Corrosion Inhibitory Effects of Mullite in Concrete Exposed to Sulfuric Acid Attack
Abstract
:1. Introduction
2. Materials and Methods
- Control concrete: cement, sand, and aggregate (ratio 3:2:5) and water.
- Control mortar: cement, sand (ratio 3:7), and water.
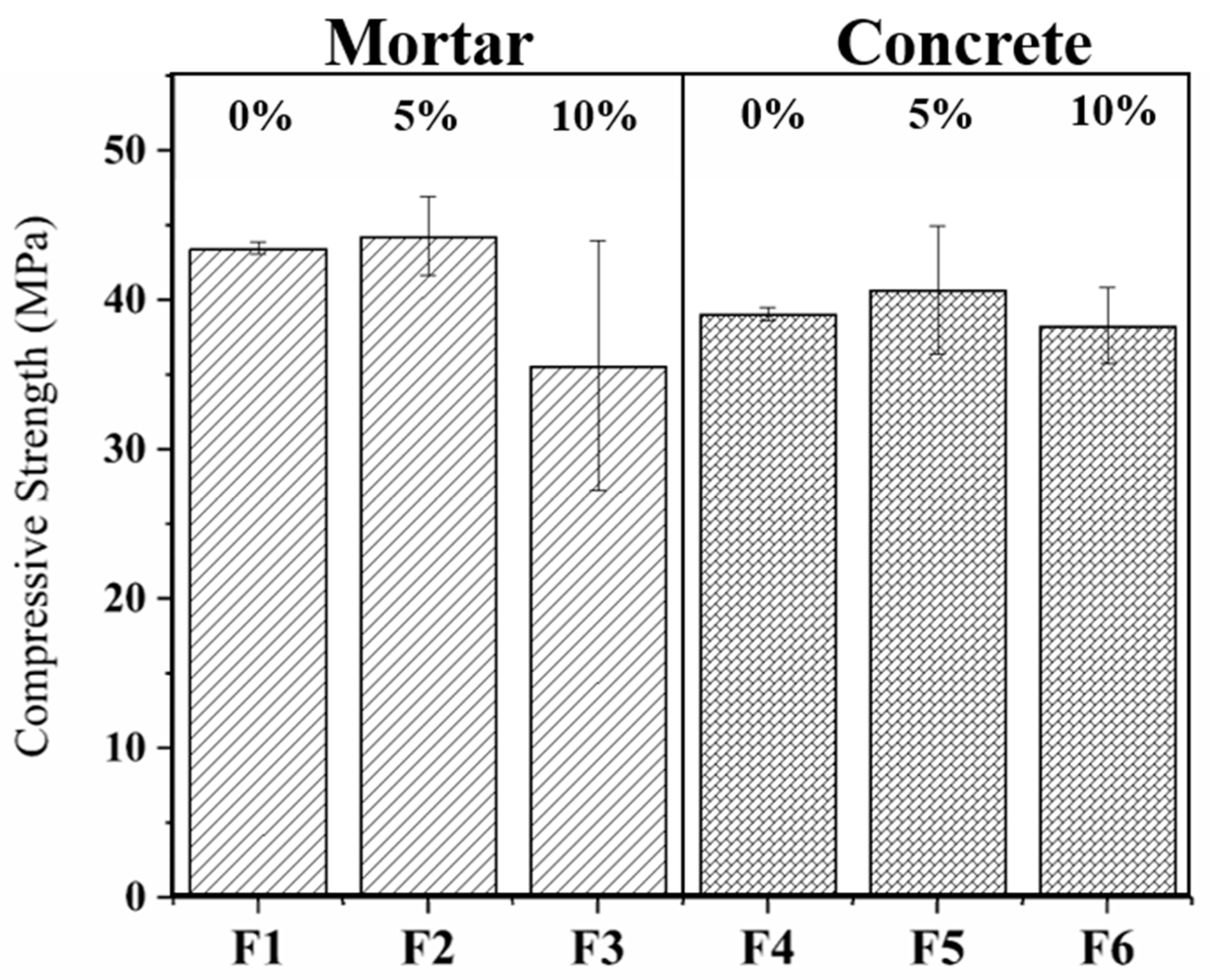
