High-Gas-Barrier and Biodegradable PPC-P/PBAT Composite Films Coated by Poly(vinyl alcohol)/borax Complexes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
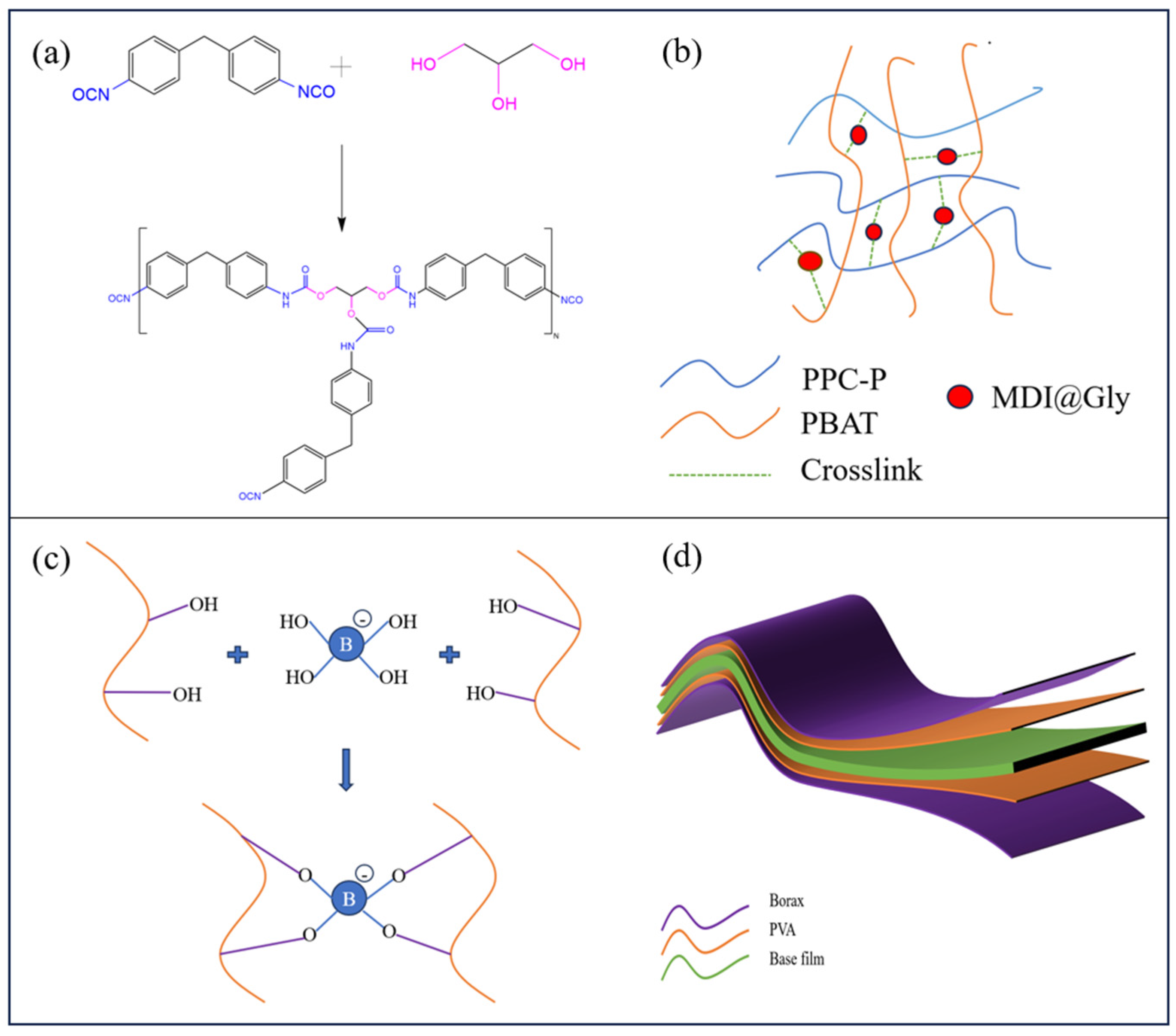
2.2. Preparation of PPC-P/PBAT Blends
2.3. Preparation of the Coating Layer
2.4. Characterization
2.4.1. Mechanical Properties
2.4.2. Thermogravimetry
2.4.3. Thermal Transition Performance
2.4.4. Microscopic Morphology
2.4.5. Optical Properties
2.4.6. Barrier Properties
3. Results
3.1. Mechanical Properties
3.2. Thermal Properties
3.2.1. Analysis of Crystallization and Melting Behavior
3.2.2. Thermal Stability Analysis
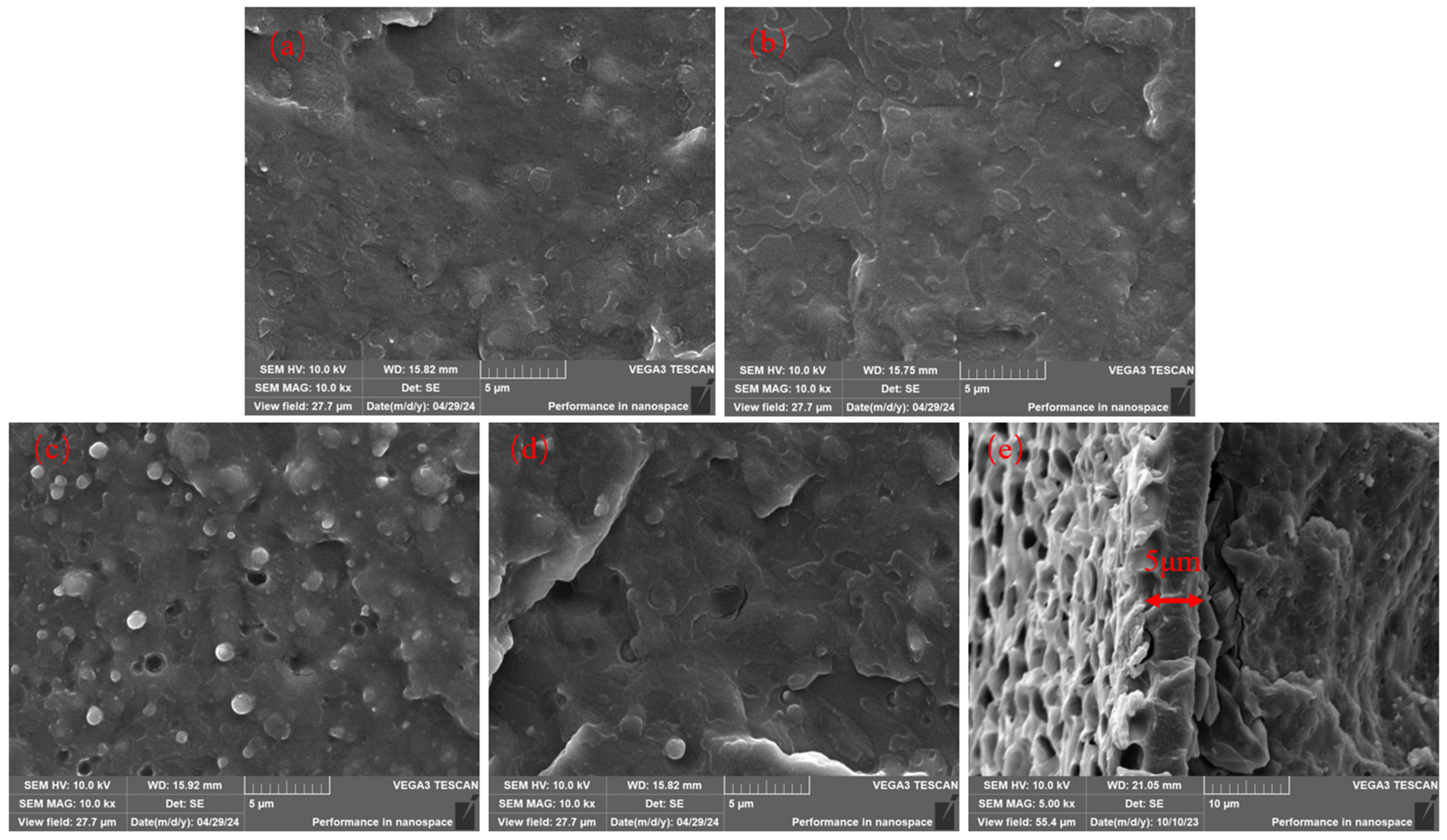
3.3. Microstructure Characterization
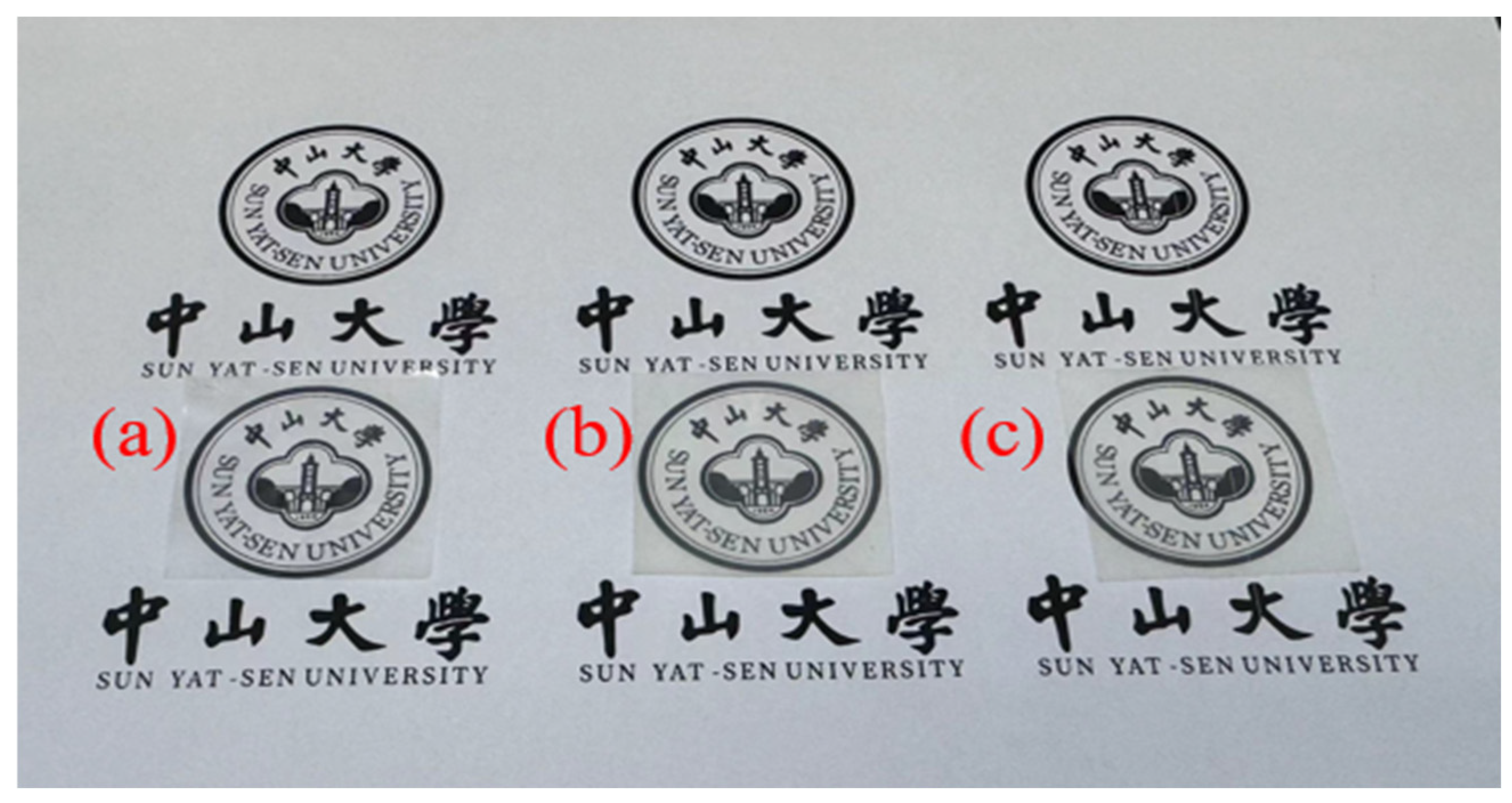
3.4. Optical Properties
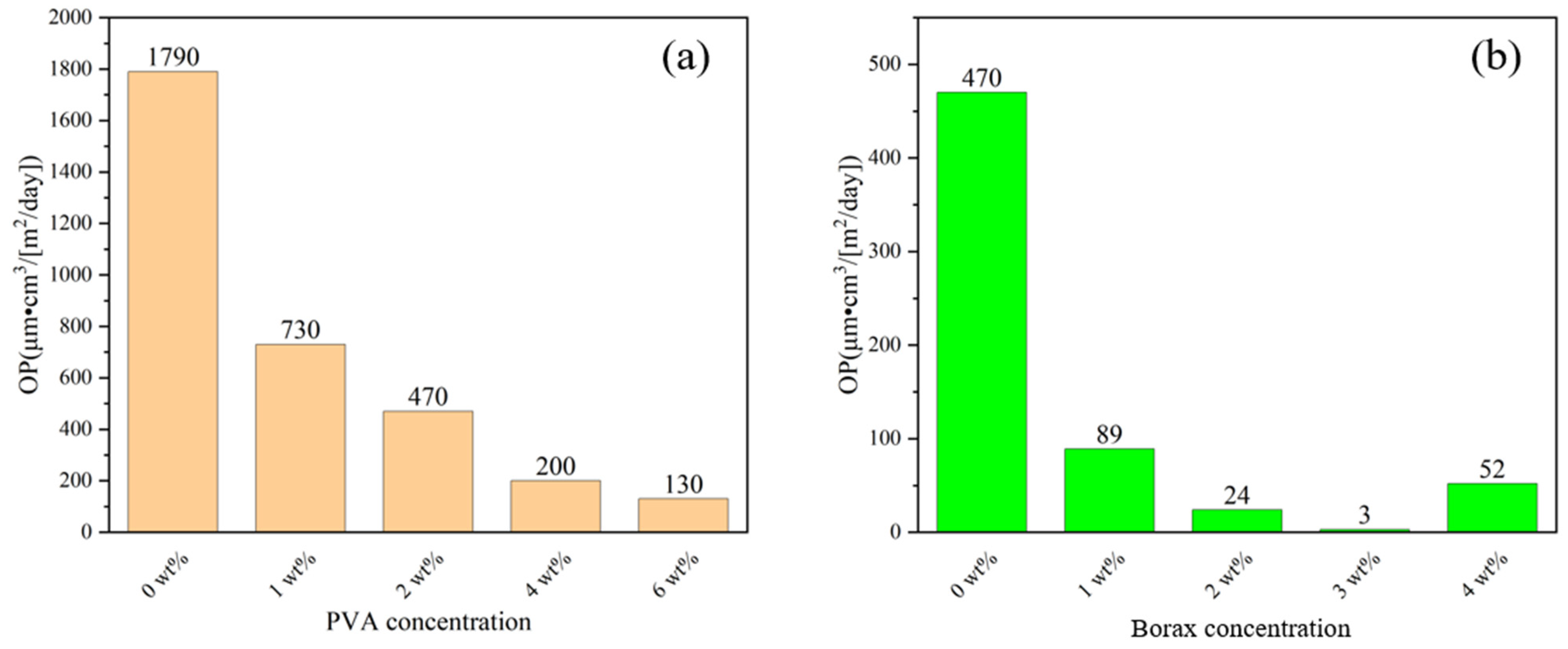
3.5. Barrier Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ribeiro, A.M.; Estevinho, B.N.; Rocha, F. Preparation and Incorporation of Functional Ingredients in Edible Films and Coatings. Food Bioprocess Technol. 2021, 14, 209–231. [Google Scholar] [CrossRef]
- Amin, U.; Khan, M.U.; Majeed, Y.; Rebezov, M.; Khayrullin, M.; Bobkova, E.; Shariati, M.A.; Chung, I.M.; Thiruvengadam, M. Potentials of polysaccharides, lipids and proteins in biodegradable food packaging applications. Int. J. Biol. Macromol. 2021, 183, 2184–2198. [Google Scholar] [CrossRef]
