Scanning Kelvin Probe Investigation of High-Strength Steel Surface after Impact of Hydrogen and Tensile Strain
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Surface Treatment
2.2. Electrochemical Treatment
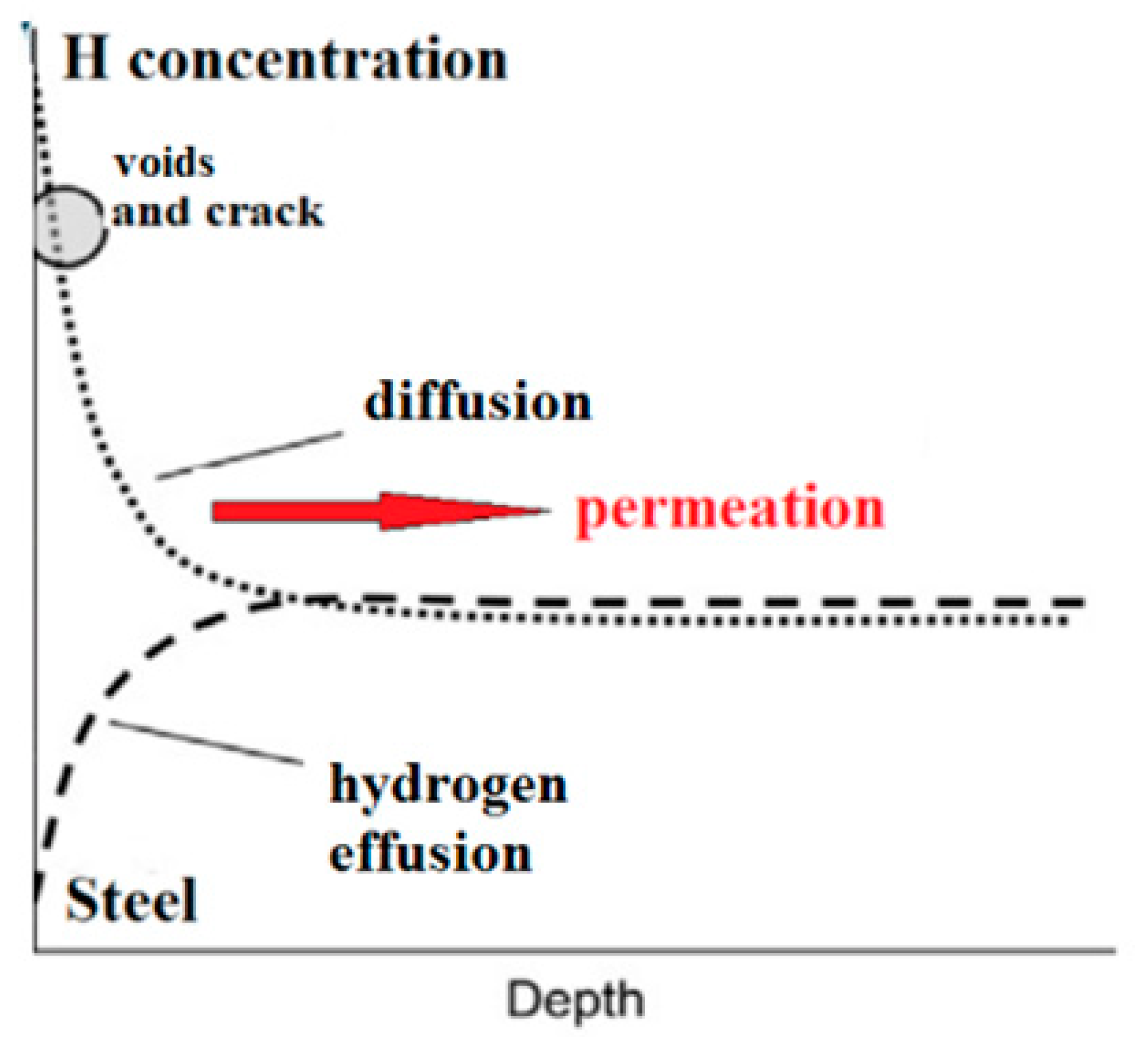
2.3. SKP Setup for Analysis of Stress and Hydrogen
3. Results and Discussion
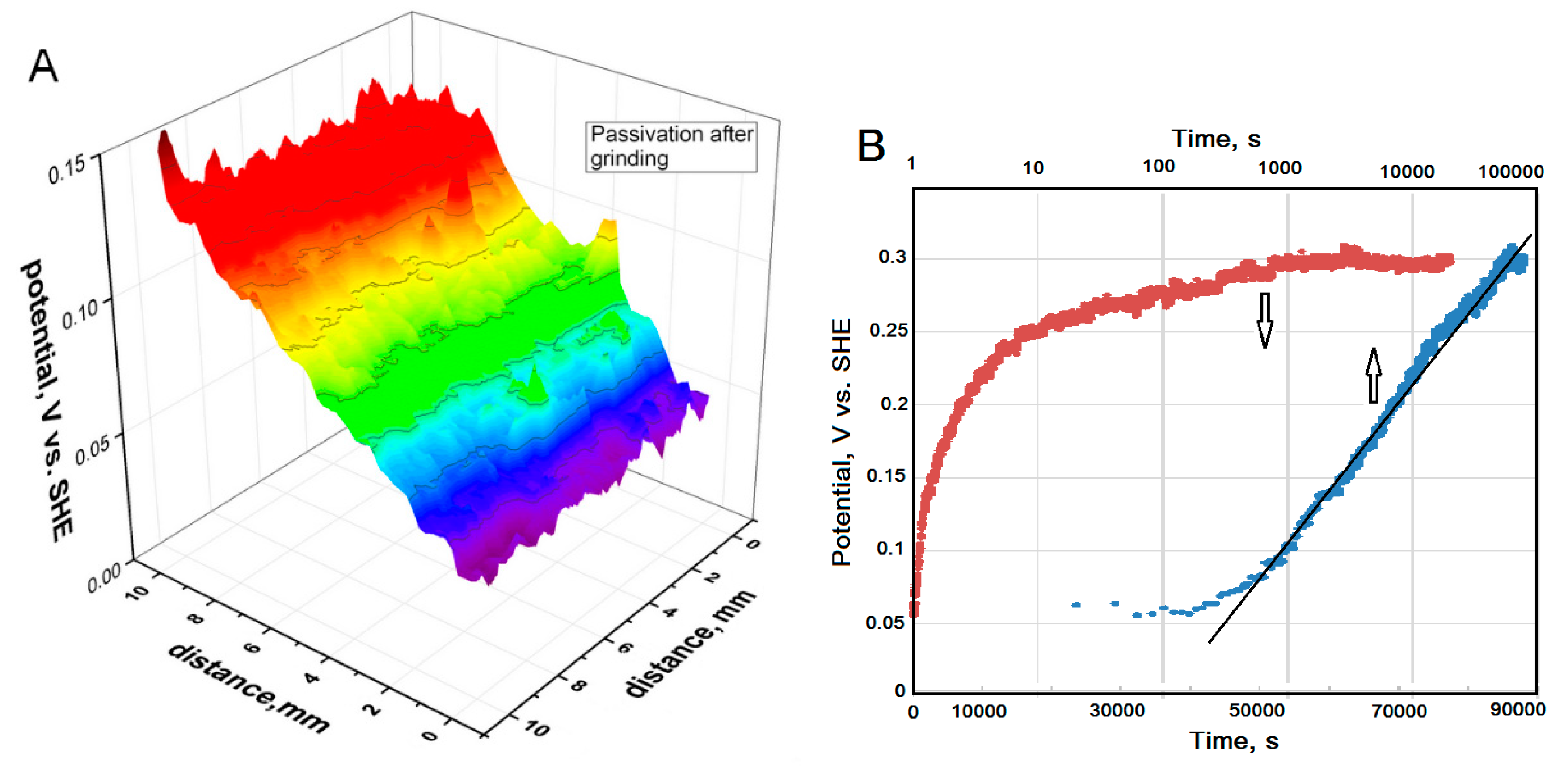