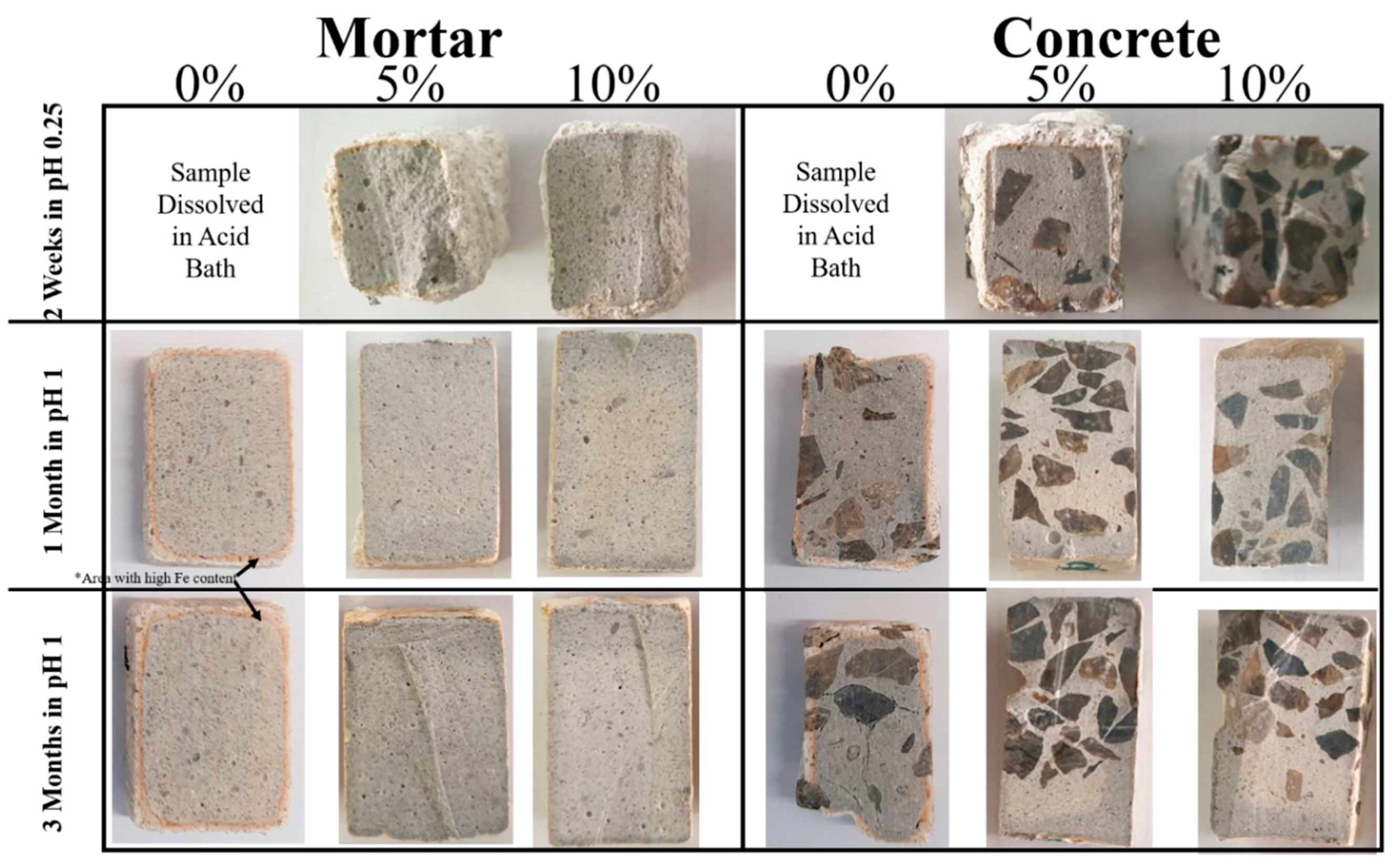
3. Results and Discussion
4. Conclusions
- ■
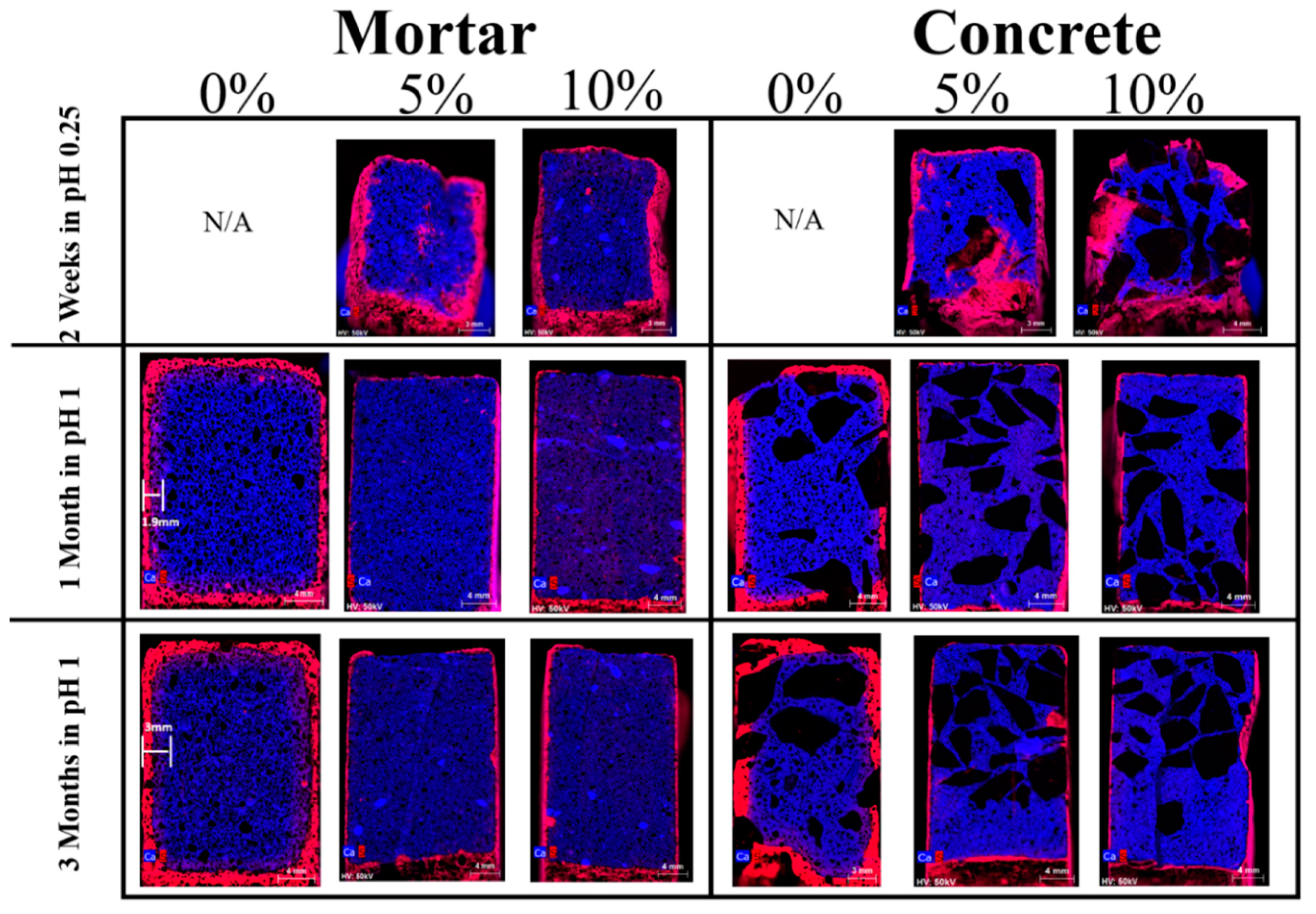
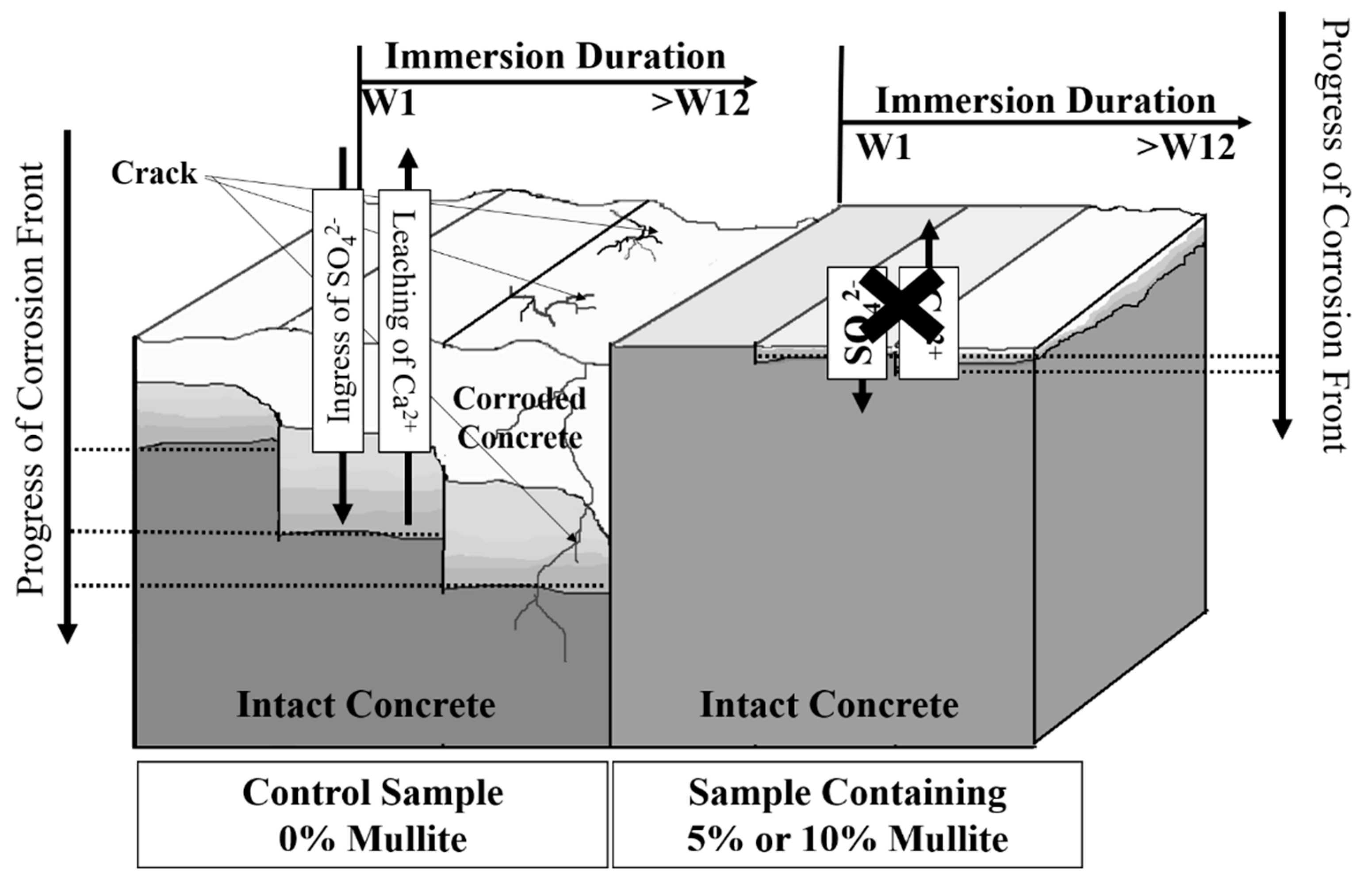
- The presence of Ca in the surface zone of 5% and 10% corroded samples is seen to be higher than in the control corroded samples. The extrinsic acid attack promotes decalcification of calcium-silicate-hydrate (C–S–H) and leaching of free Ca2+, thus the corroded areas are depleted in Ca and appeared whiter. The formation of silica-alumina gels in 5% and 10% mortar and samples seems to slow than this process and protect the samples from the ingress of invasive ions and rapid break down.
