Microstructure Refinement or Increased Copper Solubility: Factors That Contribute to the Pitting Corrosion Tendency in Aluminum–Copper Alloys
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample for Laser Surface Melting
2.2. Laser Surface Melting
2.3. Samples for Microscopy
2.4. X-Ray Diffraction
2.5. Potentiodynamic Polarization
3. Results and Discussion
3.1. Laser Surface Treatment
3.2. Microstructure Study
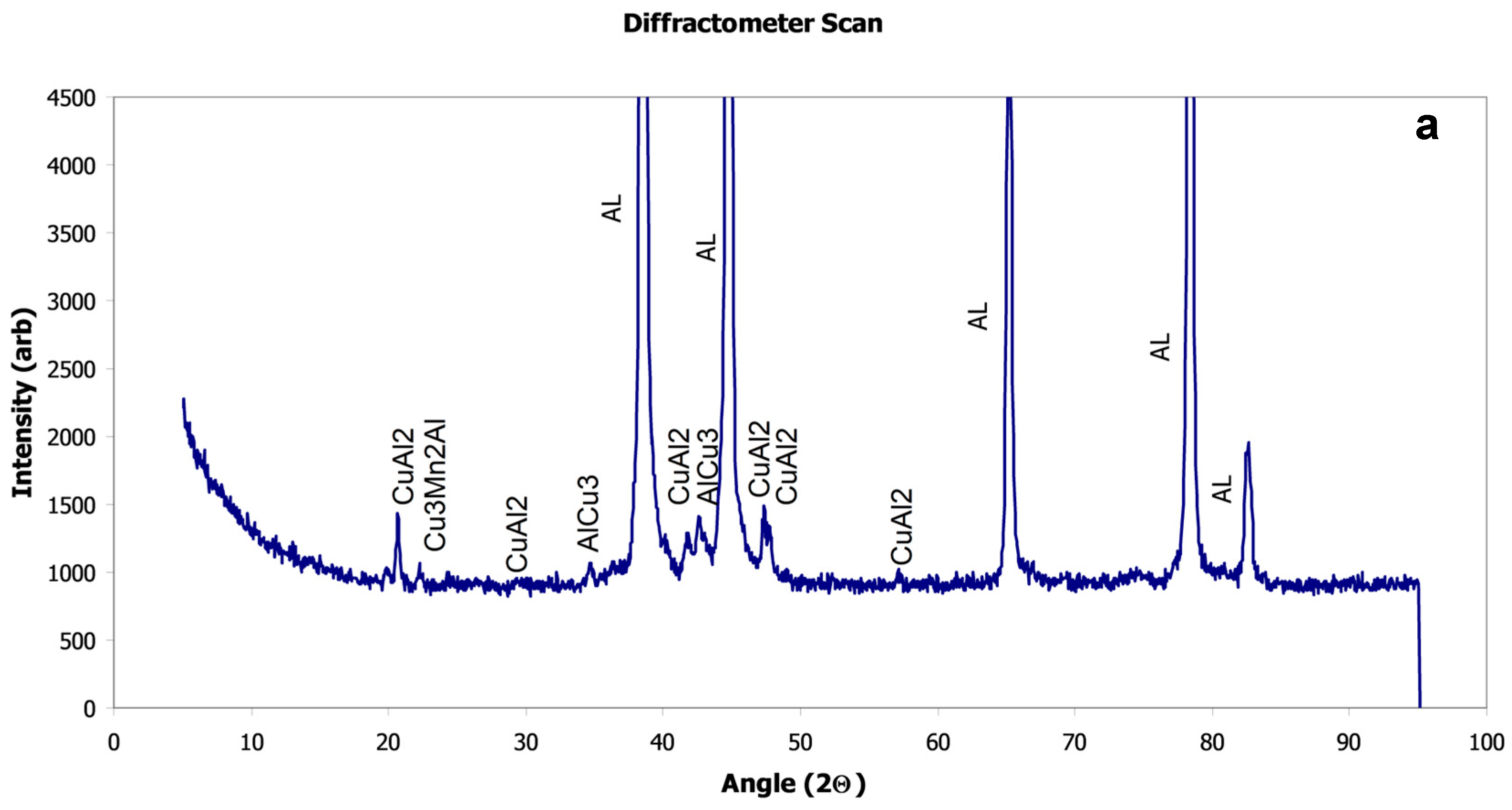
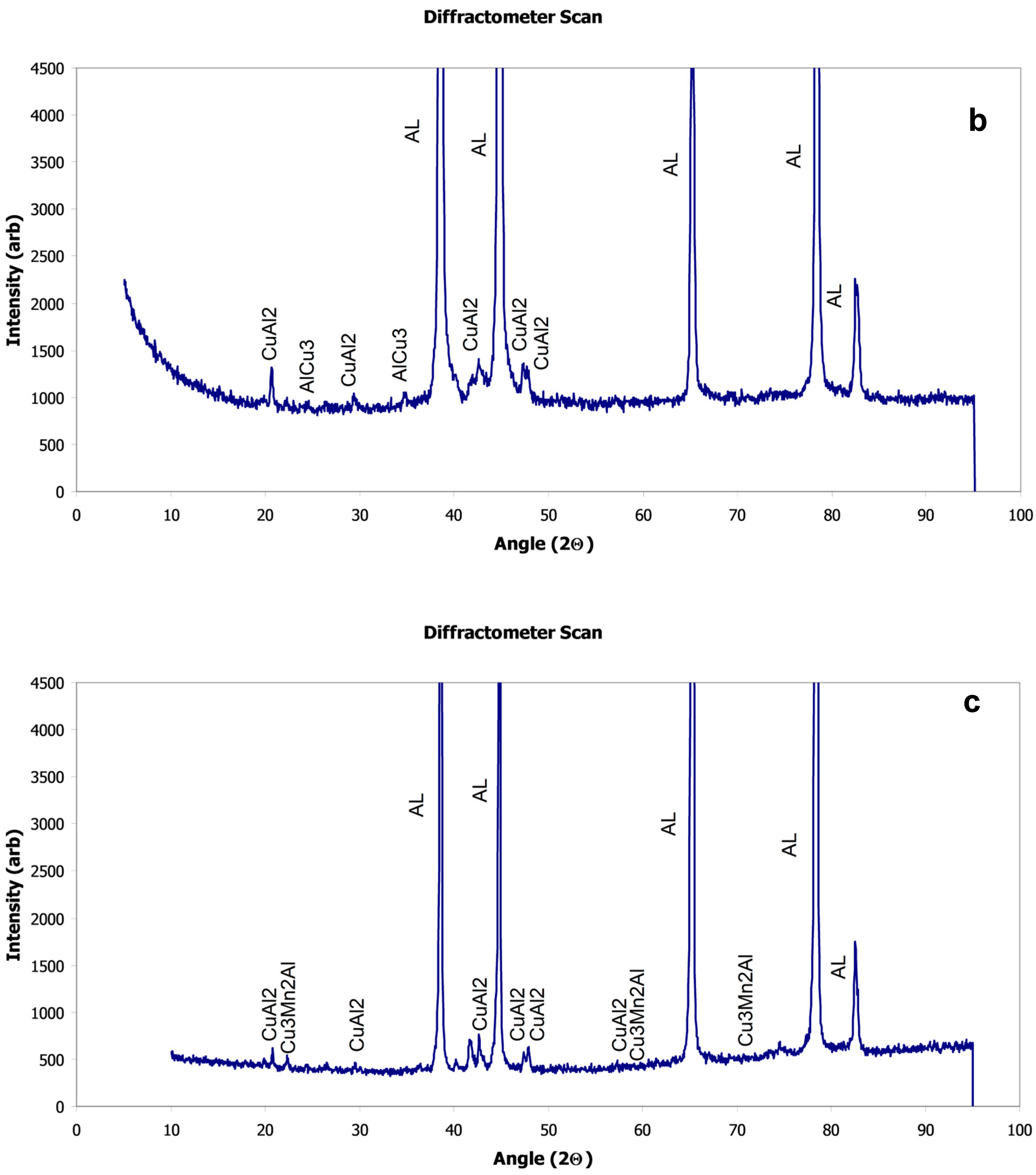
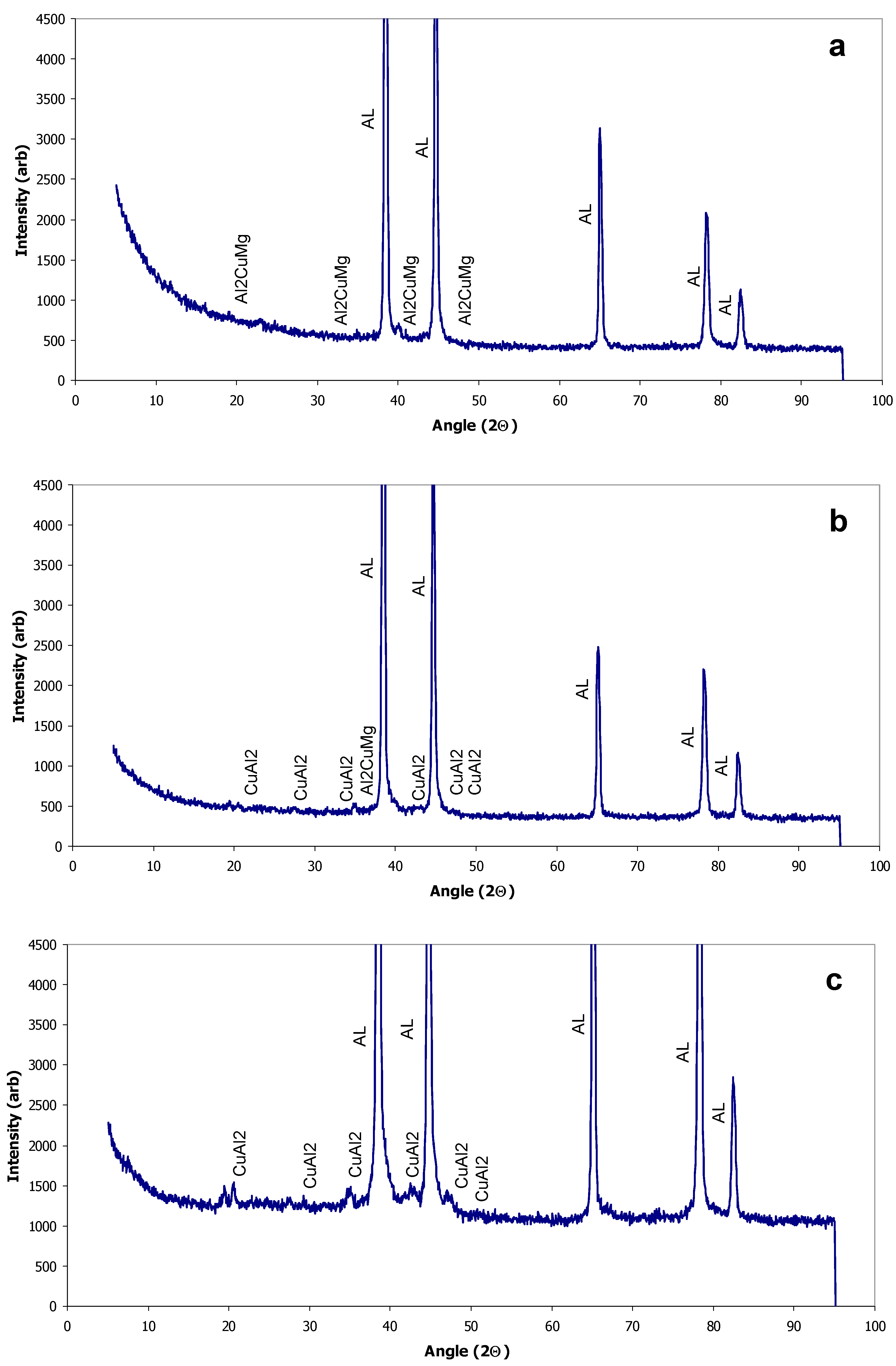
X-Ray Diffraction
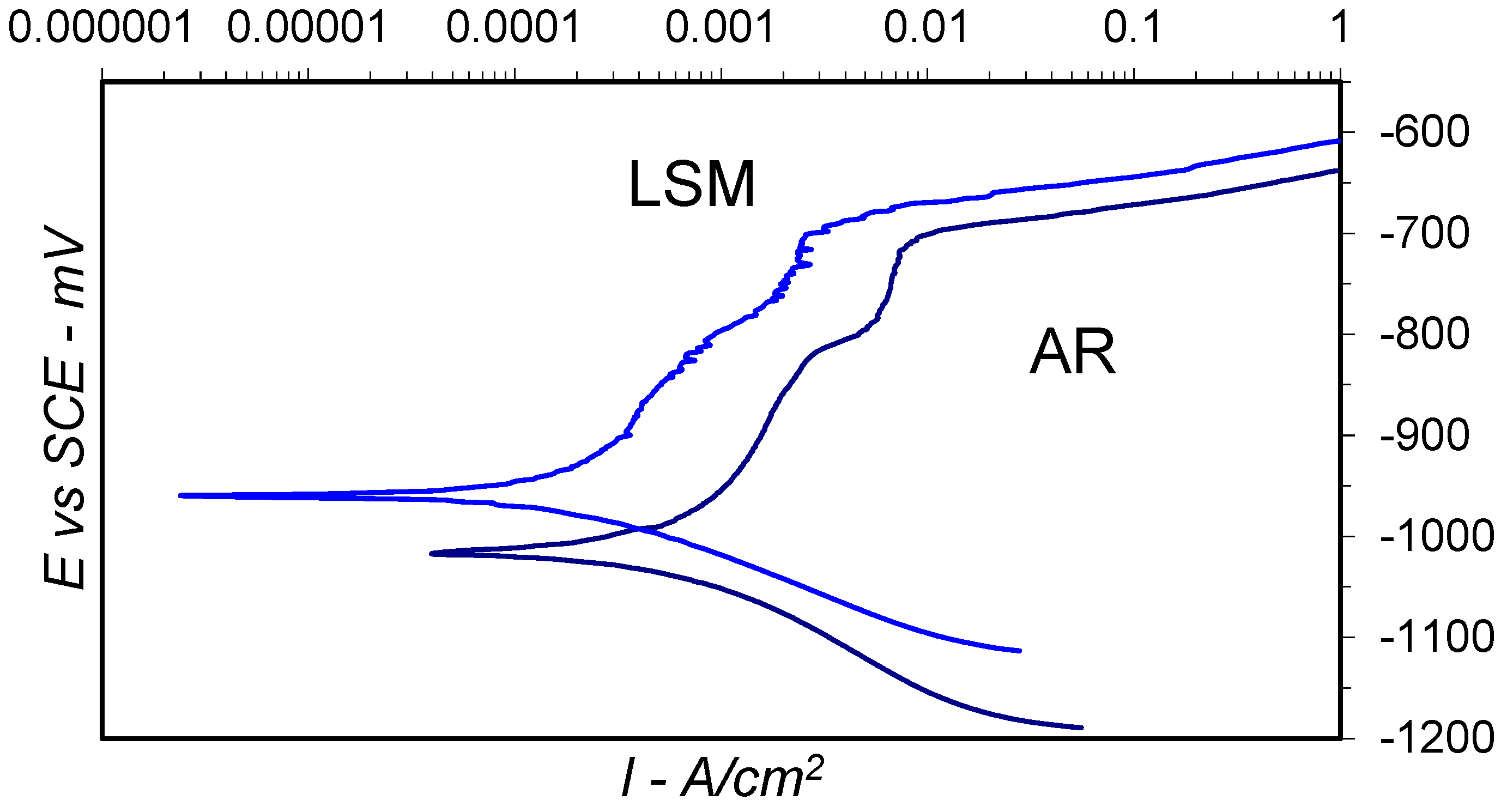
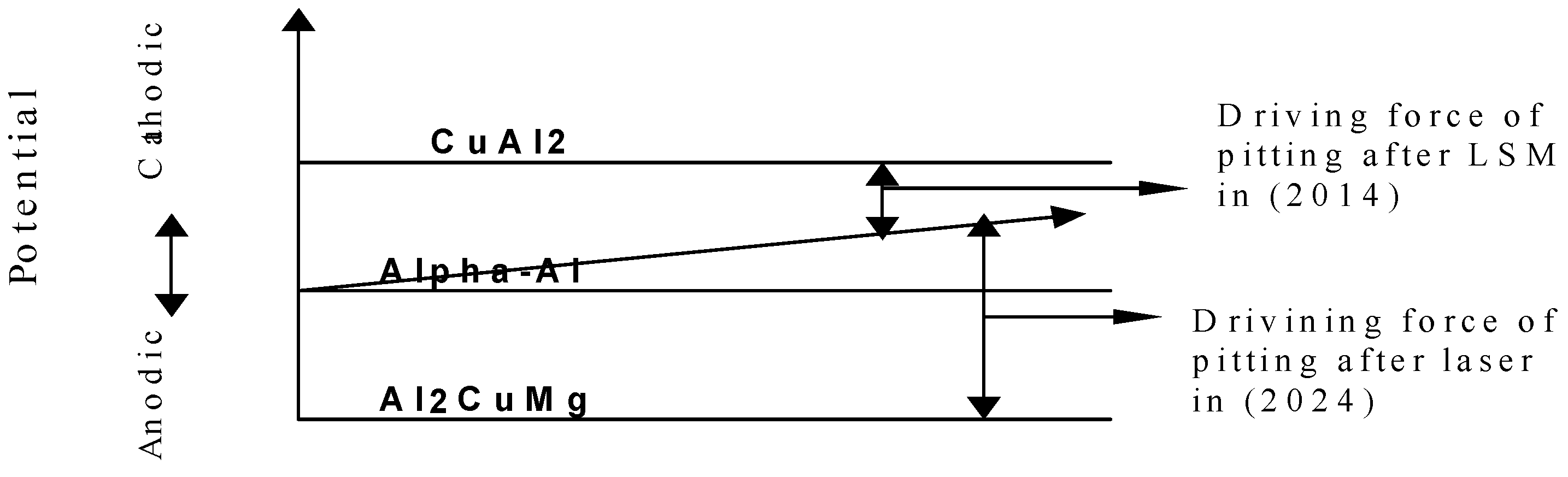
3.3. Electrochemical Testing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, H.; Li, J. Advancements in corrosion protection for aerospace aluminum alloys through surface treatment. Int. J. Electrochem. Sci. 2024, 19, 100487. [Google Scholar] [CrossRef]
- Li, S.S.; Yue, X.; Li, Q.Y.; Peng, H.L.; Dong, B.X.; Liu, T.S.; Yang, H.Y.; Fan, J.; Shu, S.L.; Qiu, F.; et al. Development and applications of aluminum alloys for aerospace industry. J. Mater. Res. Technol. 2023, 27, 944–983. [Google Scholar] [CrossRef]
- Sarker, S.; Haque, M.S.; Alvy, M.S.A.; Abir, M.M.H. The effects of solution heat treatment on the microstructure and hardness of an aluminum-4% copper alloy with added nickel and tin. J. Alloys Metall. Syst. 2023, 4, 100042. [Google Scholar] [CrossRef]
- Ikeuba, A.I.; Njoku, C.N.; Ekerenam, O.O.; Njoku, D.I.; Udoh, I.I.; Daniel, E.F.; Uzoma, P.C.; Etim, I.-I.N.; Okonkwo, B.O. A review of the electrochemical and galvanic corrosion behavior of important intermetallic compounds in the context of aluminum alloys. RSC Adv. 2024, 14, 31921–31953. [Google Scholar] [CrossRef]
- Sabry, I. Exploring the effect of friction stir welding parameters on the strength of AA2024 and A356-T6 aluminum alloys. J. Alloys Metall. Syst. 2024, 8, 100124. [Google Scholar] [CrossRef]
