Curious Corrosion Compounds Caused by Contact: A Review of Glass-Induced Metal Corrosion on Museum Exhibits (GIMME)
Abstract
:1. Introduction
2. Corrosion Factors and Their Investigation
2.1. Glass as Source of Electrolytes
2.2. Metals Prone to GIMME
2.3. Historic Objects with Contact between Glass and Metals
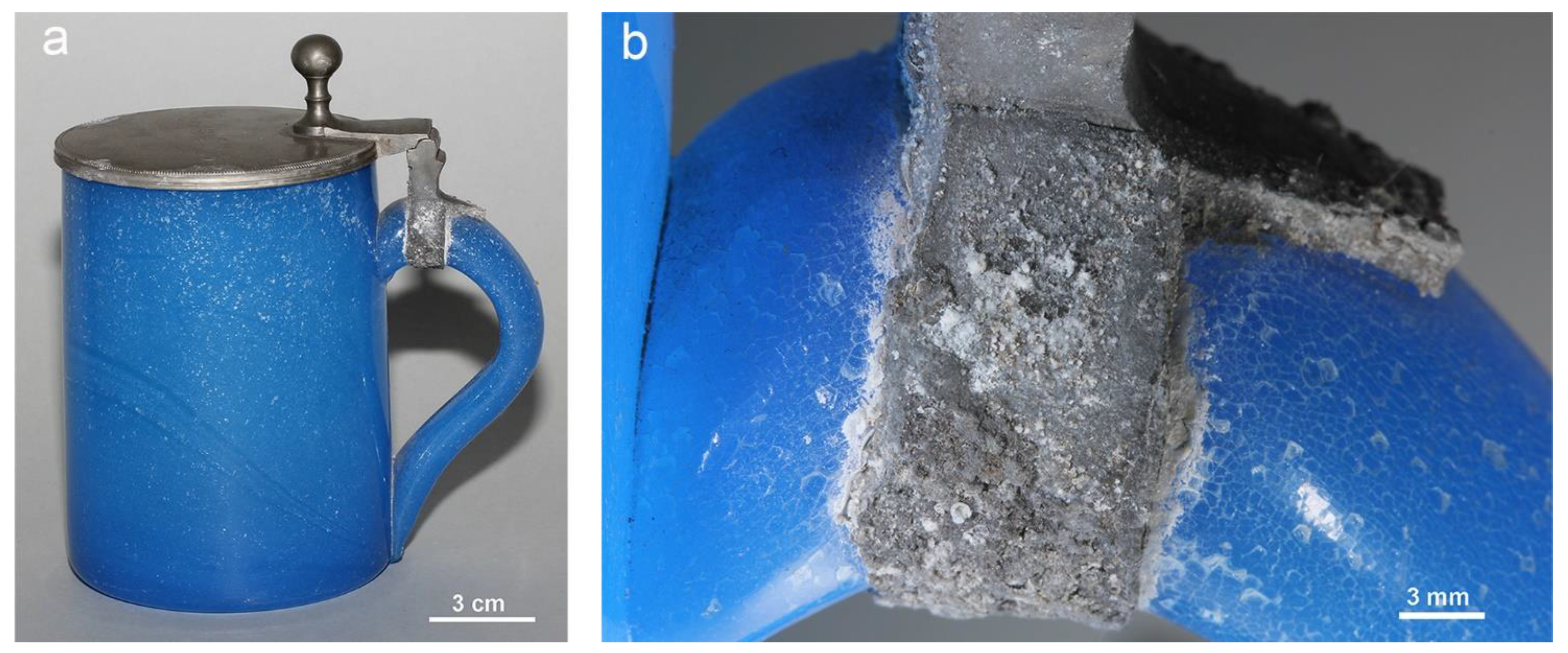
2.3.1. Fused Contact
2.3.2. Tight Mechanical Contact
2.3.3. Loose Contact
2.3.4. No Direct Contact
2.4. Analytical Identification Methods
3. GIMME Corrosion Compounds
3.1. Carbonates ①–④
3.1.1. NaPb2(CO3)2(OH) ①
3.1.2. KPb2(CO3)2(OH) ②
3.1.3. Na2[Cu(CO3)2]∙3H2O ③
3.1.4. NaCu(CH3COO)(CO3)∙nH2O ④
3.2. Formates ⑤–⑧
3.2.1. Cu4Na4O(HCOO)8(OH)2∙4H2O ⑤
3.2.2. Cu2(HCOO)(OH)3 ⑥
3.2.3. Zn(HCOO)2∙2H2O ⑦
3.2.4. Zn4Cu3(Zn1–xCux)6(HCOO)8(OH)18·6(H2O) ⑧
3.3. Uncharacterised Compounds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eggert, G. Corroding Glass, Corroding Metals: Survey of Joint Metal/Glass Corrosion Products on Historic Objects. Corros. Eng. Sci. Technol. 2010, 45, 414–419. [Google Scholar] [CrossRef]
- Eggert, G.; Fischer, A. Gefährliche Nachbarschaft: Durch Glas induzierte Metallkorrosion an Museums-Exponaten—Das GIMME-Projekt. Restauro 2012, 118, 38–43. [Google Scholar]
- Fischer, A. Glasinduzierte Metallkorrosion an Museums-Exponaten. Ph.D. Thesis, Staatliche Akademie der Bildenden Künste, Stuttgart, Germany, 19 December 2016. [Google Scholar] [CrossRef]
- Veiga, A.; Teixeira, D.; Candeias, A.; Mirão, J.; Rodrigues, P.; Teixeira, J. On the chemical signature and origin of dicoppertrihydroxyformate Cu2(OH)3HCOO) formed on copper miniatures of 17th and 18th centuries. Microsc. Microanal. 2016, 22, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Holzleitner, M.; Hietz, M.; Lenhart, E.; Anghelone, M.; Krist, G. Glass-Induced Metal Corrosion: Study and Conservation of an Enamelled Altarpiece (1954–1956) of the Collection of the University of Applied Arts Vienna. In Proceedings of the Metals 2019—Interim Meeting of the ICOM-CC Metals Working Group, Neuchâtel, Switzerland, 2–6 September 2019; Available online: https://icom-cc-publications-online.org (accessed on 30 July 2022).
