Evaluation of Photocatalytic Hydrogen Evolution in Zr-Doped TiO2 Thin Films
Abstract
1. Introduction
2. Materials and Methods
2.1. Thin Film Deposition
2.2. Characterization
2.3. Photocatalytic Reactions
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pant, B.; Park, M.; Park, S.-J. Recent Advances in TiO2 Films Prepared by Sol-Gel Methods for Photocatalytic Degradation of Organic Pollutants and Antibacterial Activities. Coatings 2019, 9, 613. [Google Scholar] [CrossRef]
- Alam, M.J.; Cameron, D.C. Preparation and Characterization of TiO2 Thin Films by Sol-Gel Method. J. Solgel Sci. Technol. 2002, 25, 137–145. [Google Scholar] [CrossRef]
- Nalajala, N.; Patra, K.K.; Bharad, P.A.; Gopinath, C.S. Why the Thin Film Form of a Photocatalyst Is Better than the Particulate Form for Direct Solar-to-Hydrogen Conversion: A Poor Man’s Approach. RSC Adv. 2019, 9, 6094–6100. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Gao, F.; Zhao, H.; Li, Y. Tailoring Cu Valence and Oxygen Vacancy in Cu/TiO2catalysts for Enhanced CO2photoreduction Efficiency. Appl. Catal. B 2013, 134–135, 349–358. [Google Scholar] [CrossRef]
- Colón, G.; Maicu, M.; Hidalgo, M.C.; Navío, J.A. Cu-Doped TiO2 Systems with Improved Photocatalytic Activity. Appl. Catal. B 2006, 67, 41–51. [Google Scholar] [CrossRef]
- Zhao, C.; Krall, A.; Zhao, H.; Zhang, Q.; Li, Y. Ultrasonic Spray Pyrolysis Synthesis of Ag/TiO2 Nanocomposite Photocatalysts for Simultaneous H2 Production and CO2 Reduction. Int. J. Hydrogen Energy 2012, 37, 9967–9976. [Google Scholar] [CrossRef]
- Li, H.; Wu, X.; Wang, J.; Gao, Y.; Li, L.; Shih, K. Enhanced Activity of AgMgOTiO2 Catalyst for Photocatalytic Conversion of CO2 and H2O into CH4. Int. J. Hydrogen Energy 2016, 41, 8479–8488. [Google Scholar] [CrossRef]
- Puga, A.V.; Forneli, A.; García, H.; Corma, A. Production of H2 by Ethanol Photoreforming on Au/TiO2. Adv. Funct. Mater. 2014, 24, 241–248. [Google Scholar] [CrossRef]
- Khatun, F.; Abd Aziz, A.; Sim, L.C.; Monir, M.U. Plasmonic Enhanced Au Decorated TiO2 Nanotube Arrays as a Visible Light Active Catalyst towards Photocatalytic CO2 Conversion to CH4. J. Environ. Chem. Eng. 2019, 7, 103233. [Google Scholar] [CrossRef]
- Galin, A.; Walendziewski, J. Photocatalytic Water Splitting over Pt-TiO2 in the Presence of Sacrificial Reagents. Energy Fuels 2005, 19, 1143–1147. [Google Scholar]