- Neuß, D.; McCarroll, I.E.; Zhang, S.; Woods, E.; Delis, W.J.; Tanure, L.; Springer, H.; Sandlöbes, S.; Yang, J.; Todorova, M.; et al. High-resolution chemical and structural characterization of the native oxide scale on a Mg-based alloy. Corros. Sci. 2024, 227, 111776. [Google Scholar] [CrossRef]
- Schneider, M.; Liebmann, T.; Langklotz, U.; Michaelis, A. Microelectrochemical investigation of anodic oxide formation on the aluminum alloy AA2024. Electrochim. Acta 2017, 249, 198–205. [Google Scholar] [CrossRef]
- Xu, J.; Song, Y.; He, K.; Zhang, H.; Dong, K.; Cai, Y.; Han, E.-H. Role of oxidants in the anodic oxidation of 2024 aluminum alloy by dominating anodic and cathodic processes. Colloids Surf. A Physicochem. Eng. Asp. 2024, 703, 135229. [Google Scholar] [CrossRef]
- Jian-jun, Y.; Shi-yang, Y.; Cai-he, F.; Ling, O.; Jia-hu, W.; Hai, P.; Bo-wen, W.; Deng, L.; Shi-yun, D.; Zai-yu, Z. Effect of voltage on structure and properties of 2024 aluminum alloy surface anodized aluminum oxide films. Surf. Coat. Technol. 2024, 479, 130508. [Google Scholar] [CrossRef]
- Erdemir, F.; Canakci, A.; Varol, T.; Ozkaya, S. Corrosion and wear behavior of functionally graded Al2024/SiC composites produced by hot pressing and consolidation. J. Alloys Compd. 2015, 644, 589–596. [Google Scholar] [CrossRef]
- Luo, C.; Albu, S.P.; Zhou, X.; Sun, Z.; Zhang, X.; Tang, Z.; Thompson, G.E. Continuous and discontinuous localized corrosion of a 2xxx aluminium–copper–lithium alloy in sodium chloride solution. J. Alloys Compd. 2016, 658, 61–70. [Google Scholar] [CrossRef]
- Chiu, L.-H.; Tsai, C.-Y.; Chen, K.-H.; Hu, C.-J.; Chang, H. Effect of Coarse Second-Phase Particles on Galvanic Corrosion of Anodized 6061 Aluminum Alloy Coupled with C1100 Copper. Int. J. Electrochem. Sci. 2015, 10, 6572–6585. [Google Scholar] [CrossRef]