- Marchetti, A.; Beltran, V.; Nuyts, G.; Borondics, F.; De Meyer, S.; Van Bos, M.; Jaroszewicz, J.; Otten, E.; Debulpaep, M.; De Wael, K. Novel optical photothermal infrared (O-PTIR) spectroscopy for the noninvasive characterization of heritage glass-metal objects. Sci. Adv. 2022, 8, eabl6769. [Google Scholar] [CrossRef]
- Saliba, N.A.; Yang, H.; Finlayson-Pitts, B.J. Reaction of gaseous nitric oxide with nitric acid on silica surfaces in the presence of water at room temperature. J. Phys. Chem. A 2001, 105, 10339–10346. [Google Scholar] [CrossRef]
- Schmutzler, B.; Eggert, G.; Kuhn-Wawrzinek, C.F. Copper(II) hydroxide on artefacts: Corrosion, conservation, colourants. Stud. Conserv. 2017, 62, 61–67. [Google Scholar] [CrossRef]
- Hatchfield, P. Pollutants in the Museum Environment, 1st ed.; Archetype: London, UK, 2002. [Google Scholar]
- Gibson, L.T.; Watt, C.M. Acetic and formic acids emitted from wood samples and their effect on selected materials in museum environments. Corros. Sci. 2010, 52, 172–178. [Google Scholar] [CrossRef]
- Thickett, D.; Ling, D. Investigation of Weeping Glass Deterioration Under Controlled Relative Humidity Conditions. Stud. Conserv. 2022, 67, 366–372. [Google Scholar] [CrossRef]
- Salthammer, T. Data on formaldehyde sources, formaldehyde concentrations and air exchange rates in European housings. Data Brief 2019, 22, 400–435. [Google Scholar] [CrossRef]
- Verhaar, G. Glass Sickness: Detection and Prevention. Ph.D. Thesis, University of Amsterdam, Amsterdam, The Netherlands, 18 October 2018. Available online: https://pure.uva.nl/ws/files/29086476/Thesis_complete_.pdf (accessed on 30 July 2022).