- Xiong, Z.; Wang, H.; Xu, N.; Li, H.; Fang, B.; Zhao, Y.; Zhang, J.; Zheng, C. Photocatalytic Reduction of CO2 on Pt2+-Pt0/TiO2 Nanoparticles under UV/Vis Light Irradiation: A Combination of Pt2+ Doping and Pt Nanoparticles Deposition. Int. J. Hydrogen Energy 2015, 40, 10049–10062. [Google Scholar] [CrossRef]
- Luna, A.L.; Dragoe, D.; Wang, K.; Beaunier, P.; Kowalska, E.; Ohtani, B.; Bahena Uribe, D.; Valenzuela, M.A.; Remita, H.; Colbeau-Justin, C. Photocatalytic Hydrogen Evolution Using Ni-Pd/TiO2: Correlation of Light Absorption, Charge-Carrier Dynamics, and Quantum Efficiency. J. Phys. Chem. C 2017, 121, 14302–14311. [Google Scholar] [CrossRef]
- Ong, W.-J.; Gui, M.M.; Chai, S.-P.; Mohamed, A.R. Direct Growth of Carbon Nanotubes on Ni/TiO2 as next Generation Catalysts for Photoreduction of CO2 to Methane by Water under Visible Light Irradiation. RSC Adv. 2013, 3, 4505. [Google Scholar] [CrossRef]
- Bolbol, A.M.; Abd-Elkader, O.H.; Elshimy, H.; Zaki, Z.I.; Shata, S.A.; Kamel, M.; Radwan, A.S.; Mostafa, N.Y. The Effect of Zr (IV) Doping on TiO2 Thin Film Structure and Optical Characteristics. Results Phys. 2022, 42, 105955. [Google Scholar] [CrossRef]
- Juma, A.; Oja Acik, I.; Oluwabi, A.T.; Mere, A.; Mikli, V.; Danilson, M.; Krunks, M. Zirconium Doped TiO2 Thin Films Deposited by Chemical Spray Pyrolysis. Appl. Surf. Sci. 2016, 387, 539–545. [Google Scholar] [CrossRef]
- Oluwabi, A.T.; Juma, A.O.; Oja Acik, I.; Mere, A.; Krunks, M. Effect of Zr Doping on the Structural and Electrical Properties of Spray Deposited TiO2 Thin Films. Proc. Est. Acad. Sci. 2018, 67, 147. [Google Scholar] [CrossRef]
- Mbiri, A.; Taffa, D.H.; Gatebe, E.; Wark, M. Zirconium Doped Mesoporous TiO2 Multilayer Thin Films: Influence of the Zirconium Content on the Photodegradation of Organic Pollutants. Catal. Today 2019, 328, 71–78. [Google Scholar] [CrossRef]
- Taechasirivichai, K.; Chiarakorn, S.; Chawengkijwanich, C.; Pongprayoon, T.; Chuangchote, S. Ceramic Tiles Coated with Zr-Ag Co-doped TiO2 Thin Film for Indoor Air Purifying and Antimicrobial Applications. Int. J. Appl. Ceram. Technol. 2022, 20, 2019–2029. [Google Scholar] [CrossRef]
- Song, J.; Wang, X.; Yan, J.; Yu, J.; Sun, G.; Ding, B. Soft Zr-Doped TiO2 Nanofibrous Membranes with Enhanced Photocatalytic Activity for Water Purification. Sci. Rep. 2017, 7, 1636. [Google Scholar] [CrossRef]
