MXene: Evolutions in Chemical Synthesis and Recent Advances in Applications
Abstract
:1. Introduction
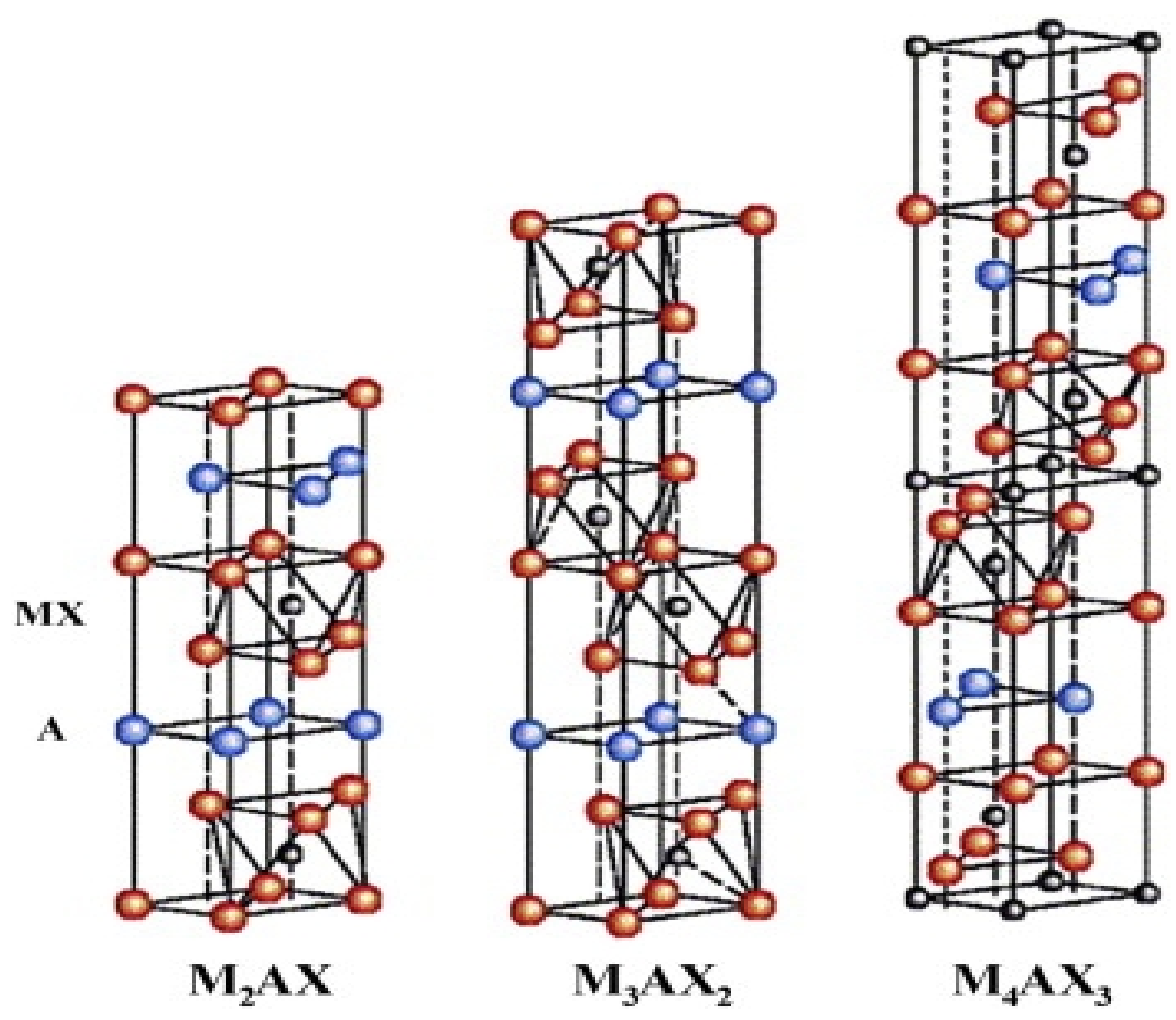
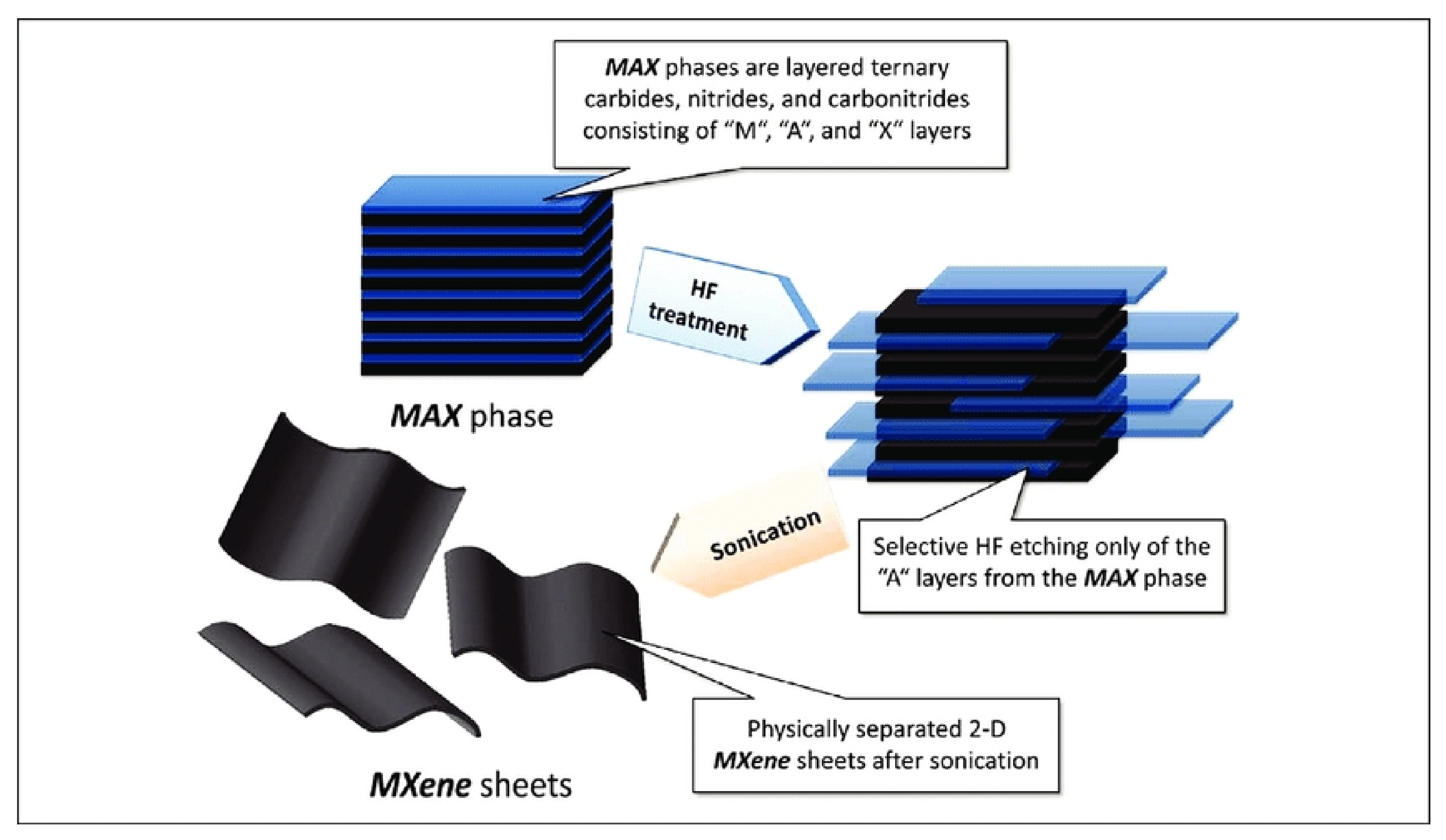
2. Synthesis of Mxene
2.1. Etching
2.1.1. Modification of Acid Etching Methods
2.1.2. Fluoride-Free Synthesis Methods
2.1.3. Molten Fluoride Salt ETCHING Method
- (a)
- Cohesive energy of Tin+1Nn < Cohesive energy of Tin+1Cn; which implies a lower stability of structure owing to this the layers dissolve in HF solution.
- (b)
- The formation energies of Tin+1Nn from Tin+1AlNn > the formation of Tin+1Cn from Tin+1AlCn, which implies requirement of more energy for extraction of Al atoms from their MAX phases.
