Microplastic-Related Leachate from Recycled Rubber Tiles: The Role of TiO2 Protective Coating
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Aging Test
2.3. Mechanical Properties—Abrasion Testing
2.4. Characterization
2.4.1. FTIR Spectroscopy
2.4.2. Carbonyl Index (C.I.)
2.5. Water Leachate Testing (LC/MS QTOF)
3. Results and Discussion
3.1. FTIR Analysis
3.2. Carbonyl Index (C.I.)
3.3. Mechanical Properties—Abrasion Testing
3.4. Water Lecheate Testing (LC/MS QTOF)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ETRMA-European Tyre & Rubber Manufacturers’ Association, (n.d.). Available online: https://www.etrma.org/ (accessed on 29 March 2024).
- Valentini, F.; Pegoretti, A. End-of-life options of tyres. A review. Adv. Ind. Eng. Polym. Res. 2022, 5, 203–213. [Google Scholar] [CrossRef]
- Armada, D.; Llompart, M.; Celeiro, M.; Garcia-WCastro, P.; Ratola, N.; Dagnac, T.; de Boer, J. Global evaluation of the chemical hazard of recycled tire crumb rubber employed on worldwide synthetic turf football pitches. Sci. Total Environ. 2022, 812, 152542. [Google Scholar] [CrossRef] [PubMed]
- Martin’s Rubber Company. (n.d.). Available online: https://www.martins-rubber.co.uk/ (accessed on 29 March 2024).
- Itoh, Y.; Gu, H. Effect of Ultraviolet Irradiation on Surface Rubber Used in Bridge Bearings. J. Struct. Eng. 2007, 53, 696–705. [Google Scholar]
- Iwase, Y.; Shindo, T.; Kondo, H.; Ohtake, Y.; Kawahara, S. Ozone degradation of vulcanized isoprene rubber as a function of humidity. Polym. Degrad. Stab. 2017, 142, 209–216. [Google Scholar] [CrossRef]
- Pourebrahimi, S.; Pirooz, M. Microplastic pollution in the marine environment: A review. J. Hazard. Mater. Adv. 2023, 10, 100327. [Google Scholar] [CrossRef]
- Kye, H.; Kim, J.; Ju, S.; Lee, J.; Lim, C.; Yoon, Y. Microplastics in water systems: A review of their impacts on the environment and their potential hazards. Heliyon 2023, 9, e14359. [Google Scholar] [CrossRef]
- Zhai, X.; Zheng, H.; Xu, Y.; Zhao, R.; Wang, W.; Guo, H. Characterization and quantification of microplastics in indoor environments. Heliyon 2023, 9, e15901. [Google Scholar] [CrossRef]
- Sutkar, P.R.; Gadewar, R.D.; Dhulap, V.P. Recent trends in degradation of microplastics in the environment: A state-of-the-art review. J. Hazard. Mater. Adv. 2023, 11, 100343. [Google Scholar] [CrossRef]
- Rasmussen, L.A.; Lykkemark, J.; Andersen, T.R.; Vollertsen, J. Permeable pavements: A possible sink for tyre wear particles and other microplastics? Sci. Total Environ. 2023, 869, 161770. [Google Scholar] [CrossRef]
- Zjačić, J.P.; Vujasinović, M.; Kovačić, M.; Božić, A.L.; Kušić, H.; Katančić, Z.; Hrnjak-Murgić, Z. From Macro to Micro Plastics; Influence of Photo-oxidative Degradation. Kem. Ind. 2023, 72, 463–471. [Google Scholar] [CrossRef]
- Enfrin, M.; Myszka, R.; Giustozzi, F. Paving roads with recycled plastics: Microplastic pollution or eco-friendly solution? J. Hazard. Mater. 2022, 437, 129334. [Google Scholar] [CrossRef]
- Österlund, H.; Blecken, G.; Lange, K.; Marsalek, J.; Gopinath, K.; Viklander, M. Microplastics in urban catchments: Review of sources, pathways, and entry into stormwater. Sci. Total Environ. 2023, 858, 159781. [Google Scholar] [CrossRef] [PubMed]
- Klun, B.; Rozman, U.; Kalčíková, G. Environmental aging and biodegradation of tire wear microplastics in the aquatic environment. J. Environ. Chem. Eng. 2023, 11, 110604. [Google Scholar] [CrossRef]
- Prasittisopin, L.; Ferdous, W.; Kamchoom, V. Microplastics in construction and built environment. Dev. Built Environ. 2023, 15, 100188. [Google Scholar] [CrossRef]
- An, L.; Liu, Q.; Deng, Y.; Wu, W.; Gao, Y.; Ling, W. Sources of Microplastic in the Environment. Handb. Environ. Chem. 2020, 95, 143–159. [Google Scholar] [CrossRef]