- Musa, A.Y.; Mohamad, A.B.; Kadhum, A.A.H.; Chee, E.P. Galvanic Corrosion of Aluminum Alloy (Al2024) and Copper in 1.0 M Nitric Acid. Int. J. Electrochem. Sci. 2011, 6, 5052–5065. [Google Scholar] [CrossRef]
- Williams, G.; Coleman, A.J.; McMurray, H.N. Inhibition of Aluminium Alloy AA2024-T3 pitting corrosion by copper complexing compounds. Electrochim. Acta 2010, 55, 5947–5958. [Google Scholar] [CrossRef]
- Wang, Z.; Qian, H.; Chang, W.; Yu, Z.; Qiao, Q.; Zhou, M.; Guo, D.; Zhang, D.; Kwok, C.T.; Tam, L.M. Microstructure and localized corrosion properties of 2219 aluminum alloy manufactured by additive friction stir deposition. Corros. Sci. 2024, 240, 112508. [Google Scholar] [CrossRef]
- Pinto, M.A.; Cheung, N.; Ierardi, M.C.F.; Garcia, A. Microstructural and hardness investigation of an aluminum–copper alloy processed by laser surface melting. Mater. Charact. 2003, 50, 249–253. [Google Scholar] [CrossRef]
- Vivas, J.; Aldanondo, E.; Dufour, P.; Delgado, C.; Fernández-Calvo, A.I. Laser beam welding of T joints for aluminum–Copper–lithium aircraft panels: Effect of filler wire on recyclability and weldability. J. Mater. Res. Technol. 2024, 33, 5809–5820. [Google Scholar] [CrossRef]
- Cheng, M.; Luo, G.; Xiao, X.; Song, L. Microstructure evolution of pulsed laser melting 2219 aluminum alloy: Solidification modes and inter-pulse thermal cycles. Mater. Lett. 2022, 313, 131768. [Google Scholar] [CrossRef]
- Stoica, A.-I.; Światowska, J.; Romaine, A.; Di Franco, F.; Qi, J.; Mercier, D.; Seyeux, A.; Zanna, S.; Marcus, P. Influence of post-treatment time of trivalent chromium protection coating on aluminium alloy 2024-T3 on improved corrosion resistance. Surf. Coat. Technol. 2019, 369, 186–197. [Google Scholar] [CrossRef]
