4-Mercaptobenzoic Acid Adsorption on TiO2 Anatase (101) and TiO2 Rutile (110) Surfaces
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. X-ray Photoelectron Spectroscopy
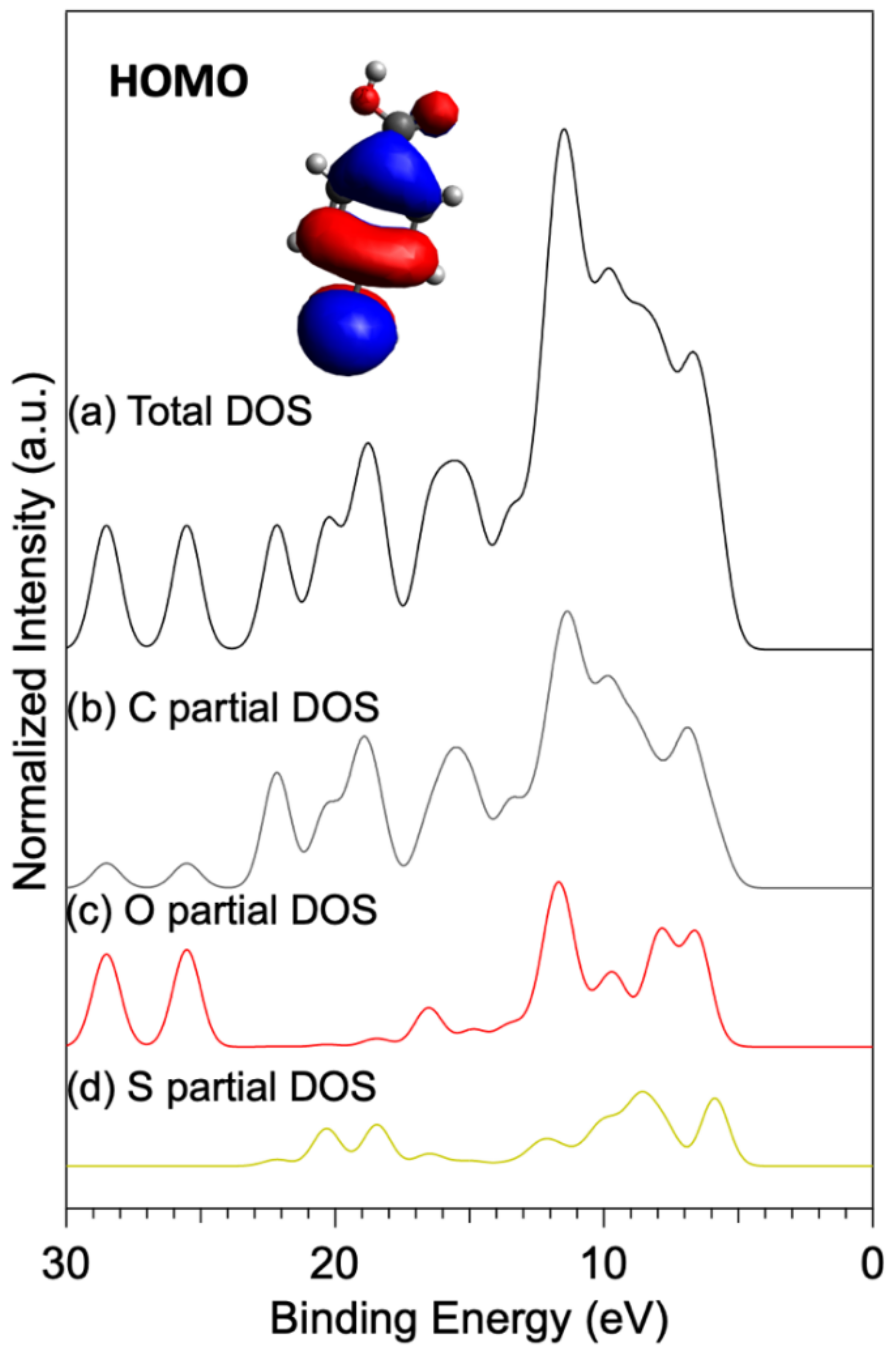
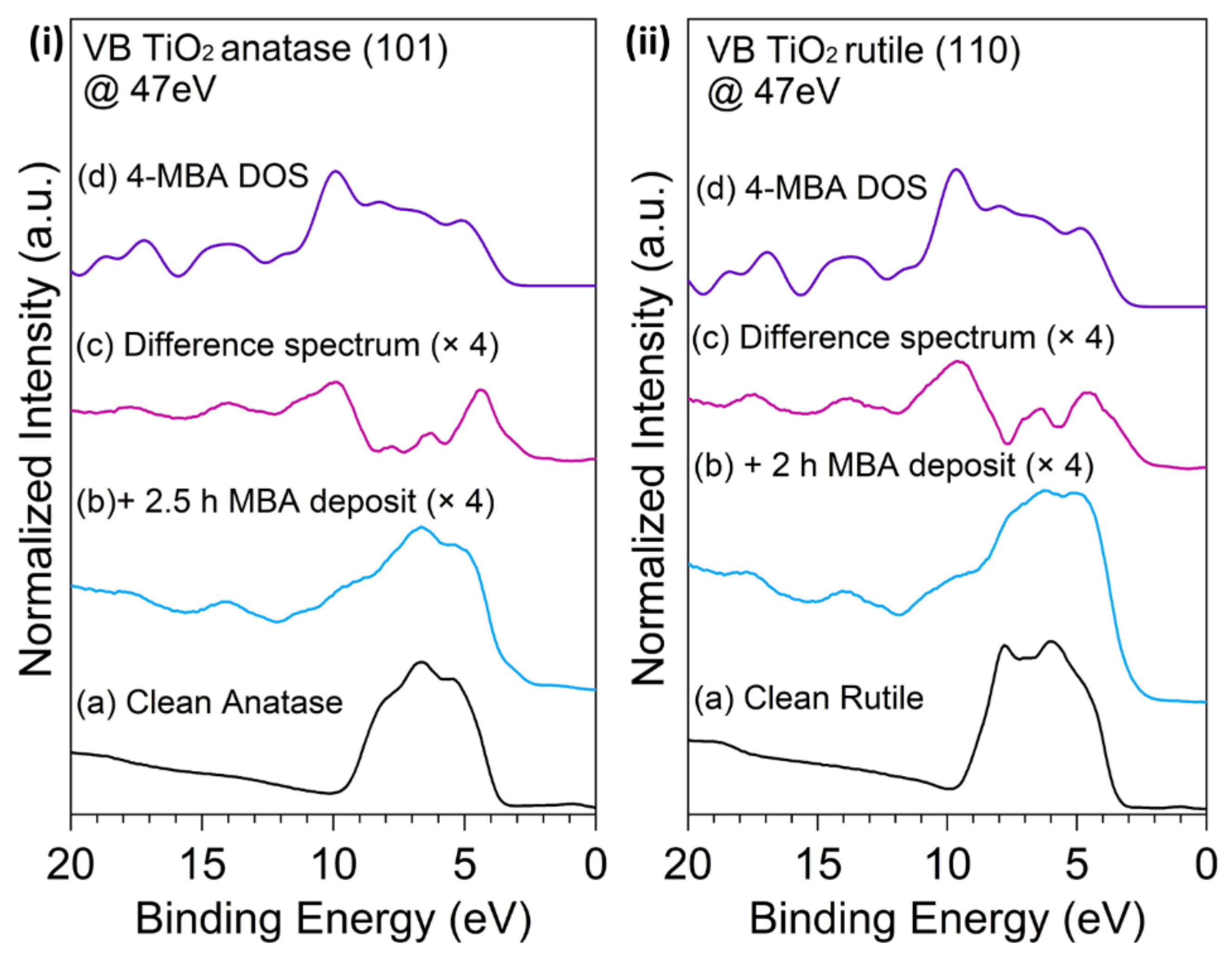
3.2. UV Photoelectron Spectroscopy
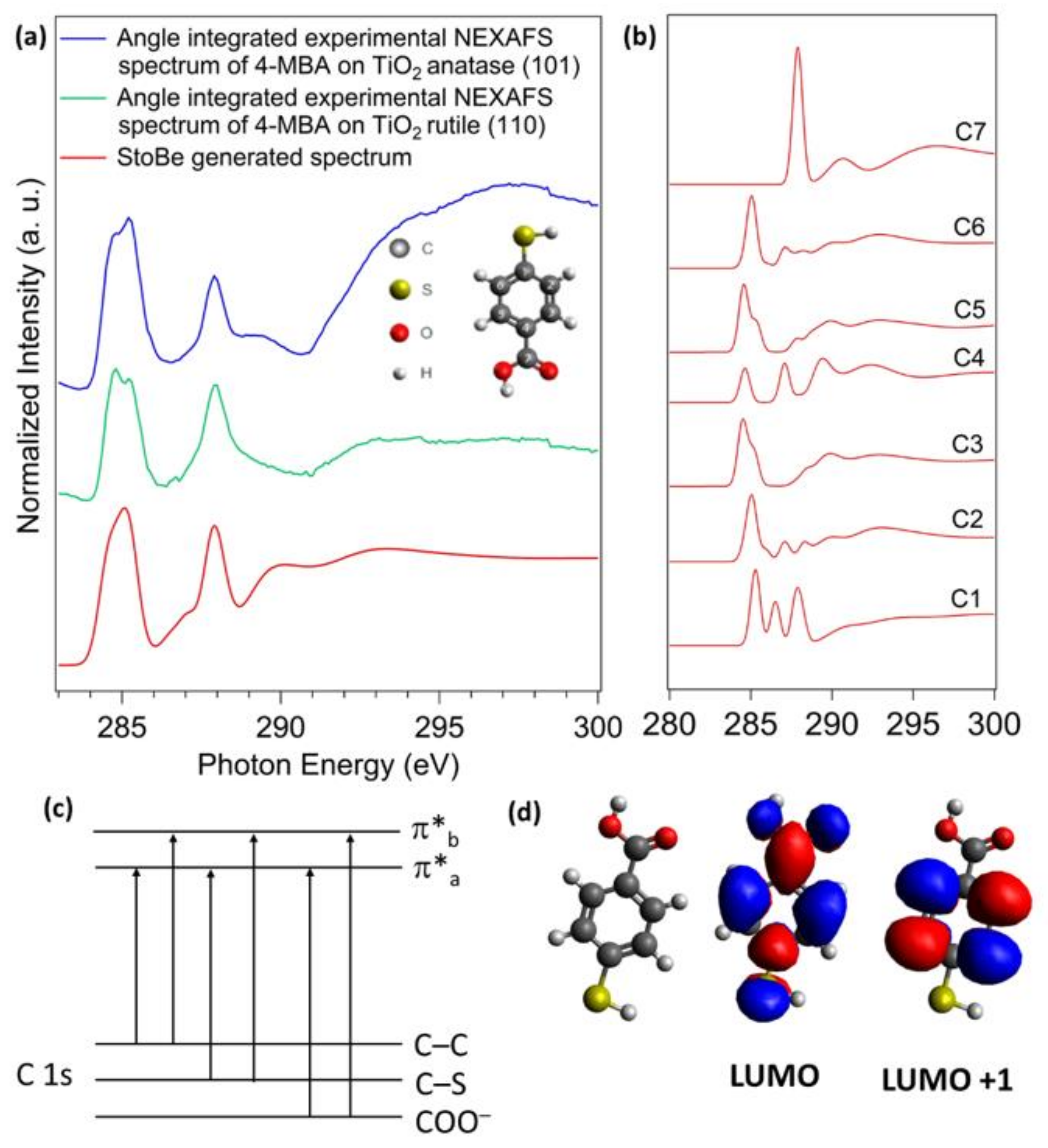
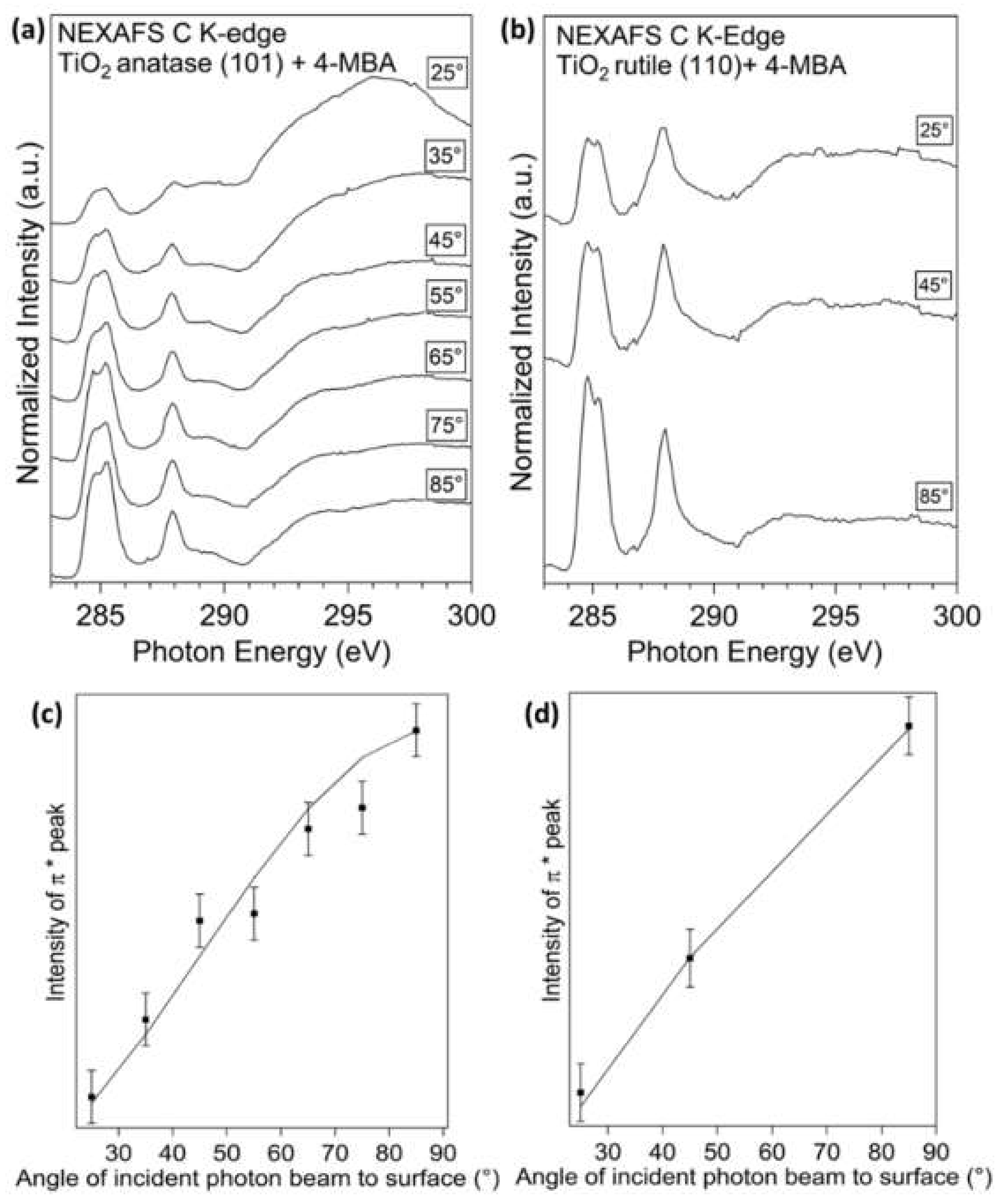
3.3. Near-Edge X-ray Absorption Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fierro, J.L.G. Metal Oxides: Chemistry and Applications; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Limo, M.J.; Sola-Rabada, A.; Boix, E.; Thota, V.; Westcott, Z.C.; Puddu, V.; Perry, C.C. Interactions between Metal Oxides and Biomolecules: From Fundamental Understanding to Applications. Chem. Rev. 2018, 118, 11118–11193. [Google Scholar] [CrossRef] [PubMed]
- Wright, M.; Uddin, A. Organic—Inorganic hybrid solar cells: A comparative review. Sol. Energy Mater. Sol. Cells 2012, 107, 87–111. [Google Scholar] [CrossRef]
- Parayil, S.K.; Baltrusaitis, J.; Wu, C.-M.; Koodali, R.T. Synthesis and characterization of ligand stabilized CdS-Trititanate composite materials for visible light-induced photocatalytic water splitting. Int. J. Hydrogen Energy 2013, 38, 2656–2669. [Google Scholar] [CrossRef]