- Eggert, G. Abschlussbericht zum DBU-Projekt AZ 33255/01. Korrosion von National Wertvollen Kulturgütern aus Glas und Metall Durch Anthropogene Carbonyl-Schadgase im Innenraum: Modellhafte Schadensdiagnose und Maßnahmen zur Prävention; Staatliche Akademie der Bildenden Künste: Stuttgart, Germany, 2019. [Google Scholar] [CrossRef]
- Eggert, G.; Bette, S.; Dinnebier, R.E. Curious compounds—Investigating the Variety and Structure of Calcium Acetate Efflorescence on Calcareous Objects by XRPD. In Proceedings of the ICOM-CC 19th Triennial Conference, Beijing, China, 17–21 May 2021; Available online: https://icom-cc-publications-online.org (accessed on 30 July 2022).
- Fischer, A.; Eggert, G.; Dinnebier, R.; Runčevski, T. When glass and metal corrode together, V: Sodium copper formate. Stud. Conserv. 2018, 63, 342–355. [Google Scholar] [CrossRef]
- Dinnebier, R.E.; Fischer, A.; Eggert, G.; Runčevski, T.; Wahlberg, N. X-ray Powder Diffraction in Conservation Science: Towards Routine Crystal Structure Determination of Corrosion Products on Heritage Art Objects. J. Vis. Exp. 2016, 112, e54109. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.; Eggert, G.; Kirchner, D.; Euler, H.; Barbier, B. When Glass and Metal Corrode Together. IV, Sodium Lead Carbonate Hydroxide. In Proceedings of the Metal 2013—Interim Meeting of the ICOM-CC Metal Working Group, Edinburgh, UK, 16–20 September 2013; Available online: https://icom-cc-publications-online.org (accessed on 30 July 2022).
- Bette, S.; Eggert, G.; Fischer, A.; Dinnebier, R.E. Glass-induced Lead Corrosion of Heritage Objects: Structural Characterization of K(OH)·2PbCO3. Inorg. Chem. 2017, 56, 5762–5770. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.; Eggert, G.; Stelzner, J. When Glass and Metal Corrode Together, VI: Chalconatronite. Stud. Conserv. 2020, 65, 152–159. [Google Scholar] [CrossRef]
- Fischer, A.; Eggert, G.; Stelzner, J.; Bette, S.; Dinnebier, R.E. When Glass and Metal Corrode Together, VII: Zinc Formates and Further Unknown Zinc Compounds. In Proceedings of the Metals 2019—Interim Meeting of the ICOM-CC Metals Working Group, Neuchâtel, Switzerland, 2–6 September 2019; Available online: https://icom-cc-publications-online.org (accessed on 30 July 2022).