2.1.4. MXenes Prepared from Other Precursors Than Al-MAX Phases
2.1.5. MXene–Polymer Composites
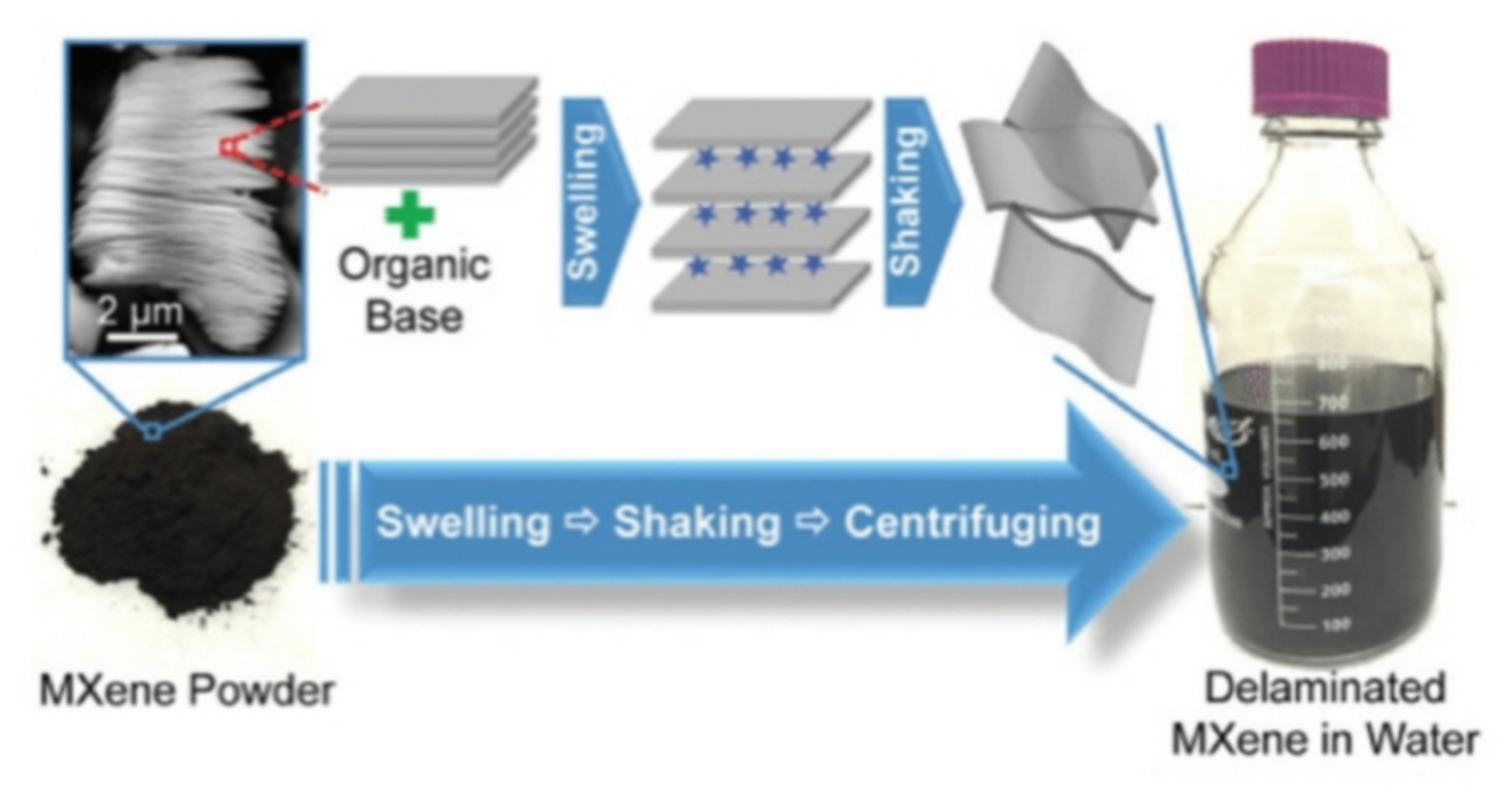
2.2. Exfoliation
3. Results
3.1. Characterization
3.2. Data of the Results of MXene Synthesis
- Etching using HF
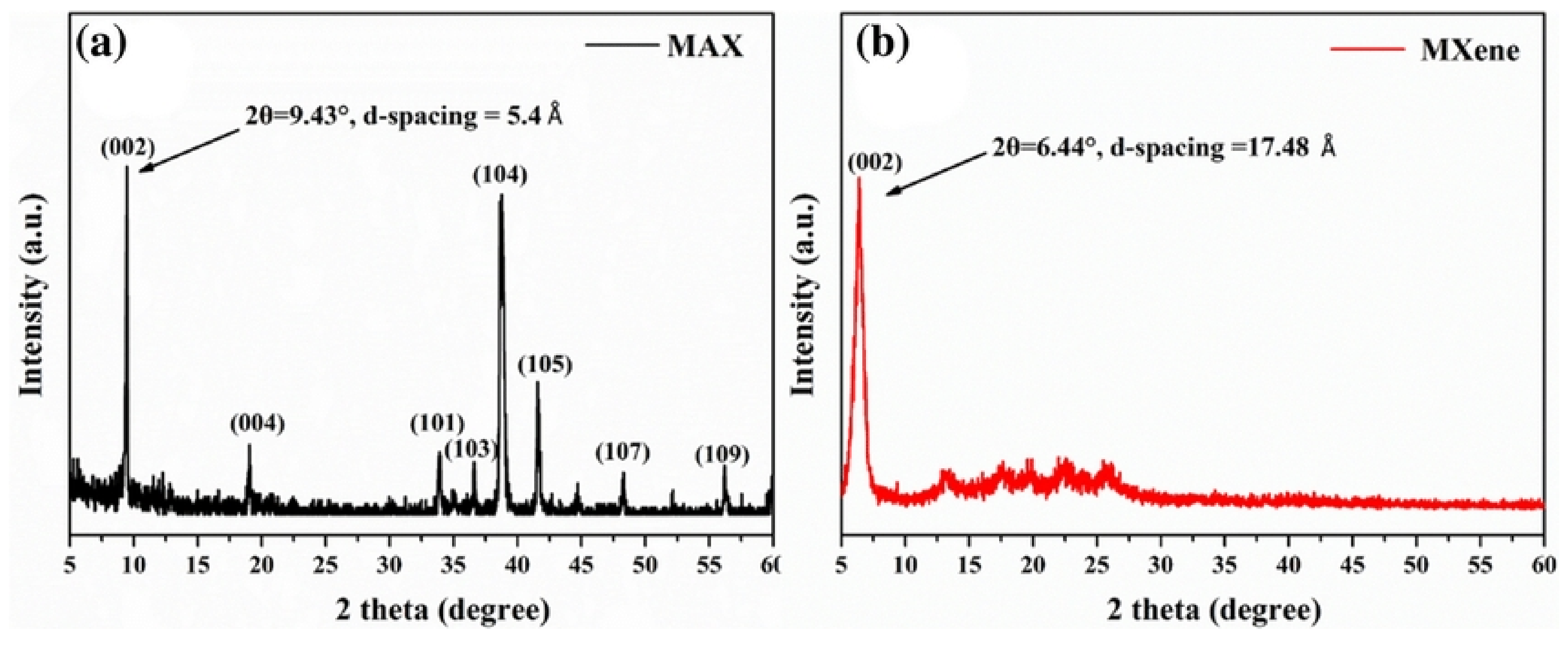
- The first MXene synthesized Ti3C2Tx using the HF method followed Reactions (1)–(3) mentioned under the synthesis section. Reactions (2) and (3) shows the Mxene surfaces terminated by –OH and –F, respectively, in a simplified manner while in reality there may be a combination of both. The dominant reactions can be studied using DFT-based calculations. When the precursor was immersed in HF solution, bubbles (presumably H2) were observed, indicating the onset of chemical reaction.
- For the XRD pattern after ultrasonication, the reaction products in methanol for 300 s depicted significant weakening of the peaks and a broadened peak 2θ = 24°. FTIR (Fourier Transform Infrared Spectroscopy) confirmed the presence of –OH groups and the final result predicted was Ti3C2(OH)2.
- Along the basal plane, the elastic modulus was around 300 GPa of single exfoliated Ti3C2(OH)2layers.
- The presence of surface functional groups OH and F groups made the band structure semiconducting in nature.
- This was obviously the first glimpse into MXenes and the impact that it can have in material science. As a new addition back then to the 2D material family, it must have been appealing to know more of the potential it possessed. The one thing that the onset of MXene experiments did is to shed some light and attention towards MAX phases. The top-down approach of synthesis seemed to be more convenient and important from a scientific point of view because it included their precursors’ MAX phases. Now there are numerous parallel works going on about MAX phase to better understand MXenes. Also, synthesis of more MAX phases means the possibility of obtaining more MXenes with versatile properties.
- Modified acid etching methods aimed at replacing HF as an etchant
- NH4HF2 as an etchant—the use of this milder etchant was approved. From XRD data, the expansion of c lattice parameter from 18.6 Armstrong (Ti3AlC2) to 19.8 Armstrong (Ti3C2) was observed (the value matched previous recorded values). There was simultaneous intercalation of species like NH3 and NH4+, which further increased the c value to 24.7 Armstrong (25% larger than HF treated samples). The presence of F and O atoms between the layers was confirmed by XPS and EDX mapping in TEM. The intercalated Ti3C2-IC films attained 90% transmittance. Also, the absorbance of the films (independent of wavelength of light) was found to be linearly dependent on thickness. The Ti3C2-IC films showed greater resistivity than non-intercalated ones.
- LiF-HCl combo as an etchant—also successfully synthesized MXene. The result was clay-like paste rolled after hydrating to produce flexible free-standing films in a very short time compared to othersproduced by laborious techniques. Highly conductive when dry and could be given any shape when wet was the exciting feature. XRD and EDX data confirmed: the MXene is indeed formed, –O or –F containing surface functional groups, and the structure showing tight stacking of layers presumably due to cationic and/or water intercalation instead of having the claimed accordion-like morphology that HF-treated samples exhibit. Simple sonication yielded 45% by mass of dispersed flakes. Owing to the mild nature of the etchant, the flakes had larger lateral dimension and no nanometer-size defects frequent in HF-etched samples. The performance of the rolled up films as supercapacitor electrodes with H2SO4 as the electrolyte was excellent with volumetric capacitance up to 900 F/cm3 or 245 F/g.
- Although HF remains the choice as etchant, other etching agents can also effectively produce MXenes as well. However, which one of these will be suitable for industrial purposes is still a question. Also, we have to notice that different etching agents produce different results. Like LiF+HCl combo, it produced the ‘clay-like texture’, far from the accordion structure that was expected. These two etching chemical combinations are modified HF ones, which means they are not fluoride free, but are milder than HF. This makes handling them easier and also implies the nature of surface terminations the product may have. The in situ HF methods also causes an increased interlayer spacing by simultaneous intercalation of Li+ ions and waterthus weakening the interaction between bonds. This is an advantage especially in energy storage applications.
- Fluoride-free synthesis methods
- CVD process—fabricated ultrathin alpha-MO2C films of regular shapes like rectangles, triangles, hexagons, octagons, nonagons, and dodecagons. At 1090 °C for a growth time of 5 min crystal sizes of ≈10 µm and thickness of 3–20 nm was observed. By increasing the growth time to 50 min, very large thin crystals having lateral sizes of ≈100 µm was achieved at 1086 °C. The crystal structure was uniform and the surface was smooth like graphene. The films exhibited superconducting behavior, which was intrinsic thickness dependent ontemperature Tmin of 35K. Before the onset of superconducting transition, the films showed metallic character and very interestingly below Tmin the data indicated insulating behavior in the normal state. According to STEM observations, the ultrathin crystals were found to be defect-free.