- Chae, E.; Yang, S.R.; Choi, S.S. Test method for abrasion behavior of tire tread compounds using the wear particles. Polym. Test. 2022, 115, 107758. [Google Scholar] [CrossRef]
- Järlskog, I.; Jaramillo-Vogel, D.; Rausch, J.; Gustafsson, M.; Strömvall, A.M.; Andersson-Sköld, Y. Concentrations of tire wear microplastics and other traffic-derived non-exhaust particles in the road environment. Environ. Int. 2022, 170, 107618. [Google Scholar] [CrossRef]
- Kovochich, M.; Oh, S.C.; Lee, J.P.; Parker, J.A.; Barber, T.; Unice, K. Characterization of tire and road wear particles in urban river samples. Environ. Adv. 2023, 12, 100385. [Google Scholar] [CrossRef]
- Rausch, J.; Jaramillo-Vogel, D.; Perseguers, S.; Schnidrig, N.; Grobéty, B.; Yajan, P. Automated identification and quantification of tire wear particles (TWP) in airborne dust: SEM/EDX single particle analysis coupled to a machine learning classifier. Sci. Total Environ. 2022, 803, 149832. [Google Scholar] [CrossRef]
- Halle, L.L.; Palmqvist, A.; Kampmann, K.; Khan, F.R. Ecotoxicology of micronized tire rubber: Past, present and future considerations. Sci. Total Environ. 2020, 706, 135694. [Google Scholar] [CrossRef]
- Wagner, S.; Hüffer, T.; Klöckner, P.; Wehrhahn, M.; Hofmann, T.; Reemtsma, T. Tire wear particles in the aquatic environment—A review on generation, analysis, occurrence, fate and effects. Water Res. 2018, 139, 83–100. [Google Scholar] [CrossRef] [PubMed]
- Järlskog, I.; Strömvall, A.M.; Magnusson, K.; Gustafsson, M.; Polukarova, M.; Galfi, H.; Aronsson, M.; Andersson-Sköld, Y. Occurrence of tire and bitumen wear microplastics on urban streets and in sweepsand and washwater. Sci. Total Environ. 2020, 729, 138950. [Google Scholar] [CrossRef] [PubMed]
- Goßmann, I.; Halbach, M.; Scholz-Böttcher, B.M. Car and truck tire wear particles in complex environmental samples—A quantitative comparison with “traditional” microplastic polymer mass loads. Sci. Total Environ. 2021, 773, 145667. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, T.; Homem, V.; Cereceda-Balic, F.; Fadic, X.; Alves, A.; Ratola, N. Are volatile methylsiloxanes in downcycled tire microplastics? Levels and human exposure estimation in synthetic turf football fields. Environ. Sci. Pollut. Res. 2024, 31, 11950–11967. [Google Scholar] [CrossRef]
- Hartmann, N.B.; Hüffer, T.; Thompson, R.C.; Hassellöv, M.; Verschoor, A.; Daugaard, A.E.; Rist, S.; Karlsson, T.; Brennholt, N.; Cole, M.; et al. Are We Speaking the Same Language? Recommendations for a Definition and Categorization Framework for Plastic Debris. Environ. Sci. Technol. 2019, 53, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Jan Kole, P.; Löhr, A.J.; Van Belleghem, F.G.A.J.; Ragas, A.M.J. Wear and tear of tyres: A stealthy source of microplastics in the environment. Int. J. Environ. Res. Public Health 2017, 14, 1265. [Google Scholar] [CrossRef]
- Rødland, E.S.; Gustafsson, M.; Jaramillo-Vogel, D.; Järlskog, I.; Müller, K.; Rauert, C.; Rausch, J.; Wagner, S. Analytical challenges and possibilities for the quantification of tire-road wear particles. TrAC Trends Anal. Chem. 2023, 165, 117121. [Google Scholar] [CrossRef]
- Baensch-Baltruschat, B.; Kocher, B.; Stock, F.; Reifferscheid, G. Tyre and road wear particles (TRWP)—A review of generation, properties, emissions, human health risk, ecotoxicity, and fate in the environment. Sci. Total Environ. 2020, 733, 137823. [Google Scholar] [CrossRef]
- Baensch-Baltruschat, B.; Kocher, B.; Kochleus, C.; Stock, F.; Reifferscheid, G. Tyre and road wear particles—A calculation of generation, transport and release to water and soil with special regard to German roads. Sci. Total Environ. 2021, 752, 141939. [Google Scholar] [CrossRef]