- ■
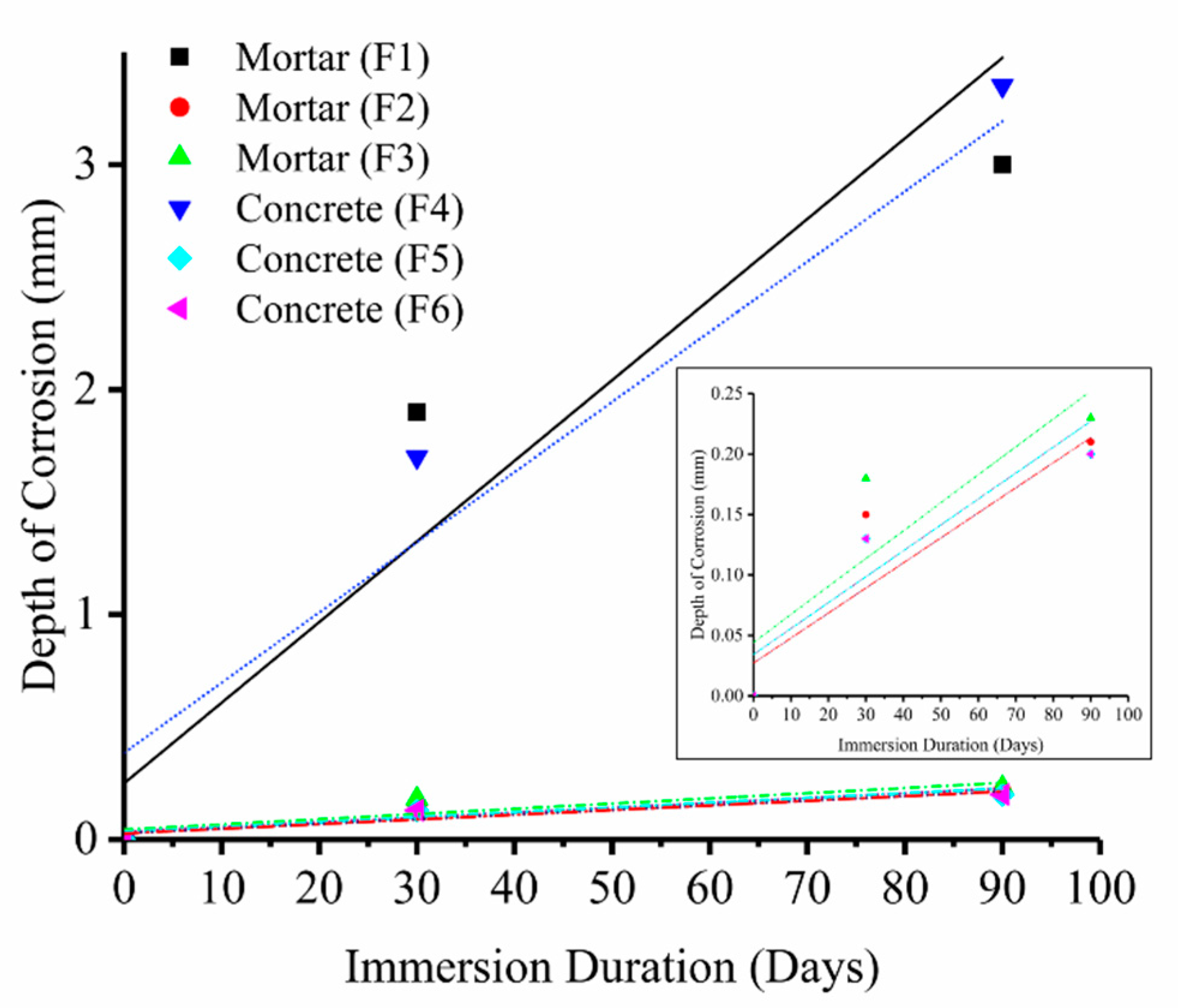
- The depth of microstructural changes, due to the formation of corrosion byproducts, in the control samples (F1 and F4, 0% mullite), was greater than 4000 µm, while in the 5% mullite formulations (F2 and F5) was around 1000 µm2, and in the 10% mullite formulations (F3 and F6), the depth of microstructural changes was limited to the first 100 µm2 from the surface.