- Hassan, F.; Mu, B.; Yang, Y. Natural polysaccharides and proteins-based films for potential food packaging and mulch applications: A review. Int. J. Biol. Macromol. 2024, 261, 129628. [Google Scholar] [CrossRef] [PubMed]
- Paul-Pont, I.; Ghiglione, J.-F.; Gastaldi, E.; Ter Halle, A.; Huvet, A.; Bruzaud, S.; Lagarde, F.; Galgani, F.; Duflos, G.; George, M.; et al. Discussion about suitable applications for biodegradable plastics regarding their sources, uses and end of life. Waste Manag. 2023, 157, 242–248. [Google Scholar] [CrossRef]
- Samir, A.; Ashour, F.H.; Hakim, A.A.A.; Bassyouni, M. Recent advances in biodegradable polymers for sustainable applications. NPJ Mater. Degrad. 2022, 6, 1–28. [Google Scholar] [CrossRef]
- Ye, S.; Wang, W.; Liang, J.; Wang, S.; Xiao, M.; Meng, Y. Metal-Free Approach for a One-Pot Construction of Biodegradable Block Copolymers from Epoxides, Phthalic Anhydride, and CO2. ACS Sustain. Chem. Eng. 2020, 8, 17860–17867. [Google Scholar] [CrossRef]
- Liang, J.; Ye, S.; Wang, W.; Fan, C.; Wang, S.; Han, D.; Liu, W.; Cui, Y.; Hao, L.; Xiao, M.; et al. Performance tailorable terpolymers synthesized from carbon dioxide, phthalic anhydride and propylene oxide using Lewis acid-base dual catalysts. J. CO2 Util. 2021, 49, 101558. [Google Scholar] [CrossRef]
- Wufuer, R.; Li, W.; Wang, S.; Duo, J. Isolation and Degradation Characteristics of PBAT Film Degrading Bacteria. Int. J. Environ. Res. Public Health IJERPH 2022, 19, 17087. [Google Scholar] [CrossRef]
