Development of an In Situ Micro-Corrosion Cell for the Investigation of Pitting Corrosion on Austenitic and Ferritic Stainless Steels
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
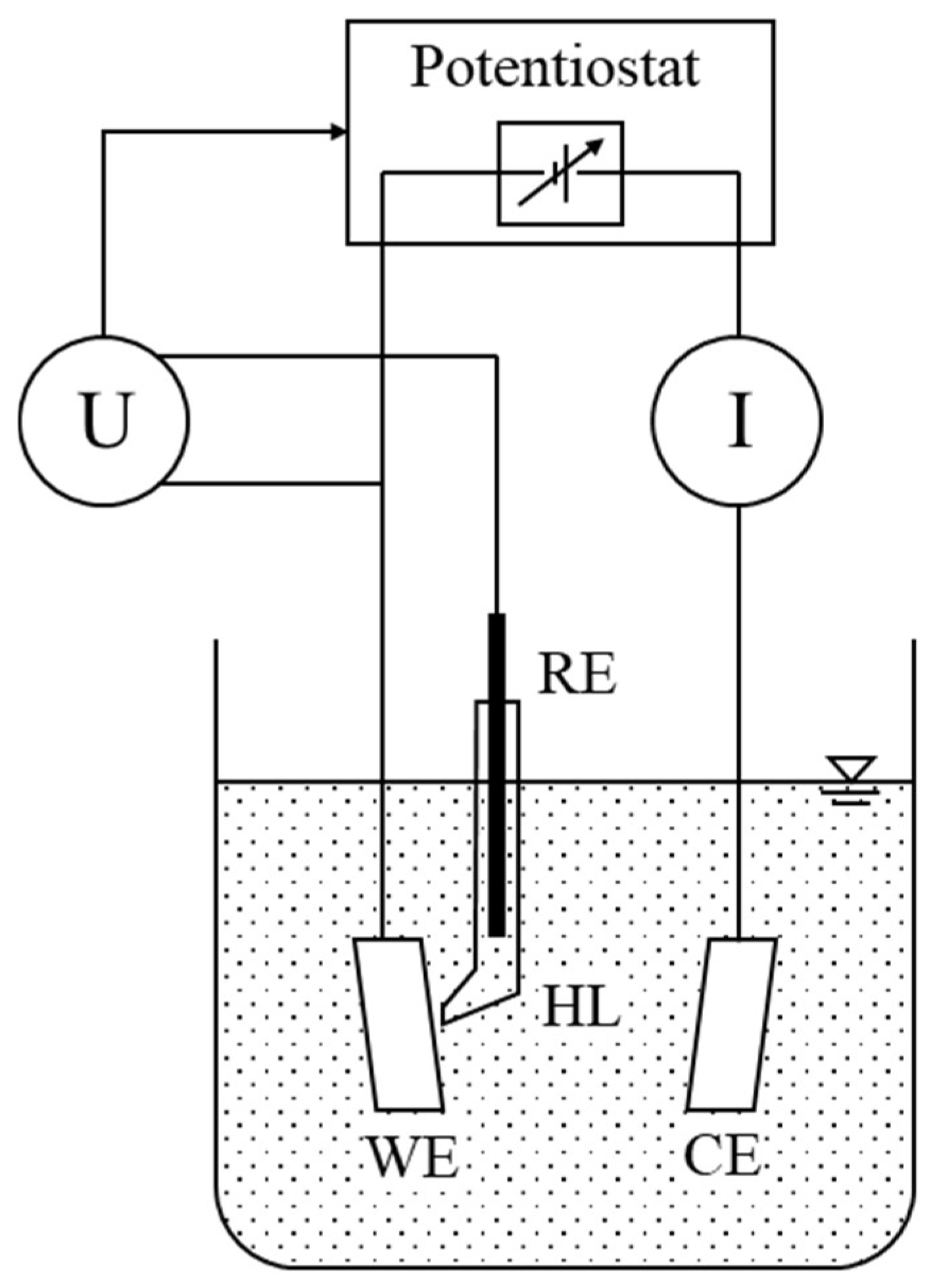