- Trinidad, C.; Światowska, J.; Zanna, S.; Seyeux, A.; Mercier, D.; Viroulaud, R.; Marcus, P. Effect of surface preparation treatments on copper enrichment on 2024 aluminium alloy surface. Appl. Surf. Sci. 2021, 560, 149991. [Google Scholar] [CrossRef]
- Boag, A.; Hughes, A.E.; Glenn, A.M.; Muster, T.H.; McCulloch, D. Corrosion of AA2024-T3 Part I: Localised corrosion of isolated IM particles. Corros. Sci. 2011, 53, 17–26. [Google Scholar] [CrossRef]
- Hughes, A.E.; Boag, A.; Glenn, A.M.; McCulloch, D.; Muster, T.H.; Ryan, C.; Luo, C.; Zhou, X.; Thompson, G.E. Corrosion of AA2024-T3 Part II: Co-operative corrosion. Corros. Sci. 2011, 53, 27–39. [Google Scholar] [CrossRef]
- Birbilis, N.; Zhu, Y.M.; Kairy, S.K.; Glenn, M.A.; Nie, J.F.; Morton, A.J.; Gonzalez-Garcia, Y.; Terryn, H.; Mol, J.M.C.; Hughes, A.E. A closer look at constituent induced localised corrosion in Al-Cu-Mg alloys. Corros. Sci. 2016, 113, 160–171. [Google Scholar] [CrossRef]
- Hashimoto, T.; Zhang, X.; Zhou, X.; Skeldon, P.; Haigh, S.J.; Thompson, G.E. Investigation of dealloying of S phase (Al2CuMg) in AA 2024-T3 aluminium alloy using high resolution 2D and 3D electron imaging. Corros. Sci. 2016, 103, 157–164. [Google Scholar] [CrossRef]
- Idrac, J.; Mankowski, G.; Thompson, G.; Skeldon, P.; Kihn, Y.; Blanc, C. Galvanic corrosion of aluminium–copper model alloys. Electrochim. Acta 2007, 52, 7626–7633. [Google Scholar] [CrossRef]
- Blanc, C.; Freulon, A.; Lafont, M.-C.; Kihn, Y.; Mankowski, G. Modelling the corrosion behaviour of Al2CuMg coarse particles in copper-rich aluminium alloys. Corros. Sci. 2006, 48, 3838–3851. [Google Scholar] [CrossRef]
- Zhu, D.; van Ooij, W.J. Corrosion protection of AA 2024-T3 by bis-[3-(triethoxysilyl)propyl]tetrasulfide in sodium chloride solution.: Part 2: Mechanism for corrosion protection. Corros. Sci. 2003, 45, 2177–2197. [Google Scholar] [CrossRef]