- Rødland, E.S.; Lind, O.C.; Reid, M.J.; Heier, L.S.; Okoffo, E.D.; Rauert, C.; Thomas, K.V.; Meland, S. Occurrence of tire and road wear particles in urban and peri-urban snowbanks, and their potential environmental implications. Sci. Total Environ. 2022, 824, 153785. [Google Scholar] [CrossRef]
- Calarnou, L.; Traïkia, M.; Leremboure, M.; Malosse, L.; Dronet, S.; Delort, A.-M.; Besse-Hoggan, P.; Eyheraguibel, B. Assessing biodegradation of roadway particles via complementary mass spectrometry and NMR analyses. Sci. Total Environ. 2023, 900, 165698. [Google Scholar] [CrossRef] [PubMed]
- Johannessen, C.; Liggio, J.; Zhang, X.; Saini, A.; Harner, T. Composition and transformation chemistry of tire-wear derived organic chemicals and implications for air pollution. Atmos. Pollut. Res. 2022, 13, 101533. [Google Scholar] [CrossRef]
- Knight, L.J.; Parker-Jurd, F.N.F.; Al-Sid-Cheikh, M.; Thompson, R.C. Tyre wear particles: An abundant yet widely unreported microplastic? Environ. Sci. Pollut. Res. 2020, 27, 18345–18354. [Google Scholar] [CrossRef] [PubMed]
- Giechaskiel, B.; Grigoratos, T.; Mathissen, M.; Quik, J.; Tromp, P.; Gustafsson, M.; Franco, V.; Dilara, P. Contribution of Road Vehicle Tyre Wear to Microplastics and Ambient Air Pollution. Sustainability 2024, 16, 522. [Google Scholar] [CrossRef]
- Rauert, C.; Rødland, E.S.; Okoffo, E.D.; Reid, M.J.; Meland, S.; Thomas, K.V. Challenges with Quantifying Tire Road Wear Particles: Recognizing the Need for Further Refinement of the ISO Technical Specification. Environ. Sci. Technol. Lett. 2021, 8, 231–236. [Google Scholar] [CrossRef]
- Rosso, B.; Gregoris, E.; Litti, L.; Zorzi, F.; Fiorini, M.; Bravo, B.; Barbante, C.; Gambaro, A.; Corami, F. Identification and quantification of tire wear particles by employing different cross-validation techniques: FTIR-ATR Micro-FTIR, Pyr-GC/MS, and SEM. Environ. Pollut. 2023, 326, 121511. [Google Scholar] [CrossRef]
- Skoczyńska, E.; Leonards, P.E.G.; Llompart, M.; de Boer, J. Analysis of recycled rubber: Development of an analytical method and determination of polycyclic aromatic hydrocarbons and heterocyclic aromatic compounds in rubber matrices. Chemosphere 2021, 276, 130076. [Google Scholar] [CrossRef]
- Ciccu, R.; Costa, G. Recycling of secondary raw materials from end-of-life car tires. WIT Trans. Ecol. Environ. 2012, 155, 1115–1126. [Google Scholar] [CrossRef]
- Patricio, J.; Andersson-Sköld, Y.; Gustafsson, M. End-of-Life Tyres Applications—Technologies and Environmental Impacts. 2021. Available online: https://www.diva-portal.org/smash/record.jsf?pid=diva2%3A1611409&dswid=9797 (accessed on 17 March 2024).
- Armada, D.; Celeiro, M.; Dagnac, T.; Llompart, M. Green methodology based on active air sampling followed by solid phase microextraction and gas chromatography-tandem mass spectrometry analysis to determine hazardous substances in different environments related to tire rubber. J. Chromatogr. A 2022, 1668, 462911. [Google Scholar] [CrossRef]
- Grynkiewicz-Bylina, B.; Słomka-Słupik, B.; Rakwic, B. Tests of Cement and Slag Mortars with SBR Rubber Granulates in Terms of Ecotoxicity and Strength. Inżynieria Miner. 2024, 2, 153–162. [Google Scholar] [CrossRef]
- Yu, H.; Bai, X.; Qian, G.; Wei, H.; Gong, X.; Jin, J.; Li, Z. Impact of Ultraviolet Radiation on the Aging Properties of SBS-Modified Asphalt Binders. Polymers 2019, 11, 1111. [Google Scholar] [CrossRef]
- Bokkers, B.G.H.; Guichelaar, S.K.; Bakker, M.I. Assessment of the Product Limit for PAHs in Rubber Articles. The Case of Shock-Absorbing Tiles. 2016. Available online: https://www.rivm.nl/bibliotheek/rapporten/2016-0184.html (accessed on 29 April 2024).