3.1. Potential Distribution above HSS after Grinding.
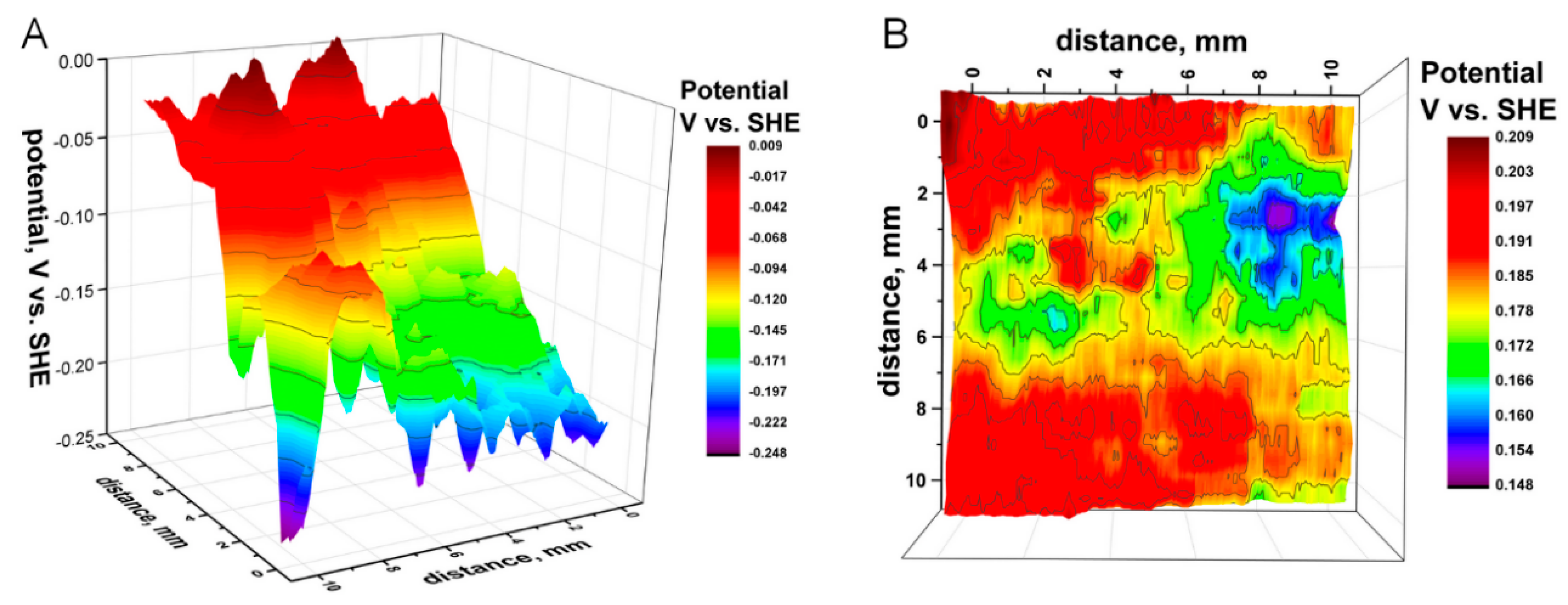
3.2. Effect of Tensile Strain on the Distribution of the Potential above HSS
3.3. SKP Assessment of Steel Surface after Cathodic Polarization in 0.1 M NaOH Aqueous Electrolyte
3.4. SKP Study of HSS Surface Interaction with Permeated Hydrogen
3.5. SKP Assessment of Stress and Hydrogen in Tensile HSS Sample
4. Conclusions
- The effect of tensile deformation on the potential of the high-strength steel was measured. Plastic strain (6%), close to the level to fracture, locally decreased the potential for 150 mV and exposure in air increased the potential. This is the result of the surface oxide breaking down with the following oxidation of the deformed surface.