2.2. Macro-Corrosion Cell for Electrochemical Corrosion Testing
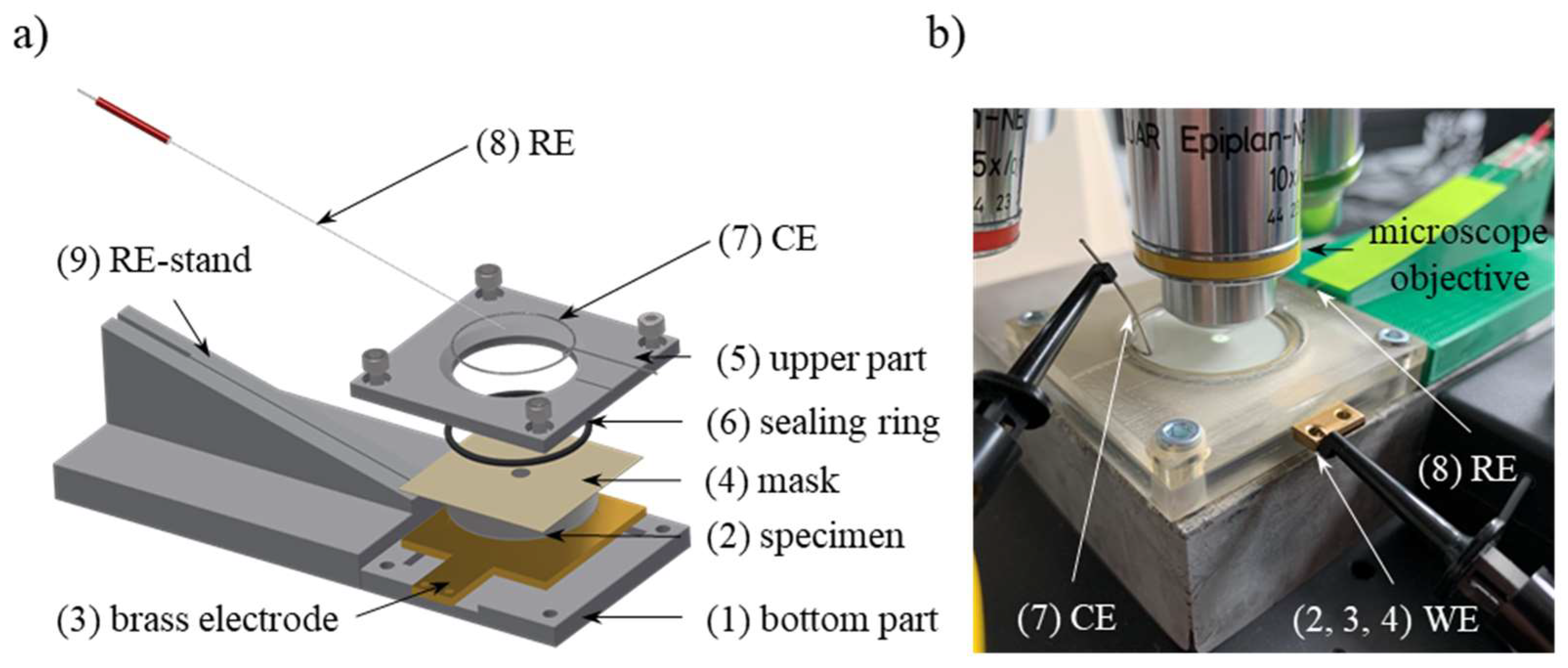
2.3. Micro-Corrosion Cell for In Situ Electrochemical Corrosion Testing
2.4. Electrochemical Test Setup