- Diebold, U. The surface science of titanium dioxide. Surf. Sci. Rep. 2003, 48, 53–229. [Google Scholar] [CrossRef]
- Park, H.; Park, Y.; Kim, W.; Choi, W. Surface modification of TiO2 photocatalyst for environmental applications. J. Photochem. Photobiol. C 2012, 15, 1–20. [Google Scholar] [CrossRef]
- Pang, C.L.; Lindsay, R.; Thornton, G. Chemical reactions on rutile TiO2(110). Chem. Soc. Rev. 2008, 37, 2328–2353. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.G.; Syres, K.L. Adsorption of organic molecules on rutile TiO2 and anatase TiO2 single crystal surfaces. Chem. Soc. Rev. 2012, 41, 4207–4217. [Google Scholar] [CrossRef]
- Moulé, A.J.; Chang, L.; Thambidurai, C.; Vidu, R.; Stroeve, P. Hybrid solar cells: Basic principles and the role of ligands. J. Mater. Chem. 2011, 22, 2351–2368. [Google Scholar] [CrossRef]
- Faure, E.; Falentin-Daudré, C.; Jérôme, C.; Lyskawa, J.; Fournier, D.; Woisel, P.; Detrembleur, C. Catechols as versatile platforms in polymer chemistry. Prog. Polym. Sci. 2012, 38, 236–270. [Google Scholar] [CrossRef]
- Khalid, W.; El Helou, M.; Murböck, T.; Yue, Z.; Montenegro, J.-M.; Schubert, K.; Göbel, G.; Lisdat, F.; Witte, G.; Parak, W.J. Immobilization of Quantum Dots via Conjugated Self-Assembled Monolayers and Their Application as a Light-Controlled Sensor for the Detection of Hydrogen Peroxide. ACS Nano 2011, 5, 9870–9876. [Google Scholar] [CrossRef]