- Cornette, P.; Zanna, S.; Seyeux, A.; Costa, D.; Marcus, P. The native oxide film on a model aluminium-copper alloy studied by XPS and ToF-SIMS. Corros. Sci. 2020, 174, 108837. [Google Scholar] [CrossRef]
- Mei, L.; Chen, X.P.; Wang, C.; Xie, J.; Liu, Q. Good combination of strength and corrosion resistance in an Al-Cu-Mg alloy processed by a short-cycled thermomechanical treatment. Mater. Charact. 2021, 181, 111469. [Google Scholar] [CrossRef]
- Liu, X.Y.; Li, M.J.; Gao, F.; Liang, S.X.; Zhang, X.L.; Cui, H.X. Effects of aging treatment on the intergranular corrosion behavior of Al–Cu–Mg–Ag alloy. J. Alloys Compd. 2015, 639, 263–267. [Google Scholar] [CrossRef]
- Qi, H.; Liu, X.Y.; Liang, S.X.; Zhang, X.L.; Cui, H.X.; Zheng, L.Y.; Gao, F.; Chen, Q.H. Mechanical properties and corrosion resistance of Al–Cu–Mg–Ag heat-resistant alloy modified by interrupted aging. J. Alloys Compd. 2016, 657, 318–324. [Google Scholar] [CrossRef]
- Kurmanaeva, L.; Topping, T.D.; Wen, H.; Sugahara, H.; Yang, H.; Zhang, D.; Schoenung, J.M.; Lavernia, E.J. Strengthening mechanisms and deformation behavior of cryomilled Al–Cu–Mg–Ag alloy. J. Alloys Compd. 2015, 632, 591–603. [Google Scholar] [CrossRef]
- Qin, J.; Zhao, W.; Yan, Z.; Wang, R.; Yu, Z.; Shi, Z.; Liu, C.; Wang, B. Intergranular corrosion and stress corrosion cracking properties evaluation and mechanism study of multi-microalloyed 2519 Al alloy. Corros. Sci. 2024, 236, 112288. [Google Scholar] [CrossRef]
- Galvele, J.R.; de De Micheli, S.M. Mechanism of intergranular corrosion of Al-Cu alloys. Corros. Sci. 1970, 10, 795–807. [Google Scholar] [CrossRef]
- Wang, X.; Li, G.; He, Q.; Xiao, E.; Jiang, J.; Shao, W.; Zhen, L. Hydrogen trapping and embrittlement susceptibility in Al-Cu-Li alloys. Corros. Sci. 2024, 226, 111628. [Google Scholar] [CrossRef]
- Kamoutsi, H.; Haidemenopoulos, G.; Bontozoglou, V.; Pantelakis, S. Corrosion-induced hydrogen embrittlement in aluminum alloy 2024. Corros. Sci. 2006, 48, 1209–1224. [Google Scholar] [CrossRef]