- Fan, L.; Ma, J.; Liu, W.; Shang, C.; Xie, Y.; Zhou, X.; Zhang, M.; Hou, J.; Feng, Y. A study on the performance, structure, composition, and release behavior changes of polybutylene adipate terephthalic acid (PBAT) film during food contact. J. Hazard. Mater. 2024, 472, 134603. [Google Scholar] [CrossRef]
- Fu, Y.; Wu, G.; Bian, X.; Zeng, J.; Weng, Y. Biodegradation Behavior of Poly(Butylene Adipate-Co-Terephthalate) (PBAT), Poly(Lactic Acid) (PLA), and Their Blend in Freshwater with Sediment. Molecules 2020, 25, 3946. [Google Scholar] [CrossRef]
- Takeno, H.; Shikano, R.; Kikuchi, R. Mechanical Performance of Corn Starch/Poly(Vinyl Alcohol) Composite Hydrogels Reinforced by Inorganic Nanoparticles and Cellulose Nanofibers. Gels 2022, 8, 514. [Google Scholar] [CrossRef] [PubMed]
- Yue, S.; Wang, S.; Han, D.; Huang, S.; Xiao, M.; Meng, Y. Perhydropolysilazane-derived-SiOx coated cellulose: A transparent biodegradable material with high gas barrier property. Cellulose 2022, 29, 8293–8303. [Google Scholar] [CrossRef]
- Zhang, M.; Jiang, C.; Wu, Q.; Zhang, G.; Liang, F.; Yang, Z. Poly(lactic acid)/Poly(butylene succinate) (PLA/PBS) Layered Composite Gas Barrier Membranes by Anisotropic Janus Nanosheets Compartibilizers. ACS Macro Lett. 2022, 11, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Ma, Y.; Li, P.; Zhang, X.; Gao, W.; Wang, B.; Xu, J.; Chen, K. Development of high-barrier composite films for sustainable reduction of non-biodegradable materials in food packaging application. Carbohydr. Polym. 