- Becker-Koch, D.; Albaladejo-Siguan, M.; Lami, V.; Paulus, F.; Xiang, H.; Chen, Z.; Vaynzof, Y. Ligand dependent oxidation dictates the performance evolution of high efficiency PbS quantum dot solar cells. Sustain. Energy Fuels 2019, 4, 108–115. [Google Scholar] [CrossRef]
- Beygi, H.; Sajjadi, S.A.; Babakhani, A.; Young, J.F.; van Veggel, F.C. Air exposure oxidation and photooxidation of solution-phase treated PbS quantum dot thin films and solar cells. Sol. Energy Mater. Sol. Cells 2019, 203, 110163. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, C.; Martín-Gago, J. Adsorption and Self-Assembly of Organic Molecules on TiO2 Substrates; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–12. [Google Scholar] [CrossRef]
- Inerbaev, T.M.; Masunov, A.E.; Khondaker, S.I.; Dobrinescu, A.; Plamadă, A.-V.; Kawazoe, Y. Quantum chemistry of quantum dots: Effects of ligands and oxidation. J. Chem. Phys. 2009, 131, 044106. [Google Scholar] [CrossRef]
- Lee, J.R.I.; Willey, T.M.; Nilsson, J.; Terminello, L.J.; De Yoreo, J.J.; van Buuren, T. Effect of Ring Substitution Position on the Structural Conformation of Mercaptobenzoic Acid Self-Assembled Monolayers on Au(111). Langmuir 2006, 22, 11134–11141. [Google Scholar] [CrossRef][Green Version]