2.5. High-Resolution Microscopy
3. Results and Discussion
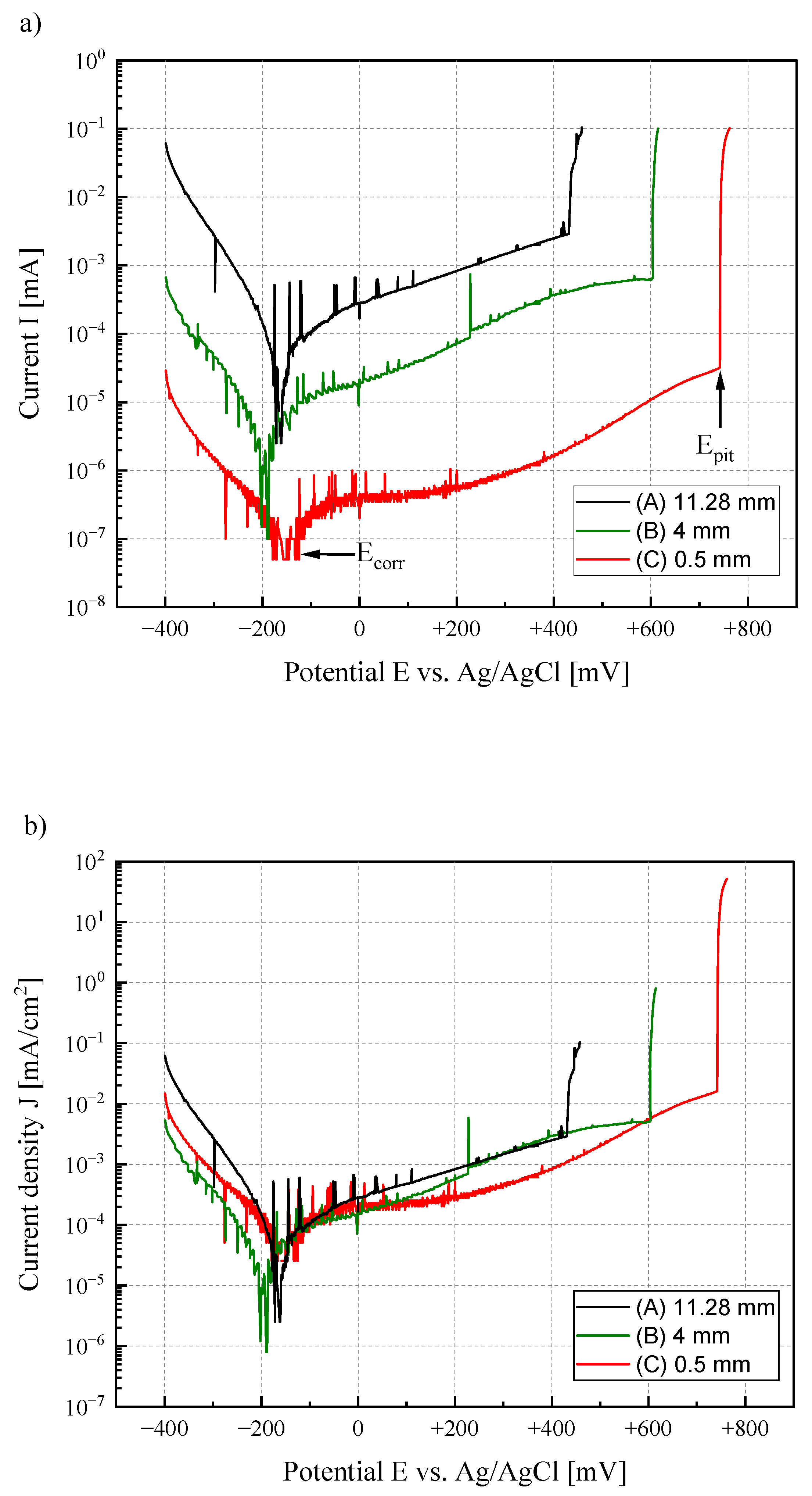
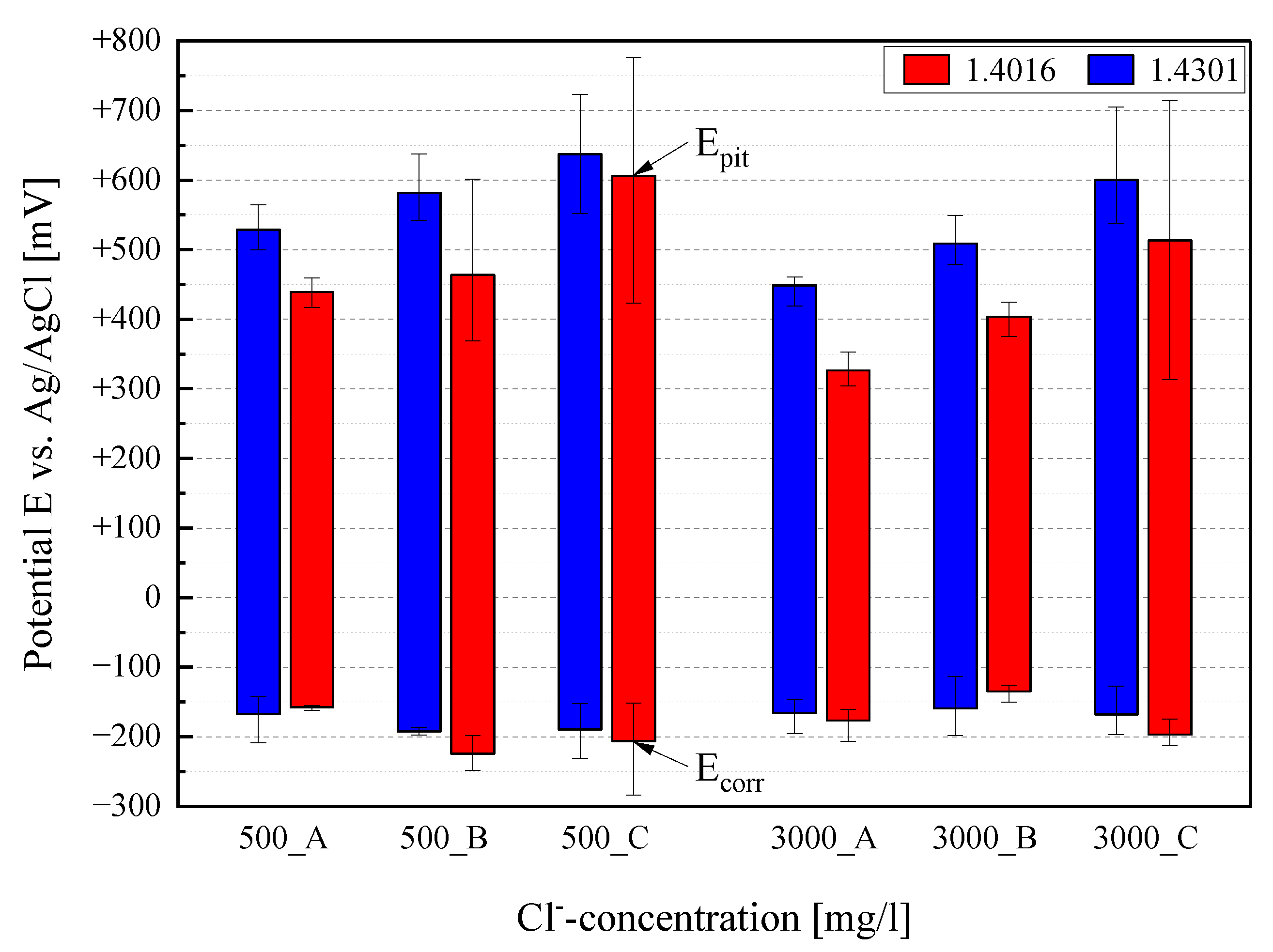
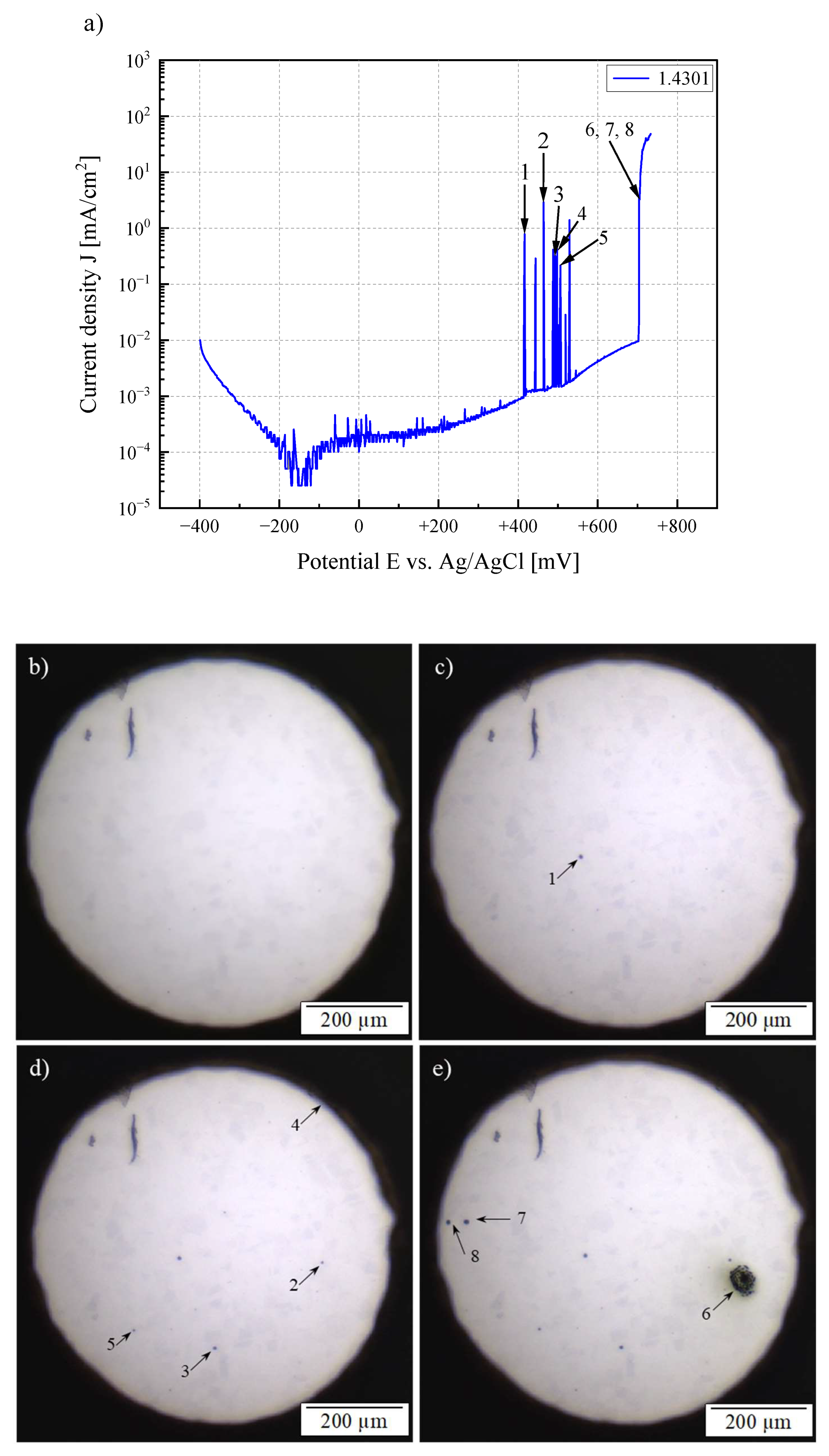
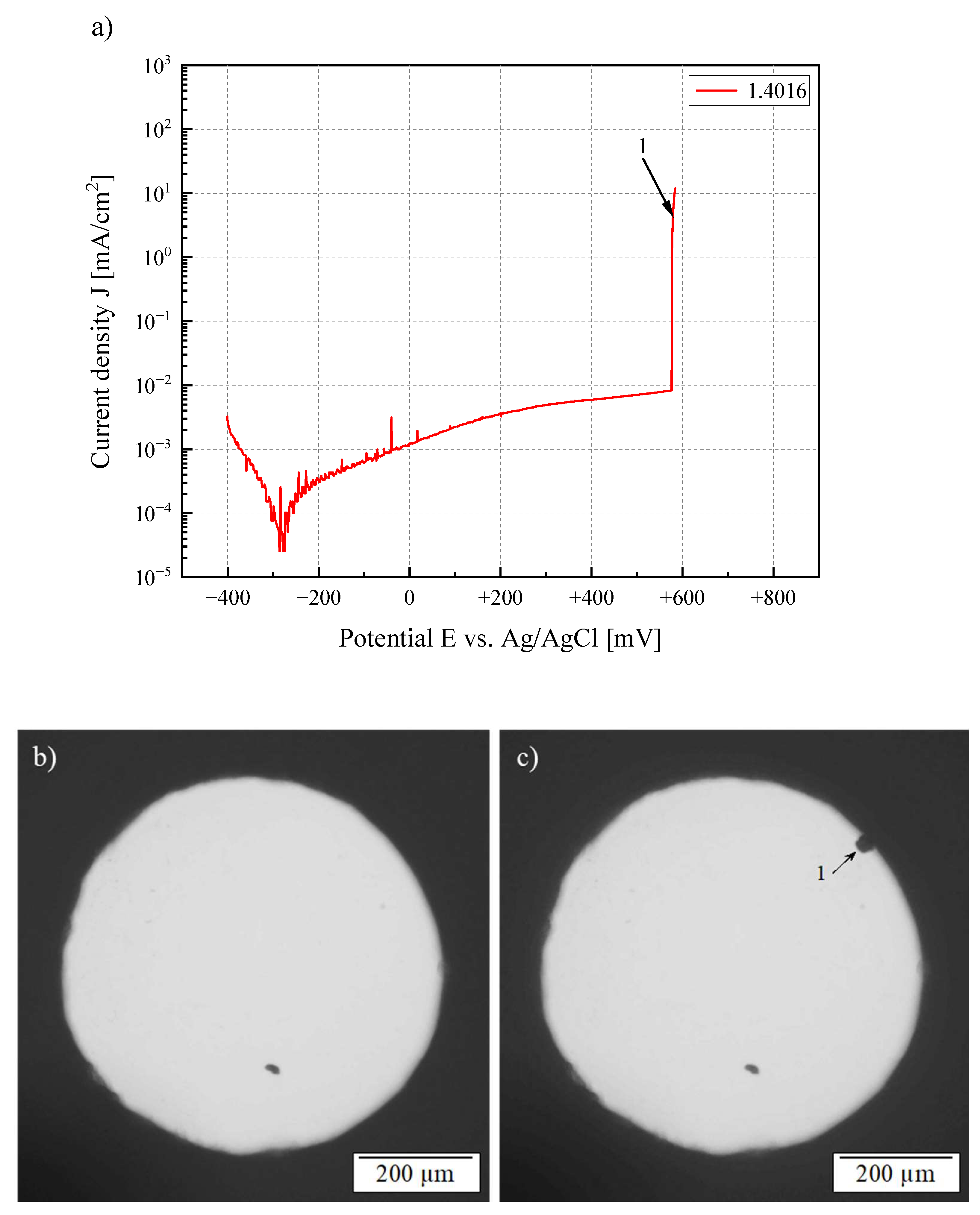