- Urea glass route—XRD data with no other crystalline side phases and elemental analysis of the synthesized powders confirmed the fabrication of alpha-Mo2C and gamma-Mo2N of high purity. Theoretically predicted values of average sizes of Mo2C and Mo2N catalysts are ≈11 and 16 nm, respectively, which was in good agreement with the TEM analysis sizes 2–17 nm and 5–25 nm, respectively. Hydrogen evolution catalyzed by Mo2C in the KOH electrolyte had turnover frequencies (TOFs) of 0.5, 0.9, and 2.5 S−1 and onset potential of 176, 200, and 250 mV, respectively. For Mo2N, HER in KOH was catalyzed with TOFs of 0.07 and 0.7 S−1 at onset potential of 250 mV and 352 mV, respectively.
- Hydrothermal method—when Ti3AlC2 is hydrothermally treated with NaOH, Ti3C2Tx (Tx = –OH, –O) was obtained taking inspiration from Bayer process. XPS and XRD data gives a planar spacing of 24 Armstrong, which is larger than 20 Armstrong reported for HF fabricated Ti3C2Tx, suggesting Na+ interlamination. The SEM images show the compact layered structure. Ti3C2Tx film electrode (52 µm thick) without any—F terminations in 1 M H2SO4 possessed a gravimetric capacitance of 314 F/g and volumetric capacitance of 511 F/cm3 at 2 mV S−1. Compared to HF-Ti3C2Tx, capacitance is higher by ≈214% and for LiF + HCl—Ti3C2Tx clay the value surpasses by ≈28.2%. With another hydrothermal route using (NaBF4, HCl) as the etching agents, two kinds of MXenes Ti3C2 and Nb2C were prepared. The resulting Ti3C2 show adsorption to cationic dye (MB), the adsorption performance being better than the traditional HF etched samples.
- Electrochemical method—Ti3C2 produced by electrochemical corrosion of Ti3AlC2 in binary aqueous electrolyte was studied for capacitative behavior and the observations stated the areal and volumetric capacitances to be 220 mF cm−2and 439 F/cm3, respectively at a scan rate of 10 mV S−1.
- HF hasa corrosive nature, which often hampers the yield of the product. Out of the four methods listed above, the hydrothermal method is the successful one in terms of producing better yield and enhanced properties compared to the HF etched products. Although one thing should be kept in mind that if we talk about synthesizing only, then the fluoride component has to play an important part. Recently, a study suggested that mechanical stress can also break the A layer bond in MAX phases, which if proven can be a huge leap towards industrial purposes. Mechanically breaking the bond will be so cost effective and harmless that it can change the entire picture. However, it is yet to be determined whether this concept will be true to every MAX phase or not because of the varying bond strength [73] (Guo, Zhu, Zhou, & Sun, 2015). Also, Mo2C fabricated by CVD process showed thermal and chemical stability in solvents like ethanol, isopropanol, and HCl. The strength of Mo2C is 20.8 GPa, which is almost comparable to a monolayer of MoS2. This makes the Mo2C MXene a choice for mechanical applications.
- Molten fluoride salt etching method
- The mixture of molten fluoride salts produced the nitride base MXene Ti4N3Tx. Spin polarized density functional theory (DFT) calculations were performed and according to density of states at the Fermi level are metallic for both bare and functionalized Ti4N3. Bare Ti4N3 is magnetic, while introducing surface termination groups lowers the total magnetic moment in unit cells. The OH introduction dramatically lowered the magnetic moment from 7.0 µB to zero.
- The most studied MXene is titanium carbide. This was a novel material discovered using the molten salt method, which had not been reported earlier. The study was made between terminated and non-terminated Ti4N3 monolayers by using spin-polarized DFT calculations. The electronic structure is explained via the partial density of states (PDOS) of bare Ti4N3and Ti4N3Tx with all the possible terminations. The bare MXene shows the same metallic character as the carbide MXenes, while introducing surface terminations tends to lower the DOS at the Fermi level. However, among the surface terminations, only –F terminated Nitride MXenes were theoretically calculated for DOS in all previous research works. Considering –OH and –O terminated ones in this paper, it was found out that comparatively –OH terminated ones are the least energetically favorable and –O terminated MXenes are most favorable. The fact that the magnetic moment can be tuned by tuning F/OH and F/O ratio opens up a completely new field of research of MXene applications. Another nitride-based MXene was synthesized (Ti2NTx) and was tested for SERS activity [30] (B. Soundiraraju & B. K. George, 2017). SERS sensitivity was confirmed in Ti2Nwhich makes it the candidate for developing self-standing flexible SERS substrates. This is a completely different direction of application in detecting traces of explosives and later can be expanded to capture other poisonous contaminants in the environment.
- MXenes prepared from other precursors than Al-based MAX phases
- 2D Zr3C2TxMXene was prepared for the first time from parent ternary-layered Zr3Al3C5, which was a fresh approach to obtain MXenes outside of Ti-C systems. It was found out that all of the three functionalized Zr3C2Tx MXenes (T = OH, F, O) exhibit metallic behavior with Zr3C2T2 having the strongest mechanical strength of calculated elastic constants as high as 392.9 GPa. It showed greater stability in argon atmosphere or complete vacuum than the titanium carbide.
- Synthesized Mo2C from gallium-based laminate Mo2Ga2C is the first instance of non-Al containing MAX phases used for fabrication.
- This is a no brainer that Ti3C2Tx is the most studied MXene, and also the Al-based MAX phases are the most wanted precursors. However, shifting from the conventional ones brings unknown properties forward, which may find a unique kind of application. Not much experimental data is present regarding this idea as synthesis of non-Al MXene itself is in progress. The main thing about all these attempts is to prove that we can customize our MXenes as per our requirements.
- MXene-Polymer composites
- For Ti3C2Tx/PVA composite, the tensile strength comparatively improved by 34%. While the mechanical strength of Ti3C2Tx (3.3 µm thick) was found to be 22 + 2 MPa or 22 − 2 MPa with a Young’s Modulus of 3.5 + 0.01 GPa or 3.5 – 0.01 GPa, the strength of Ti3C2Tx/PVA (PVA loading of 60 wt%) film enhanced four times to 91 + 10 MPa or 91 – 10 MPa. Ti3C2Tx/PDDA composites did not have any impressive or enhanced capacitate effect, but combination of PVA and KOH gel electrolyte showed significant increase in volumetric capacitance: 528 F/cm3 at 2 mV/S and 306 F/cm3 at 100 mV/S.
- For PPy/Ti3C2Tx composite, XRD and TEM data confirmed pyrrole intercalation in between layers. Composite exhibited volumetric capacitance of ≈1000 F/cm3 and capacitance retention of 92% after 25,000 cycles.
- The pyrrole composite was further improved using 1,5 naphthalene disulfonic acid (NA) and cetyltrimethylammonium bromide (CTAB). Specific capacitance calculated for pure PPy, Ti3C2Tx/PPy, NA-Ti3C2Tx/PPy, and NA-CTAB-Ti3C2Tx/PPy composite was 128, 266, 318, and 437 F g−1, respectively. This is clear that the composite yielded a higher specific capacitance. NA-CTAB-Ti3C2Tx/PPy composite showed good electrochemical properties with capacitance retention ratio of 76% after 1000 cycles compared to only 50% for the pure PPy electrodes.
- In 6 wt%MXene/PAM nanocomposite films, SEM, TEM, and XRD confirm the presence of well dispersed nanoflakes of MXenes. The conductivity increased significantly to 3.3 × 10−2 S m−1 for 1.7 vol% MXene loaded films by increasing the MXene loading.
- MXene/PANI composite showed excellent electrochemical performances including high specific capacitance of 556.2 F g−1 at 0.5 A g−1, outstanding cycling stability of almost 91.6% retention after 5000 cycles and good rate capability with 78.7% capacitance retention at 5 A g−1.
- MXene-based composites are the next step evolution in material science. They are leaving their impact on energy storage applications as well as in environmental applications, for example, water purification and sensors. Sure enough MXenes are considered as good electrode materials, but as mentioned above, the capacitate retention is more in case of MXene/PANI substrates than both pure MXene and PANI. So with the high electrical conductivity of MXene, the pseudocapacitive nature of PANI is added, which explains the good rate performance. MXene-PVA composite shows four times more mechanical strength than conventional titanium-based MXene. On the flip side, it can be said that MXenes are suitable nanofillers for obtaining high performance polymer composites. In a study, it has been reported that MXene-based composites are superior as electrodes in supercapacitors, Li-ion, Li-S, and Na-ion batteries. The large surface area of MXenes does encourage deposition of charge during each cycle of charging and discharging in supercapacitors. However, their energy density is limited and also the longevity is questionable. So, reinforcing them with polymers constitutes double layers for pseudocapacitive effect that improves the performance drastically. MXenes degradation is a big problem and can be rectified by forming composites that act as stable matrices for holding the structures and giving a good cycle stability [74] (J. Yang et al., 2019).
4. Discussion
- Resemblance of the multilayer sheets to exfoliated graphite.
- The ability to form rolled scrolls similar to sonicated graphene scrolls.
4.1. Properties
4.1.1. Mechanical Properties
- Effect of surface terminations
- 2.