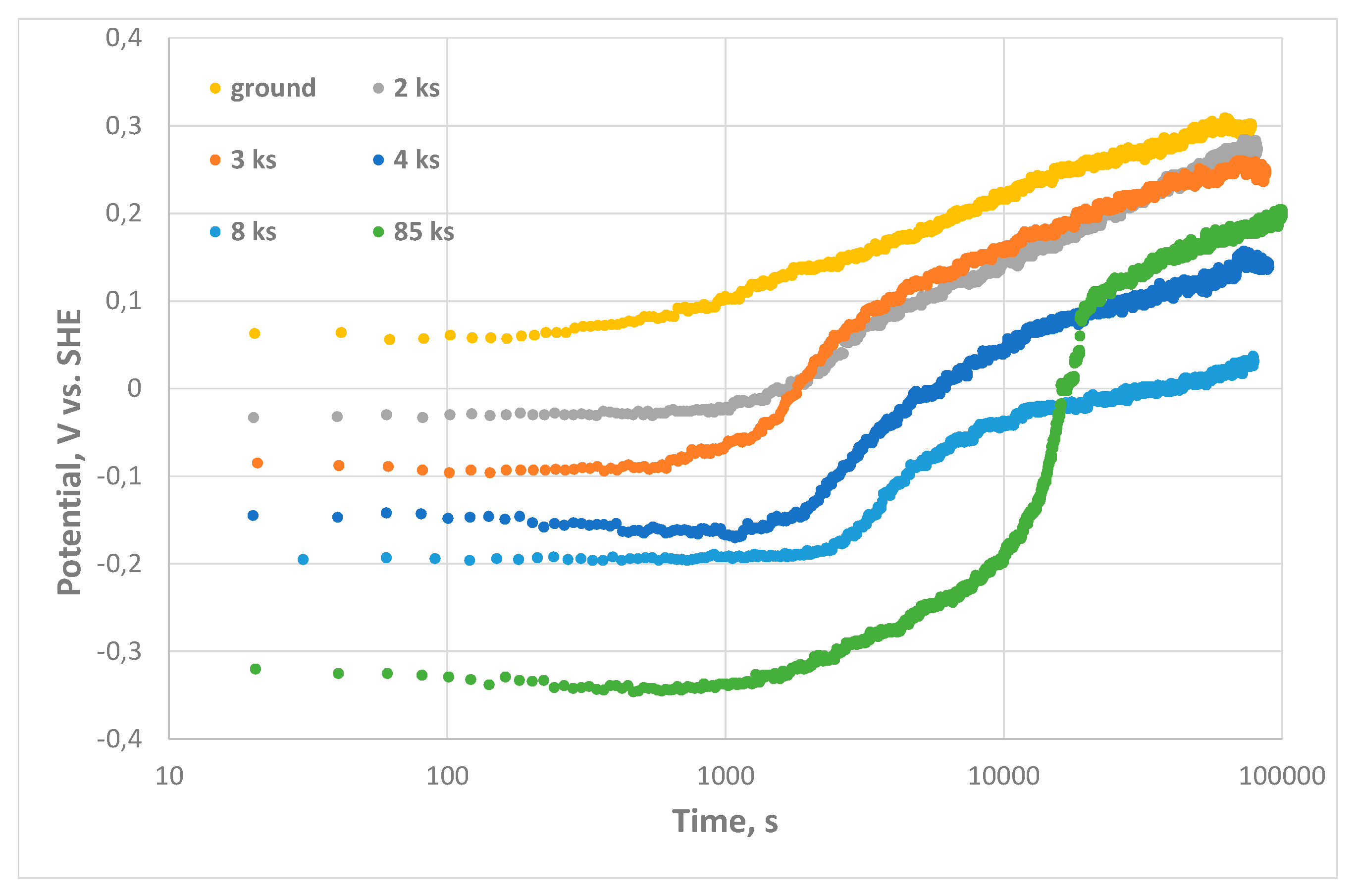
- The potential of specimen was monitored after cathodic hydrogen charging. Hydrogen effusion initially decreased the potential of steel for 300–500 mV and exposure in air and growth of surface oxide increased the potential. Effusion of subsurface hydrogen delayed the oxidation as a function of current density and duration of cathodic charging. Non-uniform distribution of the potential related to different surface reactivity was found for the case of charging with high current density.
- Hydrogen pre-charged or pre-strained surfaces showed prolonged electron reactivity. Locations containing residual stress and absorbed hydrogen showed the lowest potential (electron work function).