- Embuka, D.; Coy, A.E.; Hernandez-Barrios, C.A.; Viejo, F.; Liu, Z. Thermal stability of excimer laser melted films formed on the AA2024-T351 aluminium alloy: Microstructure and corrosion performance. Surf. Coat. Technol. 2017, 313, 214–221. [Google Scholar] [CrossRef]
- Bonelli, M.; Miotello, A.; Mosaner, P. Morphological changes induced on aluminum surfaces by excimer laser irradiation. Appl. Surf. Sci. 2002, 186, 211–215. [Google Scholar] [CrossRef]
- Guillaumin, V.; Mankowski, G. Localized corrosion of 2024 T351 aluminium alloy in chloride media. Corros. Sci. 1998, 41, 421–438. [Google Scholar] [CrossRef]
- Cui, Z.-q.; Shi, H.-x.; Wang, W.-x.; Xu, B.-s. Laser surface melting AZ31B magnesium alloy with liquid nitrogen-assisted cooling. Trans. Nonferrous Met. Soc. China 2015, 25, 1446–1453. [Google Scholar] [CrossRef]
- Khalfaoui, W.; Valerio, E.; Masse, J.E.; Autric, M. Excimer laser treatment of ZE41 magnesium alloy for corrosion resistance and microhardness improvement. Opt. Lasers Eng. 2010, 48, 926–931. [Google Scholar] [CrossRef]
- Yue, T.M.; Yan, L.J.; Chan, C.P.; Dong, C.F.; Man, H.C.; Pang, G.K.H. Excimer laser surface treatment of aluminum alloy AA7075 to improve corrosion resistance. Surf. Coat. Technol. 2004, 179, 158–164. [Google Scholar] [CrossRef]
- Xu, W.L.; Yue, T.M.; Man, H.C.; Chan, C.P. Laser surface melting of aluminium alloy 6013 for improving pitting corrosion fatigue resistance. Surf. Coat. Technol. 2006, 200, 5077–5086. [Google Scholar] [CrossRef]
- Liu, Y.; Curioni, M.; Dong, S.; Liu, Z. Understanding the effects of excimer laser treatment on corrosion behavior of biodegradable Mg-1Ca alloy in simulated body fluid. J. Magnes. Alloys 2022, 10, 1004–1023. [Google Scholar] [CrossRef]