- Schiller, R.; Weiss, C.K.; Landfester, K. Phase Stability and Photocatalytic Activity of Zr-Doped Anatase Synthesized in Miniemulsion. Nanotechnology 2010, 21, 405603. [Google Scholar] [CrossRef]
- Bathula, C.; Nissimagoudar, A.S.; Kumar, S.; Jana, A.; Sekar, S.; Lee, S.; Kim, H.-S. Enhanced Photodegradation of Methylene Blue by Zr-Doped TiO2:A Combined DFT and Experimental Investigation. Inorg. Chem. Commun. 2024, 164, 112442. [Google Scholar] [CrossRef]
- Wang, C.; Geng, A.; Guo, Y.; Jiang, S.; Qu, X.; Li, L. A Novel Preparation of Three-Dimensionally Ordered Macroporous M/Ti (M=Zr or Ta) Mixed Oxide Nanoparticles with Enhanced Photocatalytic Activity. J. Colloid Interface Sci. 2006, 301, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Divya, G.; Jaishree, G.; Sivarao, T.; Lakshmi, K.V.D. Microwave Assisted Sol–Gel Approach for Zr Doped TiO2 as a Benign Photocatalyst for Bismark Brown Red Dye Pollutant. RSC Adv. 2023, 13, 8692–8705. [Google Scholar] [CrossRef]
- Liao, X.; Ren, H.-T.; Shen, B.; Lin, J.-H.; Lou, C.-W.; Li, T.-T. Enhancing Mechanical and Photocatalytic Properties by Surface Microstructure Regulation of TiO2 Nanofiber Membranes. Chemosphere 2023, 313, 137195. [Google Scholar] [CrossRef] [PubMed]
- Lukáč, J.; Klementová, M.; Bezdička, P.; Bakardjieva, S.; Šubrt, J.; Szatmáry, L.; Bastl, Z.; Jirkovský, J. Influence of Zr as TiO2 Doping Ion on Photocatalytic Degradation of 4-Chlorophenol. Appl. Catal. B 2007, 74, 83–91. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Mondal, S.; De, G. Hierarchical Ti1−xZrxO2−y Nanocrystals with Exposed High Energy Facets Showing Co-Catalyst Free Solar Light Driven Water Splitting and Improved Light to Energy Conversion Efficiency. J. Mater. Chem. A Mater. 2017, 5, 17341–17351. [Google Scholar] [CrossRef]
- Pahi, S.; Sahu, S.; Singh, S.K.; Behera, A.; Patel, R.K. Visible Light Active Zr- and N-Doped TiO2 Coupled g-C3N4 Heterojunction Nanosheets as a Photocatalyst for the Degradation of Bromoxynil and Rh B along with the H 2 Evolution Process. Nanoscale Adv. 2021, 3, 6468–6481. [Google Scholar] [CrossRef]
- Barba-Nieto, I.; Caudillo-Flores, U.; Gómez-Cerezo, M.N.; Kubacka, A.; Fernández-García, M. Boosting Pt/TiO2 Hydrogen Photoproduction through Zr Doping of the Anatase Structure: A Spectroscopic and Mechanistic Study. Chem. Eng. J. 2020, 398, 125665. [Google Scholar] [CrossRef]