2024, 330, 121824. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Pang, Q.; Zhao, Y.; Li, Y.; Li, F.; He, H. Effects of triglycidyl isocyanurate on thermal, mechanical, and gas barrier properties of poly(butylene adipate-co-terephthalate)/poly(propylene carbonate) composites. J. Appl. Polym. Sci. 2022, 139, e52940. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, X.; Jiang, L.; Qiao, J. Advances in toughened polymer materials by structured rubber particles. Prog. Polym. Sci. 2019, 98, 101160. [Google Scholar] [CrossRef]
- Braz, Á.G.; Pulcinelli, S.H.; Santilli, C.V. Glycerol-based polyurethane-silica organic-inorganic hybrid as an anticorrosive coating. Prog. Org. Coat. 2022, 169, 106939. [Google Scholar] [CrossRef]
- Jiang, G.; Wang, F.; Zhang, S.; Huang, H. Structure and improved properties of PPC/PBAT blends via controlling phase morphology based on melt viscosity. J. Appl. Polym. Sci. 2020, 137, 48924. [Google Scholar] [CrossRef]
- Farrag, Y.; Barral, L.; Gualillo, O.; Moncada, D.; Montero, B.; Rico, M.; Bouza, R. Effect of Different Plasticizers on Thermal, Crystalline, and Permeability Properties of Poly(3–hydroxybutyrate–co−3–hydroxyhexanoate) Films. Polymers 2022, 14, 3503. [Google Scholar] [CrossRef]