- Pronk, M.E.J.; Woutersen, M.; Herremans, J.M.M. Synthetic turf pitches with rubber granulate infill: Are there health risks for people playing sports on such pitches? J. Expo. Sci. Environ. Epidemiol. 2020, 30, 567–584. [Google Scholar] [CrossRef] [PubMed]
- Celeiro, M.; Armada, D.; Ratola, N.; Dagnac, T.; de Boer, J.; Llompart, M. Evaluation of chemicals of environmental concern in crumb rubber and water leachates from several types of synthetic turf football pitches. Chemosphere 2021, 270, 128610. [Google Scholar] [CrossRef] [PubMed]
- Perkins, A.N.; Inayat-Hussain, S.H.; Deziel, N.C.; Johnson, C.H.; Ferguson, S.S.; Garcia-Milian, R.; Thompson, D.C.; Vasiliou, V. Evaluation of potential carcinogenicity of organic chemicals in synthetic turf crumb rubber. Environ. Res. 2019, 169, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Pfautsch, S.; Wujeska-Klause, A.; Walters, J. Outdoor playgrounds and climate change: Importance of surface materials and shade to extend play time and prevent burn injuries. Build. Environ. 2022, 223, 109500. [Google Scholar] [CrossRef]
- Benjak, P.; Radetić, L.; Tomaš, M.; Brnardić, I.; Radetić, B.; Špada, V.; Grčić, I. Rubber Tiles Made from Secondary Raw Materials with Immobilized Titanium Dioxide as Passive Air Protection. Processes 2023, 11, 125. [Google Scholar] [CrossRef]
- Leng, Z.; Yu, H. Novel Method of Coating Titanium Dioxide on to Asphalt Mixture Based on the Breath Figure Process for Air-Purifying Purpose. J. Mater. Civ. Eng. 2016, 28, 04015188. [Google Scholar] [CrossRef]
- Boonen, E.; Beeldens, A. Recent photocatalytic applications for air purification in Belgium. Coatings 2014, 4, 553–573. [Google Scholar] [CrossRef]
- La Russa, M.F.; Rovella, N.; De Buergo, M.A.; Belfiore, C.M.; Pezzino, A.; Crisci, G.M.; Ruffolo, S.A. Nano-TiO2 coatings for cultural heritage protection: The role of the binder on hydrophobic and self-cleaning efficacy. Prog. Org. Coatings 2016, 91, 1–8. [Google Scholar] [CrossRef]
- Gherardi, F.; Maravelaki, P.N. Advances in the application of nanomaterials for natural stone conservation. RILEM Tech. Lett. 2022, 7, 20–29. [Google Scholar] [CrossRef]
- Ruffolo, S.A.; Francesco, M.; Russa, L. Nanostructured Coatings for Stone Protection: An Overview. Front. Mater. 2019, 6, 147. [Google Scholar] [CrossRef]
- Esposito, C.; Ingrosso, C.; Petronella, F.; Comparelli, R.; Striccoli, M.; Agostiano, A.; Frigione, M.; Curri, M.L. Progress in Organic Coatings A designed UV—Vis light curable coating nanocomposite based on colloidal TiO2 NRs in a hybrid resin for stone protection. Prog. Org. Coatings 2018, 122, 290–301. [Google Scholar] [CrossRef]
- Nazir, M.; Irfan, M.; Ali, I.; Abdul, M. Photonics and Nanostructures—Fundamentals and Applications Revealing antimicrobial and contrasting photocatalytic behavior of metal chalcogenide deposited P25-TiO2 nanoparticles. Photonics Nanostruct. Fundam. Appl. 2019, 36, 100721. [Google Scholar] [CrossRef]
- Dds, A.S.; Bahador, A.; Khalil, S.; Saffar, A.; Dds, S.; Zaman, M. The effect of TiO2 and SiO2 nanoparticles on flexural strength of poly (methyl methacrylate) acrylic resins. J. Prosthodont. Res. 2013, 57, 15–19. [Google Scholar] [CrossRef]
- Salama, A.; Kamel, B.M.; Osman, T.A.; Rashad, R.M. Investigation of mechanical properties of UHMWPE composites reinforced with HAP þ TiO2 fabricated by solvent dispersing technique. J. Mater. Res. Technol. 2022, 21, 4330–4343. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Q.; Jing, D.; Zhou, S.; Shao, L. ScienceDirect Biomechanical properties of nano-TiO2 addition to a medical silicone elastomer: The effect of artificial ageing. J. Dent. 2014, 42, 475–483. [Google Scholar] [CrossRef]
- Elsaka, S.E.; Hamouda, I.M.; Swain, M.V. Titanium dioxide nanoparticles addition to a conventional glass-ionomer restorative: Influence on physical and antibacterial properties. J. Dent. 2011, 39, 589–598. [Google Scholar] [CrossRef]
- Nuzaimah, M.; Sapuan, S.M.; Nadlene, R.; Jawaid, M. Sodium hydroxide treatment ofwaste rubber crumb and its effects on properties of unsaturated polyester composites. Appl. Sci. 2020, 10, 3913. [Google Scholar] [CrossRef]
- Tawfik, M.; Tonnellier, X.; Sansom, C. Light source selection for a solar simulator for thermal applications: A review. Renew. Sustain. Energy Rev. 2018, 90, 802–813. [Google Scholar] [CrossRef]
- Krug, N.; Zarges, J.-C.; Heim, H.-P. Influence of ethylene oxide and gamma irradiation sterilization processes on the degradation behaviour of poly(lactic acid) (PLA) in the course of artificially accelerated aging. Polym. Test. 2024, 132, 108362. [Google Scholar] [CrossRef]
- Lamberti, M.; Maurel-Pantel, A.; Lebon, F. Experimental and numerical evaluation of hydro-thermal ageing’s effects on adhesive connections in offshore structures. Ocean Eng. 2023, 290, 116303. [Google Scholar] [CrossRef]
- ISO 4649:2024; Rubber, Vulcanized or Thermoplastic—Determination of Abrasion Resistance Using a Rotating Cylindrical Drum Device. International Organization for Standardization (ISO): Geneva, Switzerland, 2024.