- Bette, S.; Fischer, A.; Stelzner, J.; Eggert, G.; Dinnebier, R.E. Brass and Glass: Crystal Structure Solution and Phase Characterisation of the Corrosion Product Zn4Cu3(Zn1−xCux)6(HCOO)8(OH)18∙6(H2O). Eur. J. Inorg. Chem. 2019, 2019, 920–927. [Google Scholar] [CrossRef]
- Kutzke, H.; Heym, S.; Schönemann, A. Natriumbleihydroxidcarbonat, NaPb2(OH)(CO3)2, als Weißpigment auf einem Eisengitter in der Pfarrkirche St. Martin, Oberwesel (Rheinland). In Archäometrie und Denkmalpflege 2009; Metalla Sonderheft 2; Deutsches Bergbau-Museum: Bochum, Germany, 2009; pp. 252–253. [Google Scholar]
- Auerbach, F.; Pick, H. Umsetzungen schwerlöslicher Bleisalze. Z. Elektrochem. 1913, 19, 827–830. [Google Scholar]
- Frade, J.C.; Oliveira, M.J. Uncovering the Decoration Techniques of a Southeast Asian Lacquered Buddha Sculpture. e-Conserv. J. 2014, 2, 79–93. [Google Scholar] [CrossRef]
- Ibáñez-Insa, J.; Elvira, J.J.; Llovet, X.; Pérez-Cano, J.; Oriols, N.; Busquets-Masó, M.; Hernández, S. Abellaite, NaPb2(CO3)2(OH), a new supergene mineral from the Eureka mine, Lleida province, Catalonia, Spain. Eur. J. Mineral. 2017, 29, 915–922. [Google Scholar] [CrossRef]
- Barger, S.; White, W.B. The Daguerreotype: 19th Century Technology and Modern Science; Johns Hopkins Press: London, UK, 2000; p. 167. [Google Scholar]
- Sengupta, A.K.; Nandi, A.K. Complex Carbonates of Copper (II). J. Inorg. Nucl. Chem. 1974, 36, 2479–2484. [Google Scholar] [CrossRef]
- Eggert, G.; Fischer, A.; Dinnebier, R.E. One Heritage Corrosion Product Less: Basic Sodium Copper Carbonate. Herit. Sci. 2016, 4, 27. [Google Scholar] [CrossRef]
- Thickett, D.; Odlyha, M. Note on the Identification of an Unusual Pale Blue Corrosion Product from Egyptian Copper Alloy Artifacts. Stud. Conserv. 2000, 45, 63–67. [Google Scholar] [CrossRef]
- Paterakis, A.B. The Formation of Acetate Corrosion on Bronze Antiquities: Characterisation and Conservation. Ph.D. Thesis, University College of London, London, UK, 1 June 2011. Available online: https://discovery.ucl.ac.uk/id/eprint/1318069 (accessed on 30 July 2022).
- Eggert, G.; Fischer, A. The formation of formates: A review of metal formates on heritage objects. Herit. Sci. 2021, 9, 26. [Google Scholar] [CrossRef]
- Trentelman, K.; Stodulski, L.; Scott, D.; Back, M.; Stock, S.; Strahan, D.; Drews, A.R.; O’Neill, A.; Weber, W.H.; Chen, A.E.; et al. The Characterization of a New Pale Blue Corrosion Product Found on Copper Alloy Artifacts. Stud. Conserv. 2002, 47, 217–227. [Google Scholar] [CrossRef]
- Dinnebier, R.E.; Runčevski, T.; Fischer, A.; Eggert, G. Solid-state Structure of a Degradation Product Frequently Observed on Historic Metal Objects. Inorg. Chem. 2015, 54, 2638–2642. [Google Scholar] [CrossRef] [PubMed]
- Eggert, G.; Wollman, A.; Schwahn, B.; Hustedt-Martens, E.; Barbier, B.; Euler, H. When glass and metal corrode together. In Proceedings of the ICOM Committee for Conservation 15th Triennial Meeting, New Delhi, India, 22–26 September 2008; Available online: https://icom-cc-publications-online.org (accessed on 30 July 2022).
- Eggert, G.; Bührer, A.; Barbier, B.; Euler, H. When Glass and metal corrode together, II: A Black Forest Schäppel and Further Occurences of Socoformacite. In Glass and Ceramics Conservation 2010; Roemich, H., Ed.; Corning Museum of Glass: Corning, NY, USA, 2010; pp. 174–180. [Google Scholar]
- Eggert, G.; Haseloff, S.; Euler, H.; Barbier, B. When Glass and Metal Corrode Together, III: The Formation of Dicopper Trihydroxy-Formate. In Proceedings of the ICOM-CC 16th Ttriennial Conference, Lisbon, Portugal, 19–23 September 2011; Critério-Produção Grafica: Lisbon, Portugal,, 2011; pp. 1–9. Available online: https://icom-cc-publications-online.org (accessed on 30 July 2022).