- Song, J.; Zhou, H.; Wang, X.; Zhang, Y.; Mi, J. Role of chain extension in the rheological properties, crystallization behaviors, and microcellular foaming performances of poly (butylene adipate-co-terephthalate). J. Appl. Polym. Sci. 2019, 136, 47322. [Google Scholar] [CrossRef]
- Weligama Thuppahige, V.T.; Karim, M.A. A comprehensive review on the properties and functionalities of biodegradable and semibiodegradable food packaging materials. Comp. Rev. Food Sci. Food Safe 2022, 21, 689–718. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Saito, Y.; Yamamoto, T.; Ujihira, Y.; Nomura, K. Correlation Study between Oxygen Permeability and Free Volume of Ethylene−Vinyl Alcohol Copolymer through Positronium Lifetime Measurement. Macromolecules 2001, 34, 6153–6155. [Google Scholar] [CrossRef]
- Ari, B.; Sahiner, M.; Demirci, S.; Sahiner, N. Poly(vinyl alcohol)-tannic Acid Cryogel Matrix as Antioxidant and Antibacterial Material. Polymers 2021, 14, 70. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yu, Q.; Zhang, Y.; Wang, X.; Xu, L.; Wang, J.; Song, P. One-step assembly of poly(vinyl alcohol)/tannic acid complexes as a high oxygen barrier coating for poly(lactic acid) packaging films. Prog. Org. Coat. 2023, 184, 107880. [Google Scholar] [CrossRef]


| PPC-P/PBAT (wt%/wt%) | Tensile Strength (MPa) | Elongation at Break (%) | Young’s Modulus (MPa) |