- Effect of number of layers and thickness of layers
4.1.2. Electronic Properties
4.1.3. Optical Properties
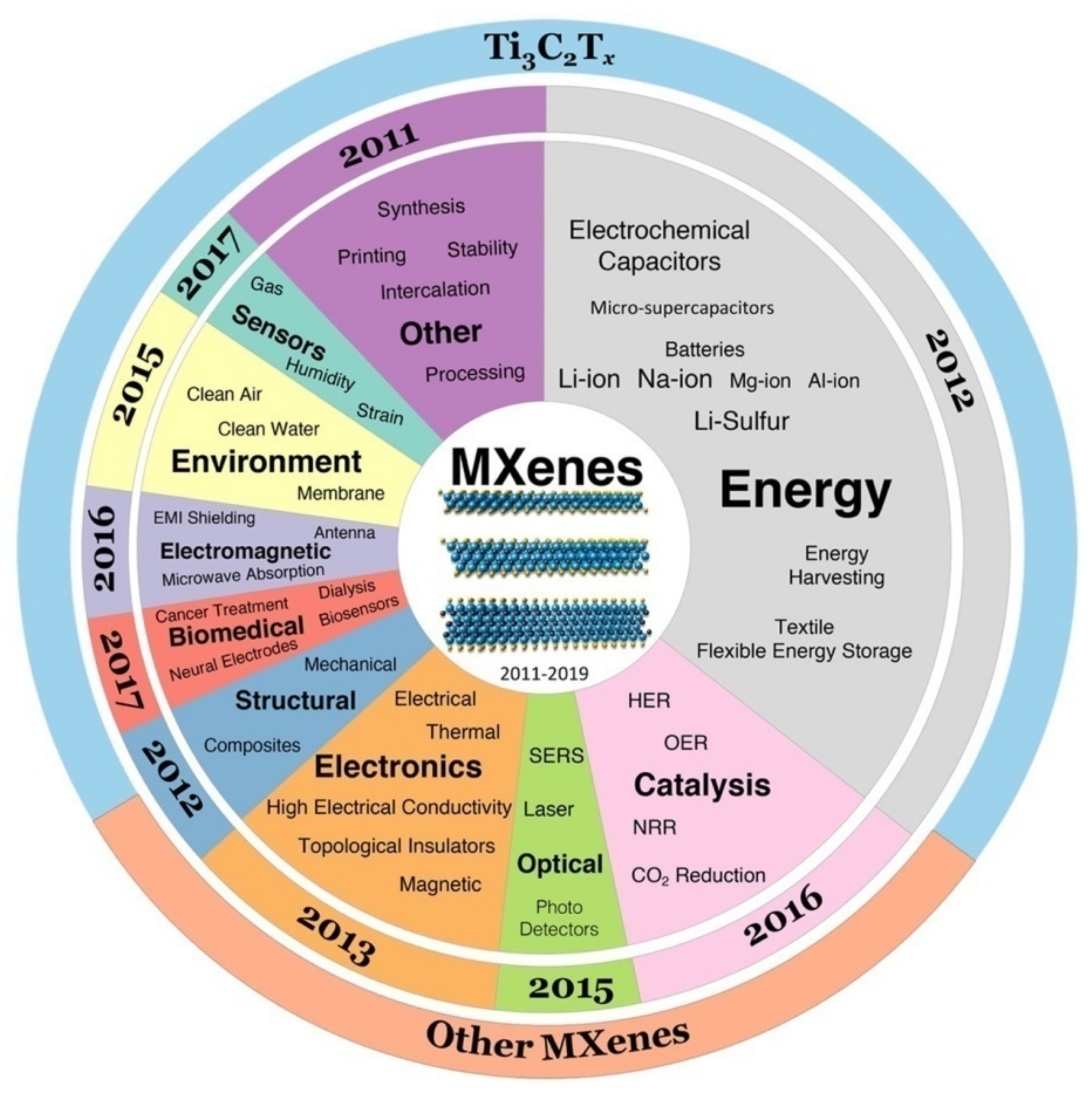
5. Applications and Recent Works
6. Future Aspects and Concluding Remarks
- Exploring simpler, feasible, and environment-friendly routes of synthesis in order to take it from small-scale laboratory synthesis to large-scale industrial production on commercial level. It is important that the experimental endeavors have successful industrial outcomes. Especially for a material to have versatile properties, we can have the ambition to make it dominate versatile sectors of applications. For that though, safer and cost effective routes of production is necessary.
- Despite the amount of theoretical studies, their mechanism especially in the field of energy storage is not fully understood. MXenes are being confirmed to have the most impact in the energy sector, for example in supercapacitors, microsupercapacitors, electrodes in batteries, etc. Still, there is clear lacuna in confirming the stability of MXenes when put under these applications. It is yet to be confirmed whether MXenes are capable of producing desirable results on their own or if they need any kind of stable base or matrix to support their longevity. So, more research works are needed to make MXenes really stand out as the best available material for use in energy storage applications at present.
- The stability of MXenes is a real struggle as MXene nanosheets degrade very quickly.The duration of activity of MXenes before they degradehas been pointed out time and again in numerous experimental papers. In order to have them incorporated in devices, stability issues need to be taken care of firsthand.
- Researching more on MXene-based Polymer composites.
- Shifting from titanium carbides to other MXenes classes. The possibilities of new properties and diverse applications cannot be denied of having other MXenes replacing titanium and focusing on nitrogen-based MXenes. There is a clear lack of evidence in order to make a comparison in properties between them. So, this area needs to be explored more.
- Analyzing mixed surface group terminations and their effect on MXene properties. We have seen how surface terminations can affect not only mechanical properties, but also the impact in various experimental works. Terminated MXenes show far better results than bare MXenes and later we found out that the nature of termination is also important. It will be more interesting to find out about mixed surface terminations, which are practically obtained when a MXene is synthesized. The hydrophilicity of MXenes is the reason of so many properties and potential applications. Mixed surface terminations can make a MXene a multitasking device.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [Green Version]
- Geim, A.; Novoselov, K. The rise of graphene, 2007. Nat. Mater. 2010, 6, 183. [Google Scholar] [CrossRef] [PubMed]
- Lukatskaya, M.R.; Halim, J.; Dyatkin, B.; Naguib, M.; Buranova, Y.S.; Barsoum, M.W.; Gogotsi, Y. Room-Temperature Carbide-Derived Carbon Synthesis by Electrochemical Etching of MAX Phases. Angew. Chem. 2014, 126, 4977–4980. [Google Scholar] [CrossRef]
- Radovic, M.; Barsoum, M.W. MAX phases: Bridging the gap between metals and ceramics. Am. Ceram. Soc. Bull. 2013, 92, 20–27. [Google Scholar]
- Zheng, L.; Wang, J.; Lu, X.; Li, F.; Wang, J.; Zhou, Y. (Ti0.5Nb0.5) 5AlC4: A NewLayered Compound Belonging to MAX Phases. J. Am. Ceram. Soc. 2010, 93, 3068–3071. [Google Scholar] [CrossRef]
- Meshkian, R.; Tao, Q.; Dahlqvist, M.; Lu, J.; Hultman, L.; Rosen, J. Theoretical stability and materials synthesis of a chemically ordered MAX phase, Mo2ScAlC2, and its two-dimensional derivate Mo2ScC2 MXene. Acta Mater. 2017, 125, 476–480. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Meng, F.; Zhang, J. New MAX-Phase Compounds in the V–Cr–Al–C System. J. Am. Ceram. Soc. 2008, 91, 1357–1360. [Google Scholar] [CrossRef]
- Barsoum, M.W.; Radovic, M. Elastic and Mechanical Properties of the MAX Phases. Annu. Rev. Mater. Res. 2011, 41, 195–227. [Google Scholar] [CrossRef]
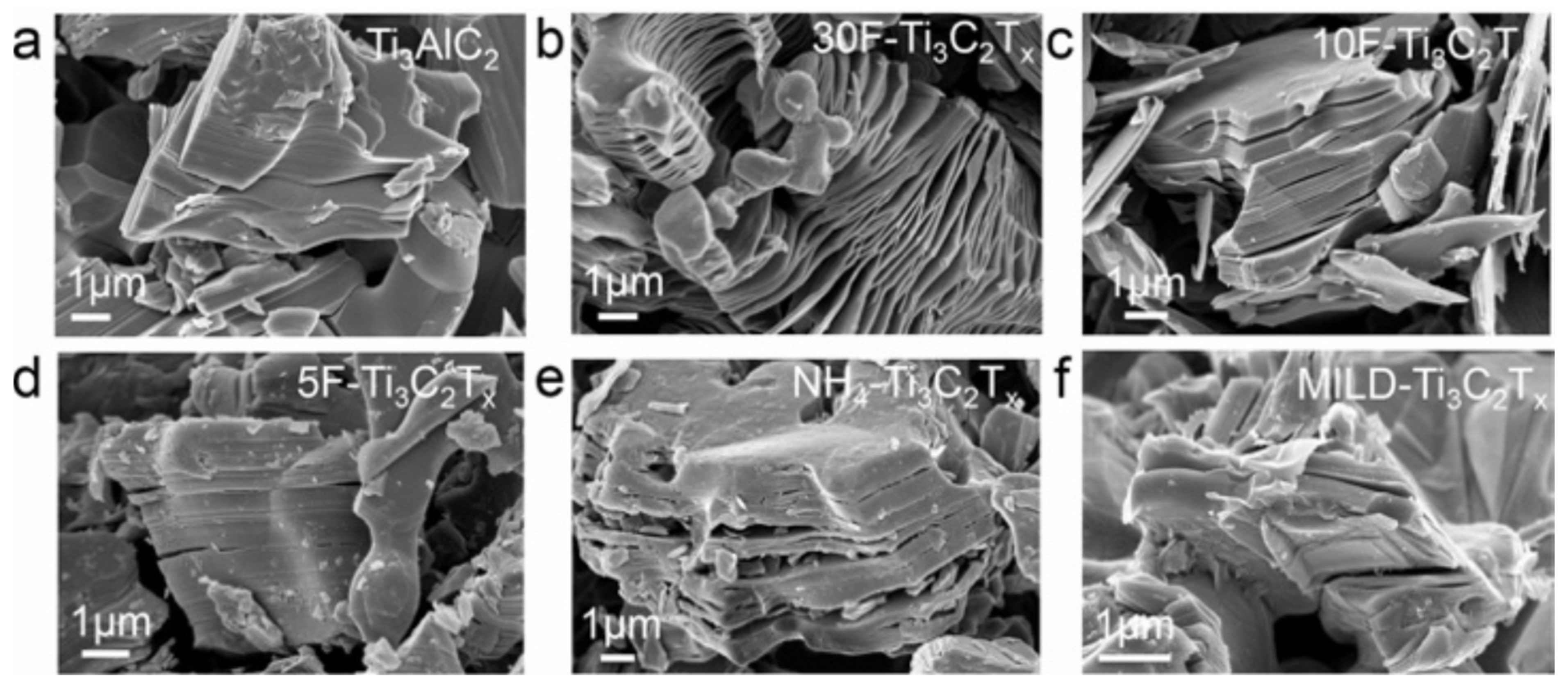
- Benchakar, M.; Loupias, L.; Garnero, C.; Bilyk, T.; Morais, C.; Canaff, C.; Guignard, N.; Morisset, S.; Pazniak, H.; Hurand, S.; et al. One MAX phase, different MXenes: A guideline to understand the crucial role of etching conditions on Ti3C2Tx surface chemistry. Appl. Surf. Sci. 2020, 530, 147209. [Google Scholar] [CrossRef]
- Wyatt, B.C.; Nemani, S.K.; Anasori, B. 2D transition metal carbides (MXenes) in metal and ceramic matrix composites. Nano Converg. 2021, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Eklund, P.; Beckers, M.; Jansson, U.; Högberg, H.; Hultman, L. The M+1AX phases: Materials science and thin-film processing. Thin Solid Films 2009, 518, 1851–1878. [Google Scholar] [CrossRef] [Green Version]
- Jin, X.; Shin, S.-J.; Kim, N.; Kang, B.; Piao, H.; Choy, J.-H.; Kim, H.; Hwang, S.-J. Superior role of MXene nanosheet as hybridization matrix over graphene in enhancing interfacial electronic coupling and functionalities of metal oxide. Nano Energy 2018, 53, 841–848. [Google Scholar] [CrossRef]
- Lukatskaya, M.R.; Mashtalir, O.; Ren, C.E.; Dall’Agnese, Y.; Rozier, P.; Taberna, P.L.; Gogotsi, Y. Cation intercalation and high volumetric capacitance of two-dimensional titanium carbide. Science 2013, 341, 1502–1505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, X.; Garsuch, A.; Nazar, L.F. Sulfur Cathodes Based on Conductive MXene Nanosheets for High-Performance Lithium-Sulfur Batteries. Angew. Chem. 2015, 127, 3979–3983. [Google Scholar] [CrossRef]
- Ren, C.E.; Hatzell, K.B.; Alhabeb, M.; Ling, Z.; Mahmoud, K.A.; Gogotsi, Y. Charge-and size-selective ion sieving through Ti3C2T x MXene membranes. J. Phys. Chem. Lett. 2015, 6, 4026–4031. [Google Scholar] [CrossRef]
- Shahzad, F.; Alhabeb, M.; Hatter, C.B.; Anasori, B.; Hong, S.M.; Koo, C.M.; Gogotsi, Y. Electromagnetic interference shielding with 2D transition metal carbides (MXenes). Science 2016, 353, 1137–1140. [Google Scholar] [CrossRef] [Green Version]
- Seh, Z.W.; Fredrickson, K.D.; Anasori, B.; Kibsgaard, J.; Strickler, A.L.; Lukatskaya, M.R.; Vojvodic, A. Two-dimensional molybdenum carbide (MXene) as an efficient electrocatalyst for hydrogen evolution. ACS Energy Lett. 2016, 1, 589–594. [Google Scholar] [CrossRef]
- Anasori, B.; Gogotsi, Û.G. 2D Metal Carbides and Nitrides (MXenes); Springer: Berlin, Germany, 2019. [Google Scholar]
- Anasori, B.; Lukatskaya, M.R.; Gogotsi, Y. 2D metal carbides and nitrides (MXenes) for energy storage. Nat. Rev. Mater. 2017, 2, 16098. [Google Scholar] [CrossRef]
- Satawara, A.M.; Shaikh, G.A.; Gupta, S.K.; Gajjar, P. Structural, electronic and optical properties of hexagonal boron-nitride (h-BN) monolayer: An Ab-initio study. Mater. Today Proc. 2020, 47, 529–532. [Google Scholar] [CrossRef]
- He, Z.; Que, W. Molybdenum disulfide nanomaterials: Structures, properties, synthesis and recent progress on hydrogen evolution reaction. Appl. Mater. Today 2016, 3, 23–56. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naguib, M.; Mochalin, V.N.; Barsoum, M.W.; Gogotsi, Y. 25th Anniversary Article: MXenes: A New Family of Two-Dimensional Materials. Adv. Mater. 2013, 26, 992–1005. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, Y. Layered machinable and electrically conductive Ti2AlC and Ti3AlC2 ceramics: A review. J. Mater. Sci. Technol. 2010, 26, 385–416. [Google Scholar] [CrossRef]