- Euler, H.; Barbier, B.; Kirfel, A.; Haseloff, S.; Eggert, G. Crystal Structure of Trihydroxydicopper Formate, Cu2(OH)3(HCOO). Z. Krist. New Cryst. Struct. 2009, 224, 609–610. [Google Scholar] [CrossRef]
- Keller, I.; Fischer, A. How Rare Is It? A Survey in the Swiss National Museum. In Glass Deterioration Colloquium—Extended Abstracts; Staatliche Akademie der Bildenden Künste: Stuttgart, Germany, 2015; pp. 41–44. [Google Scholar]
- Schorpp, A.; Braun, M.; Fischer, A.; Eggert, G. In Search of Frequency: Glass-induced Metal Corrosion in the Deutsches Bergbau-Museum Bochum. METALLA 2019, 25, 33–41. Available online: https://metalla.org/index.php/METALLA/article/view/9269/8810 (accessed on 30 July 2022). [CrossRef]
- Eggert, G. Saturated salt solutions in showcases: Humidity control and pollutant absorption. Herit. Sci. 2022, 10, 54. [Google Scholar] [CrossRef]








| Compounds | Formula | Ref. |
|---|---|---|
| Carbonates | ||
| ① Sodium lead carbonate hydroxide | NaPb2(CO3)2(OH) | [18] |
| ② Potassium lead carbonate hydroxide | KPb2(CO3)2(OH) | [19] |
| ③ Sodium dicarbonato cuprate(II) trihydrate | Na2[Cu(CO3)2]∙3H2O | [20] |
| ④ Sodium copper acetate carbonate hydrate | NaCu(CH3COO)(CO3)∙nH2O | [3] |
| Formates | ||
| ⑤ Sodium copper formate hydroxide oxide tetrahydrate | Cu4Na4O(HCOO)8(OH)2∙4H2O | [16] |
| ⑥ Copper formate trihydroxide | Cu2(HCOO)(OH)3 | [3] |
| ⑦ Zinc formate dihydrate | Zn(HCOO)2∙2H2O | [21] |
| ⑧ Zinc copper formate hydroxide hydrate | Zn4Cu3(Zn1–xCux)6(HCOO)8(OH)18·6H2O 0 ≤ x ≤ 1 | [22] |
| Uncharacterised Compounds | ||
| Compounds containing Na, K, Cu, and/or Zn, often carboxylates | [3] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eggert, G.; Fischer, A. Curious Corrosion Compounds Caused by Contact: A Review of Glass-Induced Metal Corrosion on Museum Exhibits (GIMME). Corros. Mater. Degrad. 2022, 3, 553-565. https://doi.org/10.3390/cmd3030030
Eggert G, Fischer A. Curious Corrosion Compounds Caused by Contact: A Review of Glass-Induced Metal Corrosion on Museum Exhibits (GIMME). Corrosion and Materials Degradation. 2022; 3(3):553-565. https://doi.org/10.3390/cmd3030030
Chicago/Turabian StyleEggert, Gerhard, and Andrea Fischer. 2022. "Curious Corrosion Compounds Caused by Contact: A Review of Glass-Induced Metal Corrosion on Museum Exhibits (GIMME)" Corrosion and Materials Degradation 3, no. 3: 553-565. https://doi.org/10.3390/cmd3030030
APA StyleEggert, G., & Fischer, A. (2022). Curious Corrosion Compounds Caused by Contact: A Review of Glass-Induced Metal Corrosion on Museum Exhibits (GIMME). Corrosion and Materials Degradation, 3(3), 553-565. https://doi.org/10.3390/cmd3030030