- ■
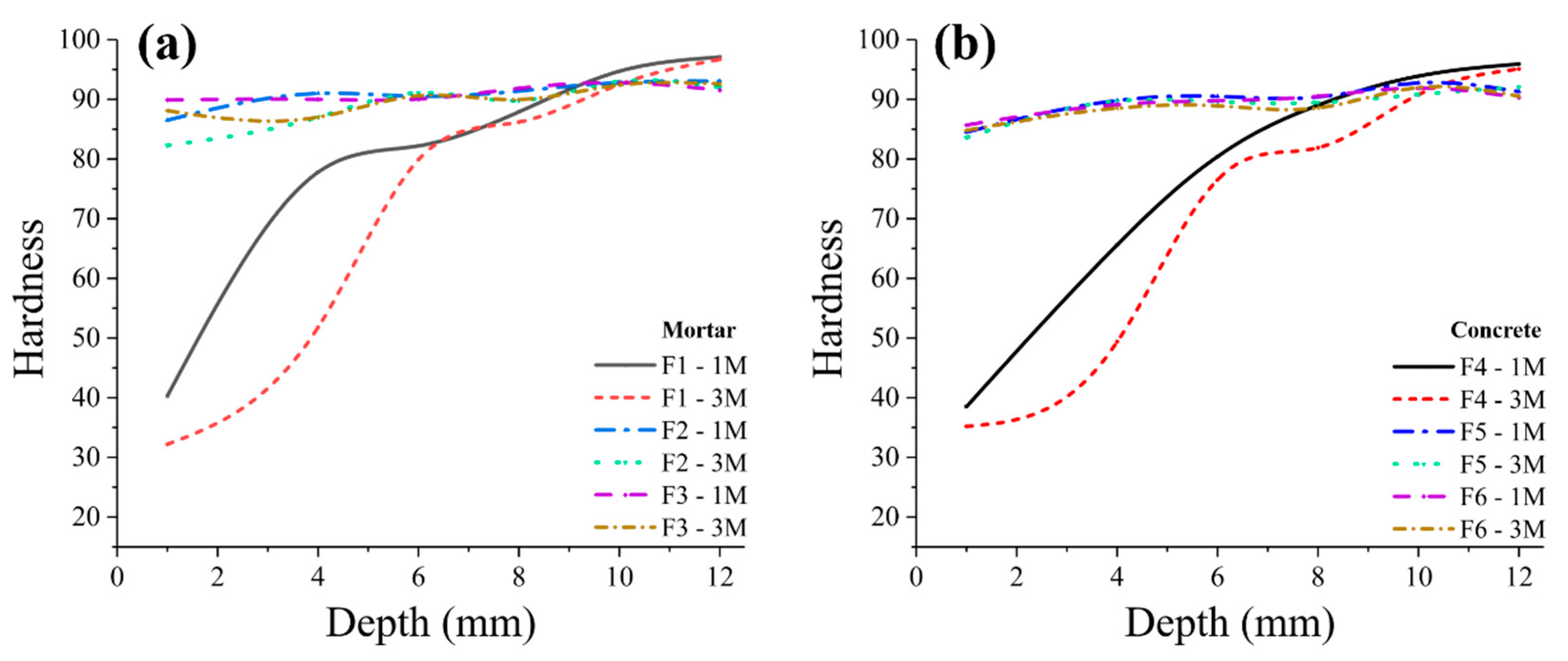
- With the increase of immersion time, control samples became softer at surface. This can be due to the formation of corrosion byproducts (such as gypsum) as well as decalcification of cement-paste. On average we detected a significant change in hardness as a function of depth in control mortar and concrete samples (0% mullite) with approximately 60% decrease in the hardness at the surface exposed to acidic media, and the depth of softening in three months corroded samples was almost up to 6 mm. Samples containing 5% and 10% mullite had a smaller corroded area compared to control samples, and that hardness did not really change with depth in those samples.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Marcos-Meson, V.; Fischer, G.; Edvardsen, C.; Skovhus, T.L.; Michel, A. Durability of Steel Fibre Reinforced Concrete (SFRC) exposed to acid attack—A literature review. Constr. Build. Mater. 2019, 200, 490–501. [Google Scholar] [CrossRef]
- Taheri, S.; Ams, M.; Bustamante, H.; Vorreiter, L.; Bevitt, J.J.; Withford, M.; Clark, S.M. Characterizing Concrete Corrosion Below Sewer Tidal Levels at Chemically Dosed Locations. Water Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Wiener, M.S.; Salas, B.V. Corrosion of the marine infrastructure in polluted seaports. Corros. Eng. Sci. Technol. 2005, 40, 137–142. [Google Scholar]
- Erbektas, A.R.; Isgor, O.B.; Weiss, W.J. Comparison of Chemical and Biogenic Acid Attack on Concrete. ACI Mater. J. 2020, 117, 255–264. [Google Scholar] [CrossRef]
- Allahverdi, A.; ŠkvÁra, F. Acidic corrosion of hydrated cement based materials. Ceram. Silik. 2000, 44, 152–160. [Google Scholar]
- Sersale, R.; Frigione, G.; Bonavita, L. Acid depositions and concrete attack: Main influences. Cem. Concr. Res. 1998, 28, 19–24. [Google Scholar] [CrossRef]
- Song, Y.; Wightman, E.; Kulandaivelu, J.; Bu, H.; Wang, Z.; Yuan, Z.; Jiang, G. Rebar corrosion and its interaction with concrete degradation in reinforced concrete sewers. Water Res. 2020, 182, 115961. [Google Scholar] [CrossRef]
- Song, Y.; Wightman, E.; Tian, Y.; Jack, K.; Li, X.; Zhong, H.; Bond, P.L.; Yuan, Z.; Jiang, G. Corrosion of reinforcing steel in concrete sewers. Sci. Total Environ. 2019, 649, 739–748. [Google Scholar] [CrossRef] [Green Version]
- Fattuhi, N.I.; Hughes, B.P. The performance of cement paste and concrete subjected to sulphuric acid attack. Cem. Concr. Res. 1988, 18, 545–553. [Google Scholar] [CrossRef]
- Beddoe, R.E.; Dorner, H.W. Modelling acid attack on concrete: Part I. The essential mechanisms. Cem. Concr. Res. 2005, 35, 2333–2339. [Google Scholar] [CrossRef]
- Romanova, A. Concrete corrosion induced by sulfuric acid. In Proceedings of the Sheffield Research Seminar, Sheffield, UK, 2 March 2016; p. 16120. [Google Scholar]
- Irico, S.; de Meyst, L.; Qvaeschning, D.; Alonso, M.C.; Villar, K.; de Belie, N. Severe Sulfuric Acid Attack on Self-Compacting Concrete with Granulometrically Optimized Blast-Furnace Slag-Comparison of Different Test Methods. Materials 2020, 13, 1431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Revertegat, E.; Richet, C.; Gegout, P. Effect of pH on the durability of cement pastes. Cem. Concr. Res. 1992, 22, 259–272. [Google Scholar] [CrossRef]
- Cao, H.T.; Bucea, L.; Ray, A.; Yozghatlian, S. The effect of cement composition and pH of environment on sulfate resistance of Portland cements and blended cements. Cem. Concr. Compos. 1997, 19, 161–171. [Google Scholar] [CrossRef]
- Panarese, W.C. Environmental Performance of Concrete. In ASM Handbook Volume 13B: Corrosion: Materials; ASM International: Cleveland, OH, USA, 2005; pp. 579–588. [Google Scholar] [CrossRef]
- Ulm, F.J.; Heukamp, F.H.; Germaine, J.T. Durability mechanics of calcium leaching of concrete and beyond. Fract. Mech. Concr. Struct. 2001, 1, 133–143. [Google Scholar]