- Hou, W.; Setvin, M.; Schmid, M.; Aschauer, U.J.; Diebold, U.; Selloni, A.; Li, Y.-F.; Scheiber, P. Reaction of O2 with Subsurface Oxygen Vacancies on TiO2 Anatase (101). Science 2013, 341, 988–991. [Google Scholar] [CrossRef]
- Quah, E.L.; Wilson, J.N.; Idriss, H. Photoreaction of the Rutile TiO2(011) Single-Crystal Surface: Reaction with Acetic Acid. Langmuir 2010, 26, 6411–6417. [Google Scholar] [CrossRef]
- Syres, K.L.; Thomas, A.G.; Graham, D.M.; Spencer, B.F.; Flavell, W.R.; Jackman, M.J.; Dhanak, V.R. Adsorption and stability of malonic acid on rutile TiO2 (110), studied by near edge X-ray absorption fine structure and photoelectron spectroscopy. Surf. Sci. 2014, 626, 14–20. [Google Scholar] [CrossRef]
- Powell, C.; Jablonski, A. NIST Electron Effective-Attenuation-Lenght Database, SRD82; U.S. Department of Commerce: Gaithersburg, MD, USA, 2011.
- Syres, K.; Thomas, A.; Bondino, F.; Malvestuto, M.; Grätzel, M. Dopamine Adsorption on Anatase TiO2(101): A Photoemission and NEXAFS Spectroscopy Study. Langmuir 2010, 26, 14548–14555. [Google Scholar] [CrossRef]
- Hermann, K.; Pettersson, L. StoBe-deMon Software, Version 2.2 of deMon. 2006.