|---|---|---|---|
| 100/0 | 42.4 | 7.3 | 1650 |
| 75/25 | 35.9 | 9.6 | 605 |
| 75/25/2 a | 33.0 | 435 | 471 |
| 75/25/4 b | 43.1 | 256 | 612 |
| 50/50 | 12.2 | 77 | 382 |
| 25/75 | 11.3 | 437 | 318 |
| 0/100 | 26.4 | 1100 | 225 |
| PPC-P/PBAT (wt%/wt%) | Tg,PBAT (°C) | Tg,PPC-P (°C) | Tm (°C) | ΔHf (J/g) | χc (%) |
|---|---|---|---|---|---|
| 100/0 | - | 54.3 | - | - | - |
| 75/25 | −35.3 | 55.4 | 124.4 | 4.73 | 16.60 |
| 75/25/2 | −28.2 | 57.0 | 102.7 | 2.11 | 7.40 |
| 75/25/4 | −27.7 | 55.6 | 104.2 | 2.43 | 8.53 |
| 50/50 | −32.2 | 56.0 | 118.4 | 8.85 | 15.53 |
| 25/75 | −31.1 | 49.7 | 120.8 | 14.59 | 17.06 |
| 0/100 | −28.7 | - | 115.9 | 24.47 | 21.46 |
| PPC-P/PBAT (wt%/wt%) | Td5% (°C) | Tdmax (°C) | ||
|---|---|---|---|---|
| Tdmax1 | Tdmax2 | Tdmax3 | ||
| 100/0 | 265.1 | 278.5 | 360.7 | - |
| 75/25 | 276.9 | 298.3 | 368.9 | 394.5 |
| 75/25/2 b | 284.3 | 297.6 | 366.5 | 392.2 |
| 75/25/4 c | 281.1 | 293.7 | 367.4 | 392.0 |
| 50/50 | 283.9 | 299.3 | 366.6 | 398.7 |
| 25/75 | 290.4 | 295.6 | 366.0 | 409.1 |
| 0/100 | 370.0 | - | - | 413.4 |
| Films | Transparency (%) | Fog (%) |
|---|---|---|
| 75PPC-P/PBAT | 86.3 | 38.5 |
| 75PPC-P/PBAT/2MDI | 85.3 | 42.9 |
| PVA–borax coating a | 84.9 | 43.2 |
| PPC-P/PBAT (wt%/wt%) | Thickness (μm) | OTR (cm3/[m2/day] | OP (μm·cm3/[m2/day]) | Thickness (μm) | WVTR (g/[m2/day]) | WVP (μm·g/[m2/day]) |
|---|---|---|---|---|---|---|