- Almond, J.; Sugumaar, P.; Wenzel, M.N.; Hill, G.; Wallis, C. Determination of the carbonyl index of polyethylene and polypropylene using speci fi ed area under band methodology with ATR-FTIR Spectroscopy. e-Polymers 2020, 20, 369–381. [Google Scholar] [CrossRef]
- Brandon, J.; Goldstein, M.; Ohman, M.D. Long-term aging and degradation of microplastic particles: Comparing in situ oceanic and experimental weathering patterns. Mar. Pollut. Bull. 2016, 110, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Berset, J.D.; Rennie, E.; Glauner, T. Screening and Identification of Emerging Contaminants in Wastewater Treatment Plant Effluents Using UHPLC/Q-TOF MS and an Accurate Mass Database and Library. 2016. Available online: https://sem.com.tr/wp-content/uploads/Screening-and-Identification-of-Emerging-Contaminants-in-Wastewater-Treatment-Plant.pdf (accessed on 17 September 2024).
- Redon, A.; Le Cam, J.B.; Robin, E.; Miroir, M.; Fralin, J.C. Aging characterization of different nitrile butadiene rubbers for sealing in a pneumatic system: Linking the change of the physicochemical state to the mechanical properties. J. Appl. Polym. Sci. 2023, 140, e54068. [Google Scholar] [CrossRef]
- Aielo, P.B.; Borges, F.A.; Romeira, K.M.; Miranda, M.C.R.; Arruda, L.B.D.; Paulo, P.N.; Drago, B.D.C.; Herculano, R.D. Evaluation of sodium diclofenac release using natural rubber latex as carrier. Mater. Res. 2014, 17, 146–152. [Google Scholar] [CrossRef]
- Ling, L.; Li, J.; Zhang, G.; Sun, R.; Wong, C.P. Self-Healing and Shape Memory Linear Polyurethane Based on Disulfide Linkages with Excellent Mechanical Property. Macromol. Res. 2018, 26, 365–373. [Google Scholar] [CrossRef]
- InstaNANO, FTIR Functional Group Database Table with Search-InstaNANO, (n.d.). Available online: https://instanano.com/ (accessed on 2 May 2024).
- Merck, IR Spectrum Table by Frequency Range. (n.d.). Available online: https://www.sigmaaldrich.com/HR/en (accessed on 2 May 2024).
- Liao, M.; Liu, Z.; Gao, Y.; Liu, L.; Xiang, S. Study on UV aging resistance of nano-TiO2/montmorillonite/styrene-butadiene rubber composite modified asphalt based on rheological and microscopic properties. Constr. Build. Mater. 2021, 301, 124108. [Google Scholar] [CrossRef]
- Gomes, F.O.; Rocha, M.R.; Alves, A.; Ratola, N. A review of potentially harmful chemicals in crumb rubber used in synthetic football pitches. J. Hazard. Mater. 2021, 409, 124998. [Google Scholar] [CrossRef]
- Paredes, M.; Viteri, R.; Castillo, T.; Caminos, C.; Enyoh, C.E. Microplastics from degradation of tires in sewer networks of the city of Riobamba, Ecuador. Environ. Eng. Res. 2020, 26, 200276. [Google Scholar] [CrossRef]
- Liu, M.; Xu, H.; Feng, R.; Gu, Y.; Bai, Y.; Zhang, N.; Wang, Q.; Ho, S.S.H.; Qu, L.; Shen, Z.; et al. Chemical composition and potential health risks of tire and road wear microplastics from light-duty vehicles in an urban tunnel in China. Environ. Pollut. 2023, 330, 121835. [Google Scholar] [CrossRef]
- Celeiro, M.; Dagnac, T.; Llompart, M. Determination of priority and other hazardous substances in football fields of synthetic turf by gas chromatography-mass spectrometry: A health and environmental concern. Chemosphere 2018, 195, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Seiwert, B.; Klöckner, P.; Wagner, S.; Reemtsma, T. Source-related smart suspect screening in the aqueous environment: Search for tire-derived persistent and mobile trace organic contaminants in surface waters. Anal. Bioanal. Chem. 2020, 412, 4909–4919. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information, Compound Summary for CID 13625, 2(3H)-Benzothiazolone, PubChem. 2024. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/13625 (accessed on 28 June 2024).
- National Center for Biotechnology Information, PubChem Compound Summary for CID 17520, 1,2-Benzisothiazol-3(2H)-one, PubChem. 2024. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/1_2-Benzisothiazol-3_2H_-one (accessed on 28 June 2024).