3.1. Electrochemical Investigation
3.2. In Situ Microscopic Analysis
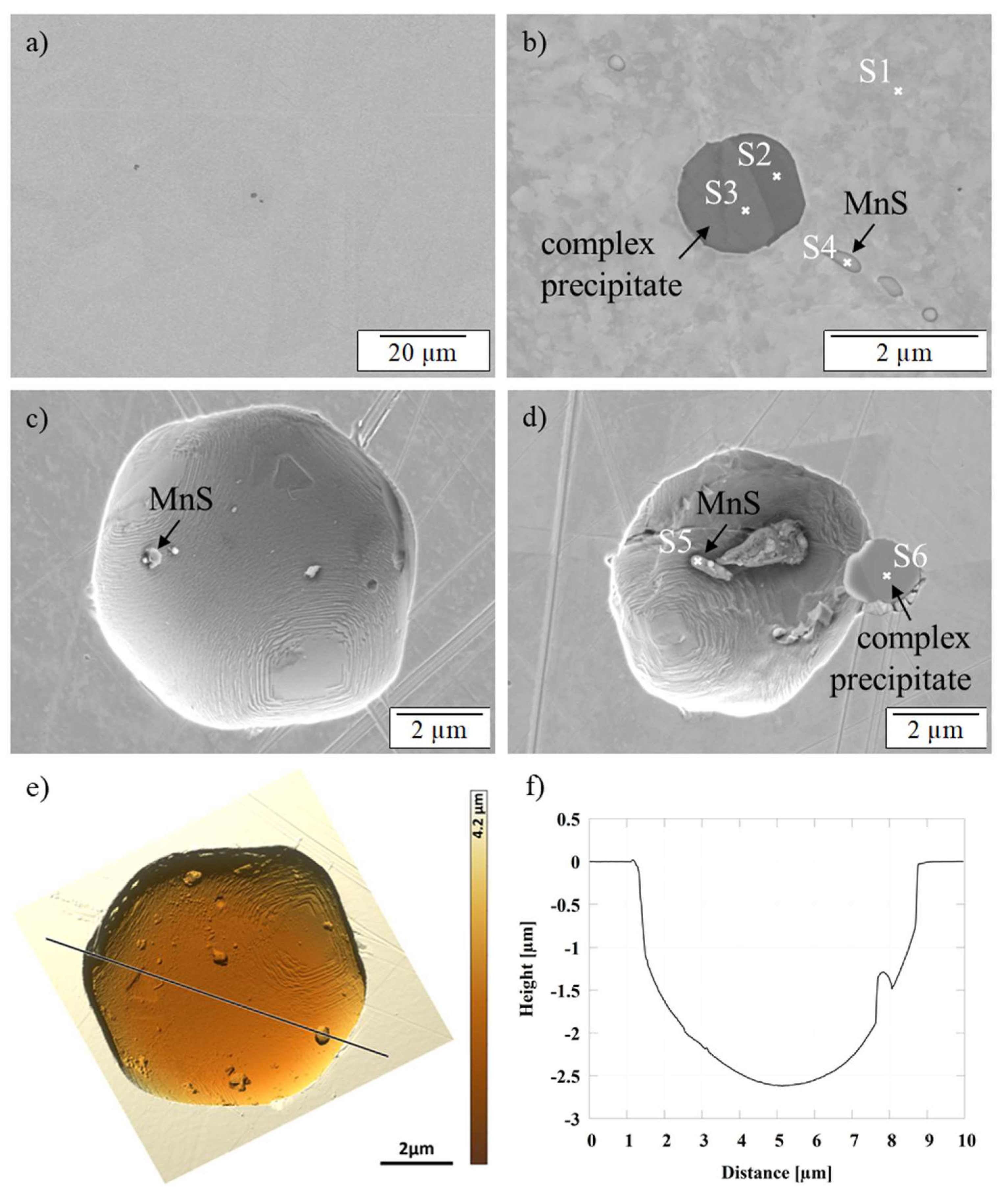
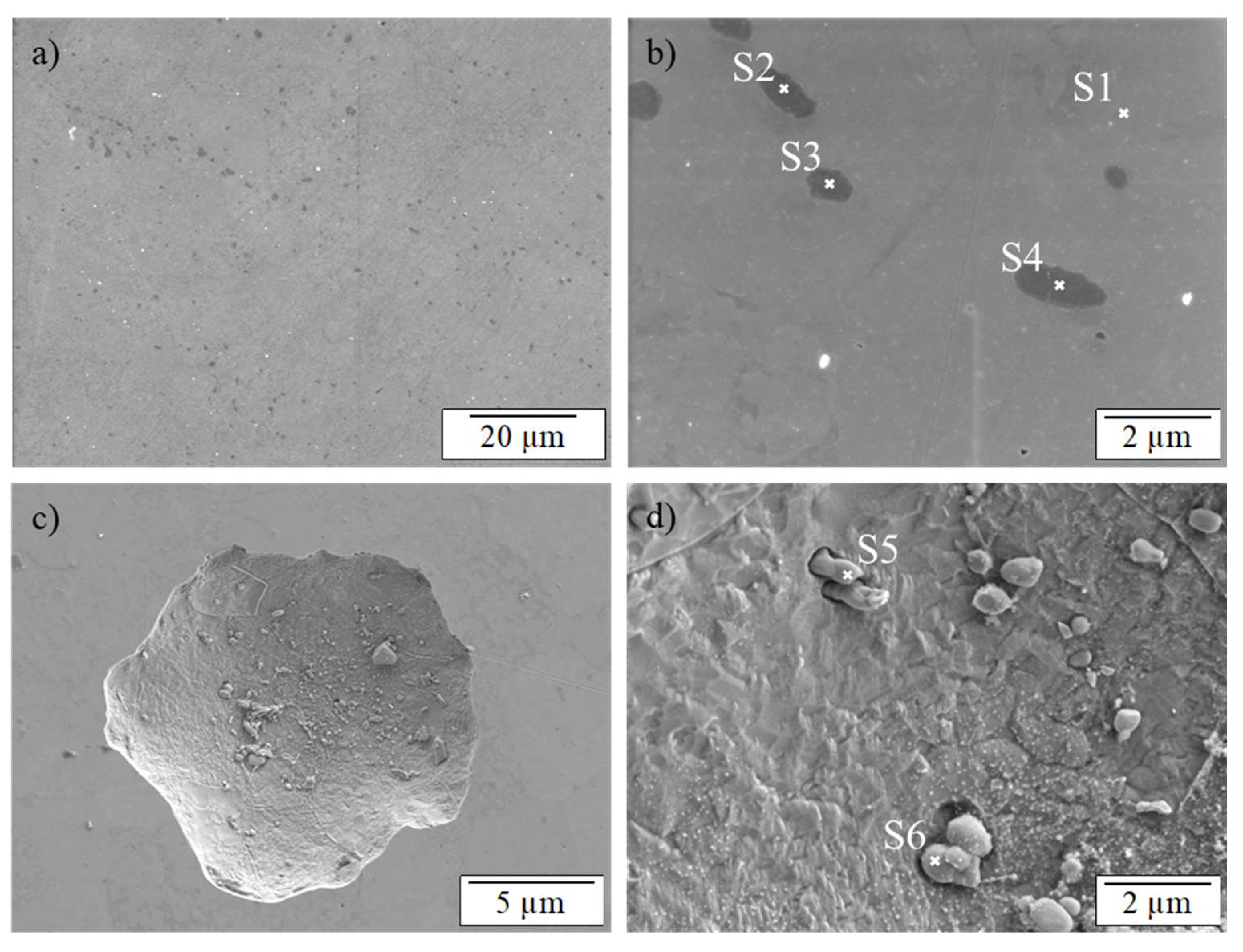
3.3. SEM/EDS and AFM Investigations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kumar, A.M.; Adesina, A.Y.; Veeramani, J.; Rahman, M.M.; Nirmal Ram, J.S. Hybrid Polyurethane/Polypyrrole Composite Coatings on Passivated 316L SS for Surface Protective Action against Corrosion in Saline Medium. Corros. Mater. Degrad. 2022, 3, 612–627. [Google Scholar] [CrossRef]
- DIN EN 10088-1; Stainless Steels—Part 1: List of Stainless Steels; German Version. European Committee for Standardization: Brüssel, Belgium, 2014.