| 100/0 | 116 | 12 | 1392 | 124 | 0.111 | 13.8 |
| 75/25 | 185 | 17 | 3217 | 174 | 0.190 | 33.2 |
| 75/25/2 | 217 | 8.2 | 1790 | 231 | 0.206 | 47.5 |
| 50/50 | 252 | 68 | 17,136 | 255 | 0.443 | 113 |
| 25/75 | 230 | 128 | 29,440 | 222 | 0.963 | 214 |
| 0/100 | 253 | 180 | 45,540 | 262 | 1.087 | 285 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, J.; Yue, S.; Xiao, M.; Huang, S.; Wang, S.; Han, D.; Meng, Y. High-Gas-Barrier and Biodegradable PPC-P/PBAT Composite Films Coated by Poly(vinyl alcohol)/borax Complexes. Surfaces 2024, 7, 517-528. https://doi.org/10.3390/surfaces7030034
Deng J, Yue S, Xiao M, Huang S, Wang S, Han D, Meng Y. High-Gas-Barrier and Biodegradable PPC-P/PBAT Composite Films Coated by Poly(vinyl alcohol)/borax Complexes. Surfaces. 2024; 7(3):517-528. https://doi.org/10.3390/surfaces7030034
Chicago/Turabian StyleDeng, Jiangtao, Shuangshuang Yue, Min Xiao, Sheng Huang, Shuanjin Wang, Dongmei Han, and Yuezhong Meng. 2024. "High-Gas-Barrier and Biodegradable PPC-P/PBAT Composite Films Coated by Poly(vinyl alcohol)/borax Complexes" Surfaces 7, no. 3: 517-528. https://doi.org/10.3390/surfaces7030034
APA StyleDeng, J., Yue, S., Xiao, M., Huang, S., Wang, S., Han, D., & Meng, Y. (2024). High-Gas-Barrier and Biodegradable PPC-P/PBAT Composite Films Coated by Poly(vinyl alcohol)/borax Complexes. Surfaces, 7(3), 517-528. https://doi.org/10.3390/surfaces7030034