- Jackman, M.J.; Thomas, A.G.; Muryn, C. Photoelectron Spectroscopy Study of Stoichiometric and Reduced Anatase TiO2(101) Surfaces: The Effect of Subsurface Defects on Water Adsorption at Near-Ambient Pressures. J. Phys. Chem. C 2015, 119, 13682–13690. [Google Scholar] [CrossRef]
- Thomas, A.G.; Jackman, M.J.; Wagstaffe, M.; Radtke, H.; Syres, K.; Adell, J.; Lévy, A.; Martsinovich, N. Adsorption Studies of p-Aminobenzoic Acid on the Anatase TiO2(101) Surface. Langmuir 2014, 30, 12306–12314. [Google Scholar] [CrossRef]
- Schnadt, J.; O’Shea, J.; Patthey, L.; Schiessling, J.; Krempaský, J.; Shi, M.; Mårtensson, N.; Brühwiler, P. Structural study of adsorption of isonicotinic acid and related molecules on rutile TiO2(110) II: XPS. Surf. Sci. 2003, 544, 74–86. [Google Scholar] [CrossRef]
- Hashimoto, S.; Tanaka, A. Alteration of Ti 2p XPS spectrum for titanium oxide by low-energy Ar ion bombardment. Surf. Interface Anal. 2002, 34, 262–265. [Google Scholar] [CrossRef]
- Pang, C.L.; Lindsay, R.; Thornton, G. Structure of Clean and Adsorbate-Covered Single-Crystal Rutile TiO2 Surfaces. Chem. Rev. 2013, 113, 3887–3948. [Google Scholar] [CrossRef] [PubMed]
- Hermann, K.; Pettersson, L.G.M.; Casida, M.E.; Daul, C.; Goursot, A.; Koester, A.; Proynov, E.; St-Amant, A.; Salahub, D.R.; Carravetta, V.; et al. StoBe-deMon, Version 3.3. 2014.