- Loto, R.T.; Mater, J. Pitting corrosion evaluation of austenitic stainless steel type 304 in acid chloride media. J. Mater. Environ. Sci. 2013, 4, 448–459. [Google Scholar]
- Frankenthal, P.; Kruger, J. Passivity of Metals. In Proceedings of the 4th International Symposium on Passivity, The Electrochemical Society, Airlie House Conference Center, Warrenton, VA, USA, 17–21 October 1977. [Google Scholar]
- Elsener, B.; Fantauzzi, M.; Rossi, A. Stainless steels: Passive film composition, pitting potentials, and critical chloride content in concrete. Mater. Corros. 2020, 71, 797–807. [Google Scholar] [CrossRef]
- McBee, C.L.; Kruger, J. Nature of passive films on iron-chromium alloys. Electrochim. Acta 1972, 17, 1337–1341. [Google Scholar] [CrossRef]
- Akpanyung, K.V.; Loto, R.T. Pitting corrosion evaluation: A review. J. Phys. Conf. Ser. 2019, 1378, 022088. [Google Scholar] [CrossRef]
- Bohni, H. Localized corrosion of passive metals. In Uhlig’s Corrosion Handbook, 3rd ed.; Winston Revie, R., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 157–169. [Google Scholar]
- Brownlie, F.; Hodgkiess, T.; Pearson, A.; Galloway, A. Electrochemical Evaluation of the Effect of Different NaCl Concentrations on Low Alloy- and Stainless Steels under Corrosion and Erosion-Corrosion Conditions. Corros. Mater. Degrad. 2022, 3, 101–126. [Google Scholar] [CrossRef]
- Williams, D.E.; Zhu, Y.Y. Explanation for Initiation of Pitting Corrosion of Stainless Steels at Sulfide Inclusions. J. Electrochem. Soc. 2000, 147, 1763–1766. [Google Scholar] [CrossRef]
- Örnek, C.; Davut, K.; Kocabas, M.; Bayatlı, A.; Ürgen, M. Understanding Corrosion Morphology of Duplex Stainless Steel Wire in Chloride Electrolyte. Corros. Mater. Degrad. 2021, 2, 397–411. [Google Scholar] [CrossRef]
- Soltis, J. Passivity breakdown, pit initiation and propagation of pits in metallic materials—Review. Corros. Sci. 2015, 90, 5–22. [Google Scholar] [CrossRef]
- Strehblow, H.H. Nucleation and Repassivation of Corrosion Pits for Pitting on Iron and Nickel. Mater. Corros 1976, 27, 792–799. [Google Scholar] [CrossRef]
- Suter, T.; Böhni, H. Microelectrodes for corrosion studies in microsystems. Electrochim. Acta 2001, 47, 191–199. [Google Scholar] [CrossRef]
- Muto, I.; Kurokawa, S.; Hara, N. Microelectrochemical Investigation of Anodic Polarization Behavior of CrS Inclusions in Stainless Steels. J. Electrochem. Soc. 2009, 156, C395–C399. [Google Scholar] [CrossRef]
- Webb, E.G.; Alkire, R.C. Pit Initiation at Single Sulfide Inclusions in Stainless Steel: I. Electrochemical Microcell Measurements. J. Electrochem. Soc. 2002, 149, B272–B279. [Google Scholar] [CrossRef]
- Stewart, J.; Williams, D.E. The initiation of pitting corrosion on austenitic stainless steel: On the role and importance of sulphide inclusions. Corros. Sci. 1992, 33, 457–474. [Google Scholar] [CrossRef]
- Streicher, M.A.; Grubb, J.F. Austenitic and Ferritic Stainless Steels. In Uhlig’s Corrosion Handbook, 3rd ed.; Winston Revie, R., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 657–693. [Google Scholar]
- Xie, Y.; Ju, Y.; Toku, Y.; Morita, Y. Fabrication of Fe2O3 nanowire arrays based on oxidation-assisted stress-induced atomic-diffusion and their photovoltaic properties for solar water splitting. RSC Adv. 2017, 7, 30548–30553. [Google Scholar] [CrossRef]
- Nečas, D.; Klapetek, P. Gwyddion: An open-source software for SPM data analysis. Cent. Eur. J. Phys. 2012, 10, 181–188. [Google Scholar] [CrossRef]
- Ida, N.; Muto, I.; Sugawara, Y.; Hara, N. Local Electrochemistry and In Situ Microscopy of Pitting at Sensitized Grain Boundary of Type 304 Stainless Steel in NaCl Solution. J. Electrochem. Soc. 2017, 164, C779–C787. [Google Scholar] [CrossRef]
- Kiremit, S.; Stallmeier, Y.; Klöcker, M.; Kordisch, T. Analysis of the corrosion behavior of stainless steel in aqueous electrolyte with different compositions. In Proceedings of the Tagung Werkstoffprüfung 2020, Online, 3–4 December 2020. [Google Scholar]
- Ibrahim, M.A.M.; Abd El Rehim, S.S.; Hamza, M.M. Corrosion behavior of some austenitic stainless steels in chloride environments. Mater. Chem. Phys. 2009, 115, 80–85. [Google Scholar] [CrossRef]