Author Contributions
Funding
Conflicts of Interest
References
- Omura, T.; Kudo, T.; Fujimoto, S. Environmental Factors Affecting Hydrogen Entry into High Strength Steel due to Atmospheric Corrosion. Mater. Trans. 2006, 47, 2956–2962. [Google Scholar] [CrossRef] [Green Version]
- Tsuru, T.; Huang, Y.; Ali, M.R.; Nishikata, A. Hydrogen entry into steel during atmospheric corrosion process. Corros. Sci. 2005, 47, 2431–2440. [Google Scholar] [CrossRef]
- So, K.H.; Kim, J.S.; Chun, Y.S.; Park, K.T.; Lee, Y.K.; Lee, C.S. Hydrogen delayed fracture properties and internal hydrogen behaviour of a Fe–18Mn–1.5Al–0.6C TWIP steel. ISIJ Int. 2009, 49, 1952–1959. [Google Scholar] [CrossRef] [Green Version]
- Hirth, J.P. Effects of hydrogen on the properties of iron and steel. Metall. Mater. Trans. A 1980, 11, 861–890. [Google Scholar] [CrossRef]
- Chiaberge, M. New Trends and Developments in Automotive System Engineering, High Mn TWIP Steels for Automotive Applications; De Cooman, B.C., Chin, K.G., Kim, J.K., Eds.; InTech: Rijeka, Croatia, 2011; pp. 101–128. [Google Scholar]
- Liu, Z.Y.; Li, X.G.; Cheng, Y.F. Mechanistic aspects of near-neutral stress corrosion cracking of pipelines under cathodic polarization. Corros. Sci. 2012, 55, 54–60. [Google Scholar] [CrossRef]
- Wang, M.; Akiyama, E.; Tsuzaki, K. Determination of the critical hydrogen concentration for delayed fracture of high strength steel by constant load test and numerical calculation. Corros. Sci. 2006, 48, 2189–2202. [Google Scholar] [CrossRef]
- Akiyama, E.; Matsukado, K.; Wang, M.; Tsuzaki, K. Evaluation of hydrogen entry into high strength steel under atmospheric corrosion. Corros. Sci. 2010, 52, 2758–2765. [Google Scholar] [CrossRef]
- Sanchez, J.; Lee, S.F.; Martin-Rengel, M.A.M.; Fullea, J.; Andrade, C.; Ruiz-Hervias, J. Measurement of hydrogen and embrittlement of high strength steel. Eng. Fail. Anal. 2016, 59, 467–477. [Google Scholar] [CrossRef]
- Nanninga, N.; Grochowsi, J.; Heldt, L.; Rundman, K. Role of microstructure, composition and hardness in resisting hydrogen embrittlement of fastener grade steels. Corros. Sci. 2010, 52, 1237–1246. [Google Scholar] [CrossRef]
- Cabrini, M.; Sinigaglia, E.; Spinelli, C.; Tarenzi, M.; Testa, C.; Bolzoni, F.M. Hydrogen Embrittlement Evaluation of Micro Alloyed Steels by Means of J-Integral Curve. Materials 2019, 12, 1843. [Google Scholar] [CrossRef] [Green Version]
- Pérez Escobar, D.; Depover, T.; Duprez, L.; Verbeken, K.; Verhaege, M. Combined thermal desorption spectroscopy, differential scanning calorimetry, scanning electron microscopy and X-ray diffraction study of hydrogen trapping in cold deformed TRIP steel. Acta Mater. 2012, 60, 2593–2605. [Google Scholar]
- Frappart, S.; Feaugas, X.; Creus, J.; Thebault, F.; Delattre, L.; Marchebois, H. Hydrogen solubility, diffusivity and trapping in a tempered Fe–C–Cr martensitic steel under various mechanical stress states. Mater. Sci. Eng. A 2012, 534, 384–393. [Google Scholar] [CrossRef]
- Koyama, M.; Rohwerder, M.; Tasan, C.C.; Bashir, A.; Akiyama, E.; Takai, K.; Raabe, D.; Tsuzaki, K. Recent progress in microstructural hydrogen mapping in steels: Quantification, kinetic analysis, and multi-scale characterization. Mater. Sci. Technol. 2017, 33, 1481–1496. [Google Scholar] [CrossRef] [Green Version]
- Ohmisawa, T.; Uchiyama, S.; Nagumo, M. Detection of hydrogen trap distribution in steel using a microprint technique. J. Alloys Compd. 2003, 356, 290–294. [Google Scholar] [CrossRef]
- Krieger, W.; Merzlikin, S.V.; Bashir, A.; Szczepaniak, A.; Springer, H.; Rohwerder, M. Spatially resolved localization and characterization of trapped hydrogen in zero to three dimensional defects inside ferritic steel. Acta Mater. 2015, 144, 236–244. [Google Scholar] [CrossRef]