- Ahuir-Torres, J.I.; Arenas, M.A.; Perrie, W.; Dearden, G.; de Damborenea, J. Surface texturing of aluminium alloy AA2024-T3 by picosecond laser: Effect on wettability and corrosion properties. Surf. Coat. Technol. 2017, 321, 279–291. [Google Scholar] [CrossRef]
- Ibatan, T.; Uddin, M.S.; Chowdhury, M.A.K. Recent development on surface texturing in enhancing tribological performance of bearing sliders. Surf. Coat. Technol. 2015, 272, 102–120. [Google Scholar] [CrossRef]
- Kromer, R.; Costil, S.; Cormier, J.; Courapied, D.; Berthe, L.; Peyre, P.; Boustie, M. Laser surface patterning to enhance adhesion of plasma sprayed coatings. Surf. Coat. Technol. 2015, 278, 171–182. [Google Scholar] [CrossRef]

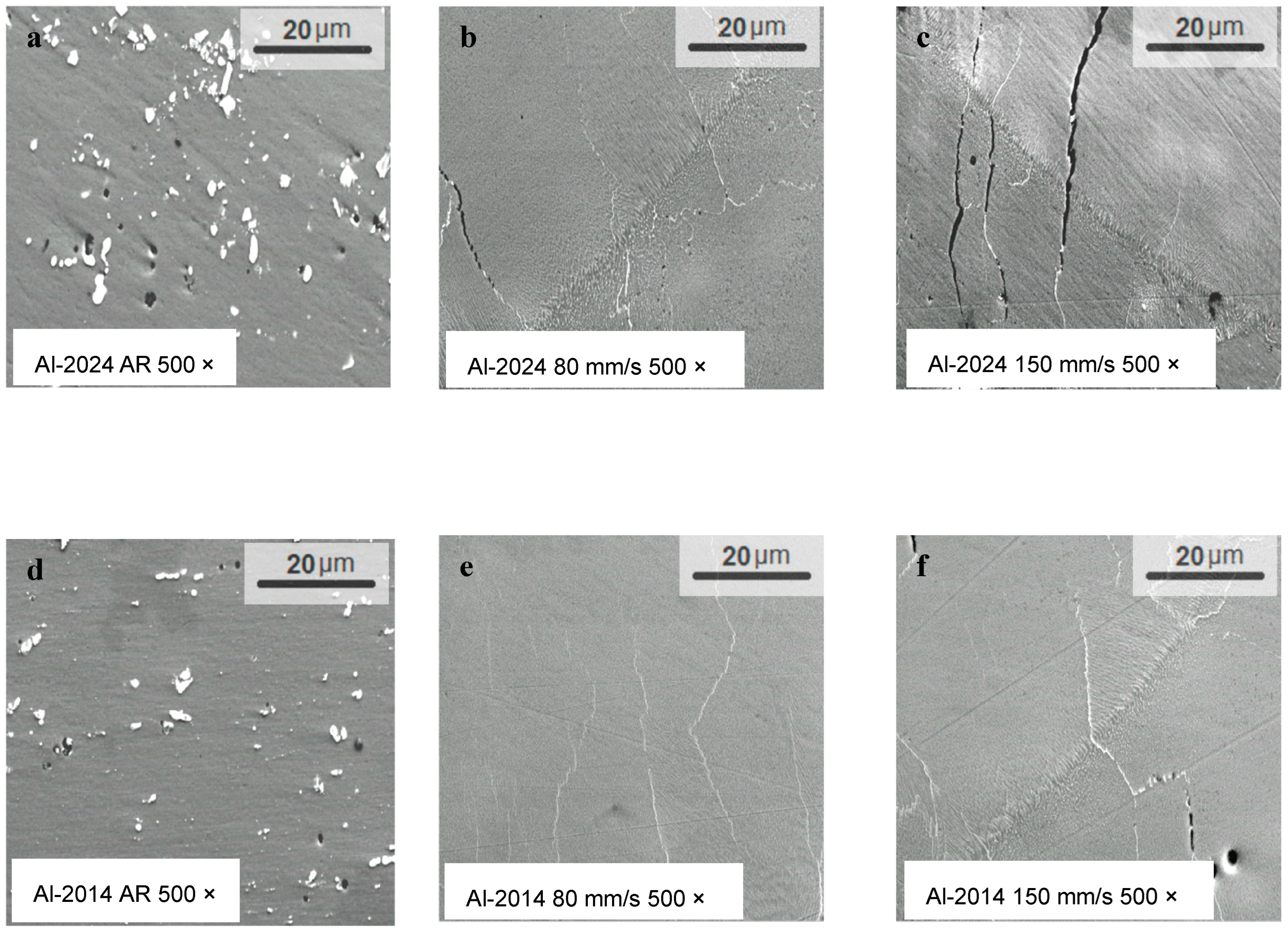
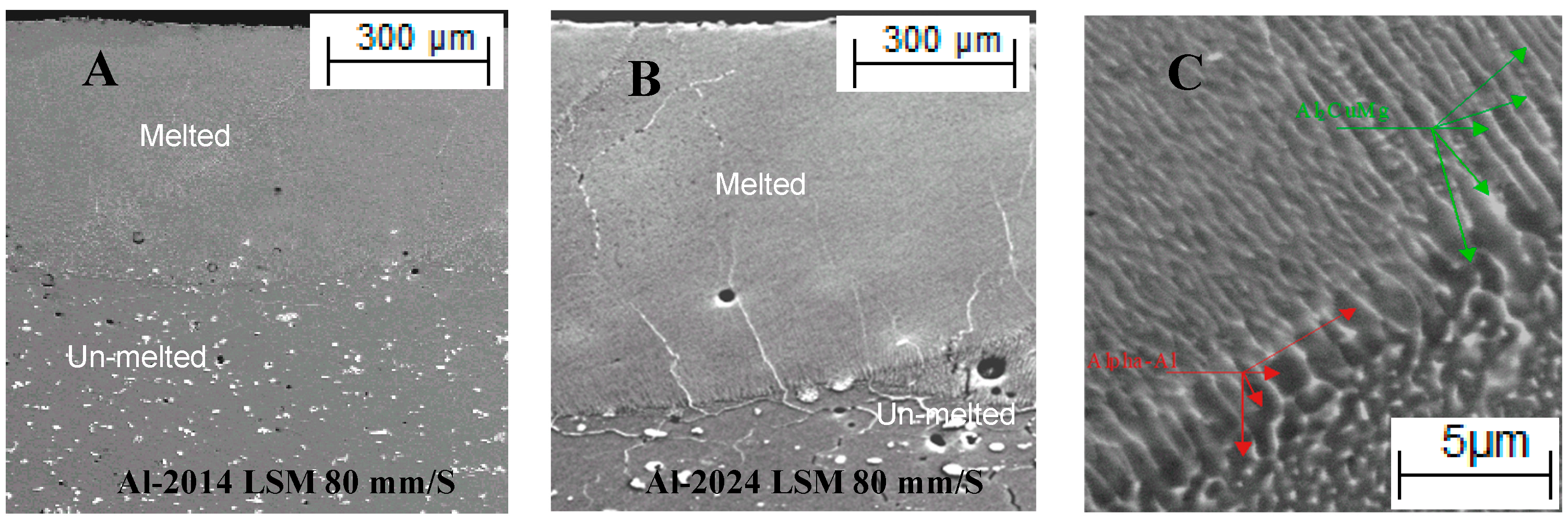

| Al-Cu 2014 Alloy | Al-Cu 2024 Alloy | ||
|---|---|---|---|
| Cu | 3.9–5 | Cu | 3.8–4.9 |
| Mg | 0.2–0.8 | Mg | 1.2–1.8 |
| Si | 0.5–1.2 | Si | 0.5 |
| Mn | 0.4–1.2 | Mn | 0.3–0.9 |
| Cr | 0.1 | Cr | 0.1 |
| Zn | 0.25 | Zn | 0.25 |
| Ti | 0.15 | Ti | 0.15 |
| Fe | 0.7 | Fe | 0.5 |
| Specimen | Power W | Overlap % Bead | Type of Laser | Shielding Gas | Scanning Speed mm/s |
|---|---|---|---|---|---|
| 2014 | 1.6 | 50 | CO2 | Argon | 20, 50, 80, 100, 120, 150 |
| 2024 | 1.6 | 50 | CO2 | Argon | 20, 50, 80, 100, 120, 150 |
| Scan Range 2θ | Step Size mm | Counting Time s/step | Incident Angle X-Ray | Copper Anode X-Ray Tube |
|---|---|---|---|---|
| 5° to 95° | 0.05 | 25 | 30° | 50 kV/40 mA |
| Electrolyte | De-Aeration Gas Purge | Potential mV | Sweep Rate mV/min | Current Limit mA/cm2 | Temperature °C | Reference Electrode |
|---|---|---|---|---|---|---|
| 1M NaCl | N2 | −200 to 1200 | 60 | 1 | 25 | Calomel |
| Scanning Speed (mm/s) | Depth of Bead (mm) | Width of Bead (mm) | Microstructure |
|---|---|---|---|
| 20 | 1.29 | 0.37 | Porosity at root bead |
| 50 | 1.24 | 0.34 | Porosity at root bead |
| 80 | 1.14 | 0.29 | Single crack, no porosity |