- Malik, A.U.; Mayan Kutty, P.C.; Siddiqi, N.A.; Andijani, I.N.; Ahmed, S. The influence of pH and chloride concentration on the corrosion behaviour of AISI 316L steel in aqueous solutions. Corros. Sci. 1992, 33, 1809–1827. [Google Scholar] [CrossRef]
- Tokuda, S.; Muto, I.; Sugawara, Y.; Hara, N. Pit initiation on sensitized Type 304 stainless steel under applied stress: Correlation of stress, Cr-depletion, and inclusion dissolution. Corros. Sci. 2020, 167, 108506. [Google Scholar] [CrossRef]
- Lillard, R.S.; Kashfipour, M.A.; Niu, W. Pit Propagation at the Boundary between Manganese Sulfide Inclusions and Austenitic Stainless Steel 303 and the Role of Copper. J. Electrochem. Soc. 2016, 163, C440–C451. [Google Scholar] [CrossRef]
- Sourisseau, T.; Chauveau, E.; Baroux, B. Mechanism of copper action on pitting phenomena observed on stainless steels in chloride media. Corros. Sci. 2005, 47, 1097–1117. [Google Scholar] [CrossRef]
- Radojković, B.; Kovačina, J.; Jegdić, B.; Bobić, B.; Alić, B.; Marunkić, D.; Simović, A. Influence of inhibitors on the corrosion behavior of welded joint X5CrNi18 10 stainless steel. Mater. Corros. 2021, 72, 694–707. [Google Scholar] [CrossRef]
- Sumita, M.; Hanawa, T.; Teoh, S.H. Development of nitrogen-containing nickel-free austenitic stainless steels for metallic biomaterials—Review. Mater. Sci. Eng. C 2004, 24, 753–760. [Google Scholar] [CrossRef]
| Material | C | Si | Mn | P | S | Cr | Mo | Ni | Cu | Ti | N |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1.4301 | 0.0457 | 0.4494 | 1.53 | 0.0438 | 0.0016 | 17.77 | 0.3353 | 9.16 | 0.3143 | 0.0105 | 0.0555 |
| 1.4016 | 0.0611 | 0.4239 | 0.3248 | 0.0309 | <0.0010 | 15.96 | 0.0572 | 0.4593 | 0.1528 | 0.0047 | 0.0458 |
| Material | Tafel-Line Values | 500 mg/L Cl− | 3000 mg/L Cl− | ||||
|---|---|---|---|---|---|---|---|
| 11.28 mm | 4 mm | 0.5 mm | 11.28 mm | 4 mm | 0.5 mm | ||
| 1.4301 | Ecorr [mV] | −167 | −192 | −190 | −166 | −159 | −168 |
| Jcorr [mA/cm2] | 1.91 × 10−4 | 1.45 × 10−5 | 4.68 × 10−5 | 1.28 × 10−4 | 1.34 × 10−5 | 3.93 × 10−5 | |
| 1.4016 | Ecorr [mV] | −158 | −224 | −206 | −177 | −135 | −197 |
| Jcorr [mA/cm2] | 7.30 × 10−5 | 2.24 × 10−5 | 5.08 × 10−5 | 9.40 × 10−5 | 1.19 × 10−5 | 4.81 × 10−5 | |
| Spectrum | Al | C | Cr | Cu | Fe | Mn | Ni | O | S | Ti |
|---|---|---|---|---|---|---|---|---|---|---|
| S1 | 0 | 6 | 17 | 0 | 63 | 2 | 11 | 0 | 0 | 0 |
| S2 | 15 | 3 | 12 | 0 | 12 | 25 | 0 | 27 | 0 | 6 |
| S3 | 2 | 2 | 6 | 0 | 5 | 8 | 0 | 33 | 0 | 44 |
| S4 | 0 | 5 | 13 | 13 | 38 | 17 | 4 | 0 | 9 | 0 |
| S5 | 0 | 5 | 11 | 36 | 30 | 5 | 4 | 0 | 9 | 0 |
| S6 | 1 | 2 | 8 | 5 | 7 | 6 | 0 | 28 | 1 | 39 |
| Spectrum | C | Cr | Fe | N |
|---|---|---|---|---|
| S1 | 2 | 19 | 79 | 0 |
| S2 | 14 | 71 | 7 | 8 |
| S3 | 11 | 64 | 17 | 7 |
| S4 | 10 | 81 | 0 | 9 |
| S5 | 4 | 42 | 45 | 6 |
| S6 | 7 | 52 | 39 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiremit, S.; Cremer, J.; Stallmeier, Y.; Sonntag, A.; Klöcker, M.; Anselmetti, D.; Hütten, A.; Kordisch, T. Development of an In Situ Micro-Corrosion Cell for the Investigation of Pitting Corrosion on Austenitic and Ferritic Stainless Steels. Corros. Mater. Degrad. 2023, 4, 104-119. https://doi.org/10.3390/cmd4010007
Kiremit S, Cremer J, Stallmeier Y, Sonntag A, Klöcker M, Anselmetti D, Hütten A, Kordisch T. Development of an In Situ Micro-Corrosion Cell for the Investigation of Pitting Corrosion on Austenitic and Ferritic Stainless Steels. Corrosion and Materials Degradation. 2023; 4(1):104-119. https://doi.org/10.3390/cmd4010007
Chicago/Turabian StyleKiremit, Sinan, Julian Cremer, Yannic Stallmeier, Adrian Sonntag, Michaela Klöcker, Dario Anselmetti, Andreas Hütten, and Thomas Kordisch. 2023. "Development of an In Situ Micro-Corrosion Cell for the Investigation of Pitting Corrosion on Austenitic and Ferritic Stainless Steels" Corrosion and Materials Degradation 4, no. 1: 104-119. https://doi.org/10.3390/cmd4010007
APA StyleKiremit, S., Cremer, J., Stallmeier, Y., Sonntag, A., Klöcker, M., Anselmetti, D., Hütten, A., & Kordisch, T. (2023). Development of an In Situ Micro-Corrosion Cell for the Investigation of Pitting Corrosion on Austenitic and Ferritic Stainless Steels. Corrosion and Materials Degradation, 4(1), 104-119. https://doi.org/10.3390/cmd4010007