- Vazquet-Duhalt, R.; Marquez-Rocha, F.; Ponce, E.; Licea, A.F.; Viana, M.T. Nonylphenol, an integrated vision of a pollutant. Scientific review. Appl. Ecol. Environ. Res. 2005, 4, 1–25. [Google Scholar] [CrossRef]
- Duque-Villaverde, A.; Armada, D.; Dagnac, T.; Llompart, M. Recycled tire rubber materials in the spotlight. Determination of hazardous and lethal substances. Sci. Total Environ. 2024, 929, 172674. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Tang, T.; Li, Y.; Wang, R.; Chen, X.; Song, D.; Du, X.; Tao, X.; Zhou, J.; Dang, Z.; et al. Non-targeted screening and photolysis transformation of tire-related compounds in roadway runoff. Sci. Total Environ. 2024, 924, 171622. [Google Scholar] [CrossRef]
- Binda, G.; Kalčíková, G.; Allan, I.J.; Hurley, R.; Rødland, E.; Spanu, D.; Nizzetto, L. Microplastic aging processes: Environmental relevance and analytical implications. TrAC Trends Anal. Chem. 2024, 172, 117566. [Google Scholar] [CrossRef]
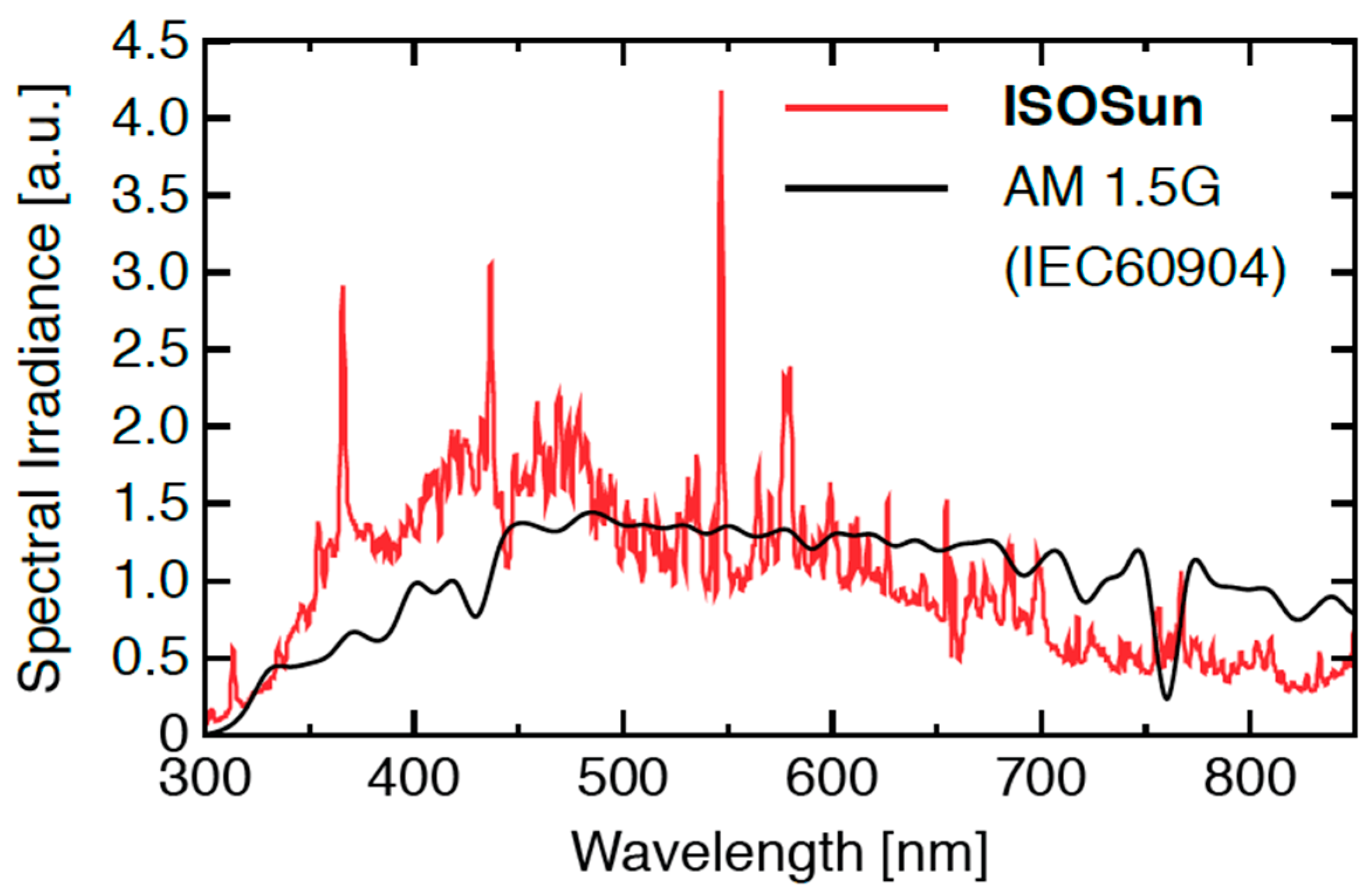

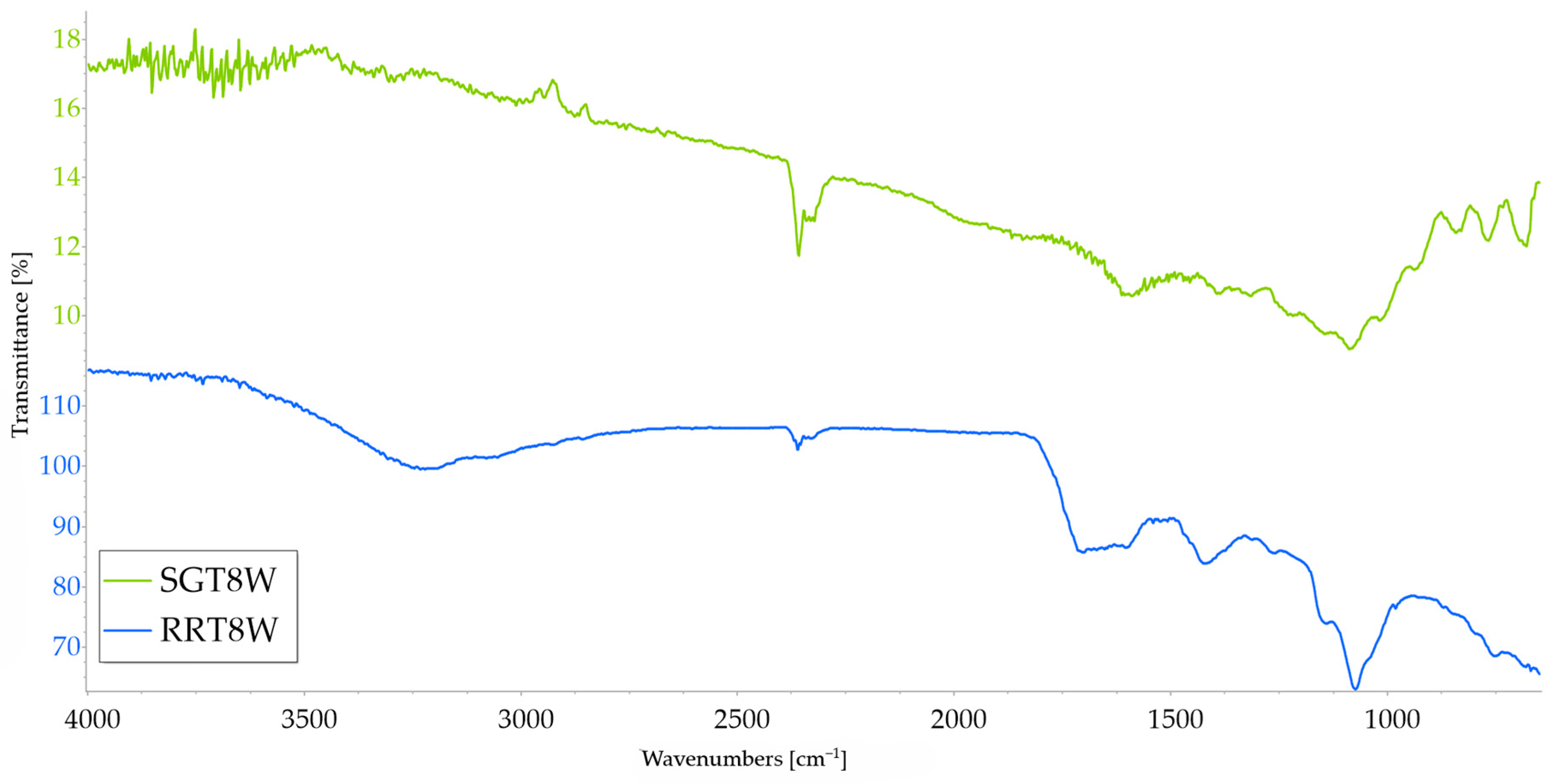

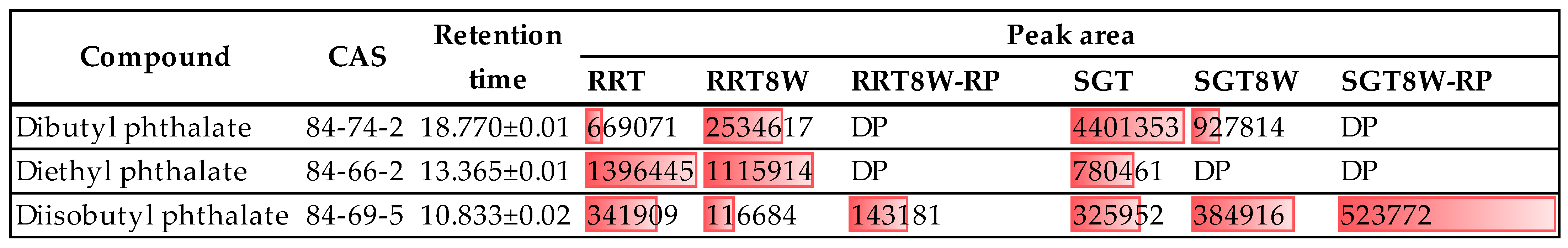
| Sample | C=O 1850–1650 | C−O 1300–1140 | Sample | C=O 1850–1650 | C−O 1300–1140 |
|---|---|---|---|---|---|
| RRT/0 | 0.6204 | 1.2262 | SGT/0 | 0.5410 | 1.1561 |
| RRT/4W/U | 1.5639 | 7.3035 | SGT/4W/U | 0.8111 | 1.3107 |
| RRT/6W/U | 0.9748 | 4.2755 | SGT/6W/U | 0.7781 | 1.2532 |
| RRT/8W/U | 1.1850 | 0.8438 | SGT/8W/U | 0.8130 | 1.2663 |
| RRT/4W/B | 1.1167 | 2.3274 | SGT/4W/B | 0.7811 | 1.0137 |