- Pérez-Larios, A.; Rico, J.L.; Anaya-Esparza, L.M.; Vargas, O.A.G.; González-Silva, N.; Gómez, R. Hydrogen Production from Aqueous Methanol Solutions Using Ti–Zr Mixed Oxides as Photocatalysts under UV Irradiation. Catalysts 2019, 9, 938. [Google Scholar] [CrossRef]
- Li, Y.; Xiang, Y.; Peng, S.; Wang, X.; Zhou, L. Modification of Zr-Doped Titania Nanotube Arrays by Urea Pyrolysis for Enhanced Visible-Light Photoelectrochemical H2 Generation. Electrochim. Acta 2013, 87, 794–800. [Google Scholar] [CrossRef]
- Touam, T.; Atoui, M.; Hadjoub, I.; Chelouche, A.; Boudine, B.; Fischer, A.; Boudrioua, A.; Doghmane, A. Effects of Dip-Coating Speed and Annealing Temperature on Structural, Morphological and Optical Properties of Sol-Gel Nano-Structured TiO2 Thin Films. Eur. Phys. J. Appl. Phys. 2014, 67, 30302. [Google Scholar] [CrossRef]
- Oh, S.H.; Kim, D.J.; Hahn, S.H.; Kim, E.J. Comparison of Optical and Photocatalytic Properties of TiO2 Thin Films Prepared by Electron-Beam Evaporation and Sol–Gel Dip-Coating. Mater. Lett. 2003, 57, 4151–4155. [Google Scholar] [CrossRef]
- Ranjitha, A.; Muthukumarasamy, N.; Thambidurai, M.; Balasundaraprabhu, R.; Agilan, S. Effect of Annealing Temperature on Nanocrystalline TiO2 Thin Films Prepared by Sol–Gel Dip Coating Method. Optik 2013, 124, 6201–6204. [Google Scholar] [CrossRef]
- Johari, N.D.; Rosli, Z.M.; Juoi, J.M.; Yazid, S.A. Comparison on the TiO2 Crystalline Phases Deposited via Dip and Spin Coating Using Green Sol–Gel Route. J. Mater. Res. Technol. 2019, 8, 2350–2358. [Google Scholar] [CrossRef]
- Teeparthi, S.R.; Awin, E.W.; Kumar, R. Dominating Role of Crystal Structure over Defect Chemistry in Black and White Zirconia on Visible Light Photocatalytic Activity. Sci. Rep. 2018, 8, 5541. [Google Scholar] [CrossRef] [PubMed]
- Shaddad, M.N.; Cardenas-Morcoso, D.; García-Tecedor, M.; Fabregat-Santiago, F.; Bisquert, J.; Al-Mayouf, A.M.; Gimenez, S. TiO2 Nanotubes for Solar Water Splitting: Vacuum Annealing and Zr Doping Enhance Water Oxidation Kinetics. ACS Omega 2019, 4, 16095–16102. [Google Scholar] [CrossRef] [PubMed]
- Godoy Junior, A.; Pereira, A.; Gomes, M.; Fraga, M.; Pessoa, R.; Leite, D.; Petraconi, G.; Nogueira, A.; Wender, H.; Miyakawa, W.; et al. Black TiO2 Thin Films Production Using Hollow Cathode Hydrogen Plasma Treatment: Synthesis, Material Characteristics and Photocatalytic Activity. Catalysts 2020, 10, 282. [Google Scholar] [CrossRef]