- Neese, F. The ORCA program system. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Neese, F. Software update: The ORCA program system, version 4.0. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2018, 8, e1327. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic che-mical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 17. [Google Scholar]
- Thomas, A.G.; Flavell, W.R.; Kumarasinghe, A.R.; Mallick, A.K.; Tsoutsou, D.; Smith, G.C.; Stockbauer, R.; Patel, S.; Grätzel, M.; Hengerer, R. Resonant photoemission of anatase (formula presented) (101) and (001) single crystals. Phys. Rev.-Condens. Matter Mater. Phys. 2003, 67, 035110. [Google Scholar] [CrossRef]
- Thomas, A.G.; Flavell, W.R.; Mallick, A.K.; Kumarasinghe, A.R.; Tsoutsou, D.; Khan, N.; Chatwin, C.; Rayner, S.; Smith, G.C.; Stockbauer, R.L.; et al. Comparison of the electronic structure of anatase and rutile TiO2 single-crystal surfaces using resonant photoemission and x-ray absorption spectroscopy. Phys. Rev. B. 2007, 75, 035105. [Google Scholar] [CrossRef]
- Jackman, M.J.; Syres, K.L.; Cant, D.J.H.; Hardman, S.J.O.; Thomas, A.G. Adsorption of Dopamine on Rutile TiO2 (110): A Photoemission and Near-Edge X-ray Absorption Fine Structure Study. Langmuir 2014, 30, 8761–8769. [Google Scholar] [CrossRef]
- Stöhr, J. NEXAFS Spectroscopy; Gomer, R.L., Mills, D., Eds.; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1992; Volume 25. [Google Scholar]