- Nagao, A.; Kuramoto, S.; Ichitani, K.; Kanno, M. Visualization of hydrogen transport in high strength steels affected by stress fields and hydrogen trapping. Scr. Mater. 2001, 45, 1227–1232. [Google Scholar] [CrossRef]
- Ozdirik, B.; Suter, T.; Hans, U.; Depover, T.; Verbeken, K.; Schmutz, P.; Jeurgens, L.P.H.; Terryn, H.; De Graeve, I. Study of the hydrogen uptake in deformed steel using the microcapillary cell technique. Corros. Sci. 2019, 155, 55–66. [Google Scholar]
- Schaller, R.F.; Thomas, S.; Birbilis, N.; Scully, J.R. Spatially resolved mapping of the relative concentration of dissolved hydrogen using the scanning electrochemical microscope. Electrochem. Commun. 2015, 51, 54–58. [Google Scholar] [CrossRef]
- Evers, S.; Senöz, C.; Rohwerder, M. Hydrogen detection in metals: A review and introduction of a Kelvin probe approach. Sci. Technol. Adv. Mater. 2013, 14, 14201. [Google Scholar] [CrossRef]
- Evers, E.; Senöz, C.; Rohwerder, M. Spatially resolved high sensitive measurement of hydrogen permeation by scanning Kelvin probe microscopy. Electrochim. Acta 2013, 110, 534–538. [Google Scholar] [CrossRef]
- Senöz, C.; Evers, S.; Stratmann, M.; Rohwerder, M. Scanning Kelvin probe as a highly sensitive tool for detecting hydrogen permeation with high local resolution. Electrochem. Commun. 2011, 13, 1542–1545. [Google Scholar] [CrossRef]
- Williams, G.; McMurray, H.N.; Newman, R.C. Surface oxide reduction by hydrogen permeation through iron foil detected using a scanning Kelvin probe. Electrochem. Commun. 2013, 27, 144–147. [Google Scholar] [CrossRef]
- Schaller, R.F.; Scully, J.R. Measurement of effective hydrogen diffusivity using the scanning Kelvin probe. Electrochem. Commun. 2014, 40, 42–44. [Google Scholar] [CrossRef]
- Schaller, R.F.; Scully, J.R. Spatial determination of diffusible hydrogen concentrations proximate to pits in a Fe–Cr–Ni–Mo steel using the Scanning Kelvin Probe. Electrochem. Commun. 2016, 63, 5–9. [Google Scholar] [CrossRef] [Green Version]
- Nazarov, A.; Vucko, F.; Thierry, D. Scanning Kelvin Probe for detection of the hydrogen induced by atmospheric corrosion of ultra-high strength steel. Electrochim. Acta 2016, 216, 130–139. [Google Scholar] [CrossRef]
- Li, M.; Guo, L.Q.; Qiao, L.; Bai, Y. The mechanism of hydrogen-induced pitting corrosion in duplex stainless steel studied by SKPFM. Corros. Sci. 2012, 60, 76–81. [Google Scholar] [CrossRef]
- Wang, G.; Yan, Y.; Yang, X.; Li, J.; Qiao, L. Investigation of hydrogen evolution and enrichment by scanning Kelvin probe force microscopy. Electrochem. Commun. 2013, 35, 100–103. [Google Scholar] [CrossRef]
- Nazarov, A.; Thierry, D. Application of Volta potential mapping to determine metal surface defects. Electrochim. Acta 2007, 52, 7689–7696. [Google Scholar] [CrossRef]
- Fuertes Casals, N.; Nazarov, A.; Vucko, F.; Pettersson, R.; Thierry, D. Influence of Mechanical Stress on the Potential Distribution on a 301 LN Stainless Steel Surface. J. Electrochem. Soc. 2015, 162, C465. [Google Scholar] [CrossRef] [Green Version]
- Nazarov, A.; Vivier, V.; Vucko, F.; Thierry, D. Effect of Tensile Stress on the Passivity Breakdown and Repassivation of AISI 304 Stainless Steel: A Scanning Kelvin Probe and Scanning Electrochemical Microscopy Study. J. Electrochem. Soc. 2019, 166, C3207–C3219. [Google Scholar] [CrossRef]
- Wang, R.J.; Lia, J.X.; Su, Y.J.; Qiao, L.J.; Volinsky, A.A. Changes of work function in different deformation stage for 2205 duplex stainless steel by SKPFM. Procedia Mater. Sci. 2014, 3, 1736–1741. [Google Scholar] [CrossRef] [Green Version]
- ASTM E8/E8M-16 Standard Test Methods for Tension Testing of Metallic Materials; ASTM International: West Conshohocken, PA, USA, 2016.