| RRT/6W/B | 2.1125 | 1.3821 | SGT/6W/B | 0.7234 | 1.5315 |
| RRT/8W/B | 0.7485 | 1.2005 | SGT/8W/B | 0.8445 | 1.1907 |
| Name | CAS | Mass | RT (min) | RSD (Mass, ppm) | Possible Origin |
|---|---|---|---|---|---|
| HOBT/2-Hydroxybenzothiazole | 934-34-9 | 151.0101 | 14.617 | 1.59 | rubber accelerator |
| BIT/Benzisothiazolinone | 2634-33-5 | 151.0101 | 14.617 | 1.59 | rubber and polymerized materials preservatives |
| 2-Mercaptobenzoxazole | 2382-96-9 | 151.0101 | 14.617 | 1.59 | rubber accelerator |
| 4-Nonylphenoxyacetic acid | 3115-49-9 | 278.1885 | 17.605 | 1.57 | Surfactant degradation |
| Benzothiazole-2-sulfonic acid | 941-57-1 | 214.9721 | 12.509 | 2.52 | rubber accelerator |
| 4-Hydroxybenzoic acid | 138.0326 | 12.576 | 2.58 | Additive, corrosion inhibitor | |
| Camphor | 152.1205 | 17.122 | 1.46 | Additive, plasticizer | |
| Isoborneol | 124-76-5 | 154.1359 | 16.247 | 5.03 | Flavor and fragrance additive |
| Dibutyl adipate | 105-99-7 | 258.1839 | 16.576 | 2.38 | Plasticizers |
| Methylsalicylate | 119-36-8 | 152.0484 | 13.839 | 1.45 | UV light stabilizer |
| 4-Methoxybenzoic acid | 100-09-4 | 180.1156 | 17.132 | 5.6 | Flavoring Agents |
| 2-tert-Butyl-4-methoxyphenol | 25013-16-5 | 192.1523 | 16.786 | 5.3 | Antioxidant, additive |
| Ionone | 256.0636 | 17.671 | 1.94 | Flavoring agent | |
| N4-Acetylsulfaguanidin (Acetamide) | 19077-97-5 | 206.1682 | 18.594 | 5.27 | solvent, plasticizer, stabilizer |