- Naguib, M.; Halim, J.; Lu, J.; Cook, K.M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. New Two-Dimensional Niobium and Vanadium Carbides as Promising Materials for Li-Ion Batteries. J. Am. Chem. Soc. 2013, 135, 15966–15969. [Google Scholar] [CrossRef]
- Zhu, K.; Jin, Y.; Du, F.; Gao, S.; Gao, Z.; Meng, X.; Gao, Y. Synthesis of Ti2CTx MXene as electrode materials for symmetric supercapacitor with capable volumetric capacitance. J. Energy Chem. 2019, 31, 11–18. [Google Scholar] [CrossRef] [Green Version]
- VahidMohammadi, A.; Hadjikhani, A.; Shahbazmohamadi, S.; Beidaghi, M. Two-dimensional vanadium carbide (MXene) as a high-capacity cathode material for rechargeable aluminum batteries. ACS Nano 2017, 11, 11135–11144. [Google Scholar] [CrossRef]
- Naguib, M.; Mashtalir, O.; Carle, J.; Presser, V.; Lu, J.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Transition Metal Carbides. ACS Nano 2012, 6, 1322–1331. [Google Scholar] [CrossRef] [PubMed]
- Munir, S.; Rasheed, A.; Rasheed, T.; Ayman, I.; Ajmal, S.; Rehman, A.; Warsi, M.F. Exploring the Influence of Critical Parameters for the Effective Synthesis of High-Quality 2D MXene. ACS Omega 2020, 5, 26845–26854. [Google Scholar] [CrossRef] [PubMed]
- Soundiraraju, B.; George, B.K. Two-dimensional titanium nitride (Ti2N) MXene: Synthesis, characterization, and potential application as surface-enhanced Raman scattering substrate. ACS Nano 2017, 11, 8892–8900. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Menga, X.; Zhu, K.; Du, F.; Chen, G.; Wei, Y.; Gogotsi, Y.; Gao, Y. Li-ion uptake and increase in interlayer spacing of Nb4C3 MXene. Energy Storage Mater. 2017, 8, 42–48. [Google Scholar] [CrossRef]
- Tran, M.H.; Schäfer, T.; Shahraei, A.; Dürrschnabel, M.; Molina-Luna, L.; Kramm, U.I.; Birkel, C.S. Adding a New Member to the MXene Family: Synthesis, Structure, and Electrocatalytic Activity for the Hydrogen Evolution Reaction of V4C3Tx. ACS Appl. Energy Mater. 2018, 1, 3908–3914. [Google Scholar] [CrossRef]
- Anasori, B.; Xie, Y.; Beidaghi, M.; Lu, J.; Hosler, B.C.; Hultman, L.; Kent, P.R.C.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional, Ordered, Double Transition Metals Carbides (MXenes). ACS Nano 2015, 9, 9507–9516. [Google Scholar] [CrossRef]
- Tao, Q.; Dahlqvist, M.; Lu, J.; Kota, S.; Meshkian, R.; Halim, J.; Palisaitis, J.; Hultman, L.; Barsoum, M.W.; Persson, P.O.; et al. Two-dimensional Mo1.33C MXene with divacancy ordering prepared from parent 3D laminate with in-plane chemical ordering. Nat. Commun. 2017, 8, 14949. [Google Scholar] [CrossRef]
- Halim, J.; Palisaitis, J.; Lu, J.; Thörnberg, J.; Moon, E.; Precner, M.; Rosen, J. Synthesis of two-dimensional Nb1. 33C (MXene) with randomly distributed vacancies by etching of the quaternary solid solution (Nb2/3Sc1/3) 2AlC MAX phase. ACS Appl. Nano Mater. 2018, 1, 2455–2460. [Google Scholar] [CrossRef] [Green Version]
- Meshkian, R.; Dahlqvist, M.; Lu, J.; Wickman, B.; Halim, J.; Thörnberg, J.; Snyder, J. W-based atomic laminates and their 2D derivative W1. 33C MXene with vacancy ordering. Adv. Mater. 2018, 30, 1706409. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zha, X.; Chen, F.Y.; Ye, Q.; Eklund, P.; Du, S.; Huang, Q. A Two-Dimensional Zirconium Carbide by Selective Etching of Al3C3from Nanolaminated Zr3Al3C5. Angew. Chem. Int. Ed. 2016, 55, 5008–5013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Zha, X.; Zhou, X.; Chen, F.; Gao, G.; Wang, S.; Eklund, P. Synthesis and electrochemical properties of two-dimensional hafnium carbide. ACS Nano 2017, 11, 3841–3850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halim, J.; Lukatskaya, M.R.; Cook, K.M.; Lu, J.; Smith, C.R.; Näslund, L.; May, S.J.; Hultman, L.; Gogotsi, Y.; Eklund, P.; et al. Transparent Conductive Two-Dimensional Titanium Carbide Epitaxial Thin Films. Chem. Mater. 2014, 26, 2374–2381. [Google Scholar] [CrossRef] [PubMed]
- Feng, A.; Yu, Y.; Jiang, F.; Wang, Y.; Mi, L.; Yu, Y.; Song, L. Fabrication and thermal stability of NH4 HF2 -etched Ti3 C2 MXene. Ceram. Int. 2017, 43, 6322–6328. [Google Scholar] [CrossRef]
- Ghidiu, M.; Lukatskaya, M.R.; Zhao, M.-Q.; Gogotsi, Y.; Barsoum, M.W. Conductive two-dimensional titanium carbide ‘clay’with high volumetric capacitance. Nature 2014, 516, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Alhabeb, M.; Maleski, K.; Anasori, B.; Lelyukh, P.; Clark, L.; Sin, S.; Gogotsi, Y. Guidelines for synthesis and processing of two-dimensional titanium carbide (Ti3C2T x MXene). Chem. Mater. 2017, 29, 7633–7644. [Google Scholar] [CrossRef]
- Wang, X.; Garnero, C.; Rochard, G.; Magne, D.; Morisset, S.; Hurand, S.; Coutanceau, C. A new etching environment (FeF 3/HCl) for the synthesis of two-dimensional titanium carbide MXenes: A route towards selective reactivity vs. water. J. Mater. Chem. A 2017, 5, 22012–22023. [Google Scholar] [CrossRef]
- Xu, C.; Wang, L.; Liu, Z.; Chen, L.; Guo, J.; Kang, N.; Ma, X.-L.; Cheng, H.-M.; Ren, W. Large-area high-quality 2D ultrathin Mo2C superconducting crystals. Nat. Mater. 2015, 14, 1135–1141. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Ting, L.R.L.; Molinari, V.; Giordano, C.; Yeo, B.S. Efficient hydrogen evolution reaction catalyzed by molybdenum carbide and molybdenum nitride nanocatalysts synthesized via the urea glass route. J. Mater. Chem. A 2015, 3, 8361–8368. [Google Scholar] [CrossRef]
- Fashandi, H.; Dahlqvist, M.; Lu, J.; Palisaitis, J.; Simak, S.I.; Abrikosov, I.A.; Rosen, J.; Hultman, L.; Andersson, M.; Spetz, A.L.; et al. Synthesis of Ti3AuC2, Ti3Au2C2 and Ti3IrC2 by noble metal substitution reaction in Ti3SiC2 for high-temperature-stable Ohmic contacts to SiC. Nat. Mater. 2017, 16, 814–818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fashandi, H.; Lai, C.-C.; Dahlqvist, M.; Lu, J.; Rosen, J.; Hultman, L.; Greczynski, G.; Andersson, M.; Spetz, A.L.; Eklund, P. Ti2Au2C and Ti3Au2C2 formed by solid state reaction of gold with Ti2AlC and Ti3AlC2. Chem. Commun. 2017, 53, 9554–9557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Cheng, J.; Zhu, S.; Ma, J.; Qiao, Z.; Yang, J.; Liu, W. A novel route to prepare a Ti3SnC2/Al2O3 composite. Scr. Mater. 2017, 131, 80–83. [Google Scholar] [CrossRef]
- Li, M.; Lu, J.; Luo, K.; Li, Y.; Chang, K.; Chen, K.; Huang, Q. Element Replacement Approach by Reaction with Lewis Acidic Molten Salts to Synthesize Nanolaminated MAX Phases and MXenes. J. Am. Chem. Soc. 2019, 141, 4730–4737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.; Zhang, P.; Wang, F.; Ricciardulli, A.G.; Lohe, M.R.; Blom, P.W.M.; Feng, X. Fluoride-Free Synthesis of Two-Dimensional Titanium Carbide (MXene) Using A Binary Aqueous System. Angew. Chem. Int. Ed. 2018, 57, 15491–15495. [Google Scholar] [CrossRef] [PubMed]