- Hewayde, E.; Nehdi, M.; Allouche, E.; Nakhla, G. Effect of mixture design parameters and wetting-drying cycles on resistance of concrete to sulfuric acid attack. J. Mater. Civ. Eng. 2007, 19, 155–163. [Google Scholar] [CrossRef]
- Zhou, Y.; Tian, H.; Sui, L.; Xing, F.; Han, N. Strength deterioration of concrete in sulfate environment: An experimental study and theoretical modeling. Adv. Mater. Sci. Eng. 2015, 951209. [Google Scholar] [CrossRef] [Green Version]
- Islander, R.L.; Devinny, J.S.; Mansfeld, F.; Postyn, A.; Shih, H. Microbial ecology of crown corrosion in sewers. J. Environ. Eng. 1991, 117, 751–770. [Google Scholar] [CrossRef]
- Barton, L.L.; Tomei, F.A. Characteristics and activities of sulfate-reducing bacteria. In Sulfate-Reducing Bacteria; Springer: Boston, MA, USA, 1995; pp. 1–32. [Google Scholar]
- Huber, B.; Herzog, B.; Drewes, J.E.; Koch, K.; Müller, E. Characterization of sulfur oxidizing bacteria related to biogenic sulfuric acid corrosion in sludge digesters. BMC Microbiol. 2016, 16, 153. [Google Scholar] [CrossRef] [Green Version]
- Yuan, H.; Dangla, P.; Chatellier, P.; Chaussadent, T. Degradation modeling of concrete submitted to biogenic acid attack. Cem. Concr. Res. 2015, 70, 29–38. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; O’Moore, L.; Song, Y.; Bond, P.L.; Yuan, Z.; Wilkie, S.; Hanzic, L.; Jiang, G. The rapid chemically induced corrosion of concrete sewers at high H2S concentration. Water Res. 2019, 162, 95–104. [Google Scholar] [CrossRef]
- Fattuhi, N.I.; Hughes, B.P. Ordinary Portland cement mixes with selected admixtures subjected to sulfuric acid attack. Mater. J. 1988, 85, 512–518. [Google Scholar]
- Apté, N. Know your sewer-corrosion protection of sewer assets. In Proceedings of the 78th WIOA Victorian Water Industry Operations Conference & Exhibition Bendigo Exhibition Centre, Bendigo, Australia, 2–3 September 2015. [Google Scholar]
- Ganigue, R.; Gutierrez, O.; Rootsey, R.; Yuan, Z. Chemical dosing for sulfide control in Australia: An industry survey. Water Res. 2011, 45, 6564–6574. [Google Scholar] [CrossRef] [PubMed]
- Hewlett, P.; Liska, M. Lea’s Chemistry of Cement and Concrete; Butterworth-Heinemann: Oxford, UK, 2019. [Google Scholar]
- Mehta, P.K. Durability of concrete in marine environment—A review. Spec. Publ. 1980, 65, 1–20. [Google Scholar]
- Dahhou, M.; Arshad, M.A.; el Moussaouiti, M. Synthesis and characterisation of Portland cement clinker by exploiting waste oyster shells and alumina sludge. Eur. J. Environ. Civ. Eng. 2019, 1–17. [Google Scholar] [CrossRef]
- Chesley, J.; Burnet, G. A two-stage reaction sequence for C3S formation. Cem. Concr. Res. 1989, 19, 837–847. [Google Scholar] [CrossRef]
- Tan, B.; Okoronkwo, M.U.; Kumar, A.; Ma, H. Durability of calcium sulfoaluminate cement concrete. J. Zhejiang Univ. Sci. A 2020, 21, 118–128. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, S.; Lan, M.; Tian, G.; Liu, L. Influence of characteristics of alumina-silicate raw materials on the formation process of clinker. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2014, 29, 966–971. [Google Scholar] [CrossRef]
- Gartner, E.; Sui, T. Alternative cement clinkers. Cem. Concr. Res. 2018, 114, 27–39. [Google Scholar] [CrossRef]
- Liu, K.W.; Deng, M.; Mo, L.W. Effect of Fly Ash on Resistance to Sulfate Attack of Cement-based Materials. In Key Engineering Materials; Trans Tech Publications Ltd.: Bäch SZ, Switzerland, 2013; pp. 124–129. [Google Scholar]
- Goyal, S.; Kumar, M.; Sidhu, D.S.; Bhattacharjee, B. Resistance of mineral admixture concrete to acid attack. J. Adv. Concr. Technol. 2009, 7, 273–283. [Google Scholar] [CrossRef] [Green Version]
- Kiliswa, M.W.; Scrivener, K.L.; Alexander, M.G. The corrosion rate and microstructure of Portland cement and calcium aluminate cement-based concrete mixtures in outfall sewers: A comparative study. Cem. Concr. Res. 2019, 124, 105818. [Google Scholar] [CrossRef]