| 4-tert-Octylphenol | 140-66-9 | 125.9992 | 5.377 | 1.84 | rubber additives, antioxidant |
| Ethyl sulfate | 540-82-9 | 135.0155 | 12.801 | 1.44 | Environmental contaminant |
| 4-tert-Butylbenzoic acid | 98-73-7 | 144.1147 | 16.283 | 2.86 | Regulator of polymerization, inhibitor of corrosion |
| Benzothiazole | 166.9865 | 15.414 | 1.56 | rubber accelerator | |
| MBT/2-Mercaptobenzothiazole | 149-30-4 | 198.1414 | 17.767 | 4.38 | Rubber accelerator |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benjak, P.; Radetić, L.; Presečki, I.; Brnardić, I.; Sakač, N.; Grčić, I. Microplastic-Related Leachate from Recycled Rubber Tiles: The Role of TiO2 Protective Coating. Surfaces 2024, 7, 786-800. https://doi.org/10.3390/surfaces7030051
Benjak P, Radetić L, Presečki I, Brnardić I, Sakač N, Grčić I. Microplastic-Related Leachate from Recycled Rubber Tiles: The Role of TiO2 Protective Coating. Surfaces. 2024; 7(3):786-800. https://doi.org/10.3390/surfaces7030051
Chicago/Turabian StyleBenjak, Paula, Lucija Radetić, Ivana Presečki, Ivan Brnardić, Nikola Sakač, and Ivana Grčić. 2024. "Microplastic-Related Leachate from Recycled Rubber Tiles: The Role of TiO2 Protective Coating" Surfaces 7, no. 3: 786-800. https://doi.org/10.3390/surfaces7030051
APA StyleBenjak, P., Radetić, L., Presečki, I., Brnardić, I., Sakač, N., & Grčić, I. (2024). Microplastic-Related Leachate from Recycled Rubber Tiles: The Role of TiO2 Protective Coating. Surfaces, 7(3), 786-800. https://doi.org/10.3390/surfaces7030051