- Toušek, J. Discussion of “Occurrence of the Salt Film during Initiation and Growth of Corrosion Pits” [T. R. Beck and R. C. Alkire (pp. 1662–1666, Vol. 126, No. 10)]. J. Electrochem. Soc. 1980, 127, 1322–1323. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, H.; Wang, B.; Shen, C.; Zhang, C.; Hu, Q.; Zhou, A.; Liu, B. Synthesis and electrochemical performance of Ti3C2Tx with hydrothermal process. Electron. Mater. Lett. 2016, 12, 702–710. [Google Scholar] [CrossRef]
- Peng, C.; Wei, P.; Chen, X.; Zhang, Y.; Zhu, F.; Cao, Y.; Wang, H.; Yu, H.; Peng, F. A hydrothermal etching route to synthesis of 2D MXene (Ti3C2, Nb2C): Enhanced exfoliation and improved adsorption performance. Ceram. Int. 2018, 44, 18886–18893. [Google Scholar] [CrossRef]
- Li, T.; Yao, L.; Liu, Q.; Gu, J.; Luo, R.; Li, J.; Yan, X.; Wang, W.; Liu, P.; Chen, B.; et al. Fluorine-Free Synthesis of High-Purity Ti 3 C 2 T x (T=OH, O) via Alkali Treatment. Angew. Chem. Int. Ed. 2018, 57, 6115–6119. [Google Scholar] [CrossRef]
- Adamson, A.; Bloore, E.; Carr, A. Basic Principles of Bayer Process Design. In Essential Readings in Light Metals; Springer: Cham, Switzerland, 2016; pp. 100–117. [Google Scholar]
- Urbankowski, P.; Anasori, B.; Makaryan, T.; Er, D.; Kota, S.; Walsh, P.L.; Zhao, M.; Shenoy, V.B.; Barsoum, M.W.; Gogotsi, Y. Synthesis of two-dimensional titanium nitride Ti4N3(MXene). Nanoscale 2016, 8, 11385–11391. [Google Scholar] [CrossRef]
- Chen, J.; Chen, K.; Tong, D.; Huang, Y.; Zhang, J.; Xue, J.; Chen, T. CO2 and temperature dual responsive “Smart” MXene phases. Chem. Commun. 2015, 51, 314–317. [Google Scholar] [CrossRef] [PubMed]
- Boota, M.; Anasori, B.; Voigt, C.; Zhao, M.Q.; Barsoum, M.W.; Gogotsi, Y. Pseudocapacitive electrodes produced by oxidant-free polymerization of pyrrole between the layers of 2D titanium carbide (MXene). Adv. Mater. 2016, 28, 1517–1522. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Huang, Y.; Deng, Q.; Zhou, J.; Pei, Z.; Xue, Q.; Wang, Z.; Li, H.; Huang, Q.; Zhi, C. Highly Flexible, Freestanding Supercapacitor Electrode with Enhanced Performance Obtained by Hybridizing Polypyrrole Chains with MXene. Adv. Energy Mater. 2016, 6, 1600969. [Google Scholar] [CrossRef]
- Ling, Z.; Ren, C.E.; Zhao, M.-Q.; Yang, J.; Giammarco, J.M.; Qiu, J.; Barsoum, M.W.; Gogotsi, Y. Flexible and conductive MXene films and nanocomposites with high capacitance. Proc. Natl. Acad. Sci. USA 2014, 111, 16676–16681. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Boota, M.; Xie, X.; Zhao, M.; Anasori, B.; Ren, C.E.; Miao, L.; Jiang, J.; Gogotsi, Y. Charge transfer induced polymerization of EDOT confined between 2D titanium carbide layers. J. Mater. Chem. A 2017, 5, 5260–5265. [Google Scholar] [CrossRef]
- Cao, J.; Han, Y.; Zheng, X.; Wang, Q. Preparation and electrochemical performance of modified Ti3C2Tx/polypyrrole composites. J. Appl. Polym. Sci. 2019, 136, 47003. [Google Scholar] [CrossRef]
- Naguib, M.; Saito, T.; Lai, S.; Rager, M.S.; Aytug, T.; Paranthaman, M.P.; Gogotsi, Y. Ti3C2Tx (MXene)–polyacrylamide nanocomposite films. RSC Adv. 2016, 6, 72069–72073. [Google Scholar] [CrossRef]
- Xu, H.; Zheng, D.; Liu, F.; Li, W.; Lin, J. Synthesis of an MXene/polyaniline composite with excellent electrochemical properties. J. Mater. Chem. A 2020, 8, 5853–5858. [Google Scholar] [CrossRef]
- Zhu, J.; Ha, E.; Zhao, G.; Zhou, Y.; Huang, D.; Yue, G.; Hu, L.; Sun, N.; Wang, Y.; Lee, L.Y.S.; et al. Recent advance in MXenes: A promising 2D material for catalysis, sensor and chemical adsorption. Coord. Chem. Rev. 2017, 352, 306–327. [Google Scholar] [CrossRef]
- Verger, L.; Natu, V.; Carey, M.; Barsoum, M.W. MXenes: An Introduction of Their Synthesis, Select Properties, and Applications. Trends Chem. 2019, 1, 656–669. [Google Scholar] [CrossRef]
- Mashtalir, O.; Naguib, M.; Mochalin, V.; Dall’Agnese, Y.; Heon, M.; Barsoum, M.W.; Gogotsi, Y. Intercalation and delamination of layered carbides and carbonitrides. Nat. Commun. 2013, 4, 1716. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.; Unocic, R.R.; Armstrong, B.L.; Nanda, J. Large-scale delamination of multi-layers transition metal carbides and carbonitrides “MXenes”. Dalton Trans. 2015, 44, 9353–9358. [Google Scholar] [CrossRef]
- Mashtalir, O.; Lukatskaya, M.R.; Zhao, M.-Q.; Barsoum, M.W.; Gogotsi, Y. Amine-Assisted Delamination of Nb2C MXene for Li-Ion Energy Storage Devices. Adv. Mater. 2015, 27, 3501–3506. [Google Scholar] [CrossRef]
- Shekhirev, M.; Shuck, C.E.; Sarycheva, A.; Gogotsi, Y. Characterization of MXenes at Every Step, from Their Precursors to Single Flakes and Assembled Films. Prog. Mater. Sci. 2020, 120, 100757. [Google Scholar] [CrossRef]
- Sheng, X.; Li, S.; Huang, H.; Zhao, Y.; Chen, Y.; Zhang, L.; Xie, D. Anticorrosive and UV-blocking waterborne polyurethane composite coating containing novel two-dimensional Ti3C2 MXene nanosheets. J. Mater. Sci. 2020, 56, 4212–4224. [Google Scholar] [CrossRef]
- Shuck, C.E.; Sarycheva, A.; Anayee, M.; Levitt, A.; Zhu, Y.; Uzun, S.; Balitskiy, V.; Zahorodna, V.; Gogotsi, O.; Gogotsi, Y. Scalable Synthesis of Ti3C2Tx MXene. Adv. Eng. Mater. 2020, 22, 1901241. [Google Scholar] [CrossRef]
- Guo, Z.; Zhu, L.; Zhou, J.; Sun, Z. Microscopic origin of MXenes derived from layered MAX phases. RSC Adv. 2015, 5, 25403–25408. [Google Scholar] [CrossRef]
- Yang, J.; Bao, W.; Jaumaux, P.; Zhang, S.; Wang, C.; Wang, G. MXene-Based Composites: Synthesis and Applications in Rechargeable Batteries and Supercapacitors. Adv. Mater. Interfaces 2019, 6, 1802004. [Google Scholar] [CrossRef]
- Caffrey, N.M. Effect of mixed surface terminations on the structural and electrochemical properties of two-dimensional Ti3C2T2 and V2CT2 MXenes multilayers. Nanoscale 2018, 10, 13520–13530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Z.; Zhou, J.; Si, C.; Sun, Z. Flexible two-dimensional Tin+1Cn(n = 1, 2 and 3) and their functionalized MXenes predicted by density functional theories. Phys. Chem. Chem. Phys. 2015, 17, 15348–15354. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Fu, Z.; Zhang, R.; Zhang, Q.; Tian, H.; Legut, D.; Germann, T.C.; Guo, Y.; Du, S.; Francisco, J.S. Designing flexible 2D transition metal carbides with strain-controllable lithium storage. Proc. Natl. Acad. Sci. USA 2017, 114, E11082–E11091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipatov, A.; Lu, H.; Alhabeb, M.; Anasori, B.; Gruverman, A.; Gogotsi, Y.; Sinitskii, A. Elastic properties of 2D Ti3C2Tx MXene monolayers and bilayers. Sci. Adv. 2018, 4, eaat0491. [Google Scholar] [CrossRef] [Green Version]
- Yorulmaz, U.; Ozden, A.; Perkgoz, N.K.; Ay, F.; Sevik, C. Vibrational and mechanical properties of single layer MXene structures: A first-principles investigation. Nanotechnology 2016, 27, 335702. [Google Scholar] [CrossRef]
- Borysiuk, V.N.; Mochalin, V.; Gogotsi, Y. Molecular dynamic study of the mechanical properties of two-dimensional titanium carbides Tin+1Cn (MXenes). Nanotechnology 2015, 26, 265705. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, Y.; Mohamed, A.; AbdelGawad, A.M.; Eid, K.; Abdullah, A.M.; Elzatahry, A. The Recent Advances in the Mechanical Properties of Self-Standing Two-Dimensional MXene-Based Nanostructures: Deep Insights into the Supercapacitor. Nanomaterials 2020, 10, 1916. [Google Scholar] [CrossRef]
- Chakraborty, P.; Das, T.; Nafday, D.; Boeri, L.; Saha-Dasgupta, T. Manipulating the mechanical properties of Ti2C MXene: Effect of substitutional doping. Phys. Rev. B 2017, 95, 184106. [Google Scholar] [CrossRef]