- Li, G.; Zhang, A.; Song, Z.; Shi, C.; Wang, Y.; Zhang, J. Study on the resistance to seawater corrosion of the cementitious systems containing ordinary Portland cement or/and calcium aluminate cement. Construct. Build. Mater. 2017, 157, 852–859. [Google Scholar] [CrossRef]
- Binici, H.; Aksoğan, O. Sulfate resistance of plain and blended cement. Cem. Concr. Compos. 2006, 28, 39–46. [Google Scholar] [CrossRef]
- Vuk, T.; Gabrovšek, R.; Kaučič, V. The influence of mineral admixtures on sulfate resistance of limestone cement pastes aged in cold MgSO4 solution. Cem. Concr. Res. 2002, 32, 943–948. [Google Scholar] [CrossRef]
- Harilal, M.; Uthaman, S.; Anandkumar, B.; Lahiri, B.B.; George, R.P.; Philip, J.; Amarendra, G. Fungal resistance of nanomodifiers and corrosion inhibitor amended fly ash concrete. Int. Biodeterior. Biodegrad. 2019, 143, 104725. [Google Scholar] [CrossRef]
- Rashad, A.M.; Seleem, H.E.-D.H.; Shaheen, A.F. Effect of Silica Fume and Slag on Compressive Strength and Abrasion Resistance of HVFA Concrete. Int. J. Concr. Struct. Mater. 2014, 8, 69–81. [Google Scholar] [CrossRef]
- Siddique, R. Utilization of silica fume in concrete: Review of hardened properties. Resour. Conserv. Recycl. 2011, 55, 923–932. [Google Scholar] [CrossRef]
- Jackson, M.D.; Chae, S.R.; Mulcahy, S.R.; Meral, C.; Taylor, R.; Li, P.; Emwas, A.-H.; Moon, J.; Yoon, S.; Vola, G. Unlocking the secrets of Al-tobermorite in Roman seawater concrete. Am. Mineral. 2013, 98, 1669–1687. [Google Scholar] [CrossRef] [Green Version]
- Jackson, M.D.; Mulcahy, S.R.; Chen, H.; Li, Y.; Li, Q.; Cappelletti, P.; Wenk, H.-R. Phillipsite and Al-tobermorite mineral cements produced through low-temperature water-rock reactions in Roman marine concrete. Am. Mineral. 2017, 102, 1435–1450. [Google Scholar] [CrossRef] [Green Version]
- Nagrockienė, D.; Girskas, G.; Skripkiūnas, G.; Daugėla, A. Properties of concrete modified by amorphous alumina silicate. Eng. Struct. Technol. 2014, 6, 178–183. [Google Scholar] [CrossRef]
- Ma, B.; Lothenbach, B. Synthesis, characterization, and thermodynamic study of selected Na-based zeolites. Cem. Concr. Res. 2020, 135, 106111. [Google Scholar] [CrossRef]
- Tran, Y.T.; Lee, J.; Kumar, P.; Kim, K.-H.; Lee, S.S. Natural zeolite and its application in concrete composite production. Compos. Part B Eng. 2019, 165, 354–364. [Google Scholar] [CrossRef]
- Ranjbar, N.; Kuenzel, C. Cenospheres: A review. Fuel 2017, 207, 1–12. [Google Scholar] [CrossRef]
- Schneider, H.; Schreuer, J.; Hildmann, B. Structure and properties of mullite—A review. J. Eur. Ceram. Soc. 2008, 28, 329–344. [Google Scholar] [CrossRef]
- ASTM. C39/C39M-18. Standard Test Method for Compressive Strength of Cylindrical Concrete Specimens; ASTM International: West Conshohocken, PA, USA, 2018. [Google Scholar]
- ASTM. C187-16. Standard Test Method for Amount of Water Required for Normal Consistency of Hydraulic Cement Paste; ASTM International: West Conshohocken, PA, USA, 2016. [Google Scholar]
- Reddy, M.S.; Neeraja, D. Mechanical and durability aspects of concrete incorporating secondary aluminium slag. Resour. Eff. Technol. 2016, 2, 225–232. [Google Scholar] [CrossRef] [Green Version]
- Isfahani, F.T.; Redaelli, E.; Lollini, F.; Li, W.; Bertolini, L. Effects of nanosilica on compressive strength and durability properties of concrete with different water to binder ratios. Adv. Mater. Sci. Eng. 2016, 8453567. [Google Scholar] [CrossRef] [Green Version]
- Gorse, C.; Johnston, D.; Pritchard, M. A Dictionary of Construction, Surveying, and Civil Engineering; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- Kaempfer, W.; Berndt, M. Estimation of Service Life of Concrete Pipes in Sewer Networks. Concrete Pipes in Sewer Networks. Available online: https://www.irbnet.de/daten/iconda/CIB2045.pdf (accessed on 30 July 2020).