| 100 | 1.04 | 0.24 | Multiple cracks |
| 120 | 1.01 | 0.23 | |
| 150 | 0.99 | 0.21 | |
| 180 | 0.97 | 0.2 |
| Element wt. % | Copper | Magnesium | Silicon | Manganese |
|---|---|---|---|---|
| Melted (2014) | 5.25 ± 0.15 | 0.11 ± 0.02 | 1.00 ± 0.12 | 0.59 ± 0.08 |
| Un-melted (2014) | 4.78 ± 0.75 | 0.04 ± 0.01 | 0.37 ± 0.02 | 0.14 ± 0.02 |
| Melted (2024) | 4.87 ± 0.21 | 1.28 ± 0.12 | 0.11 ± 0.05 | 0.62 ± 0.03 |
| Un-melted (2024) | 3.97 ± 0.01 | 0.59 ± 0.03 | 0.04 ± 0.01 | 0.46 ± 0.01 |
| Specimen | Ecorr (mV) | Icorr (A/cm2) | Epit (mV) | Phases Present |
|---|---|---|---|---|
| Al-20014 AR | −1017 | 8.31 × 10−3 | −708 | α-Al, Cu3Mn2Al, CuAl2 |
| Al-2014 LSM | −960 | 5.51 × 10−3 | −679 | α-Al, Cu3Mn2Al, CuAl2 |
| Al-2024 AR | −1029 | 11.27 × 10−3 | −633 | α-Al, Al2CuMg |
| Al-2024 LSM | −1072 | 8.6 × 10−3 | −678 | α-Al, Al2CuMg, CuAl2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niaz, A.; Alwi, M.M.A. Microstructure Refinement or Increased Copper Solubility: Factors That Contribute to the Pitting Corrosion Tendency in Aluminum–Copper Alloys. Surfaces 2025, 8, 9. https://doi.org/10.3390/surfaces8010009
Niaz A, Alwi MMA. Microstructure Refinement or Increased Copper Solubility: Factors That Contribute to the Pitting Corrosion Tendency in Aluminum–Copper Alloys. Surfaces. 2025; 8(1):9. https://doi.org/10.3390/surfaces8010009
Chicago/Turabian StyleNiaz, Akbar, and Muhammad Mudassir Ahmad Alwi. 2025. "Microstructure Refinement or Increased Copper Solubility: Factors That Contribute to the Pitting Corrosion Tendency in Aluminum–Copper Alloys" Surfaces 8, no. 1: 9. https://doi.org/10.3390/surfaces8010009
APA StyleNiaz, A., & Alwi, M. M. A. (2025). Microstructure Refinement or Increased Copper Solubility: Factors That Contribute to the Pitting Corrosion Tendency in Aluminum–Copper Alloys. Surfaces, 8(1), 9. https://doi.org/10.3390/surfaces8010009