- Andrew, R.C.; Mapasha, R.E.; Ukpong, A.M.; Chetty, N. Erratum: Mechanical properties of graphene and boronitrene [Phys. Rev. B 85, 125428 (2012)]. Phys. Rev. B 2019, 100, 209901. [Google Scholar] [CrossRef] [Green Version]
- Almayyali, A.O.M.; Kadhim, B.B.; Jappor, H.R. Stacking impact on the optical and electronic properties of two-dimensional MoSe2/PtS2 heterostructures formed by PtS2 and MoSe2 monolayers. Chem. Phys. 2020, 532, 110679. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Barsoum, M.W. Two-Dimensional Nanocrystals: Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2 (Adv. Mater. 37/2011). Adv. Mater. 2011, 23, 4207. [Google Scholar] [CrossRef]
- Shein, I.; Ivanovskii, A. Graphene-like titanium carbides and nitrides Tin+1Cn, Tin+1Nn (n=1, 2, and 3) from de-intercalated MAX phases: First-principles probing of their structural, electronic properties and relative stability. Comput. Mater. Sci. 2012, 65, 104–114. [Google Scholar] [CrossRef]
- Khazaei, M.; Arai, M.; Sasaki, T.; Chung, C.-Y.; Venkataramanan, N.S.; Estili, M.; Sakka, Y.; Kawazoe, Y. Novel Electronic and Magnetic Properties of Two-Dimensional Transition Metal Carbides and Nitrides. Adv. Funct. Mater. 2012, 23, 2185–2192. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, H.; Goddard, W.A., III. Schottky-barrier-free contacts with two-dimensional semiconductors by surface-engineered MXenes. J. Am. Chem. Soc. 2016, 138, 15853–15856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khazaei, M.; Ranjbar, A.; Arai, M.; Sasaki, T.; Yunoki, S. Electronic properties and applications of MXenes: A theoretical review. J. Mater. Chem. C 2017, 5, 2488–2503. [Google Scholar] [CrossRef] [Green Version]
- Khazaei, M.; Ranjbar, A.; Arai, M.; Yunoki, S. Topological insulators in the ordered double transition metalsM2′M″C2MXenes (M′=Mo, W;M″=Ti, Zr, Hf). Phys. Rev. B 2016, 94, 125152. [Google Scholar] [CrossRef] [Green Version]
- Hu, T.; Zhang, H.; Wang, J.; Li, Z.; Hu, M.; Tan, J.; Hou, P.; Li, F.; Wang, X. Anisotropic electronic conduction in stacked two-dimensional titanium carbide. Sci. Rep. 2015, 5, 16329. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.; Hu, M.; Ling, H.; Zhu, X. MXenes: Focus on optical and electronic properties and corresponding applications. Nanophotonics 2020, 9, 1601–1620. [Google Scholar] [CrossRef]
- Sinopoli, A.; Othman, Z.; Rasool, K.; Mahmoud, K.A. Electrocatalytic/photocatalytic properties and aqueous media applications of 2D transition metal carbides (MXenes). Curr. Opin. Solid State Mater. Sci. 2019, 23, 100760. [Google Scholar] [CrossRef]
- Wong, Z.M.; Tan, T.L.; Yang, S.-W.; Xu, G.Q. Enhancing the Photocatalytic Performance of MXenes via Stoichiometry Engineering of Their Electronic and Optical Properties. ACS Appl. Mater. Interfaces 2018, 10, 39879–39889. [Google Scholar] [CrossRef] [PubMed]
- Morales-García, Á.; Calle-Vallejo, F.; Illas, F. MXenes: New Horizons in Catalysis. ACS Catal. 2020, 10, 13487–13503. [Google Scholar] [CrossRef]
- Bai, Y.; Zhou, K.; Narasimalu, S.; Pang, J.; He, X.; Wang, R. Dependence of elastic and optical properties on surface terminated groups in two-dimensional MXene monolayers a first-principles study-RSC Advances. RSC Adv. 2016, 6, 35731–35739. [Google Scholar] [CrossRef]
- Lashgari, H.; Abolhassani, M.; Boochani, A.; Elahi, S.; Khodadadi, J. Electronic and optical properties of 2D graphene-like compounds titanium carbides and nitrides: DFT calculations. Solid State Commun. 2014, 195, 61–69. [Google Scholar] [CrossRef]
- Ying, G.; Dillon, A.D.; Fafarman, A.T.; Barsoum, M.W. Transparent, conductive solution processed spincast 2D Ti2CTx (MXene) films. Mater. Res. Lett. 2017, 5, 391–398. [Google Scholar] [CrossRef] [Green Version]
- Dillon, A.D.; Ghidiu, M.J.; Krick, A.L.; Griggs, J.; May, S.J.; Gogotsi, Y.; Barsoum, M.W.; Fafarman, A.T. Highly Conductive Optical Quality Solution-Processed Films of 2D Titanium Carbide. Adv. Funct. Mater. 2016, 26, 4162–4168. [Google Scholar] [CrossRef]
- Nair, R.R.; Blake, P.; Grigorenko, A.N.; Novoselov, K.S.; Booth, T.J.; Stauber, T.; Peres, N.M.R.; Geim, A.K. Fine Structure Constant Defines Visual Transparency of Graphene. Science 2008, 320, 1308. [Google Scholar] [CrossRef] [Green Version]
- Ying, G.; Kota, S.; Dillon, A.D.; Fafarman, A.T.; Barsoum, M.W. Conductive transparent V2CTx (MXene) films. FlatChem 2018, 8, 25–30. [Google Scholar] [CrossRef]
- Aierken, Y.; Sevik, C.; Gülseren, O.; Peeters, F.M.; Çakır, D. MXenes/graphene heterostructures for Li battery applications: A first principles study. J. Mater. Chem. A 2018, 6, 2337–2345. [Google Scholar] [CrossRef]
- Tang, H.; Hu, Q.; Zheng, M.; Chi, Y.; Qin, X.; Pang, H.; Xu, Q. MXene–2D layered electrode materials for energy storage. Prog. Nat. Sci. 2018, 28, 133–147. [Google Scholar] [CrossRef]
- Garg, R.; Agarwal, A.; Agarwal, M. A review on MXene for energy storage application: Effect of interlayer distance. Mater. Res. Express 2020, 7, 022001. [Google Scholar] [CrossRef]
- Lin, Z.; Shao, H.; Xu, K.; Taberna, P.-L.; Simon, P. MXenes as High-Rate Electrodes for Energy Storage. Trends Chem. 2020, 2, 654–664. [Google Scholar] [CrossRef]
- Balach, J.M.; Giebeler, L. MXenes and the progress of Li–S battery development—A perspective. J. Phys. Energy 2020, 3, 021002. [Google Scholar] [CrossRef]
- Gogotsi, Y.; Anasori, B. The Rise of MXenes. ACS Nano 2019, 13, 8491–8494. [Google Scholar] [CrossRef] [Green Version]
- Beidaghi, M.; Gogotsi, Y. Capacitive energy storage in micro-scale devices: Recent advances in design and fabrication of micro-supercapacitors. Energy Environ. Sci. 2014, 7, 867–884. [Google Scholar] [CrossRef]
- Zheng, S.; Shi, X.; Das, P.; Wu, Z.; Bao, X. The Road Towards Planar Microbatteries and Micro-Supercapacitors: From 2D to 3D Device Geometries. Adv. Mater. 2019, 31, e1900583. [Google Scholar] [CrossRef] [PubMed]
- Chmiola, J.; Largeot, C.; Taberna, P.-L.; Simon, P.; Gogotsi, Y. Monolithic Carbide-Derived Carbon Films for Micro-Supercapacitors. Science 2010, 328, 480–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, J.; Zhang, C.; Yan, Z.; Zhu, Y.; Peng, Z.; Hauge, R.H.; Natelson, D.; Tour, J.M. 3-Dimensional Graphene Carbon Nanotube Carpet-Based Microsupercapacitors with High Electrochemical Performance. Nano Lett. 2012, 13, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Mondal, A.K.; Wang, D.; Iacopi, F. Graphene-Based Planar Microsupercapacitors: Recent Advances and Future Challenges. Adv. Mater. Technol. 2018, 4, 1800200. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Han, Y.; Shao, C.; Chen, N.; Sun, G.; Jin, X.; Gao, J.; Ji, B.; Yang, H.; Qu, L. Processing and manufacturing of graphene-based microsupercapacitors. Mater. Chem. Front. 2018, 2, 1750–1764. [Google Scholar] [CrossRef]
- Kim, M.S.; Hsia, B.; Carraro, C.; Maboudian, R. Flexible micro-supercapacitors with high energy density from simple transfer of photoresist-derived porous carbon electrodes. Carbon 2014, 74, 163–169. [Google Scholar] [CrossRef]