- Flores-Caballero, A.A.; Manzo-Robledo, A.; Alonso-Vante, N. The Cerium/Boron Insertion Impact in Anatase Nano-Structures on the Photo-Electrochemical and Photocatalytic Response. Surfaces 2021, 4, 54–65. [Google Scholar] [CrossRef]
- Naumenko, A.; Gnatiuk, I.; Smirnova, N.; Eremenko, A. Characterization of Sol–Gel Derived TiO2/ZrO2 Films and Powders by Raman Spectroscopy. Thin Solid Film. 2012, 520, 4541–4546. [Google Scholar] [CrossRef]
- Ji, C.H.; Kim, K.T.; Oh, S.Y. High-Detectivity Perovskite-Based Photodetector Using a Zr-Doped TiOx Cathode Interlayer. RSC Adv. 2018, 8, 8302–8309. [Google Scholar] [CrossRef]
- Bensouici, F.; Bououdina, M.; Dakhel, A.A.; Souier, T.; Tala-Ighil, R.; Toubane, M.; Iratni, A.; Liu, S.; Cai, W. Al Doping Effect on the Morphological, Structural and Photocatalytic Properties of TiO2 Thin Layers. Thin Solid Film. 2016, 616, 655–661. [Google Scholar] [CrossRef]
- Lee, S.-M.; Joo, Y.-H.; Kim, C.-I. Influences of Film Thickness and Annealing Temperature on Properties of Sol–Gel Derived ZnO–SnO2 Nanocomposite Thin Film. Appl. Surf. Sci. 2014, 320, 494–501. [Google Scholar] [CrossRef]
- Nakanishi, Y.; Kato, K.; Horikawa, M.; Yonekura, M. Influence of Zn-Sn Ratio on Optical Property and Microstructure of Zn-Sn-O Films Deposited by Magnetron Sputtering. Thin Solid Film. 2016, 612, 231–236. [Google Scholar] [CrossRef]
- Sanchez-Martinez, A.; Koop-Santa, C.; Ceballos-Sanchez, O.; López-Mena, E.R.; González, M.A.; Rangel-Cobián, V.; Orozco-Guareño, E.; García-Guaderrama, M. Study of the Preparation of TiO2 Powder by Different Synthesis Methods. Mater. Res. Express 2019, 6, 085085. [Google Scholar] [CrossRef]
- Haryński, Ł.; Olejnik, A.; Grochowska, K.; Siuzdak, K. A Facile Method for Tauc Exponent and Corresponding Electronic Transitions Determination in Semiconductors Directly from UV–Vis Spectroscopy Data. Opt. Mater. 2022, 127, 112205. [Google Scholar] [CrossRef]
- Schubert, E.F. Physical Foundations of Solid-State Devices, 2022nd ed.; E. Fred Schubert: New York, NY, USA, 2020. [Google Scholar]
- Fang, D.; Huang, K.; Liu, S.; Huang, J. Fabrication and Photoluminiscent Properties of Titanium Oxide Nanotube Arrays. J. Braz. Chem. Soc. 2008, 19, 1059–1064. [Google Scholar] [CrossRef]