- Sato, N.; Noda, T. Ion migration in anodic barrier oxide films on iron in acidic phosphate solutions. Electrochim. Acta 1977, 22, 839–843. [Google Scholar]
- Grosvenor, A.P.; Kobe, B.A.; McIntyre, N.S. Studies of the oxidation of iron by air after being exposed to water vapour using angle-resolved X-ray photoelectron spectroscopy and QUASES. Surf. Interface Anal. 2004, 36, 1637–1641. [Google Scholar] [CrossRef]
- Nazarov, A.; Vivier, V.; Thierry, D.; Vucko, F.; Tribollet, B. Effect of Mechanical Stress on the Properties of Steel Surfaces: Scanning Kelvin Probe and Local Electrochemical Impedance Study. J. Electrochem. Soc. 2017, 164, C66–C74. [Google Scholar] [CrossRef]
- Burstein, G.T.; Kearns, M.A. Accelerated Evolution of Hydrogen on Freshly Generated Metal Surfaces in Aqueous Solution. J. Electrochem. Soc. 1984, 131, 991–997. [Google Scholar] [CrossRef]
- Lufrano, J.; Sofronis, P. Enhanced hydrogen concentrations ahead of rounded notches and cracks–competition between plastic strain and hydrostatic stress. J. Acta Mater. 1998, 46, 1519–1526. [Google Scholar] [CrossRef]
- ISO 17081:2014 Method of Measurement of Hydrogen Permeation and Determination of Hydrogen Uptake and Transport in Metals by an Electrochemical Technique; ISO: Geneva, Switzerland, 2014.
- Masuda, H. SKFM observation of SCC on SUS304 stainless steel. Corros. Sci. 2007, 49, 120–129. [Google Scholar] [CrossRef]




| Material | Fe | C | Si | Mn | P | S | Al | Nb + Ti |
|---|---|---|---|---|---|---|---|---|
| MS 1500 | Bal. | <0.3 | <0.4 | <0.1 | <0.02 | 0.01 | >0.015 | <0.1 |
| Material | Yield Strength (MPa) | Ultimate Tensile Strength (MPa) | Elongation (%) |
|---|---|---|---|
| MS1500 | 1200 | 1500 | 7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazarov, A.; Vucko, F.; Thierry, D. Scanning Kelvin Probe Investigation of High-Strength Steel Surface after Impact of Hydrogen and Tensile Strain. Corros. Mater. Degrad. 2020, 1, 187-197. https://doi.org/10.3390/cmd1010009
Nazarov A, Vucko F, Thierry D. Scanning Kelvin Probe Investigation of High-Strength Steel Surface after Impact of Hydrogen and Tensile Strain. Corrosion and Materials Degradation. 2020; 1(1):187-197. https://doi.org/10.3390/cmd1010009
Chicago/Turabian StyleNazarov, Andrei, Flavien Vucko, and Dominique Thierry. 2020. "Scanning Kelvin Probe Investigation of High-Strength Steel Surface after Impact of Hydrogen and Tensile Strain" Corrosion and Materials Degradation 1, no. 1: 187-197. https://doi.org/10.3390/cmd1010009
APA StyleNazarov, A., Vucko, F., & Thierry, D. (2020). Scanning Kelvin Probe Investigation of High-Strength Steel Surface after Impact of Hydrogen and Tensile Strain. Corrosion and Materials Degradation, 1(1), 187-197. https://doi.org/10.3390/cmd1010009






