Green Version]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, L.; Zhang, Y.-Y.; Pantelides, S.T.; Du, S. Tuning the Catalytic Activity of a Quantum Nutcracker for Hydrogen Dissociation. Surfaces 2020, 3, 40-47. https://doi.org/10.3390/surfaces3010004
Tao L, Zhang Y-Y, Pantelides ST, Du S. Tuning the Catalytic Activity of a Quantum Nutcracker for Hydrogen Dissociation. Surfaces. 2020; 3(1):40-47. https://doi.org/10.3390/surfaces3010004
Chicago/Turabian StyleTao, Lei, Yu-Yang Zhang, Sokrates T. Pantelides, and Shixuan Du. 2020. "Tuning the Catalytic Activity of a Quantum Nutcracker for Hydrogen Dissociation" Surfaces 3, no. 1: 40-47. https://doi.org/10.3390/surfaces3010004
APA StyleTao, L., Zhang, Y.-Y., Pantelides, S. T., & Du, S. (2020). Tuning the Catalytic Activity of a Quantum Nutcracker for Hydrogen Dissociation. Surfaces, 3(1), 40-47. https://doi.org/10.3390/surfaces3010004