- Méndez-López, A.; Zelaya-Ángel, O.; Toledano-Ayala, M.; Torres-Pacheco, I.; Pérez-Robles, J.F.; Acosta-Silva, Y.J. The Influence of Annealing Temperature on the Structural and Optical Properties of ZrO2 Thin Films and How Affects the Hydrophilicity. Crystals 2020, 10, 454. [Google Scholar] [CrossRef]
- Nabi, G.; Raza, W.; Tahir, M.B. Green Synthesis of TiO2 Nanoparticle Using Cinnamon Powder Extract and the Study of Optical Properties. J. Inorg. Organomet. Polym. Mater. 2020, 30, 1425–1429. [Google Scholar] [CrossRef]
- Komaraiah, D.; Radha, E.; Sivakumar, J.; Ramana Reddy, M.V.; Sayanna, R. Photoluminescence and Photocatalytic Activity of Spin Coated Ag+ Doped Anatase TiO2 Thin Films. Opt. Mater. 2020, 108, 110401. [Google Scholar] [CrossRef]
- Hou, L.; Zhang, M.; Guan, Z.; Li, Q.; Yang, J. Effect of Annealing Ambience on the Formation of Surface/Bulk Oxygen Vacancies in TiO2 for Photocatalytic Hydrogen Evolution. Appl. Surf. Sci. 2018, 428, 640–647. [Google Scholar] [CrossRef]
- Goswami, P.; Ganguli, J.N. Tuning the Band Gap of Mesoporous Zr-Doped TiO2 for Effective Degradation of Pesticide Quinalphos. Dalton Trans. 2013, 42, 14480. [Google Scholar] [CrossRef] [PubMed]
- Tudu, B.; Nalajala, N.; Saikia, P.; Gopinath, C.S. Cu–Ni Bimetal Integrated TiO2 Thin Film for Enhanced Solar Hydrogen Generation. Sol. RRL 2020, 4, 1900557. [Google Scholar] [CrossRef]
- Cruz, M.R.A.; Sanchez-Martinez, D.; Torres-Martínez, L.M. Optical Properties of TiO2 Thin Films Deposited by DC Sputtering and Their Photocatalytic Performance in Photoinduced Process. Int. J. Hydrogen Energy 2019, 44, 20017–20028. [Google Scholar] [CrossRef]
- Alfaro Cruz, M.R.; Sanchez-Martinez, D.; Torres-Martínez, L.M. TiO2 Nanorods Grown by Hydrothermal Method and Their Photocatalytic Activity for Hydrogen Production. Mater. Lett. 2019, 237, 310–313. [Google Scholar] [CrossRef]
- Zdorovets, M.; Kozlovskiy, A.; Tishkevich, D.; Zubar, T.; Trukhanov, A. The Effect of Doping of TiO2 Thin Films with Low-Energy O2+ Ions on Increasing the Efficiency of Hydrogen Evolution in Photocatalytic Reactions of Water Splitting. J. Mater. Sci. Mater. Electron. 2020, 31, 21142–21153. [Google Scholar] [CrossRef]
- Khan, M.A.; Sinatra, L.; Oufi, M.; Bakr, O.M.; Idriss, H. Evidence of Plasmonic Induced Photocatalytic Hydrogen Production on Pd/TiO2 Upon Deposition on Thin Films of Gold. Catal. Lett. 2017, 147, 811–820. [Google Scholar] [CrossRef]