- Briggs, D.; Seah, M.P. Practical Surface Analysis (Second Edition), Volume 1—Auger and X-ray Photoelectron Spectroscopy, 2nd ed.; John Wiley and Sons Ltd.: Chichester, UK, 1990. [Google Scholar]
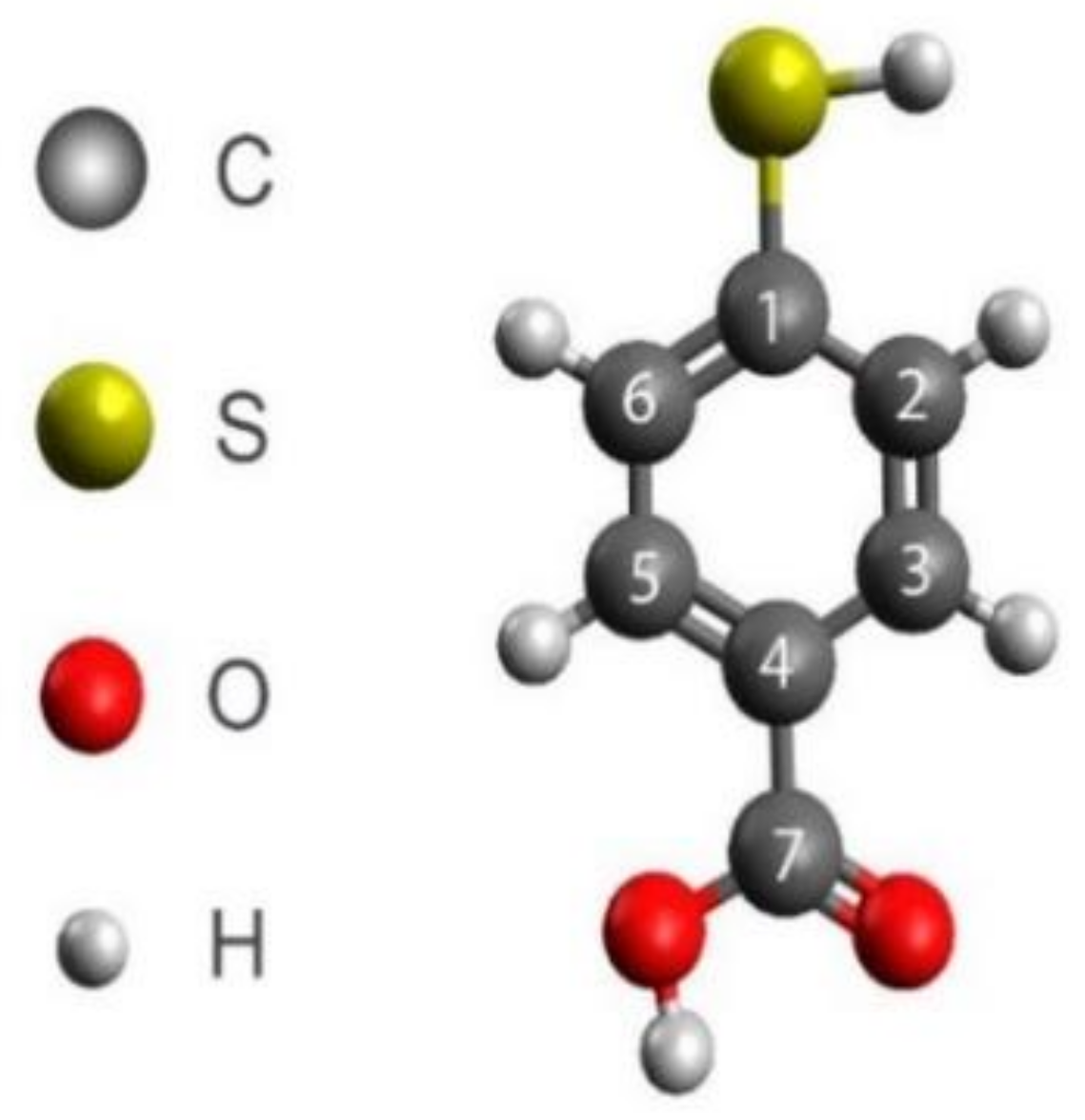
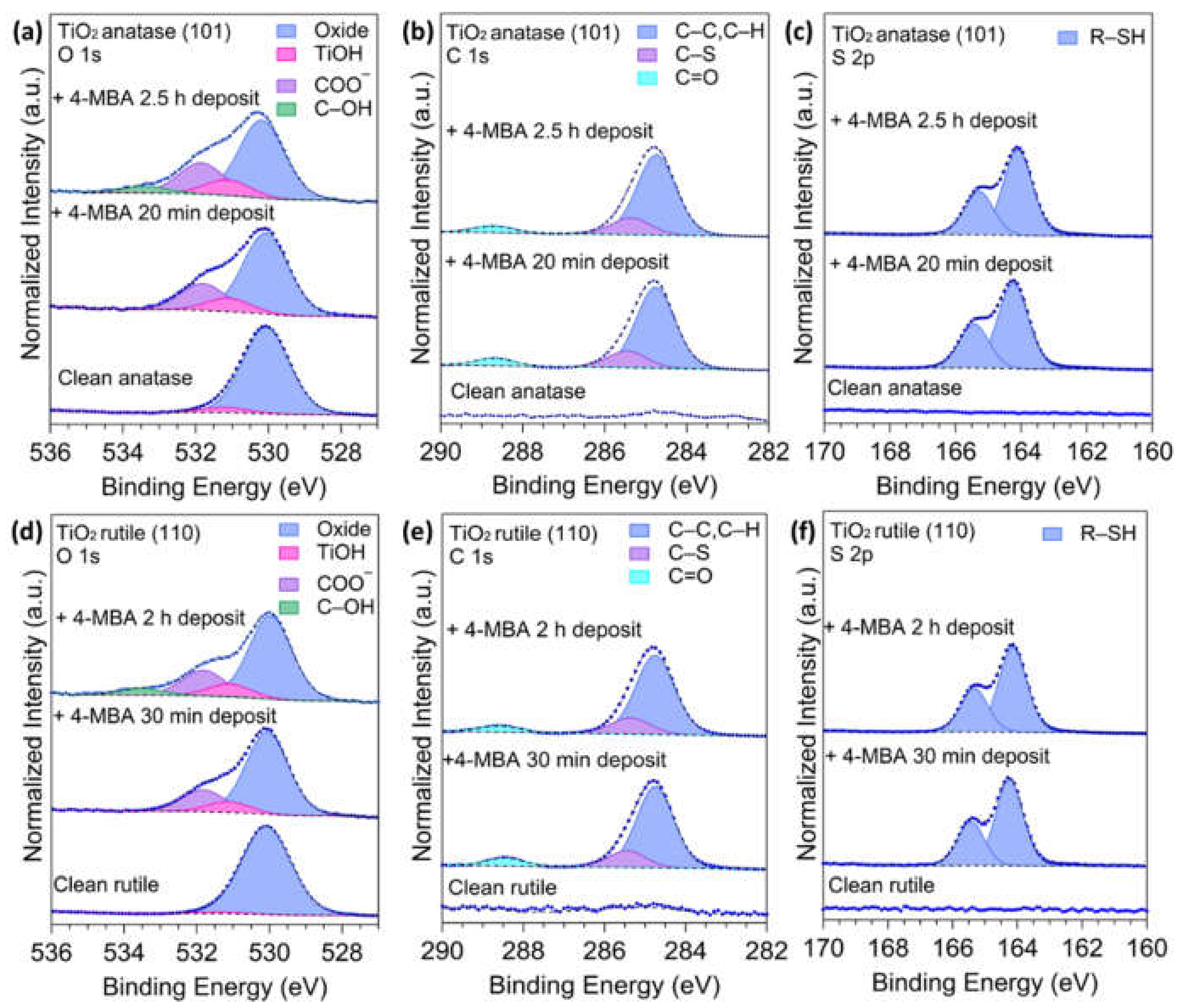
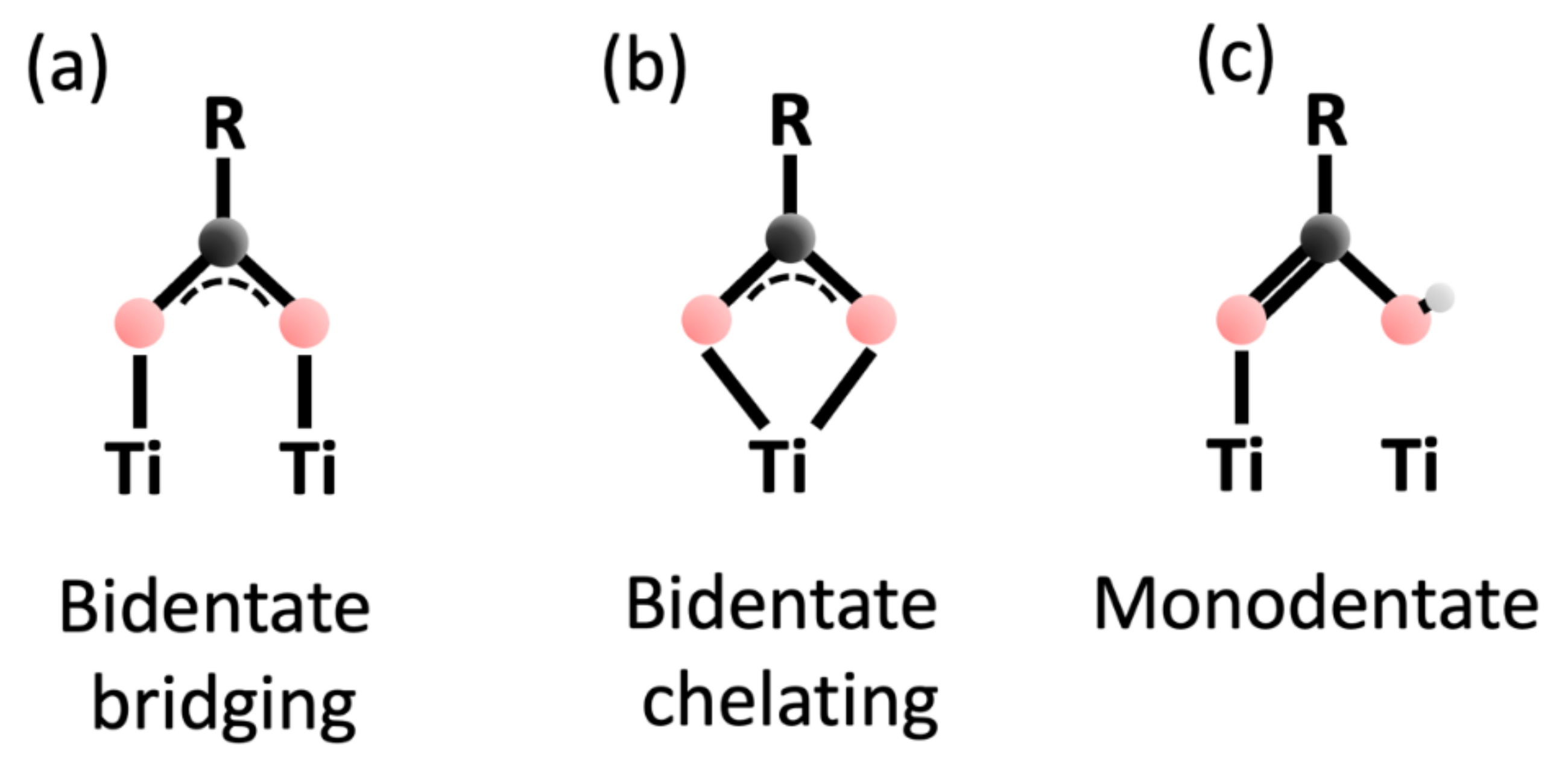
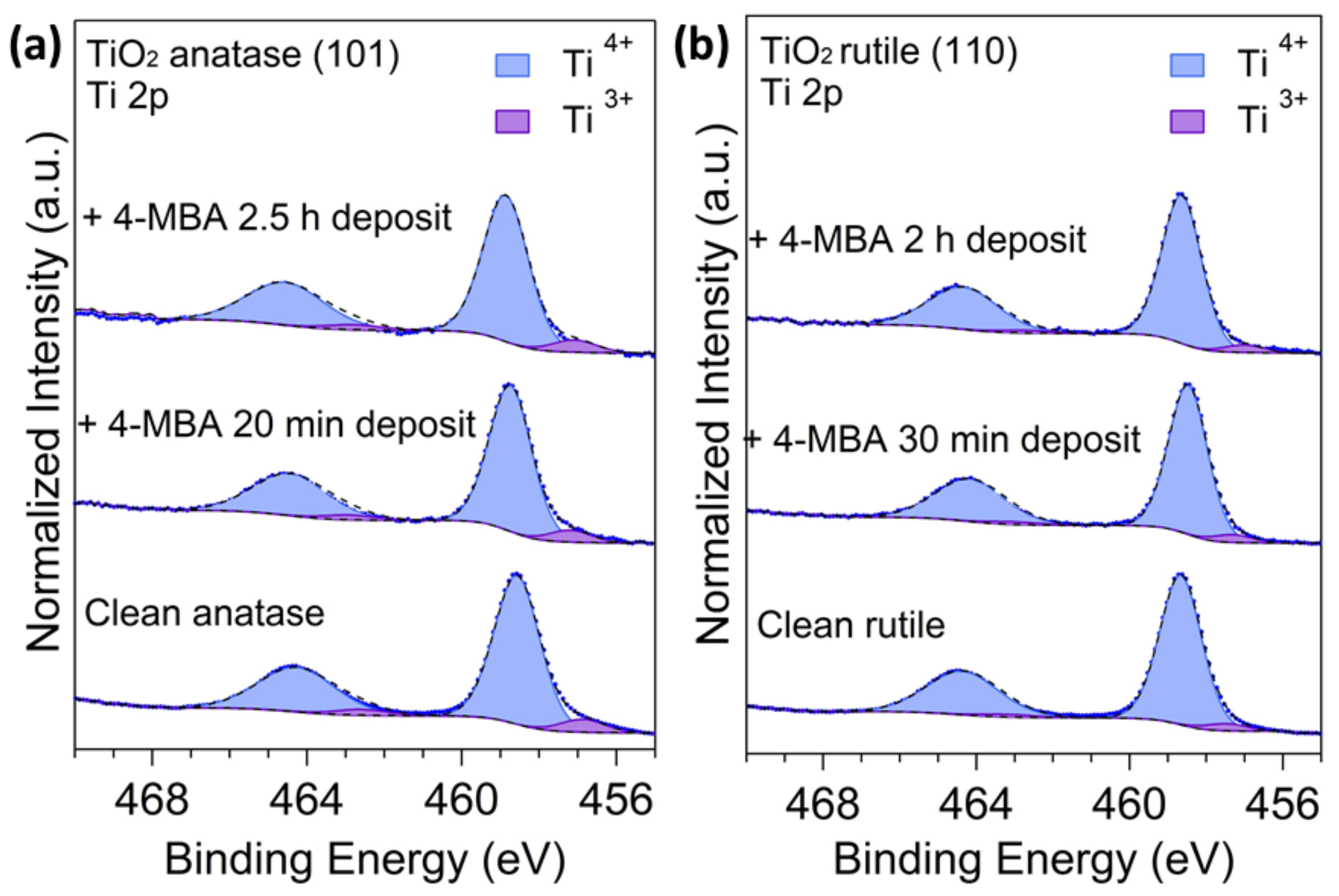
| Species | Assignment | Clean Anatase | 20 min 4-MBA Deposit | 2.5 h 4-MBA Deposit | |||
|---|---|---|---|---|---|---|---|
| BE (eV) ± 0.1 | % | BE (eV) ± 0.1 | % | BE (eV) ± 0.1 | % | ||
| Ti 2p ** | Ti 3+ | 456.9 | 8.1 | 457.2 | 7.6 | 457.1 | 7.8 |
| Ti 4+ | 458.6 | 91.9 | 458.7 | 92.4 | 458.9 | 92.2 | |
| O 1s | O Oxide | 530.1 | 93.8 | 530.0 | 72.1 | 530.2 | 68.9 |
| TiOH * | 531.2 | 6.2 | 531.1 | 9.31 | 531.1 | 8.6 | |
| COO− | 531.8 | 18.6 | 531.8 | 17.5 | |||
| C–OH | - | - | - | - | 533.4 | 5.0 | |
| C 1s | C2–6 (C–C, C–H) | - | - | 284.8 | 77.1 | 284.7 | 77.7 |
| C1 (C–S) | - | - | 285.5 | 15.9 | 285.4 | 16.0 | |
| C7 (C–O) | - | - | 288.7 | 7.0 | 288.7 | 6.3 | |
| S 2p ** | R–SH | - | - | 164.3 | 100 | 164.1 | 100 |
| Species | Assignment | Clean Rutile | 30 min MBA Deposit | 2 h MBA Deposit | |||
|---|---|---|---|---|---|---|---|
| BE (eV) ± 0.1 | % | BE (eV) ± 0.1 | % | BE (eV) ± 0.1 | % | ||
| Ti 2p ** | Ti 3+ | 457.5 | 4.6 | 457.3 | 5.0 | 457.0 | 4.9 |
| Ti 4+ | 458.7 | 95.4 | 458.5 | 95.0 | 458.6 | 95.1 | |
| O 1s | O Oxide | 530.1 | 97.8 | 530.0 | 72.98 | 530.0 | 66.09 |