- Pech, D.; Brunet, M.; Durou, H.; Huang, P.; Mochalin, V.; Gogotsi, Y.; Taberna, P.-L.; Simon, P. Ultrahigh-power micrometre-sized supercapacitors based on onion-like carbon. Nat. Nanotechnol. 2010, 5, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Hsia, B.; Carraro, C.; Maboudian, R. High-performance all solid-state micro-supercapacitor based on patterned photoresist-derived porous carbon electrodes and an ionogel electrolyte. J. Mater. Chem. A 2014, 2, 7997–8002. [Google Scholar] [CrossRef]
- Kurra, N.; Hota, M.; Alshareef, H.N. Conducting polymer micro-supercapacitors for flexible energy storage and Ac line-filtering. Nano Energy 2015, 13, 500–508. [Google Scholar] [CrossRef]
- Meng, C.; Maeng, J.; John, S.W.M.; Irazoqui, P.P. Ultrasmall Integrated 3D Micro-Supercapacitors Solve Energy Storage for Miniature Devices. Adv. Energy Mater. 2013, 4, 1301269. [Google Scholar] [CrossRef]
- Augustyn, V.; Come, J.; Lowe, M.A.; Kim, J.W.; Taberna, P.-L.; Tolbert, S.H.; Abruña, H.D.; Simon, P.; Dunn, B. High-rate electrochemical energy storage through Li+ intercalation pseudocapacitance. Nat. Mater. 2013, 12, 518–522. [Google Scholar] [CrossRef]
- Choi, D.; Blomgren, G.E.; Kumta, P.N. Fast and Reversible Surface Redox Reaction in Nanocrystalline Vanadium Nitride Supercapacitors. Adv. Mater. 2006, 18, 1178–1182. [Google Scholar] [CrossRef]
- Peng, Y.-Y.; Akuzum, B.; Kurra, N.; Zhao, M.-Q.; Alhabeb, M.; Anasori, B.; Kumbur, E.C.; Alshareef, H.N.; Ger, M.-D.; Gogotsi, Y. All-MXene (2D titanium carbide) solid-state microsupercapacitors for on-chip energy storage. Energy Environ. Sci. 2016, 9, 2847–2854. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Q.; Kurra, N.; Maleski, K.; Lei, Y.; Liang, H.; Zhang, Y.; Gogotsi, Y.; Alshareef, H.N. On-Chip MXene Microsupercapacitors for AC-Line Filtering Applications. Adv. Energy Mater. 2019, 9, 1901061. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Q.; Li, L.; Yang, L.; Wang, Y.; Wang, X.; Fang, H. Microsupercapacitors: Femtosecond Laser-Etched MXene Microsupercapacitors with Double-Side Configuration via Arbitrary On-and Through-Substrate Connections (Adv. Energy Mater. 24/2020). Adv. Energy Mater. 2020, 10. [Google Scholar] [CrossRef]
- Sharma, A.; Rout, C.S. Two-Dimensional MXene Based Materials for Micro-Supercapacitors; Headquarters IntechOpen Limited: London, UK, 2021. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, J.; Huang, F. Enhanced visible light photocatalytic H2 evolution of metal-free g-C3N4 /SiC heterostructured photocatalysts. Appl. Surf. Sci. 2017, 391, 449–456. [Google Scholar] [CrossRef]
- Li, Y.; Jin, Z.; Zhang, L.; Fan, K. Controllable design of Zn-Ni-P on g-C3N4 for efficient photocatalytic hydrogen production. Chin. J. Catal. 2019, 40, 390–402. [Google Scholar] [CrossRef]
- Li, X.; Bai, Y.; Shi, X.; Su, N.; Nie, G.; Zhang, R.; Nie, H.; Ye, L. Applications of MXene (Ti3C2Tx) in photocatalysis: A review. Mater. Adv. 2021, 2, 1570–1594. [Google Scholar] [CrossRef]
- Kuang, P.; Low, J.; Cheng, B.; Yu, J.; Fan, J. MXene-based photocatalysts. J. Mater. Sci. Technol. 2020, 56, 18–44. [Google Scholar] [CrossRef]
- Guo, Z.; Zhou, J.; Zhu, L.; Sun, Z. MXene: A promising photocatalyst for water splitting. J. Mater. Chem. A 2016, 4, 11446–11452. [Google Scholar] [CrossRef]
- Takanabe, K. Photocatalytic Water Splitting: Quantitative Approaches toward Photocatalyst by Design. ACS Catal. 2017, 7, 8006–8022. [Google Scholar] [CrossRef]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2008, 38, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Morales-García, Á.; Mayans-Llorach, M.; Viñes, F.; Illas, F. Thickness biased capture of CO2 on carbide MXenes. Phys. Chem. Chem. Phys. 2019, 21, 23136–23142. [Google Scholar] [CrossRef]
- Chen, J.; Huang, Q.; Huang, H.; Mao, L.; Liu, M.; Zhang, X.; Wei, Y. Recent progress and advances in the environmental applications of MXene related materials. Nanoscale 2020, 12, 3574–3592. [Google Scholar] [CrossRef]
- Ding, L.; Wei, Y.; Li, L.; Zhang, T.; Wang, H.; Xue, J.; Ding, L.-X.; Wang, S.; Caro, J.; Gogotsi, Y. MXene molecular sieving membranes for highly efficient gas separation. Nat. Commun. 2018, 9, 155. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Si, C.; Zhou, J.; Sun, Z. MXene and MXene-based composites: Synthesis, properties and environment-related applications. Nanoscale Horizons 2019, 5, 235–258. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, C.; Guo, S.; Mu, T.; Wei, L.; Lu, Y. CO2 capture and conversion to value-added products promoted by MXene-based materials. Green Energy Environ. 2020. [Google Scholar] [CrossRef]



| Year | Synthesis Methods Development Timeline |
|---|---|
| 2011 | Discovery of Ti3C2MXene via HF etching method. |
| 2012 | Emergence of MXene family including Ti2C, Ta4C3, Ti3CN, (V0.5Cr0.5)3C2, (Ti0.5Nb0.5)2C. |
| 2013 | Delamination of MXenes. |
| 2014 | (i) Bifluoride NH4HF2etching method; (ii) LiF/HCl etching method producing clay (small flakes); (iii) MXene-polymer composite. |
| 2015 | (i) TBAOH and amine-assisted delamination; (ii) Urea Glass Route to produce MO2C and MO2N Mxene; (iii) Chemical vapor deposition method, theoretical predictions of double-ordered MXenes. |
| 2016 | (i) Molten salt etching method to produce nitride-based MXene Ti4N3; (ii) Room temperature synthesis of new MXene Zr3C2. |
| 2018 | (i) Hydrothermal synthesis method using NaOH; (ii) Electrochemical fluoride free synthesis of MXene using a binary aqueous system. |
| 2019 | (i) Advances in MXene-composite synthesis; (ii) Lewis acid molten salts etching method to synthesize Mxenes |
| 2020 | Synthesis of MXene/PANI composite. |
| S. No. | Precursors | Mxene | Conditions | ||
|---|---|---|---|---|---|
| Time (h) | Yield (%) | Ref. | |||
| 1. | Ti2AlC | Ti2CTx | 10 | 80 | [26] |
| 2. | V2AlC | V2CTx | 90 | 60 | [27] |
| 3. | Nb2AlC | Nb2CTx | 90 | 100 | [25] |
| 4. | Ti2AlN | Ti2NTx | 24 | NA | [30] |
| 5. | Mo2Ga2C | Mo2CTx | 3 | NA | |
| 6. | (Ti0.5Nb0.5)2AlC | (Ti0.5Nb0.5)2CTx | 28 | 80 | [28] |
| 7. | Ti3AlC2 | Ti3C2Tx | 2 | 100 | [22] |
| 8. | (V0.5Cr0.5)3AlC2 | (V0.5Cr0.5)3C2Tx | 69 | NA | [28] |
| 9. | Ta4AlC3 | Ta4C3Tx | 72 | 90 | [28] |
| 10. | Nb4AlC3 | Nb4C3Tx | 96 | 77 | [31] |
| 11. | V4AlC3 | V4C3Tx | 165 | NA | [32] |
| 12. | Ti3AlCN | Ti3CNTx | 18 | 80 | [28] |
| 13. | Mo2TiAlC2 | Mo2TiC2Tx | 48 | 100 | [33] |
| 14. | Mo2Ti2AlC3 | Mo2Ti2C3Tx | 90 | 100 | [33] |
| 15. | (Mo2/3Y1/3)2AlC | Mo4/3CTx | 6072 | NA | [34] |
| 16. | (Nb2/3Sc1/3)2AlC | Nb4/3CTx | 30 | NA | [35] |
| 17. | (W2/3Sc1/3)2AlC | W4/3CTx | 30 | NA | [36] |
| 18. | Zr3Al3C5 | Zr3C2Tx | 60 | NA | [37] |
| 19. | Hf3[Al(Si)]4C6 | Hf3C2Tx | 60 | NA | [38] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biswas, S.; Alegaonkar, P.S. MXene: Evolutions in Chemical Synthesis and Recent Advances in Applications. Surfaces 2022, 5, 1-34. https://doi.org/10.3390/surfaces5010001
Biswas S, Alegaonkar PS. MXene: Evolutions in Chemical Synthesis and Recent Advances in Applications. Surfaces. 2022; 5(1):1-34. https://doi.org/10.3390/surfaces5010001
Chicago/Turabian StyleBiswas, Sayani, and Prashant S. Alegaonkar. 2022. "MXene: Evolutions in Chemical Synthesis and Recent Advances in Applications" Surfaces 5, no. 1: 1-34. https://doi.org/10.3390/surfaces5010001
APA StyleBiswas, S., & Alegaonkar, P. S. (2022). MXene: Evolutions in Chemical Synthesis and Recent Advances in Applications. Surfaces, 5(1), 1-34. https://doi.org/10.3390/surfaces5010001