- Naseri, N.; Kim, H.; Choi, W.; Moshfegh, A.Z. Optimal Ag Concentration for H2 Production via Ag:TiO2 Nanocomposite Thin Film Photoanode. Int. J. Hydrogen Energy 2012, 37, 3056–3065. [Google Scholar] [CrossRef]
- Alenzi, N.; Liao, W.-S.; Cremer, P.S.; Sanchez-Torres, V.; Wood, T.K.; Ehlig-Economides, C.; Cheng, Z. Photoelectrochemical Hydrogen Production from Water/Methanol Decomposition Using Ag/TiO2 Nanocomposite Thin Films. Int. J. Hydrogen Energy 2010, 35, 11768–11775. [Google Scholar] [CrossRef]
- Dholam, R.; Patel, N.; Santini, A.; Miotello, A. Efficient Indium Tin Oxide/Cr-Doped-TiO2 Multilayer Thin Films for H2 Production by Photocatalytic Water-Splitting. Int. J. Hydrogen Energy 2010, 35, 9581–9590. [Google Scholar] [CrossRef]
- Liu, E.; Kang, L.; Yang, Y.; Sun, T.; Hu, X.; Zhu, C.; Liu, H.; Wang, Q.; Li, X.; Fan, J. Plasmonic Ag Deposited TiO2 Nano-Sheet Film for Enhanced Photocatalytic Hydrogen Production by Water Splitting. Nanotechnology 2014, 25, 165401. [Google Scholar] [CrossRef]
- Strataki, N.; Lianos, P. Optimization of Parameters for Hydrogen Production by Photocatalytic Alcohol Reforming in the Presence of Pt/TiO2 Nanocrystalline Thin Films. J. Adv. Oxid. Technol. 2008, 11, 111–115. [Google Scholar] [CrossRef]
- Dholam, R.; Patel, N.; Adami, M.; Miotello, A. Physically and Chemically Synthesized TiO2 Composite Thin Films for Hydrogen Production by Photocatalytic Water Splitting. Int. J. Hydrogen Energy 2008, 33, 6896–6903. [Google Scholar] [CrossRef]
- Ibarra-Rodríguez, L.I.; Garay-Rodríguez, L.F.; Hernández-Majalca, B.C.; Domínguez-Arvizu, J.L.; López-Ortiz, A.; Torres-Martínez, L.M.; Collins-Martínez, V.H. N–TiO2/MO (M: Ni, Cu) Films for Hydrogen Production Using Visible Light. Int. J. Hydrogen Energy, 2024; in press. [Google Scholar] [CrossRef]
- Méndez, F.J.; Barrón-Romero, D.; Pérez, O.; Flores-Cruz, R.D.; Rojas-Challa, Y.; García-Macedo, J.A. A Highly Efficient and Recyclable Pt/TiO2 Thin Film Photocatalytic System for Sustainable Hydrogen Production. Mater. Chem. Phys. 2023, 305, 127925. [Google Scholar] [CrossRef]
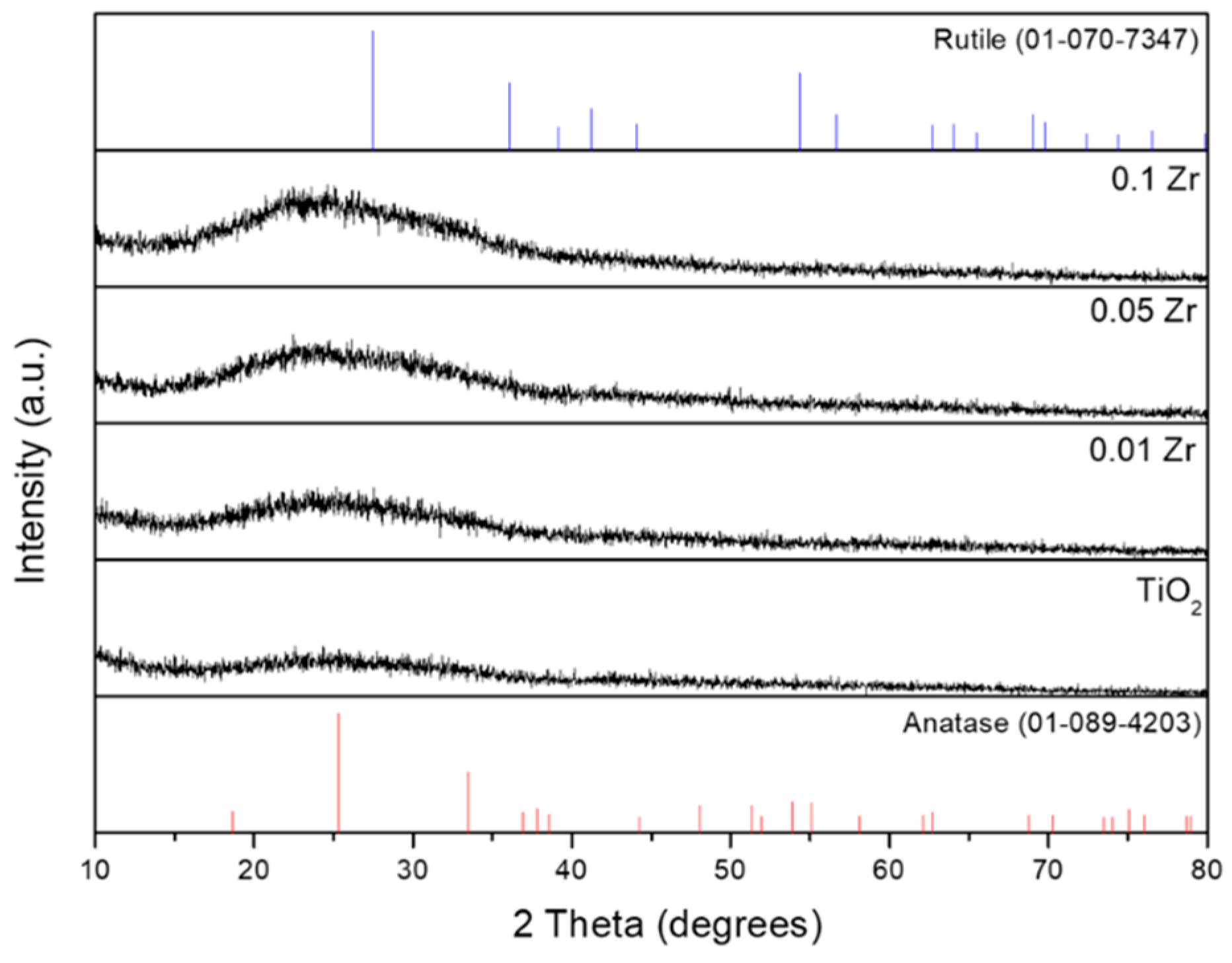

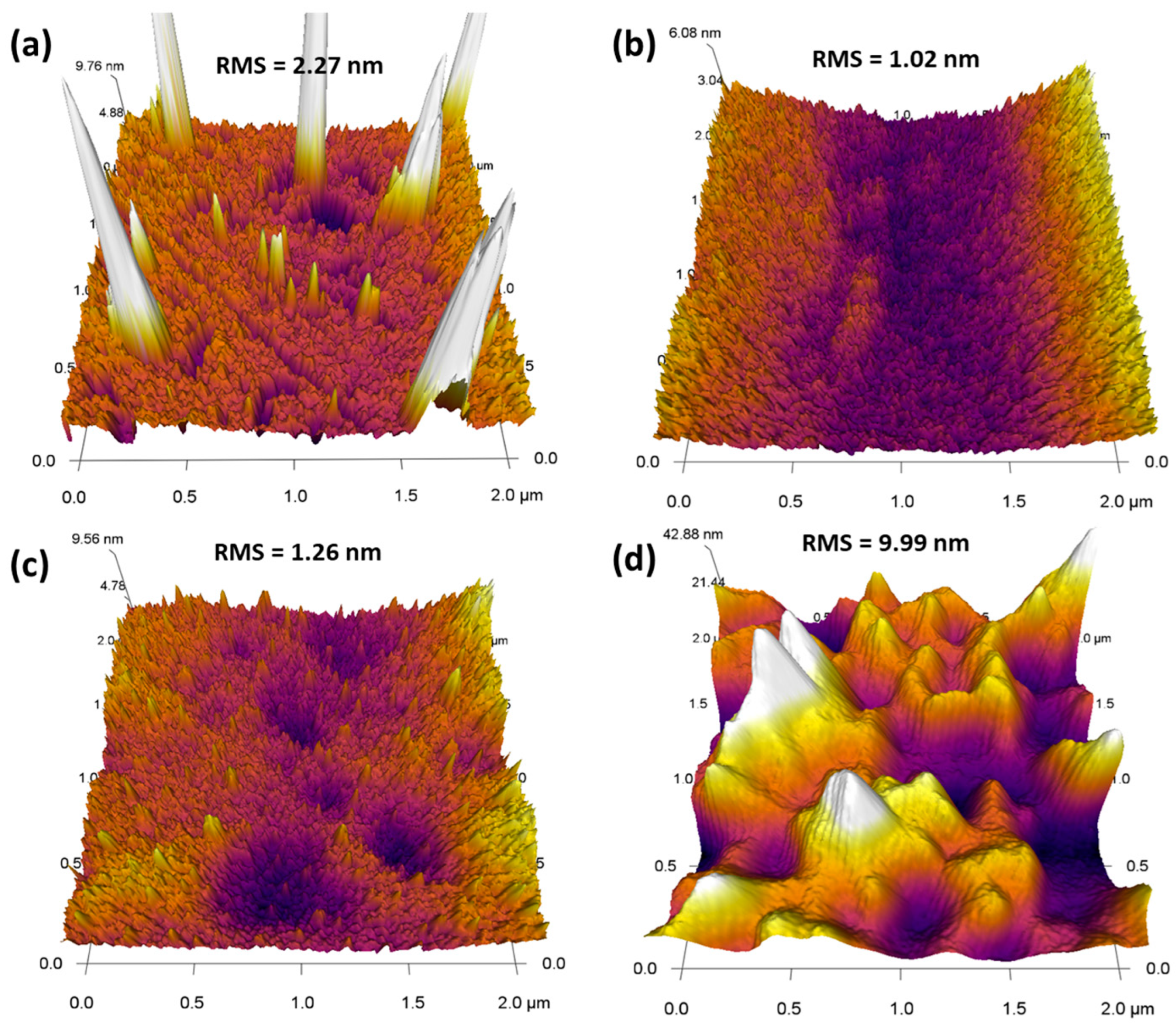
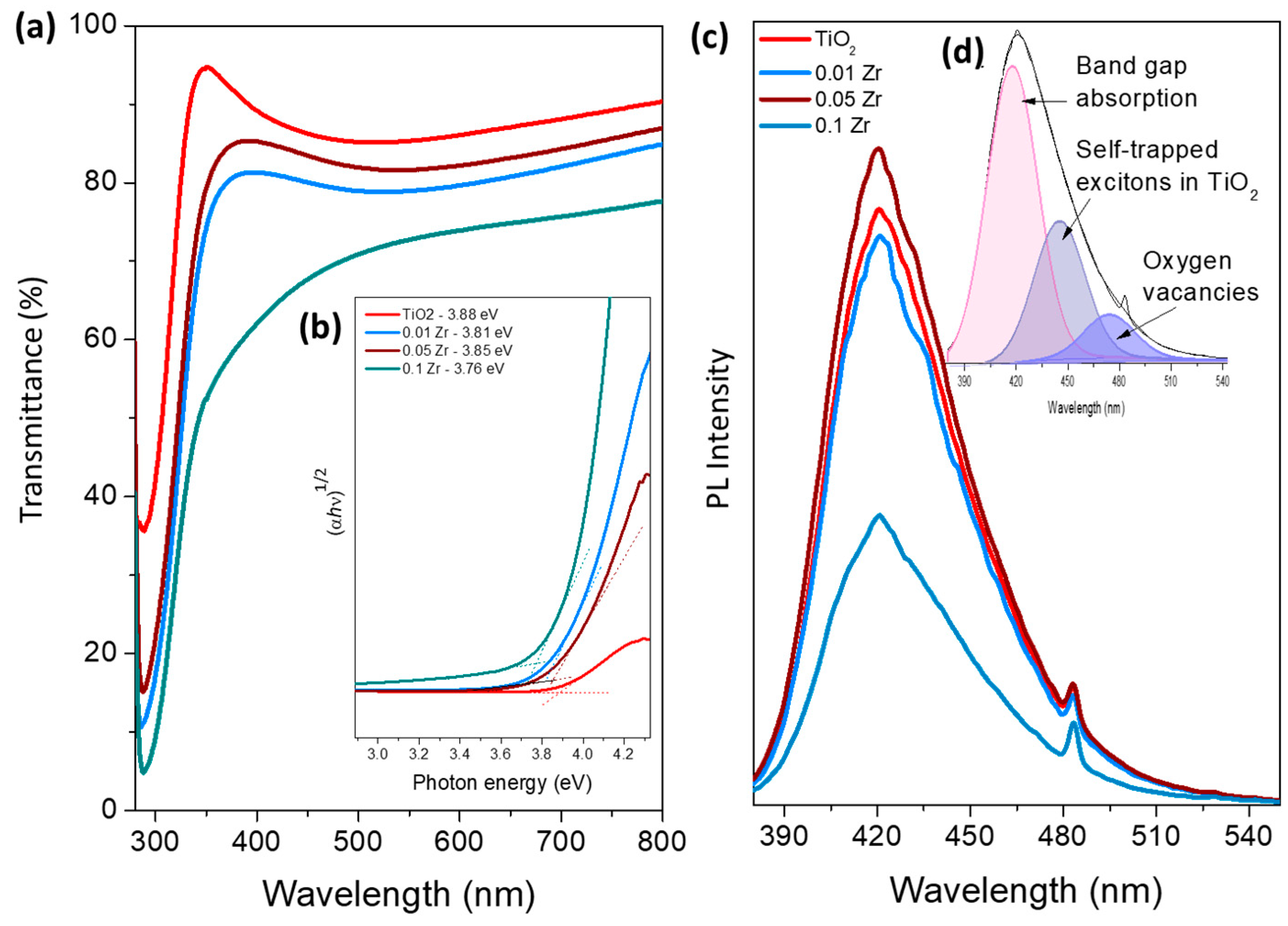

| Photocatalyst | Dopant | Deposition Method | Illumination | Sacrificial Agent | Hydrogen Production | Ref. |