| TiOH * | 531.4 | 2.2 | 531.1 | 7.58 | 531.1 | 8.01 | |
| COO− | 531.8 | 19.44 | 531.8 | 20.54 | |||
| C–OH | - | - | - | - | 533.5 | 5.37 | |
| C 1s | C2–6 (C–C, C–H) | - | - | 284.8 | 76.6 | 284.8 | 77.9 |
| C1 (C–S) | - | - | 285.4 | 15.9 | 285.4 | 16.1 | |
| C7 (C–O) | - | - | 288.5 | 7.5 | 288.6 | 6.1 | |
| S 2p ** | R–SH | - | - | 164.3 | 96.9 | 164.2 | 98.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Compeán-González, C.L.; Thomas, A.G.; Syres, K.L.; Cole, J.; Li, Z. 4-Mercaptobenzoic Acid Adsorption on TiO2 Anatase (101) and TiO2 Rutile (110) Surfaces. Surfaces 2022, 5, 238-250. https://doi.org/10.3390/surfaces5020017
Compeán-González CL, Thomas AG, Syres KL, Cole J, Li Z. 4-Mercaptobenzoic Acid Adsorption on TiO2 Anatase (101) and TiO2 Rutile (110) Surfaces. Surfaces. 2022; 5(2):238-250. https://doi.org/10.3390/surfaces5020017
Chicago/Turabian StyleCompeán-González, Claudia Lorena, Andrew Guy Thomas, Karen Louise Syres, Jordan Cole, and Zheshen Li. 2022. "4-Mercaptobenzoic Acid Adsorption on TiO2 Anatase (101) and TiO2 Rutile (110) Surfaces" Surfaces 5, no. 2: 238-250. https://doi.org/10.3390/surfaces5020017
APA StyleCompeán-González, C. L., Thomas, A. G., Syres, K. L., Cole, J., & Li, Z. (2022). 4-Mercaptobenzoic Acid Adsorption on TiO2 Anatase (101) and TiO2 Rutile (110) Surfaces. Surfaces, 5(2), 238-250. https://doi.org/10.3390/surfaces5020017