- Poole, A.B.; Sims, I. Concrete Petrography: A Handbook of Investigative Techniques; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Mori, T.; Nonaka, T.; Tazaki, K.; Koga, M.; Hikosaka, Y.; Noda, S. Interactions of nutrients, moisture and pH on microbial corrosion of concrete sewer pipes. Water Res. 1992, 26, 29–37. [Google Scholar] [CrossRef]
- Santhanam, M.; Cohen, M.D.; Olek, J. Mechanism of sulfate attack: A fresh look. Cem. Concr. Res. 2003, 33, 341–346. [Google Scholar] [CrossRef]
- Santhanam, M.; Cohen, M.D.; Olek, J. Effects of gypsum formation on the performance of cement mortars during external sulfate attack. Cem. Concr. Res. 2003, 33, 325–332. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, J.; Wang, J.; Guan, X. Effects of Aluminum Sulfate and Quicklime/Fluorgypsum Ratio on the Properties of Calcium Sulfoaluminate (CSA) Cement-Based Double Liquid Grouting Materials. Materials 2019, 12, 1222. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.J.; Thomas, J.J.; Jennings, H.M. Decalcification shrinkage of cement paste. Cem. Concr. Res. 2006, 36, 801–809. [Google Scholar] [CrossRef]


| Properties | Test Method | Results |
|---|---|---|
| SiO2 | AS2350.2 | 19.10% |
| Al2O3 | AS2350.2 | 5.10% |
| Fe2O3 | AS2350.2 | 3.00% |
| CaO | AS2350.2 | 63.60% |
| MgO | AS2350.2 | 1.40% |
| Na2O | AS2350.2 | 0.50% |
| Equivalent Loss on Ignition | AS2350.2 | 4.20% |
| Fineness Index Normal | AS2350.8 | 375 m2/kg |
| Chloride Ion | BH-TM-0507 | 0.01% |
| Parameter Unit | Al3+ mg/L | Ca2+ mg/L | Fe2+ mg/L | Mg2+ mg/L | Si2+ mg/L | pH | Temperature °C |
|---|---|---|---|---|---|---|---|
| Results | 0.257 | 265.624 | 0.0282 | 14.730 | 3.535 | 7.8 | 23 |
| Material/ID | Mortar | Concrete | ||||
|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | F6 | |
| Cement | 30 | 25 | 20 | 30 | 25 | 20 |
| Sand | 70 | 70 | 70 | 20 | 20 | 20 |
| Aggregate | – | – | – | 50 | 50 | 50 |
| Mullite | – | 5 | 10 | – | 5 | 10 |
| Total | 100 | 100 | 100 | 100 | 100 | 100 |
| Water | 13 | 18.7 | 19.7 | 12.5 | 16 | 16.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taheri, S.; Delgado, G.P.; Agbaje, O.B.A.; Giri, P.; Clark, S.M. Corrosion Inhibitory Effects of Mullite in Concrete Exposed to Sulfuric Acid Attack. Corros. Mater. Degrad. 2020, 1, 282-295. https://doi.org/10.3390/cmd1020014
Taheri S, Delgado GP, Agbaje OBA, Giri P, Clark SM. Corrosion Inhibitory Effects of Mullite in Concrete Exposed to Sulfuric Acid Attack. Corrosion and Materials Degradation. 2020; 1(2):282-295. https://doi.org/10.3390/cmd1020014
Chicago/Turabian StyleTaheri, Shima, Gerardo Pareja Delgado, Oluwatoosin B. A. Agbaje, Paritosh Giri, and Simon Martin Clark. 2020. "Corrosion Inhibitory Effects of Mullite in Concrete Exposed to Sulfuric Acid Attack" Corrosion and Materials Degradation 1, no. 2: 282-295. https://doi.org/10.3390/cmd1020014