|---|---|---|---|---|---|---|
| TiO2 | Cu-Ni | Drop casting | 300 W Xe lamp | Methanol 25% | 41,690 μmol/gh | [53] |
| TiO2 | -- | DC sputtering | UV 254 nm lamp | -- | 38 μmol | [54] |
| TiO2 | -- | Hydrothermal | UV 254 nm lamp | -- | 132 μmol | [55] |
| TiO2 | -- | RF sputtering | 300 W Xe lamp | -- | 0.55 μmol/hcm2 | [56] |
| TiO2 | Au-Pd | Spin coating | 300 W Xe lamp | Glycerol 5% | 0.014 mL/min | [57] |
| TiO2 | Ag | Sol–gel/dip coating | 5000 W Xe lamp | KOH 1 M | 580 μmol | [58] |
| TiO2 | Ag | Drop casting | 420 W Hg lamp | Water/methanol 1:1 | 148 μmol/gh | [59] |
| TiO2 | Cr | RF sputtering | 250 W W lamp | NaOH 1 M | 24 μmol/h | [60] |
| TiO2 | Ag | Hydrothermal | 16 W Hg lamp | Ethanol 10% | 8.1 μmol/cm2 | [61] |
| TiO2 | Pt | Dip coating | Black light lamps | Ethanol 50% | 9 μmol/min | [62] |
| TiO2 | Pt | RF sputtering Sol–gel/spin coating | 250 W W lamp | NaOH 1M | 12.5 μmol/h 4.3 μmol/h | [63] |
| TiO2 | N-NiO N-CuO | Sol–gel/dip coating | UV 254 nm lamp | -- | 62,000 μmol/g | [64] |
| TiO2 | Pt | Dip coating | 13 W UV lamp | Water/methanol 1:1 | 349.6 μmol/gh | [65] |
| TiO2 | Zr | Anodization method | 500 W Xe lamp | Artificial sea water/ethilenglycol | 15 μmol | [30] |
| TiO2 | Zr | Sol–gel/dip coating | UV 254 lamp | ethanol | 38,600 μmol/cm2 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garay-Rodríguez, L.F.; Alfaro Cruz, M.R.; González-Ibarra, J.; Torres-Martínez, L.M.; Kim, J.H. Evaluation of Photocatalytic Hydrogen Evolution in Zr-Doped TiO2 Thin Films. Surfaces 2024, 7, 560-570. https://doi.org/10.3390/surfaces7030038
Garay-Rodríguez LF, Alfaro Cruz MR, González-Ibarra J, Torres-Martínez LM, Kim JH. Evaluation of Photocatalytic Hydrogen Evolution in Zr-Doped TiO2 Thin Films. Surfaces. 2024; 7(3):560-570. https://doi.org/10.3390/surfaces7030038
Chicago/Turabian StyleGaray-Rodríguez, Luis F., M. R. Alfaro Cruz, Julio González-Ibarra, Leticia M. Torres-Martínez, and Jin Hyeok Kim. 2024. "Evaluation of Photocatalytic Hydrogen Evolution in Zr-Doped TiO2 Thin Films" Surfaces 7, no. 3: 560-570. https://doi.org/10.3390/surfaces7030038
APA StyleGaray-Rodríguez, L. F., Alfaro Cruz, M. R., González-Ibarra, J., Torres-Martínez, L. M., & Kim, J. H. (2024). Evaluation of Photocatalytic Hydrogen Evolution in Zr-Doped TiO2 Thin Films. Surfaces, 7(3), 560-570. https://doi.org/10.3390/surfaces7030038








